Sustainable Agriculture through the Enhancement of Microbial Biocontrol Agents: Current Challenges and New Perspectives
Abstract
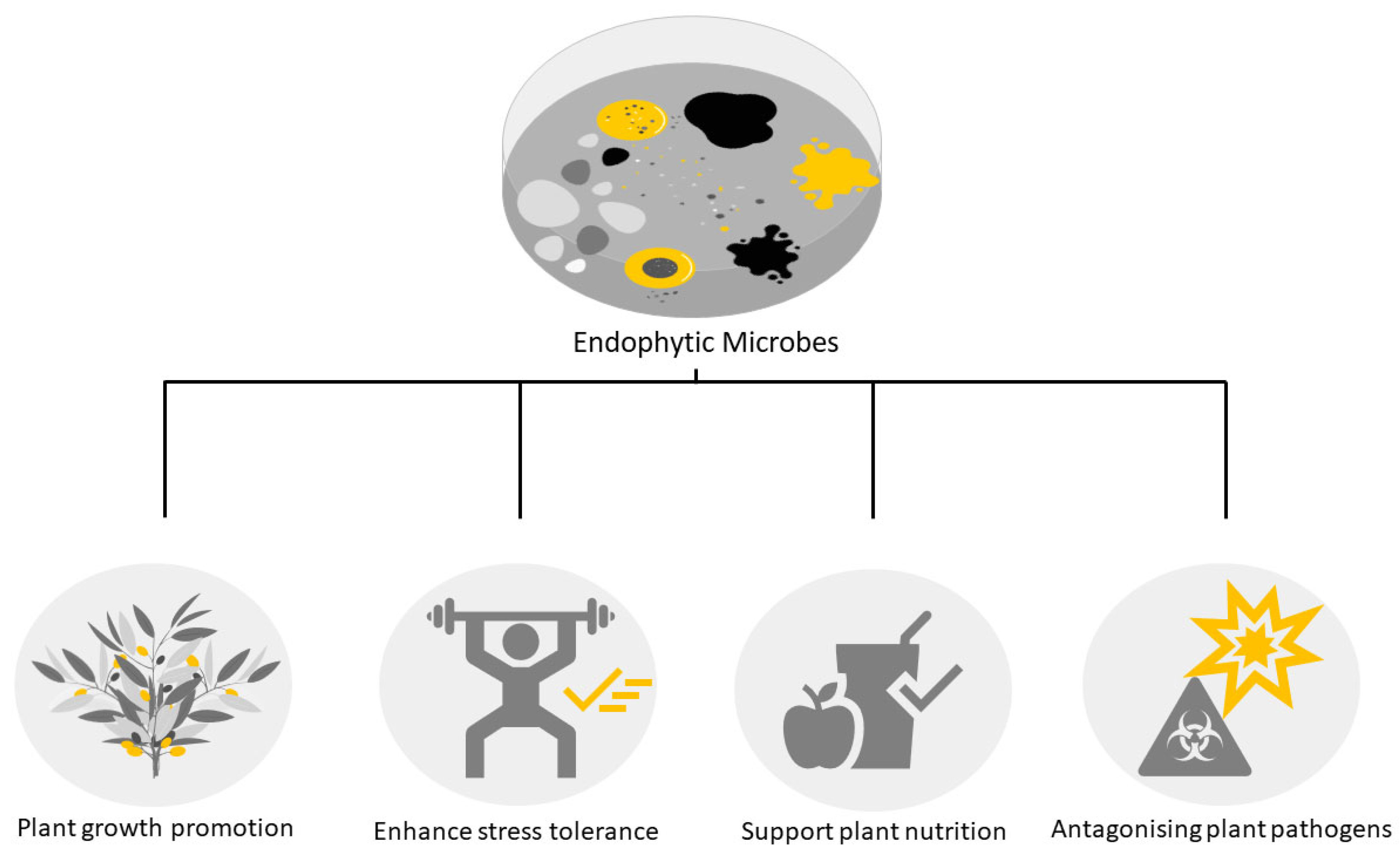
1. Introduction
2. Challenges Associated with Isolating Microbial Biocontrols
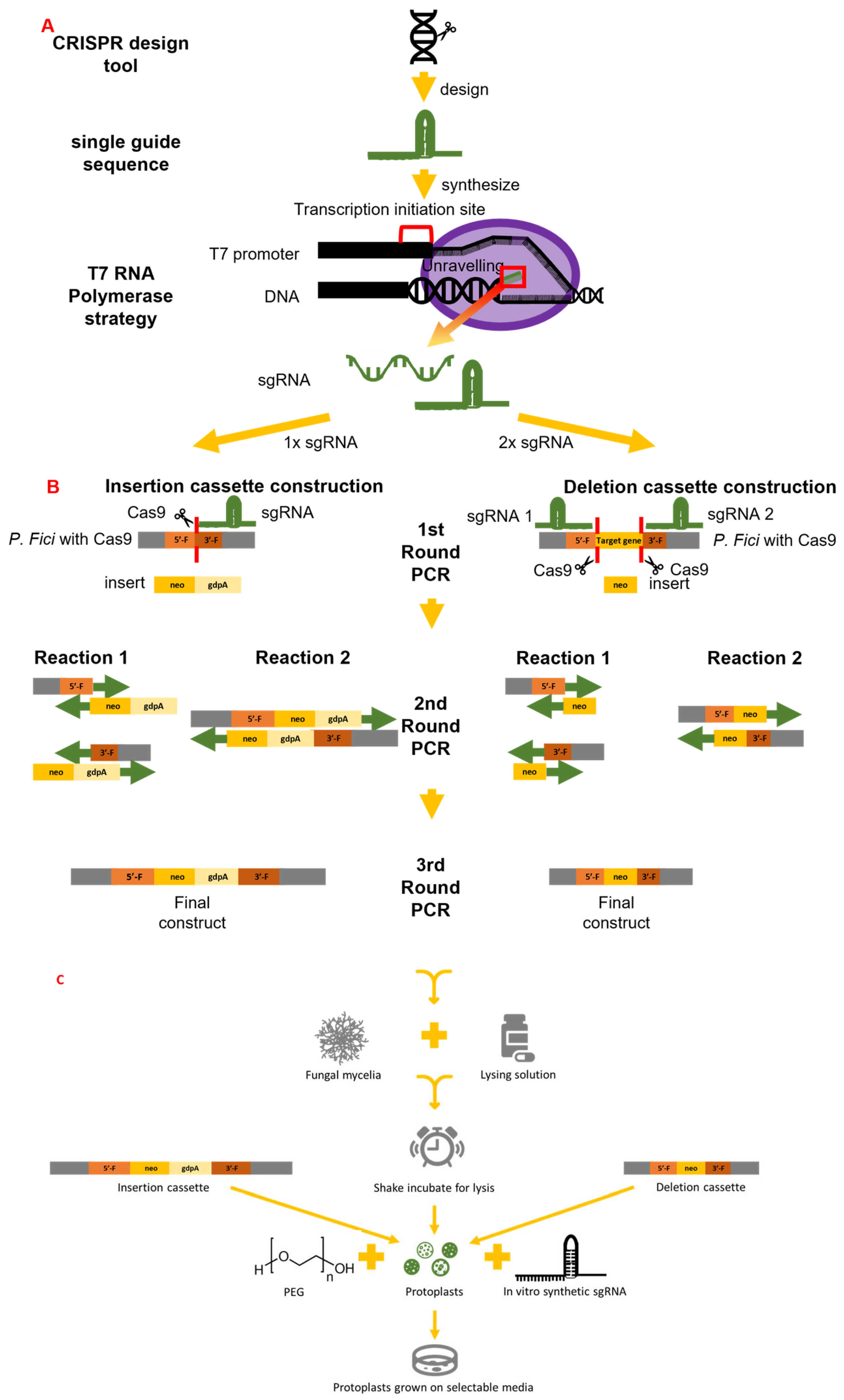
3. The Use of the CRISPR/Cas9 System in Endophytic Research for Enhanced Biocontrol or Improved Plant Growth
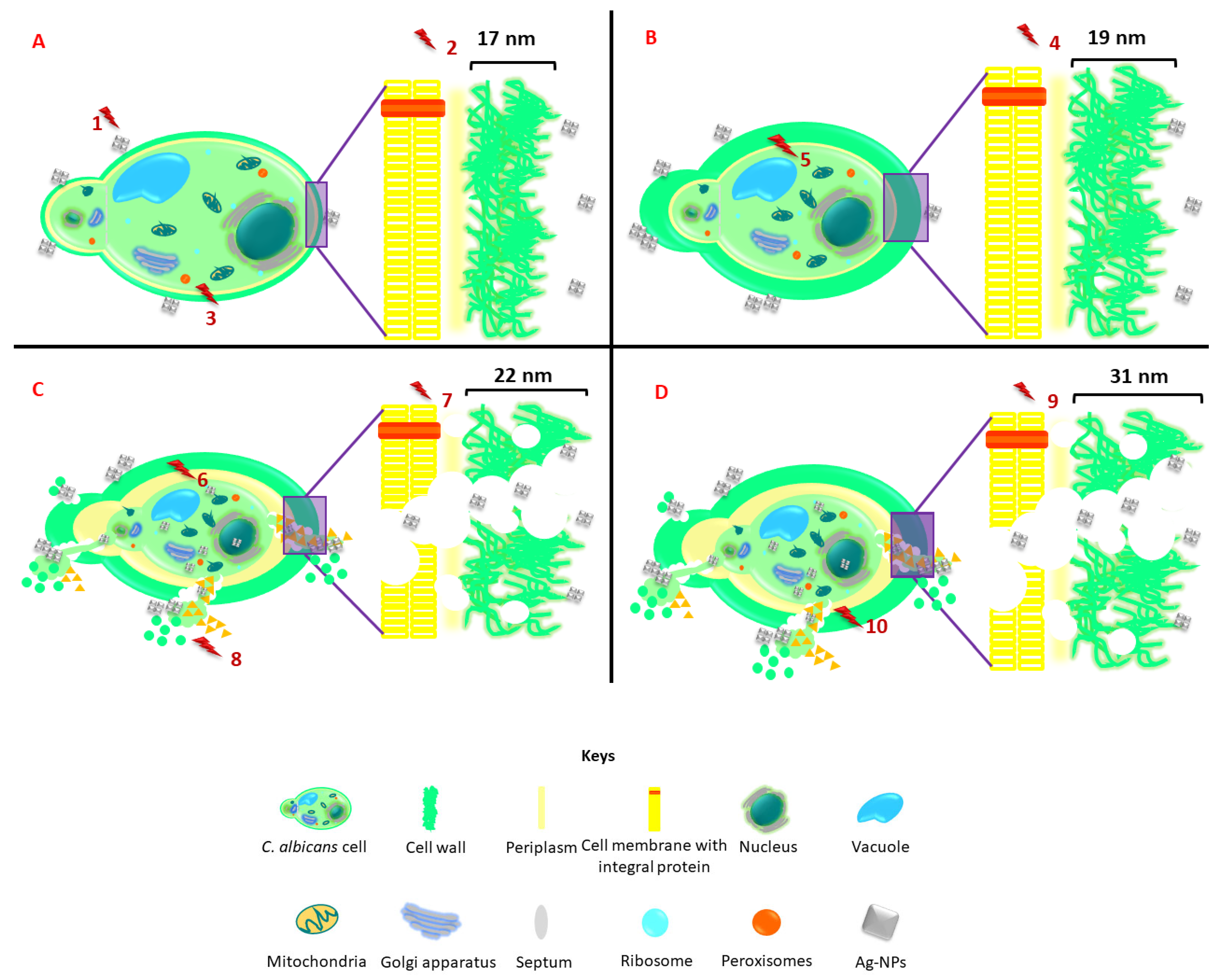
4. The Use of Nanotechnology for the Improvement of Endophytic Biocontrol Agents
5. Integrating Multi-Omics Approaches for Studying Endophytic Research and Improving Biocontrol Activity
6. Screening Techniques for Identifying Secondary Metabolites Associated with Endophytic Microbes and Biocontrol Activity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gossen, B.D.; McDonald, M.R. New technologies could enhance natural biological control and disease management and reduce reliance on synthetic pesticides. Can. J. Plant Pathol. 2020, 42, 30–40. [Google Scholar] [CrossRef]
- Tosi, M.; Gaiero, J.; Linton, N.; Mafa-Attoye, T.; Castillo, A.; Dunfield, K. Bacterial Endophytes: Diversity, Functional Importance, and Potential for Manipulation. In Rhizosphere Biology Interacteractions Between Microbes Plants; Springer: Singapore, 2021; pp. 1–49. [Google Scholar] [CrossRef]
- Chen, Y.; Hu, B.; Xing, J.; Li, C. Endophytes: The novel sources for plant terpenoid biosynthesis. Appl. Microbiol. Biotechnol. 2021, 105, 4501–4513. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, X.; Zhang, W.; Lang, D.; Zhang, X.; Cui, G.; Zhang, X. Interactions between Endophytes and Plants: Beneficial Effect of Endophytes to Ameliorate Biotic and Abiotic Stresses in Plants. J. Plant Biol. 2019, 62, 1–13. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A Treasure House of Bioactive Compounds of Medicinal Importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef]
- Xia, Y.; Liu, J.; Chen, C.; Mo, X.; Tan, Q.; He, Y.; Wang, Z.; Yin, J.; Zhou, G. The Multifunctions and Future Prospects of Endophytes and Their Metabolites in Plant Disease Management. Microorganisms 2022, 10, 1072. [Google Scholar] [CrossRef]
- Raymaekers, K.; Ponet, L.; Holtappels, D.; Berckmans, B.; Cammue, B.P.A. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control 2020, 144, 104240. [Google Scholar] [CrossRef]
- Jiao, X.; Takishita, Y.; Zhou, G.; Smith, D.L. Plant Associated Rhizobacteria for Biocontrol and Plant Growth Enhancement. Front. Plant Sci. 2021, 12, 17. [Google Scholar] [CrossRef]
- Pereyra, M.M.; Díaz, M.A.; Soliz-Santander, F.F.; Poehlein, A.; Meinhardt, F.; Daniel, R.; Dib, J.R. Screening Methods for Isolation of Biocontrol Epiphytic Yeasts against Penicillium digitatum in Lemons. J. Fungi 2021, 7, 166. [Google Scholar] [CrossRef]
- Moral, J.; Garcia-Lopez, M.T.; Camiletti, B.X.; Jaime, R.; Michailides, T.J.; Bandyopadhyay, R.; Ortega-Beltran, A. Present Status and Perspective on the Future Use of Aflatoxin Biocontrol Products. Agronomy 2020, 10, 491. [Google Scholar] [CrossRef]
- Nešić, K.; Habschied, K.; Mastanjević, K. Possibilities for the Biological Control of Mycotoxins in Food and Feed. Toxins 2021, 13, 198. [Google Scholar] [CrossRef]
- Gouveia, D.; Pible, O.; Culotta, K.; Jouffret, V.; Geffard, O.; Chaumot, A.; Degli-Esposti, D.; Armengaud, J. Combining proteogenomics and metaproteomics for deep taxonomic and functional characterization of microbiomes from a non-sequenced host. NPJ Biofilms Microbiomes 2020, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Sadiq, M.B. Lactic Acid Bacteria as Potential Probiotics. In Probiotics, Prebiotics and Synbiotics: Technological Advancement towards Safety and Industrial Applications, 1st ed.; Wiley: Hoboken, NJ, USA, 2022; pp. 57–72. [Google Scholar] [CrossRef]
- Ahmad, S.Z.N.; Wan Salleh, W.N.; Ismail, A.F.; Yusof, N.; Mohd Yusop, M.Z.; Aziz, F. Adsorptive removal of heavy metal ions using graphene-based nanomaterials: Toxicity, roles of functional groups and mechanisms. Chemosphere 2020, 248, 126008. [Google Scholar] [CrossRef] [PubMed]
- Afshar, P.; Shokrzadeh, M.; Raeisi, S.N.; Ghorbani-HasanSaraei, A.; Nasiraii, L.R. Aflatoxins biodetoxification strategies based on probiotic bacteria. Toxicon 2020, 178, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, B.; Zhang, Z.; Chen, Y.; Tian, S. Antagonistic Yeasts: A Promising Alternative to Chemical Fungicides for Controlling Postharvest Decay of Fruit. J. Fungi 2020, 6, 158. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Wang, W.; Yuan, Z.; Sederoff, R.R.; Sederoff, H.; Chiang, V.L.; Borriss, R. Microbial Interactions Within Multiple-Strain Biological Control Agents Impact Soil-Borne Plant Disease. Front. Microbiol. 2020, 11, 585404. [Google Scholar] [CrossRef]
- Vedamurthy, A.B.; Varsha, S.L.; Shruthi, S.D. Regulatory requirement for commercialization of biocontrol agents. In Biocontrol Agents and Secondary Metablites, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 659–675. [Google Scholar] [CrossRef]
- Colombo, M.; Masiero, S.; Rosa, S.; Caporali, E.; Toffolatti, S.L.; Mizzotti, C.; Tadini, L.; Rossi, F.; Pellegrino, S.; Musetti, R.; et al. NoPv1: A synthetic antimicrobial peptide aptamer targeting the causal agents of grapevine downy mildew and potato late blight. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Leneveu-Jenvrin, C.; Charles, F.; Barba, F.J.; Remize, F. Role of biological control agents and physical treatments in maintaining the quality of fresh and minimally-processed fruit and vegetables. Crit. Rev. Food Sci. Nutr. 2020, 60, 2837–2855. [Google Scholar] [CrossRef]
- Rodríguez-Rodríguez, D.R.; Ramírez-Solís, R.; Garza-Elizondo, M.A.; Garza-Rodríguez, M.D.L.; Barrera-Saldaña, H.A. Genome editing: A perspective on the application of CRISPR/Cas9 to study human diseases (Review). Int. J. Mol. Med. 2019, 43, 1559–1574. [Google Scholar] [CrossRef]
- Chen, K.; Wang, Y.; Zhang, R.; Zhang, H.; Gao, C. CRISPR/Cas Genome Editing and Precision Plant Breeding in Agriculture. Annu. Rev. Plant Biol. 2019, 70, 667–697. [Google Scholar] [CrossRef]
- Naeem, M.; Majeed, S.; Hoque, M.Z.; Ahmad, I. Latest Developed Strategies to Minimize the Off-Target Effects in CRISPR-Cas-Mediated Genome Editing. Cells 2020, 9, 1608. [Google Scholar] [CrossRef]
- Etminani, F.; Harighi, B. Isolation and Identification of Endophytic Bacteria with Plant Growth Promoting Activity and Biocontrol Potential from Wild Pistachio Trees. Plant Pathol. J. 2018, 34, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Hereme, R.; Morales-Navarro, S.; Ballesteros, G.; Barrera, A.; Ramos, P.; Gundel, P.E.; Molina-Montenegro, M.A. Fungal Endophytes Exert Positive Effects on Colobanthus quitensis Under Water Stress but Neutral Under a Projected Climate Change Scenario in Antarctica. Front. Microbiol. 2020, 11, 264. [Google Scholar] [CrossRef]
- Huang, P.W.; Yang, Q.; Zhu, Y.L.; Zhou, J.; Sun, K.; Mei, Y.Z.; Dai, C.C. The construction of CRISPR-Cas9 system for endophytic Phomopsis liquidambaris and its PmkkA-deficient mutant revealing the effect on rice. Fungal Genet. Biol. 2020, 136, 103301. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Huang, R.; Yin, W.B. An optimized and efficient crispr/cas9 system for the endophytic fungus Pestalotiopsis fici. J. Fungi 2021, 7, 809. [Google Scholar] [CrossRef] [PubMed]
- Chowdhary, K.; Arora, H.; Sharma, S. CRISPR/Cas9-Based Genome Editing as a Way Ahead for Inducing Production of Bioactive Metabolites in Endophytes. Natl. Acad. Sci. Lett. 2022, 45, 275–280. [Google Scholar] [CrossRef]
- Zhu, Y.L.; Zhang, M.Q.; Wang, L.S.; Mei, Y.Z.; Dai, C.C. Overexpression of chitinase in the endophyte Phomopsis liquidambaris enhances wheat resistance to Fusarium graminearum. Fungal Genet. Biol. 2022, 158, 103650. [Google Scholar] [CrossRef]
- Raimi, A.; Adeleke, R. Bioprospecting of endophytic microorganisms for bioactive compounds of therapeutic importance. Arch. Microbiol. 2021, 203, 1917–1942. [Google Scholar] [CrossRef]
- Pramanik, P.; Krishnan, P.; Maity, A.; Mridha, N.; Mukherjee, A.; Rai, V. Application of Nanotechnology in Agriculture. Environ. Nanotechnol. 2020, 4, 317–348. [Google Scholar] [CrossRef]
- Meena, M.; Zehra, A.; Swapnil, P.; Marwal, A.; Yadav, G.; Sonigra, P.; Ghosh, S.; Patil, P.; Keswani, C. Endophytic Nanotechnology: An Approach to Study Scope and Potential Applications. Front. Chem. 2021, 9, 25. [Google Scholar] [CrossRef]
- Elbahnasawy, M.A.; Shehabeldine, A.M.; Khattab, A.M.; Amin, B.H.; Hashem, A.H. Green biosynthesis of silver nanoparticles using novel endophytic Rothia endophytica: Characterization and anticandidal activity. J. Drug Deliv. Sci. Technol. 2021, 62, 102401. [Google Scholar] [CrossRef]
- Elmer, W.; White, J.C. The Future of Nanotechnology in Plant Pathology. Annu. Rev. Phytopathol. 2018, 56, 111–133. [Google Scholar] [CrossRef]
- Zhao, H.; Maruthupandy, M.; Al-mekhlafi, F.A.; Chackaravarthi, G.; Ramachandran, G.; Chelliah, C.K. Biological synthesis of copper oxide nanoparticles using marine endophytic actinomycetes and evaluation of biofilm producing bacteria and A549 lung cancer cells. J. King Saud Univ. Sci. 2022, 34, 101866. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Siddiqui, M.I. Anticandidal activity of biosynthesized silver nanoparticles: Effect on growth, cell morphology, and key virulence attributes of Candida species. Int. J. Nanomed. 2019, 14, 4667–4679. [Google Scholar] [CrossRef] [PubMed]
- Mosquera-Sánchez, L.P.; Arciniegas-Grijalba, P.A.; Patiño-Portela, M.C.; Guerra-Sierra, B.E.; Muñoz-Florez, J.E.; Rodríguez-Páez, J.E. Antifungal effect of zinc oxide nanoparticles (ZnO-NPs) on Colletotrichum sp., causal agent of anthracnose in coffee crops. Biocatal. Agric. Biotechnol. 2020, 25, 101579. [Google Scholar] [CrossRef]
- Abdelaziz, A.M.; Salem, S.S.; Khalil, A.M.A.; El, D.A.; Fouda, H.M.; Hashem, A.H. Potential of biosynthesized zinc oxide nanoparticles to control Fusarium wilt disease in eggplant (Solanum melongena) and promote plant growth. BioMetals 2022, 35, 601–616. [Google Scholar] [CrossRef]
- Fouda, A.; Hassan, E.-D.; Abdo, A.M.; El-Gamal, M.S. Antimicrobial, Antioxidant and Larvicidal Activities of Spherical Silver Nanoparticles Synthesized by Endophytic Streptomyces spp. Biol. Trace Elem. Res. 2011, 195, 707–724. [Google Scholar] [CrossRef] [PubMed]
- Srinivasa, C.; Mellappa, G.; Patil, S.M.; Ramu, R.; Shreevatsa, B.; Dharmashekar, C.; Kollur, S.P.; Syed, A.; Shivamallu, C. Plants and endophytes—A partnership for the coumarin production through the microbial systems. Mycology 2022, 13, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Khare, E.; Mishra, J.; Arora, N.K. Multifaceted Interactions Between Endophytes and Plant: Developments and Prospects. Front. Microbiol. 2018, 9, 15. [Google Scholar] [CrossRef]
- Howitz, K.T.; Sinclair, D.A. Xenohormesis: Sensing the chemical cues of other species. Cell 2008, 133, 387–391. [Google Scholar] [CrossRef]
- Pitakbut, T.; Spiteller, M.; Kayser, O. Genome Mining and Gene Expression Reveal Maytansine Biosynthetic Genes from Endophytic Communities Living inside Gymnosporia heterophylla (Eckl. and Zeyh.) Loes. and the Relationship with the Plant Biosynthetic Gene, Friedelin Synthase. Plants 2022, 11, 321. [Google Scholar] [CrossRef]
- Zou, K.; Liu, X.; Hu, Q.; Zhang, D.; Fu, S.; Zhang, S.; Huang, H.; Lei, F.; Zhang, G.; Miao, B.; et al. Root Endophytes and Ginkgo biloba Are Likely to Share and Compensate Secondary Metabolic Processes, and Potentially Exchange Genetic Information by LTR-RTs. Front. Plant Sci. 2021, 12, 1370. [Google Scholar] [CrossRef]
- Adeleke, B.S.; Babalola, O.O. Roles of Plant Endosphere Microbes in Agriculture—A Review. J. Plant Growth Regul. 2021, 41, 1411–1428. [Google Scholar] [CrossRef]
- Hasin, Y.; Seldin, M.; Lusis, A. Multi-omics approaches to disease. Genome Biol. 2017, 18, 1–15. [Google Scholar] [CrossRef]
- Manzoni, C.; Kia, D.A.; Vandrovcova, J.; Hardy, J.; Wood, N.W.; Lewis, P.A.; Ferrari, R. Genome, transcriptome and proteome: The rise of omics data and their integration in biomedical sciences. Brief. Bioinform. 2018, 19, 286. [Google Scholar] [CrossRef] [PubMed]
- Roy Choudhury, A.; Roy, S.K.; Trivedi, P.; Choi, J.; Cho, K.; Yun, S.H.; Walitang, D.I.; Park, J.H.; Kim, K.; Sa, T. Label-free proteomics approach reveals candidate proteins in rice (Oryza sativa L.) important for ACC deaminase producing bacteria-mediated tolerance against salt stress. Environ. Microbiol. 2022, 24, 3612–3624. [Google Scholar] [CrossRef] [PubMed]
- Arefian, M.; Vessal, S.; Malekzadeh-Shafaroudi, S.; Siddique, K.H.M.; Bagheri, A. Comparative proteomics and gene expression analyses revealed responsive proteins and mechanisms for salt tolerance in chickpea genotypes. BMC Plant Biol. 2019, 19, 300. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Liu, X.; Huang, Z.; Niu, S.; Xu, T.; Zeng, J.; Li, H.; Wang, T.; Gao, Y.; et al. Physiological, biochemical and proteomic insight into integrated strategies of an endophytic bacterium Burkholderia cenocepacia strain YG-3 response to cadmium stress. Metallomics 2019, 11, 1252–1264. [Google Scholar] [CrossRef] [PubMed]
- Dautt-Castro, M.; Jijon Moreno, S.; Gómez-Hernández, N.; González-López, M.; Hernández-Hernández, E.; Rosendo-Vargas, M.; Rebolledo-Prudencio, O.; Casas-Flores, S. New Insights on the Duality of Trichoderma as a Phytopathogen Killer and a Plant Protector Based on an Integrated Multi-Omics Perspective; Springer: Cham, Switzerland, 2022; pp. 137–189. ISBN 978-3-030-91649-7. [Google Scholar]
- Oukala, N.; Pastor, V.; Aissat, K. Bacterial Endophytes: The Hidden Actor in Plant Immune Responses against Biotic Stress. Plants 2021, 10, 1012. [Google Scholar] [CrossRef]
- Pelo, S.P.; Adebo, O.A.; Green, E. Chemotaxonomic profiling of fungal endophytes of Solanum mauritianum (alien weed) using gas chromatography high resolution time-of-flight mass spectrometry (GC-HRTOF-MS). Metabolomics 2021, 17, 43. [Google Scholar] [CrossRef]
- Farag, M.A.; Khaled, S.E.; El Gingeehy, Z.; Shamma, S.N.; Zayed, A. Comparative Metabolite Profiling and Fingerprinting of Medicinal Cinnamon Bark and Its Commercial Preparations via a Multiplex Approach of GC–MS, UV, and NMR Techniques. Metabolites 2022, 12, 614. [Google Scholar] [CrossRef]
- Tchameni, S.N.; Cotârleț, M.; Ghinea, I.O.; Bedine, M.A.B.; Sameza, M.L.; Borda, D.; Bahrim, G.; Dinică, R.M. Involvement of lytic enzymes and secondary metabolites produced by Trichoderma spp. in the biological control of Pythium myriotylum. Int. Microbiol. 2020, 23, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Timchenko, Y.V. Advantages and Disadvantages of High-Performance Liquid Chromatography (HPCL) Brief Report. J. Environ. Anal. 2021, 8, 10. [Google Scholar]
- Zhao, D.; Zhu, X.; Chen, L.; Liu, W.; Chen, J.; Wang, S.; Zang, J.; Duan, Y.; Liu, X. Toxicity of a secondary metabolite produced by Simplicillium chinense Snef5 against the root-knot nematode Meloidogyne incognita. Acta Agric. Scand. Sect. B Soil Plant Sci. 2020, 70, 550–555. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gokul, A.; Mabaso, J.; Henema, N.; Otomo, L.; Bakare, O.O.; Klein, A.; Daniel, A.I.; Omolola, A.; Niekerk, L.-A.; Nkomo, M.; et al. Sustainable Agriculture through the Enhancement of Microbial Biocontrol Agents: Current Challenges and New Perspectives. Appl. Sci. 2023, 13, 6507. https://doi.org/10.3390/app13116507
Gokul A, Mabaso J, Henema N, Otomo L, Bakare OO, Klein A, Daniel AI, Omolola A, Niekerk L-A, Nkomo M, et al. Sustainable Agriculture through the Enhancement of Microbial Biocontrol Agents: Current Challenges and New Perspectives. Applied Sciences. 2023; 13(11):6507. https://doi.org/10.3390/app13116507
Chicago/Turabian StyleGokul, Arun, Jabulani Mabaso, Nontuthuko Henema, Laetitia Otomo, Olalekan Olanrewaju Bakare, Ashwil Klein, Augustine Innalegwu Daniel, Aina Omolola, Lee-Ann Niekerk, Mbukeni Nkomo, and et al. 2023. "Sustainable Agriculture through the Enhancement of Microbial Biocontrol Agents: Current Challenges and New Perspectives" Applied Sciences 13, no. 11: 6507. https://doi.org/10.3390/app13116507
APA StyleGokul, A., Mabaso, J., Henema, N., Otomo, L., Bakare, O. O., Klein, A., Daniel, A. I., Omolola, A., Niekerk, L.-A., Nkomo, M., & Keyster, M. (2023). Sustainable Agriculture through the Enhancement of Microbial Biocontrol Agents: Current Challenges and New Perspectives. Applied Sciences, 13(11), 6507. https://doi.org/10.3390/app13116507











