Insights into the Antimicrobial Potential of Acorn Extracts (Quercus ilex and Quercus suber)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Acorns
2.2. Extract Production
2.3. Total Phenolic Content Determination
2.4. Microorganisms
2.5. Antimicrobial Assays
2.5.1. Well-Diffusion Assay
2.5.2. Minimum Bactericidal Concentration (MBC)
2.5.3. Time-Death Curves
2.6. Biofilm Formation Inhibition
2.7. Statistical Analysis
3. Results and Discussion
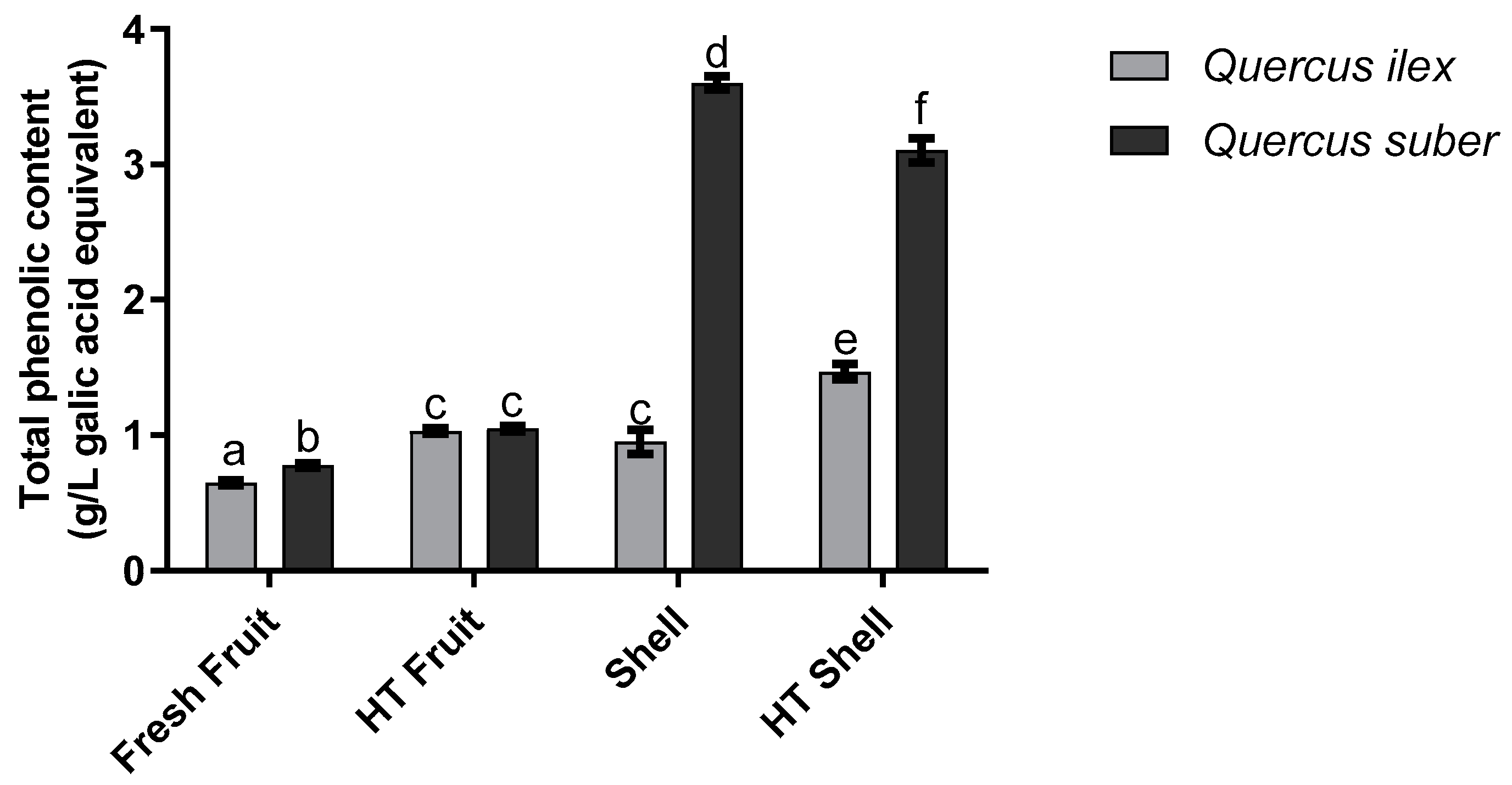
3.1. Total Phenolic Content
3.2. Well-Diffusion Assay
3.3. MBC
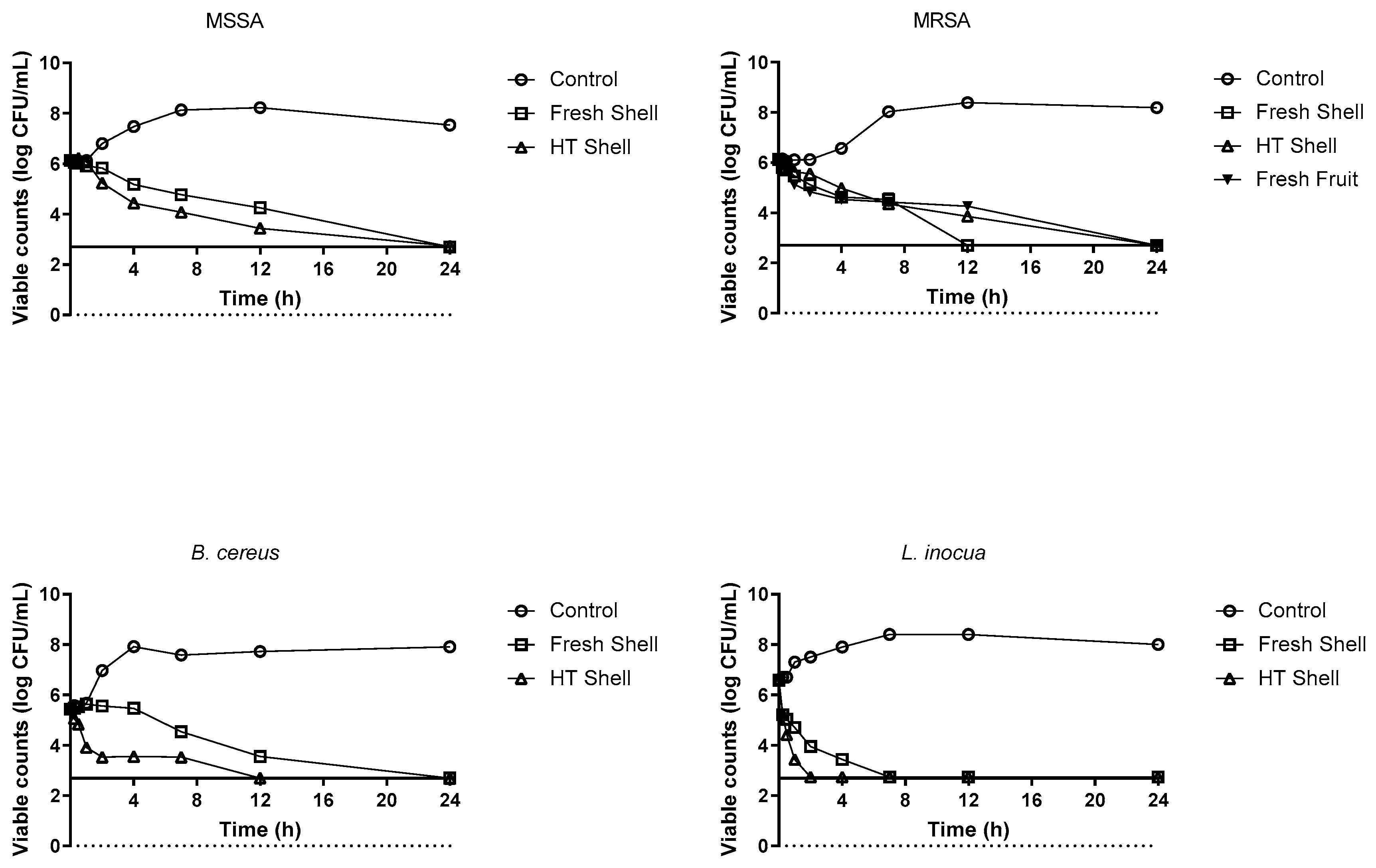
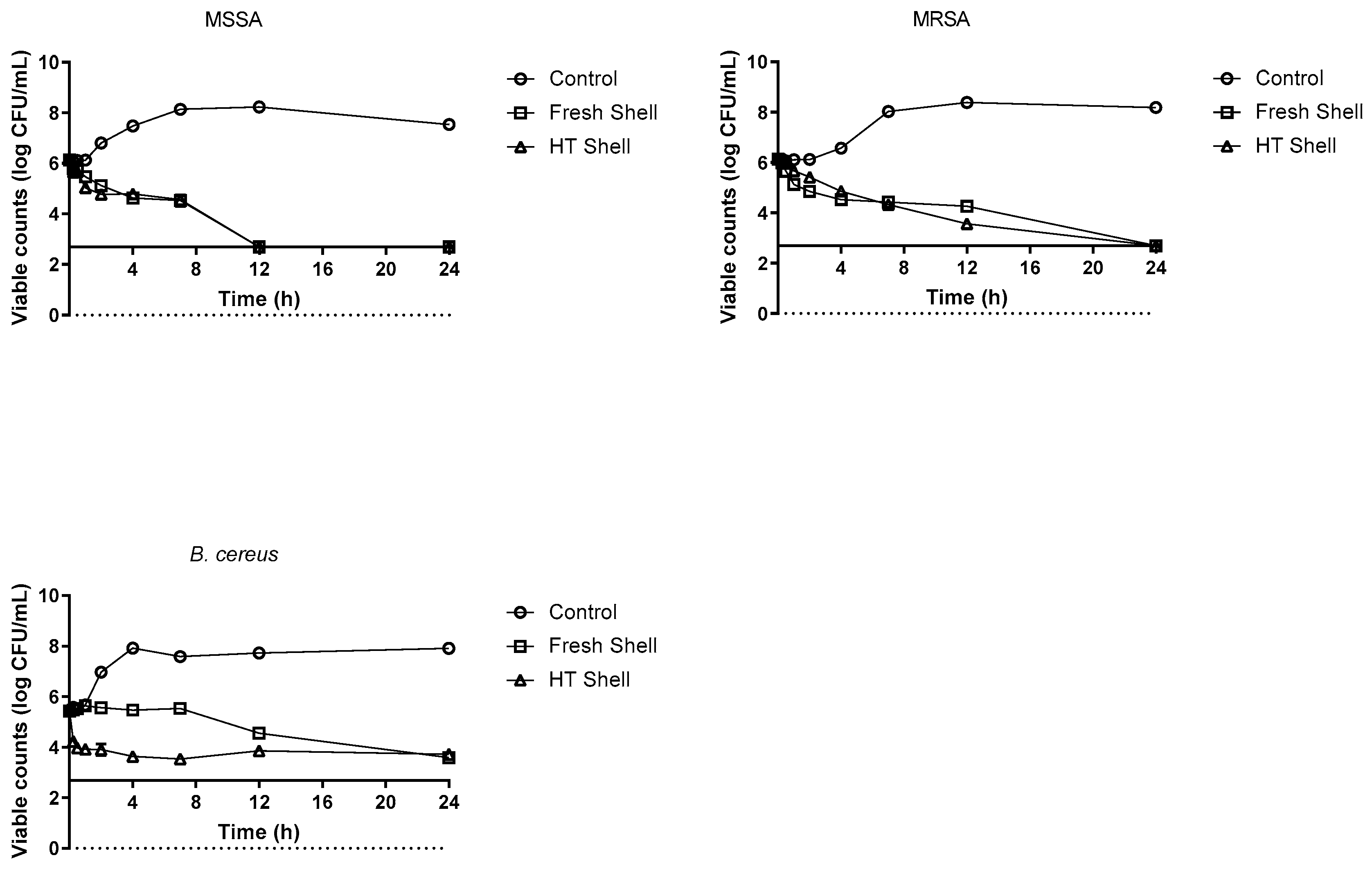
3.4. Time-Death Curves
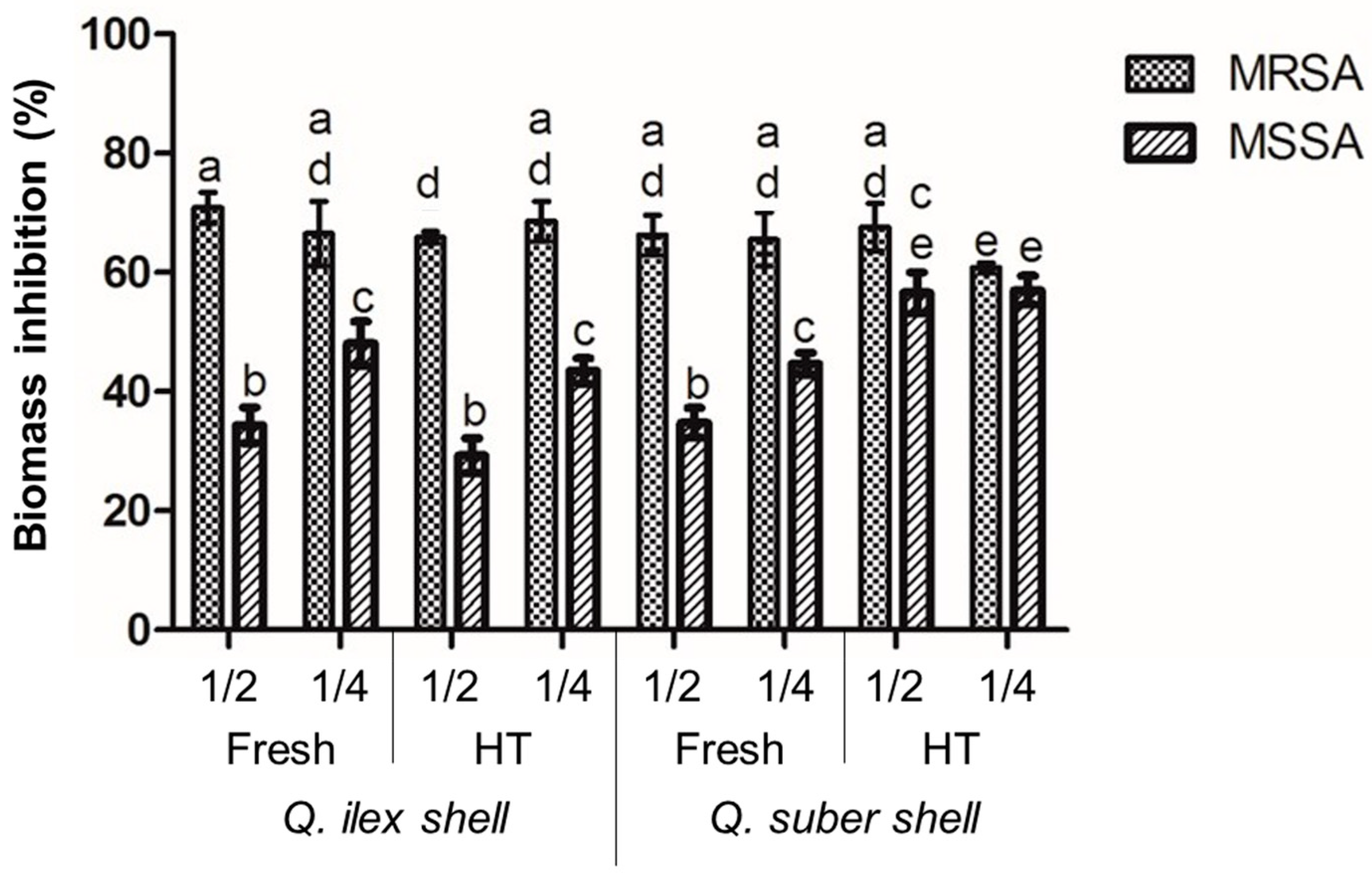
3.5. Antibiofilm Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dogan, A.; Celik, I.; Kaya, M.S. Antidiabetic properties of lyophilized extract of acorn (Quercus brantii Lindl.) on experimentally STZ-induced diabetic rats. J. Ethnopharmacol. 2015, 176, 243–251. [Google Scholar]
- Yarani, R.; Mansouri, K.; Mohammadi-Motlagh, H.R.; Mahnam, A.; Emami Aleagha, M.S. In vitro inhibition of angiogenesis by hydroalcoholic extract of oak (Quercus infectoria) acorn shell via suppressing VEGF, MMP-2, and MMP-9 secretion. Pharm. Biol. 2013, 51, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Gezici, S.; Sekeroglu, N. Neuroprotective potential and phytochemical composition of acorn fruits. Ind. Crops Prod. 2019, 128, 13–17. [Google Scholar] [CrossRef]
- Bahar, Z.; Ghotaslou, R.; Taheri, S. In vitro anti-biofilm activity of Quercus brantii subsp. persica on human pathogenic bacteria. Res. J. Pharm. 2017, 4, 67–73. [Google Scholar]
- Basri, D.; Fan, S. The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J. Pharmacol. 2005, 37, 26–29. [Google Scholar] [CrossRef] [Green Version]
- Berahou, A.; Auhmani, A.; Fdil, N.; Benharref, A.; Jana, M.; Gadhi, C.A. Antibacterial activity of Quercus ilex bark’s extracts. J. Ethnopharmacol. 2007, 112, 426–429. [Google Scholar] [CrossRef] [PubMed]
- Chusri, S.; Phatthalung, P.N.; Voravuthikunchai, S.P. Anti-biofilm activity of Quercus infectoria G. Olivier against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2012, 54, 511–517. [Google Scholar] [PubMed]
- Güllüce, M.; Adıgüzel, A.; Öğütçü, H.; Şengül, M.; Karaman, I.; Şahin, F. Antimicrobial effects of Quercus ilex L. extract. Phytother. Res. 2004, 18, 208–211. [Google Scholar] [CrossRef]
- Khurram, M.; Hameed, A.; Khan, M.A.; Amin, M.U.; Hassan, M.; Ullah, N.; Manzoor, W.; Qayum, A.; Bilal, M.; Najeeb, U.; et al. Antibacterial potentials of Quercus baloot Griff. J. Med. Plants Res. 2012, 6, 1244–1249. [Google Scholar]
- Soon, L.K.; Hasni, E.; Law, K.S.; Waliullah, S.S.; Farid, C.G.; Mohsin, S.S. Ultrastructural findings and elemental analysis of Quercus infectoria Oliv. Ann. Microsc. 2007, 7, 32–37. [Google Scholar]
- Umachigi, S.P.; Jayaveera, K.N.; Kumar, C.A.; Kumar, G.S.; Kumar, D.K. Studies on wound healing properties of Quercus infectoria. Trop. J. Pharm. Res. 2008, 7, 913–919. [Google Scholar] [CrossRef] [Green Version]
- Aroonrerk, N.; Kamkaen, N. Anti-inflammatory activity of Quercus infectoria, Glycyrrhiza uralensis, Kaempferia galanga and Coptis chinensis, the main components of Thai herbal remedies for aphthous ulcer. J. Health Res. 2009, 23, 17–22. [Google Scholar]
- Mezni, F.; Stiti, B.; Fkiri, S.; Ayari, F.; Slimane, L.B.; Ksouri, R.; Khaldi, A. Phenolic profile and in vitro anti-diabetic activity of acorn from four African Quercus species (Q. suber, Q. canariensis, Q. coccifera and Q. ilex). S. Afr. J. Bot. 2022, 146, 771–775. [Google Scholar] [CrossRef]
- Lobiuc, A.; Pavăl, N.E.; Mangalagiu, I.I.; Gheorghiță, R.; Teliban, G.C.; Amăriucăi-Mantu, D.; Stoleru, V. Future antimicrobials: Natural and functionalized phenolics. Molecules 2023, 28, 1114. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Borges, A.; Carvalho, A.P.; Monteiro, M.J.; Pintado, M.M.E. Nutritional characterization of acorn flour (a traditional component of the Mediterranean gastronomical folklore). J. Food Meas. Charact. 2016, 10, 584–588. [Google Scholar] [CrossRef]
- Gião, M.S.; González-Sanjosé, M.L.; Rivero-Pérez, M.D.; Pereira, C.I.; Pintado, M.E.; Malcata, F.X. Infusions of Portuguese medicinal plants: Dependence of final antioxidant capacity and phenol content on extraction features. J. Sci. Food Agric. 2007, 87, 2638–2647. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Calhau, C.; Morais, R.M.; Pintado, M.M.E. Production of a food grade blueberry extract rich in anthocyanins: Selection of solvents, extraction conditions and purification method. J. Food Meas. Charact. 2017, 11, 1248–1253. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Pereira, M.F.; Costa, M.R.; Pintado, M.E. Evaluation of the antimicrobial activity of aqueous extracts from dry Vaccinium corymbosum extracts upon food microorganism. Food Control. 2013, 34, 645–650. [Google Scholar] [CrossRef]
- Silva, S.; Costa, E.M.; Machado, M.; Morais, R.; Calhau, C.; Pintado, M. Antiadhesive and Antibiofilm Effect of Malvidin-3-Glucoside and Malvidin-3-Glucoside/Neochlorogenic Acid Mixtures upon Staphylococcus. Metabolites 2022, 12, 1062. [Google Scholar] [CrossRef]
- Costa, E.M.; Silva, S.; Tavaria, F.K.; Pintado, M. Insights into the Biocompatibility and Biological Potential of a Chitosan Nanoencapsulated Textile Dye. Int. J. Mol. Sci. 2022, 23, 14234. [Google Scholar] [CrossRef]
- Cantos, E.; Espín, J.C.; López-Bote, C.; de la Hoz, L.; Ordóñez, J.A.; Tomás-Barberán, F.A. Phenolic Compounds and Fatty Acids from Acorns (Quercus spp.), the Main Dietary Constituent of Free-Ranged Iberian Pigs. J. Agric. Food Chem. 2003, 51, 6248–6255. [Google Scholar] [PubMed]
- Coelho, M.; Silva, S.; Rodríguez-Alcalá, L.M.; Oliveira, A.; Costa, E.M.; Borges, A.; Martins, C.; Rodrigues, A.S.; Pintado, M.M.E. Quercus based coffee-like beverage: Effect of roasting process and functional characterization. J. Food Meas. Charact. 2018, 12, 471–479. [Google Scholar] [CrossRef]
- Rakić, S.; Petrović, S.; Kukić, J.; Jadranin, M.; Tešević, V.; Povrenović, D.; Šiler-Marinković, S. Influence of thermal treatment on phenolic compounds and antioxidant properties of oak acorns from Serbia. Food Chem. 2007, 104, 830–834. [Google Scholar] [CrossRef]
- Silliker, J.H. (Ed.) Microbial Ecology of Foods: Factors Affecting Life and Death of Microorganisms; Academic Press: Cambridge, MA, USA, 1980. [Google Scholar]
- Huang, C.B.; Ebersole, J.L. A novel bioactivity of omega-3 polyunsaturated fatty acids and their ester derivatives. Mol. Oral Microbiol. 2010, 25, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.B.; George, B.; Ebersole, J.L. Antimicrobial activity of n-6, n-7 and n-9 fatty acids and their esters for oral microorganisms. Arch. Oral Biol. 2010, 55, 555–560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, S.M.; Dias, R.O.; Franco, O.L. Phenolic Compounds in Antimicrobial Therapy. J. Med. Food 2017, 20, 1031–1038. [Google Scholar] [CrossRef]
- Burdulis, D.; Sarkinas, A.; Jasutiene, I.; Stackevicené, E.; Nikolajevas, L.; Janulis, V. Comparative study of anthocyanin composition, antimicrobial and antioxidant activity in bilberry (Vaccinium myrtillus L.) and blueberry (Vaccinium corymbosum L.) fruits. Acta Pol. Pharm. 2009, 66, 399–408. [Google Scholar]
- Doyle, M.P.; Beuchat, L.R. Food Microbiology: Fundamentals and Frontiers; ASM Press: Washington, DC, USA, 2007. [Google Scholar]
- de Souza, E.L.; de Barros, J.C.; de Oliveira, C.E.V.; da Conceição, M.L. Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. Int. J. Food Microbiol. 2010, 137, 308–311. [Google Scholar]
- Vattem, D.A.; Lin, Y.T.; Labbe, R.G.; Shetty, K. Phenolic antioxidant mobilization in cranberry pomace by solid-state bioprocessing using food grade fungus Lentinus edodes and effect on antimicrobial activity against select food borne pathogens. Innov. Food Sci. Emerg. Technol. 2004, 5, 81–91. [Google Scholar] [CrossRef]
- Sung, S.H.; Kim, K.H.; Jeon, B.T.; Cheong, S.H.; Park, J.H.; Kim, D.H.; Kweon, H.J.; Moon, S.H. Antibacterial and antioxidant activities of tannins extracted from agricultural by-products. J. Med. Plants Res. 2012, 6, 3072–3079. [Google Scholar] [CrossRef]
- Boulekbache-Makhlouf, L.; Slimani, S.; Madani, K. Total phenolic content, antioxidant and antibacterial activities of fruits of Eucalyptus globulus cultivated in Algeria. Ind. Crops Prod. 2013, 41, 85–89. [Google Scholar] [CrossRef]
- Akiyama, H.; Fujii, K.; Yamasaki, O.; Oono, T.; Iwatsuki, K. Antibacterial action of several tannins against Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 487–491. [Google Scholar] [CrossRef] [Green Version]
- Silva, S.; Costa, E.M.; Horta, B.; Calhau, C.; Morais, R.M.; Pintado, M.M. Anti-biofilm potential of phenolic acids: The influence of environmental pH and intrinsic physico-chemical properties. Biofouling 2016, 32, 853–860. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T.; et al. Phenolic Compounds from Humulus lupulus as Natural Antimicrobial Products: New Weapons in the Fight against Methicillin Resistant Staphylococcus aureus, Leishmania mexicana and Trypanosoma brucei Strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miklasińska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus Aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landini, P. Cross-talk mechanisms in biofilm formation and responses to environmental and physiological stress in Escherichia coli. Res. Microbiol. 2009, 160, 259–266. [Google Scholar] [CrossRef]
| Q. ilex | Q. suber | |||||||
|---|---|---|---|---|---|---|---|---|
| Fruit | Shell | Fruit | Shell | |||||
| Fresh | HT | Fresh | HT | Fresh | HT | Fresh | HT | |
| C. albicans | NI | NI | NI | NI | NI | NI | NI | NI |
| B. cereus | 9.3 ± 1.0 | 9.8 ± 1 | 11.5 ± 0.6 | 11.8 ± 0.5 | 8.8 ± 1.3 | NI | 9.5 ± 2.5 | 9.8 ± 0.5 |
| E. coli | NI | NI | NI | NI | NI | NI | NI | NI |
| L. inocua | NI | NI | 10.0 ± 0.8 | 8.8 ± 1.0 | NI | NI | NI | NI |
| MRSA | 10.8 ± 1.9 | 11.0 ± 1.4 | 11.5 ± 1.2 | 10.5 ± 1.3 | NI | 10.5 ± 1.3 | 9.0 ± 2.0 | 10.8 ± 0.5 |
| MSSA | 10.8 ± 1.3 | 13.3 ± 1.0 | 13.3 ± 1.0 | 11.5 ± 0.6 | NI | NI | 11.3 ± 1.1 | 11.3 ± 1.0 |
| P. aeruginosa | NI | NI | NI | NI | NI | NI | NI | NI |
| S. enteritidis | NI | NI | NI | NI | NI | NI | NI | NI |
| Q. ilex | Q. suber | |||||||
|---|---|---|---|---|---|---|---|---|
| Fruit | Shell | Fruit | Shell | |||||
| Fresh | HT | Fresh | HT | Fresh | HT | Fresh | HT | |
| B. cereus | >20 | >20 | 10 | 10 | >20 | NT | 10 | 10 |
| L. inocua | NT | NT | 10 | 10 | NT | NT | NT | NT |
| MRSA | 10 | >20 | 10 | 5 | NT | >20 | 5 | 5 |
| MSSA | >20 | >20 | 10 | 5 | NT | NT | 5 | 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, S.; Machado, M.; Coelho, M.; Costa, E.M.; Pintado, M. Insights into the Antimicrobial Potential of Acorn Extracts (Quercus ilex and Quercus suber). Appl. Sci. 2023, 13, 6820. https://doi.org/10.3390/app13116820
Silva S, Machado M, Coelho M, Costa EM, Pintado M. Insights into the Antimicrobial Potential of Acorn Extracts (Quercus ilex and Quercus suber). Applied Sciences. 2023; 13(11):6820. https://doi.org/10.3390/app13116820
Chicago/Turabian StyleSilva, Sara, Manuela Machado, Marta Coelho, Eduardo M. Costa, and Manuela Pintado. 2023. "Insights into the Antimicrobial Potential of Acorn Extracts (Quercus ilex and Quercus suber)" Applied Sciences 13, no. 11: 6820. https://doi.org/10.3390/app13116820







