Improvement Effect of Membrane-Free Stem Cell Extract on Atopic Dermatitis in NC/Nga Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of MFSCE
2.3. Experimental Animals
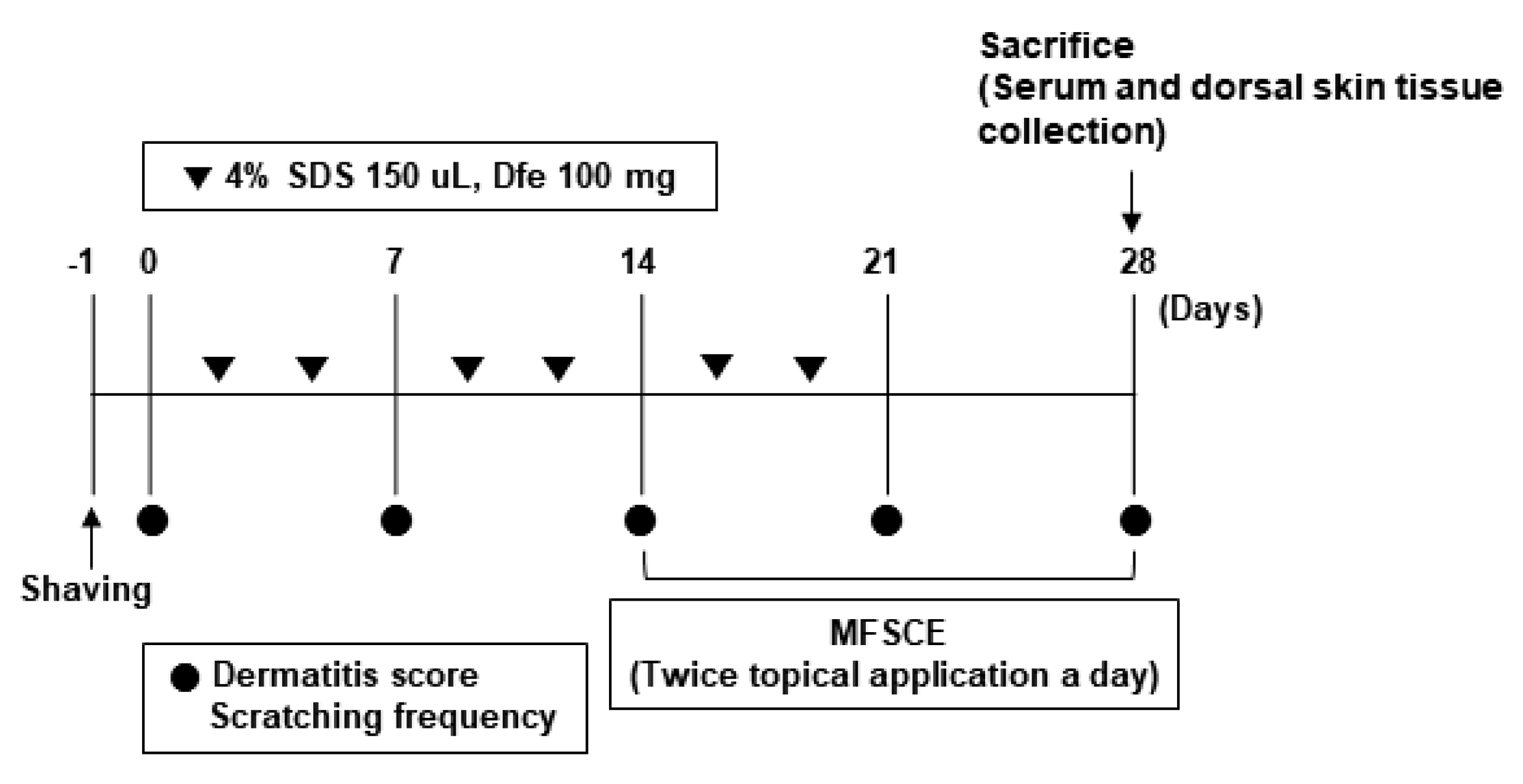
2.4. Induction of AD-like Skin Inflammation
2.5. Design of Experimental Group
2.6. AD Inflammation Score Evaluation
2.7. Evaluation of Serum Immunoglobulin (IgE) and Inflammatory Cytokines
2.8. Histological Analysis
2.9. Protein Expression of Inflammatory Cytokines
2.10. Statistical Analysis
3. Results
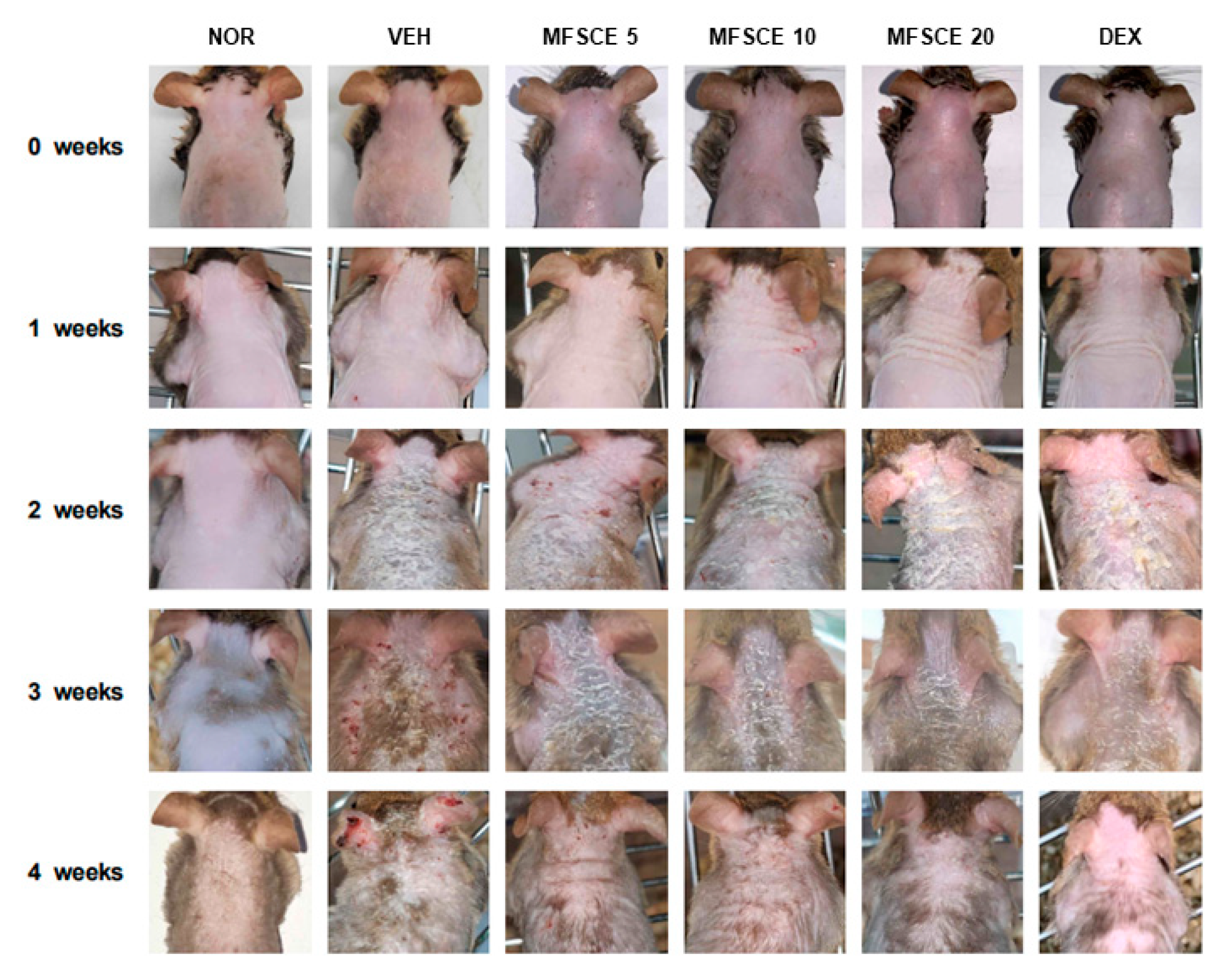
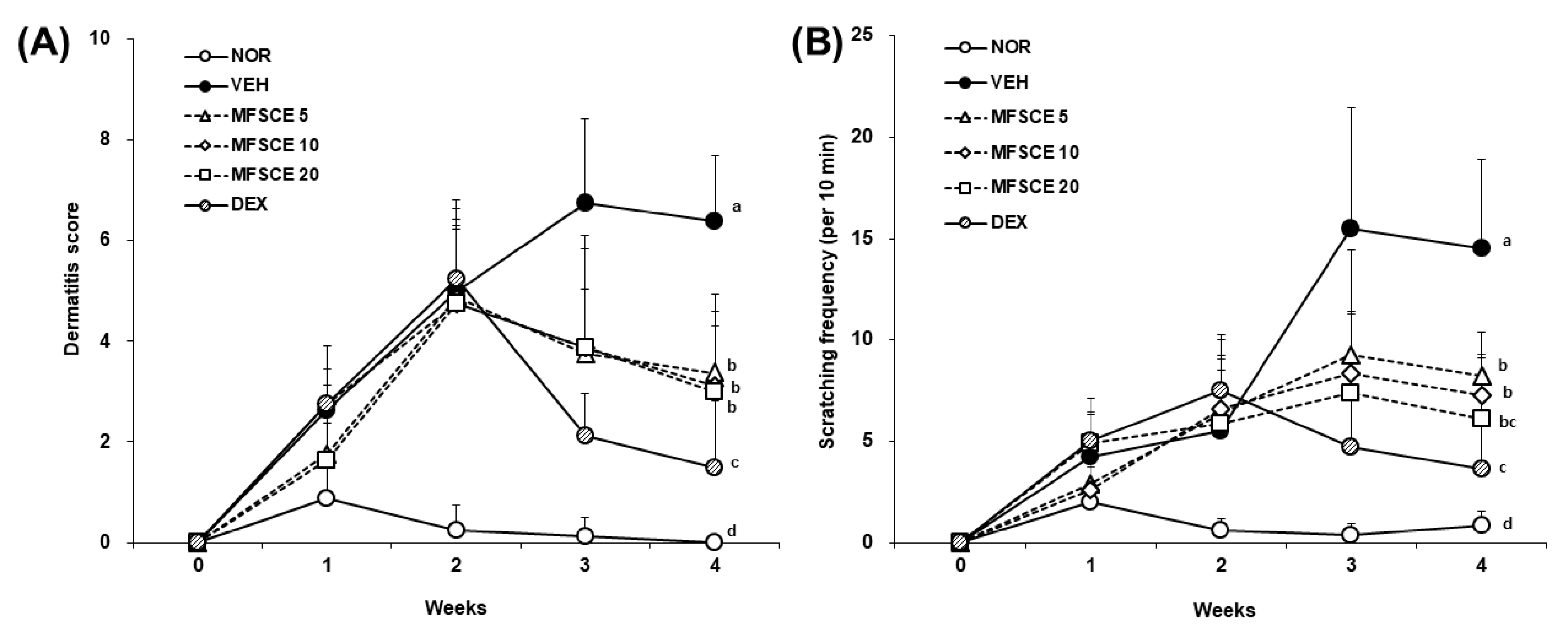
3.1. Clinical Severity and Scratching Behavior on Dorsal Skin of HDM-Sensitized NC/Nga Mice
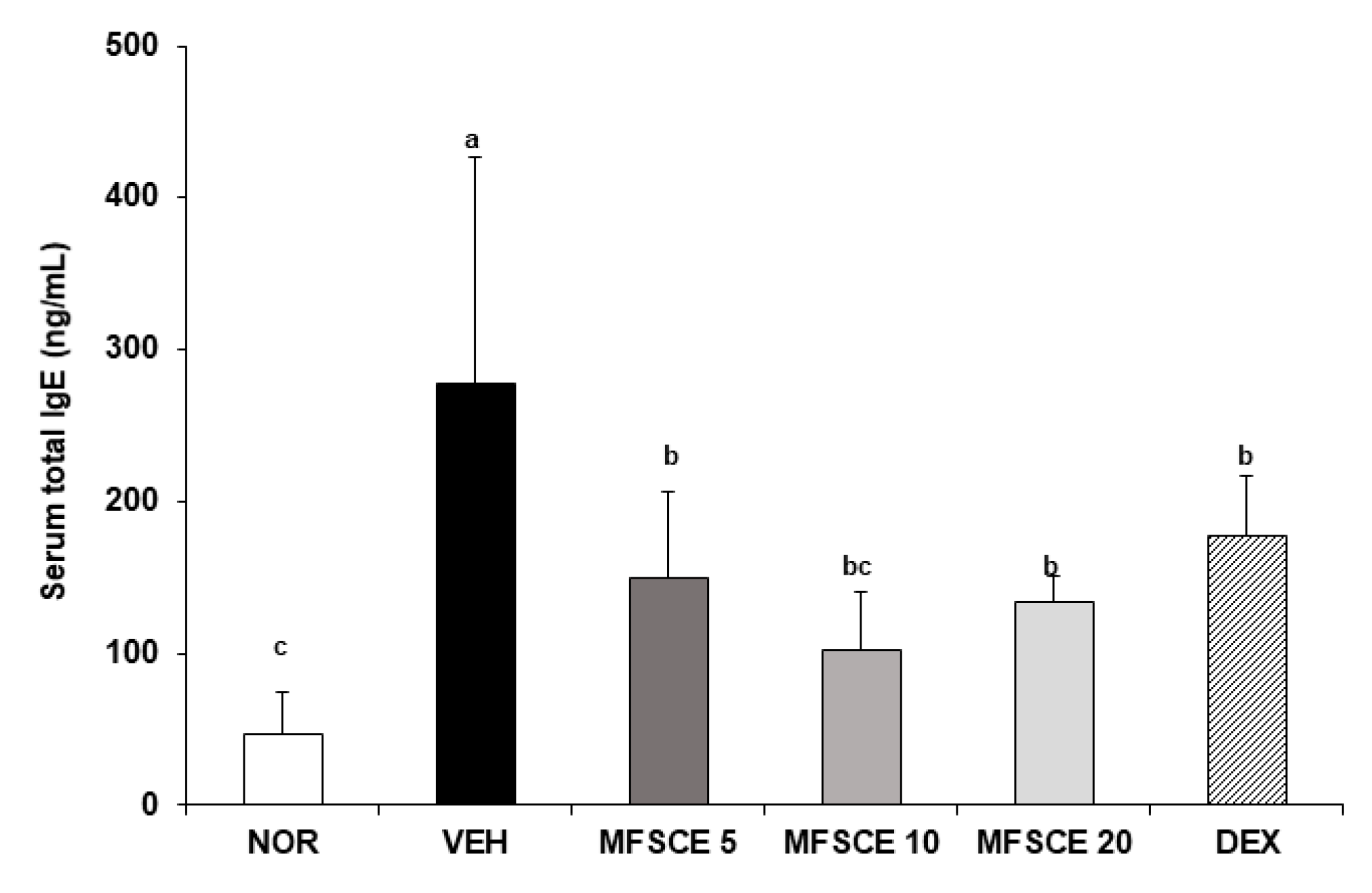
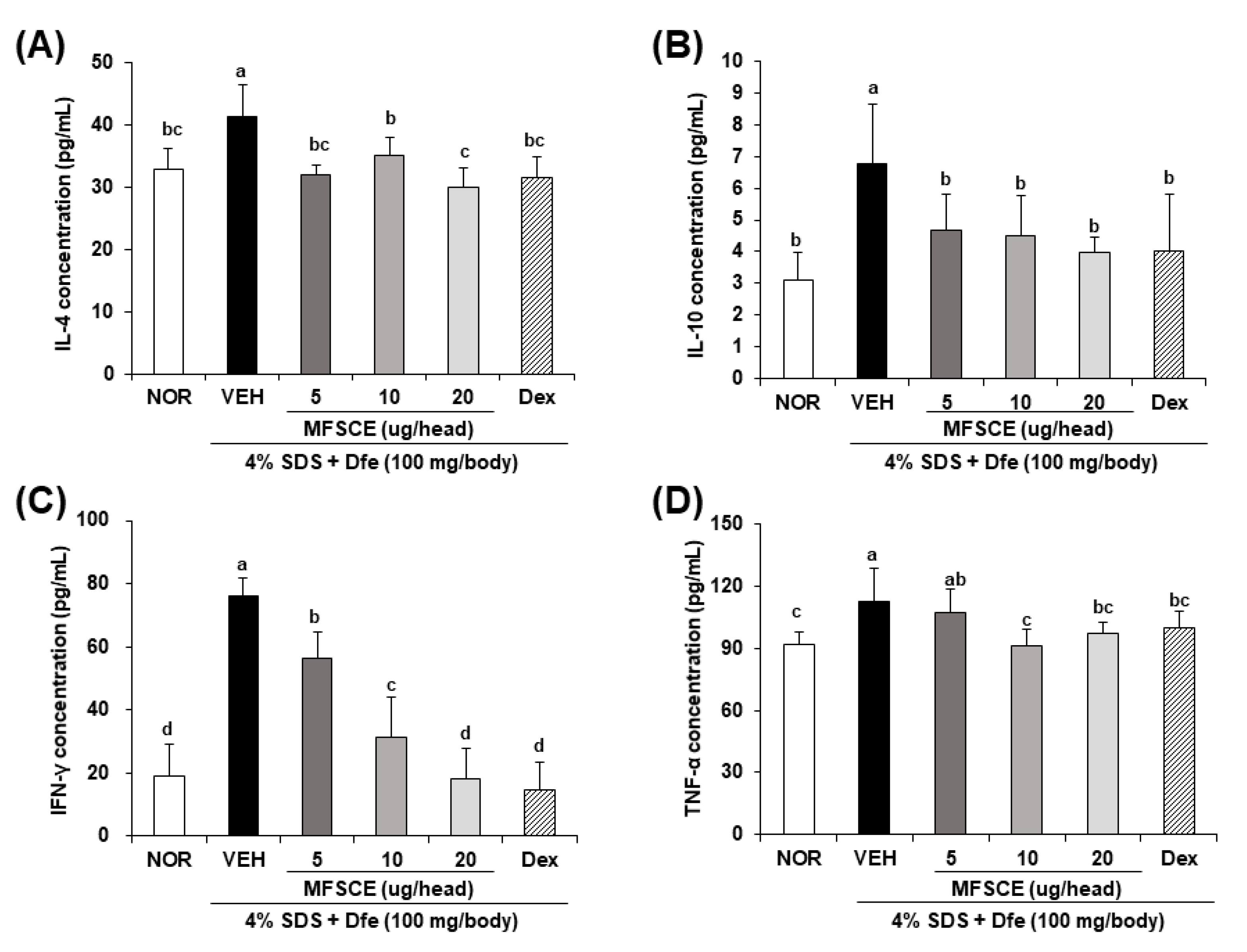
3.2. Serum Levels of IgE and Inflammatory Cytokines in HDM-Sensitized NC/Nga Mice
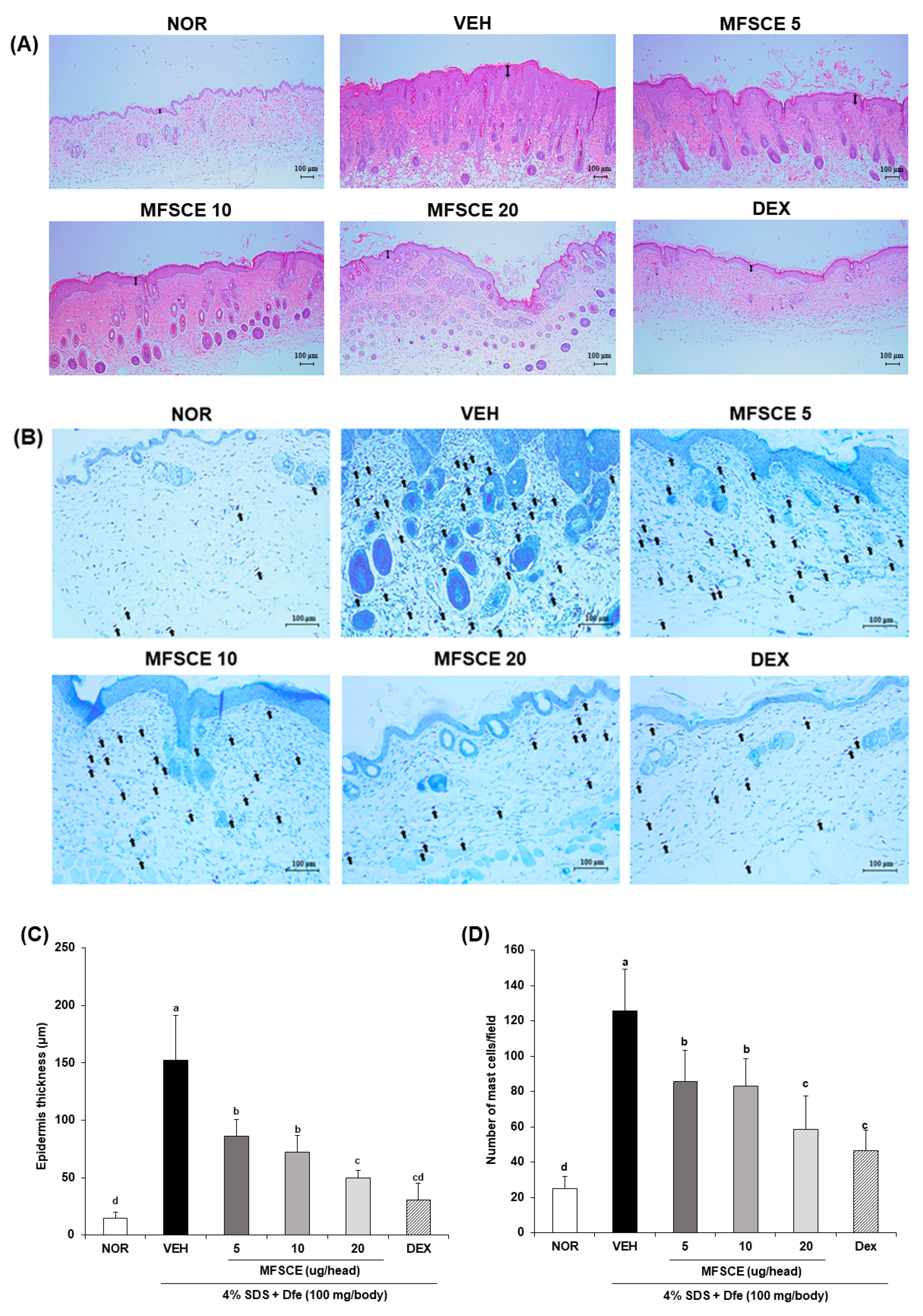
3.3. Histological Change and Mast Cell Infiltration in Dorsal Skin of HDM-Sensitized NC/Nga Mice
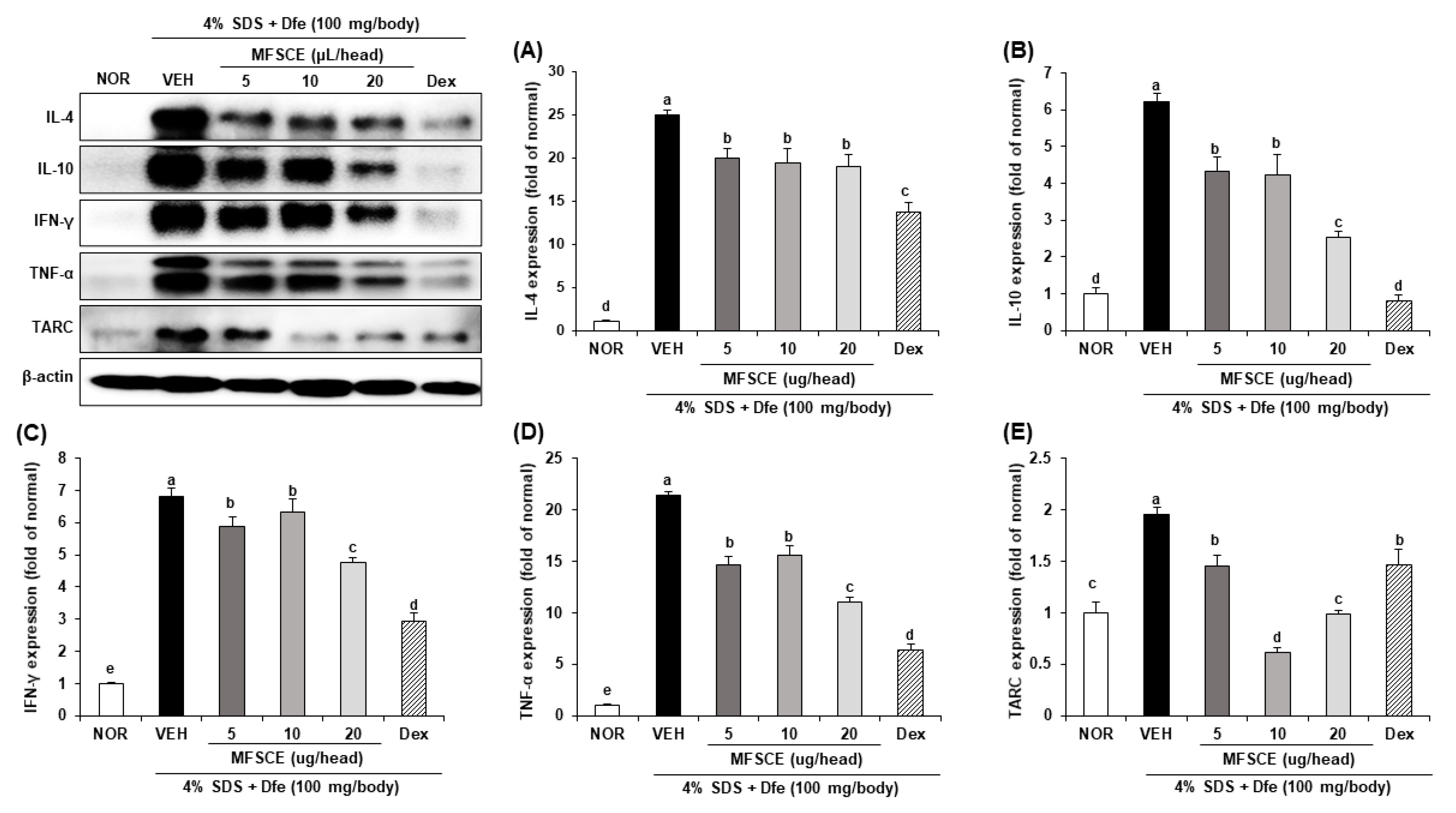
3.4. Inflammatory Cytokine Expressions in Dorsal Skin of HDM-Sensitized NC/Nga Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suga, H.; Sato, S. Novel topical and systemic therapies in atopic dermatitis. Immunol. Med. 2019, 42, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Bratu, D.; Boda, D.; Caruntu, C. Reflectance confocal microscopy in monitoring atopic dermatitis treated with topical calcineurin inhibitors. Healthcare 2023, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Mandlik, D.S.; Mandlik, S.K. Atopic dermatitis: New insight into the etiology, pathogenesis, diagnosis and novel treatment strategies. Immunopharmacol. Immunotoxicol. 2021, 43, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Bumbacea, R.S.; Corcea, S.L.; Ali, S.; Dinica, L.C.; Fanfaret, I.S.; Boda, D. Mite allergy and atopic dermatitis: Is there a clear link? (Review). Exp. Ther. Med. 2020, 20, 3554–3560. [Google Scholar] [CrossRef] [PubMed]
- Peng, W.; Novak, N. Pathogenesis of atopic dermatitis. Clin. Exp. Allergy 2015, 45, 566–574. [Google Scholar] [CrossRef]
- Bieber, T.; Paller, A.S.; Kabashima, K.; Feely, M.; Rueda, M.J.; Ross Terres, J.A.; Wollenberg, A. Atopic dermatitis: Pathomechanisms and lessons learned from novel systemic therapeutic options. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1432–1449. [Google Scholar] [CrossRef]
- Yang, J.; Kuang, H.; Li, N.; Hamdy, A.M.; Song, J. The modulation and mechanism of probiotic-derived polysaccharide capsules on the immune response in allergic diseases. Crit. Rev. Food Sci. Nutr. 2022, 1–13. [Google Scholar] [CrossRef]
- Guttman-Yassky, E.; Zhou, L.; Krueger, J.G. The skin as an immune organ: Tolerance versus effector responses and applications to food allergy and hypersensitivity reactions. J. Allergy Clin. Immunol. 2019, 144, 362–374. [Google Scholar] [CrossRef]
- Kim, W.-S.; Park, B.-S.; Sung, J.-H. The wound-healing and antioxidant effects of adipose-derived stem cells. Expert Opin. Biol. Ther. 2009, 9, 879–887. [Google Scholar] [CrossRef]
- Zhang, W.; Bai, X.; Zhao, B.; Li, Y.; Zhang, Y.; Li, Z.; Wang, X.; Luo, L.; Han, F.; Zhang, J.; et al. Cell-free therapy based on adipose tissue stem cell-derived exosomes promotes wound healing via the PI3K/Akt signaling pathway. Exp. Cell Res. 2018, 370, 333–342. [Google Scholar] [CrossRef]
- Venkatarame Gowda Saralamma, V.; Vetrivel, P.; Kim, S.M.; Ha, S.E.; Lee, H.J.; Lee, S.J.; Kim, Y.S.; Pak, J.E.; Lee, H.J.; Heo, J.D. Proteome profiling of membrane-free stem cell components by nano-LS/MS analysis and its anti-inflammatory activity. Evid. Based Complement. Altern. Med. 2019, 2019, 4683272. [Google Scholar] [CrossRef]
- Camussi, G.; Deregibus, M.C.; Cantaluppi, V. Role of stem-cell-derived microvesicles in the paracrine action of stem cells. Biochem. Soc. Trans. 2013, 41, 283–287. [Google Scholar] [CrossRef]
- Lai, R.C.; Chen, T.S.; Lim, S.K. Mesenchymal stem cell exosome: A novel stem cell-based therapy for cardiovascular disease. Regen. Med. 2011, 6, 481–492. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, J.H.; Park, H.S.; Kim, Y.S.; Cho, E.J. Protective effect of membrane-free stem cell extract against oxidative stress in LLC-PK1 cells. J. Korea Acad-Ind. Coop. Soc. 2019, 20, 303–312. [Google Scholar]
- Park, H.S.; Pang, Q.Q.; Kim, Y.S.; Kim, J.H.; Cho, E.J. Neuroprotective effect of membrane-free stem cell extract against amyloid beta25–35-induced neurotoxicity in SH-SY5Y cells. Appl. Sci. 2021, 11, 2219. [Google Scholar] [CrossRef]
- He, M.T.; Park, H.S.; Kim, Y.S.; Lee, A.Y.; Cho, E.J. Protective effect of membrane-free stem cells against lipopolysaccharide and interferon-gamma-stimulated inflammatory responses in RAW 264.7 macrophages. Int. J. Mol. Sci. 2021, 22, 6894. [Google Scholar] [CrossRef]
- Yamamoto, M.; Haruna, T.; Yasui, K.; Takahashi, H.; Iduhara, M.; Takaki, S.; Deguchi, M.; Arimura, A. A novel atopic dermatitis model induced by topical application with dermatophagoides farinae extract in NC/Nga mice. Allergol. Int. 2007, 56, 139–148. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, S.M.; Moon, Y.G.; Jung, Y.S.; Lee, J.H.; Venkatarame Gowda Saralamma, V.; Kim, Y.S.; Pak, J.E.; Lee, H.J.; Kim, G.S. Membrane-free stem cell components inhibit interleukin-1α-stimulated inflammation and cartilage degradation in vitro and in vivo: A rat model of osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4869. [Google Scholar] [CrossRef]
- Ha, S.E.; Vetrivel, P.; Kim, S.M.; Bhosale, P.B.; Kim, H.H.; Pak, J.E.; Heo, J.D.; Kim, Y.S.; Kim, G.S. Inhibitory effect of membrane-free stem cell components derived from adipose tissues on skin inflammation in keratinocytes. Mol. Med. Rep. 2022, 25, 125. [Google Scholar] [CrossRef]
- Suto, H.; Matsuda, H.; Mitsuishi, K.; Hira, K.; Uchida, T.; Unno, T.; Ogawa, H.; Ra, C. NC/Nga mice: A mouse model for atopic dermatitis. Int. Arch. Allergy Immunol. 1999, 120, 70–75. [Google Scholar] [CrossRef]
- Tanaka, A.; Matsuda, H. Evaluation of itch by using NC/NgaTnd mice: A model of human atopic dermatitis. J. Biomed. Biotechnol. 2011, 2011, 790436. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, H.; Watanabe, N.; Geba, G.P.; Sperl, J.; Tsudzuki, M.; Hiroi, J.; Matsumoto, M.; Ushio, H.; Saito, S.; Askenase, P.W.; et al. Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int. Immunol. 1997, 9, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Matsuoka, H.; Maeda, N.; Atsuta, Y.; Ando, K.; Chinzei, Y. Seasonal fluctuations of Dermatophagoides mite population in house dust. Jpn. J. Med. Sci. Biol. 1995, 48, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Jang, S.; Kim, H.K. Gardenia jasminoides extract ameliorates DfE-induced atopic dermatitis in mice through restoration of barrier function and T-helper 2-mediated immune response. Biomed. Pharmacother. 2022, 145, 112344. [Google Scholar] [CrossRef]
- Kang, H.; Lee, C.H.; Kim, J.R.; Kwon, J.Y.; Son, M.-J.; Kim, J.-E.; Lee, K.W. Theobroma cacao extract attenuates the development of Dermatophagoides farinae-induced atopic dermatitis-like symptoms in NC/Nga mice. Food Chem. 2017, 216, 19–26. [Google Scholar] [CrossRef]
- Chopra, R.; Silverberg, J.I. Assessing the severity of atopic dermatitis in clinical trials and practice. Clin. Dermatol. 2018, 36, 606–615. [Google Scholar] [CrossRef]
- Jeong, D.-Y.; Ryu, M.-S.; Yang, H.-J.; Jeong, S.-Y.; Zhang, T.; Yang, H.J.; Kim, M.J.; Park, S. Pediococcus acidilactici intake decreases the clinical severity of atopic dermatitis along with increasing mucin production and improving the gut microbiome in Nc/Nga mice. Biomed. Pharmacother. 2020, 129, 110488. [Google Scholar] [CrossRef]
- Wu, X.X.; Siu, W.S.; Wat, C.L.; Chan, C.L.; Koon, C.M.; Li, X.; Cheng, W.; Ma, H.; Tsang, M.S.M.; Lam, C.W.-K.; et al. Effects of topical application of a tri-herb formula on inflammatory dry-skin condition in mice with oxazolone-induced atopic dermatitis. Phytomedicine 2021, 91, 153691. [Google Scholar] [CrossRef]
- Sung, Y.-Y.; Yang, W.-K.; Lee, A.Y.; Kim, D.-S.; Jin Nho, K.; Kim, Y.S.; Kim, H.K. Topical application of an ethanol extract prepared from Illicium verum suppresses atopic dermatitis in NC/Nga mice. J. Ethnopharmacol. 2012, 144, 151–159. [Google Scholar] [CrossRef]
- Klunker, S.; Trautmann, A.; Akdis, M.; Verhagen, J.; Schmid-Grendelmeier, P.; Blaser, K.; Akdis, C.A. A second step of chemotaxis after transendothelial migration: Keratinocytes undergoing apoptosis release IFN-γ-inducible protein 10, monokine induced by IFN-γ, and IFN-γ-inducible α-chemoattractant for T cell chemotaxis toward epidermis in atopic dermatitis. J. Immunol. 2003, 171, 1078–1084. [Google Scholar]
- Sumiyoshi, K.; Nakao, A.; Ushio, H.; Mitsuishi, K.; Okumura, K.; Tsuboi, R.; Ra, C.; Ogawa, H. Transforming growth factor-β1 suppresses atopic dermatitis-like skin lesions in NC/Nga mice. Clin. Exp. Allergy 2002, 32, 309–314. [Google Scholar] [CrossRef]
- Mekori, Y.A.; Metcalfe, D.D. Mast cell–T cell interactions. J. Allergy Clin. Immunol. 1999, 104, 517–523. [Google Scholar] [CrossRef]
- Kawakami, T.; Ando, T.; Kimura, M.; Wilson, B.S.; Kawakami, Y. Mast cells in atopic dermatitis. Curr. Opin. Immunol. 2009, 21, 666–678. [Google Scholar] [CrossRef]
- Masuda, A.; Yoshikai, Y.; Aiba, K.; Matsuguchi, T. Th2 cytokine production from mast cells is directly induced by lipopolysaccharide and distinctly regulated by c-Jun N-terminal kinase and p38 pathways. J. Immunol. 2002, 169, 3801–3810. [Google Scholar] [CrossRef]
- Hijnen, D.; de Bruin-Weller, M.; Oosting, B.; Lebre, C.; de Jong, E.; Bruijnzeel-Koomen, C.; Knol, E. Serum thymus and activation-regulated chemokine (TARC) and cutaneous T cell–attracting chemokine (CTACK) levels in allergic diseases: TARC and CTACK are disease-specific markers for atopic dermatitis. J. Allergy Clin. Immunol. 2004, 113, 334–340. [Google Scholar] [CrossRef]
- Saeki, H.; Tamaki, K. Thymus and activation regulated chemokine (TARC)/CCL17 and skin diseases. J. Dermatol. Sci. 2006, 43, 75–84. [Google Scholar] [CrossRef]
- Yu, B.; Koga, T.; Urabe, K.; Moroi, Y.; Maeda, S.; Yanagihara, Y.; Furue, M. Differential regulation of thymus- and activation-regulated chemokine induced by IL-4, IL-13, TNF-α and IFN-γ in human keratinocyte and fibroblast. J. Dermatol. Sci. 2002, 30, 29–36. [Google Scholar] [CrossRef]
- Jung, M.; Lee, T.H.; Bang, M.-H.; Kim, H.; Son, Y.; Chung, D.K.; Kim, J. Suppression of thymus- and activation-regulated chemokine (TARC/CCL17) production by 3-O-β-d-glucopyanosylspinasterol via blocking NF-κB and STAT1 signaling pathways in TNF-α and IFN-γ-induced HaCaT keratinocytes. Biochem. Biophys. Res. Commun. 2012, 427, 236–241. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, Q.Q.; Noh, B.W.; Park, H.S.; Kim, Y.S.; Kim, J.-H.; Cho, E.J. Improvement Effect of Membrane-Free Stem Cell Extract on Atopic Dermatitis in NC/Nga Mice. Appl. Sci. 2023, 13, 4542. https://doi.org/10.3390/app13074542
Pang QQ, Noh BW, Park HS, Kim YS, Kim J-H, Cho EJ. Improvement Effect of Membrane-Free Stem Cell Extract on Atopic Dermatitis in NC/Nga Mice. Applied Sciences. 2023; 13(7):4542. https://doi.org/10.3390/app13074542
Chicago/Turabian StylePang, Qi Qi, Byeong Wook Noh, Hye Sook Park, Young Sil Kim, Ji-Hyun Kim, and Eun Ju Cho. 2023. "Improvement Effect of Membrane-Free Stem Cell Extract on Atopic Dermatitis in NC/Nga Mice" Applied Sciences 13, no. 7: 4542. https://doi.org/10.3390/app13074542
APA StylePang, Q. Q., Noh, B. W., Park, H. S., Kim, Y. S., Kim, J.-H., & Cho, E. J. (2023). Improvement Effect of Membrane-Free Stem Cell Extract on Atopic Dermatitis in NC/Nga Mice. Applied Sciences, 13(7), 4542. https://doi.org/10.3390/app13074542




