Abstract
This article presents the results of experimental research on the possible use of construction and demolition waste (CDW) to improve the properties of unburnt tailings originating from the thermally active spoil heap in Heřmanice (Ostrava, Czech Republic). Mining activity anywhere in the world generally entails a lot of negative impacts on the environment, which are of a long-term nature. One of the most pressing challenges in the remediation of the consequences of mining activity is the thermal activity of spoil heaps associated with the high acidity of the tailings. Active acidity (pH/H2O), exchangeable acidity (pH/CaCl2), hydrolytic acidity (Ha), and elemental composition of tailings and CDW have been monitored. Based on an acidity study, it has been proven that compared to burnt tailings (pH/H2O = 8.4, pH/CaCl2 = 8.9 and Ha = 1.4 mmol kg−1), unburnt tailings show acidic properties (pH/H2O = 3.7, pH/CaCl2 = 3.6 and Ha = 205 mmol kg−1). The bioavailability of two selected potentially toxic elements (PTEs), namely Al and Fe, was examined based on the elemental composition. BCR sequential extraction analysis was used to determine their bioavailability. It has been proven that mixing CDW with tailings has a positive effect on the pH value, which has a positive effect on the further development of the entire site. The increase in the pH value is provably dependent on the amount of construction waste added, so it can be said that the increasing amount of construction waste will result in improved parameters of the burnt tailings. The results of the BCR analysis show that aluminum from the tailings will be released both from the reducible and oxidisable fractions, where it will be mainly bound to sulphides. The relatively high concentration of Fe in the oxidisable fraction (2002 mg Fe kg−1) suggests that Fe is bound to sulphides in the tailings, and it is due to the high residual pyrite and sulphide content in the dumped material, as expected. This work has found no limits where CDW no longer positively affects the acidity of unburnt tailings. For practical application, however, it is important that the mixture of CDW and tailings is properly mixed and then used for remediation.
1. Introduction
Coal mining and the recent mass closure of mines are associated with a negative impact on natural objects and technical structures. In addition to the extraction of mineral raw materials, the mining activity also produces a large amount of waste material, which is generated during all the phases of development and mining activity in the mines, as well as all the technological operations related to the processes of enrichment and purification of the extracted raw material. In the past, most of these waste materials were deposited on the surface. That is why numerous spoil heaps were created near coal mines or in their immediate vicinity. A huge amount of tailings and other types of waste materials were deposited in these spoil heaps. In coal basins around the world, there are still hundreds of objects representing the locations with waste from coal mining [1,2,3]. For example, there are approximately 281 spoil tips and 46 spoil heaps in the Ostrava-Karvina Coalfield. These are anthropogenic tailing spoil heaps formed at different times during the nearly 200-year long tradition of coal mining in the Czech parts of the Upper Silesian Coal Basin [4]. However, there are countries in which this number is even higher. For example, in neighbouring Poland, there are approximately 250 such objects, and in China, the number of sites where tailings are stored exceeds 1700 [2].
Spoil heaps of mine tailings significantly affect the character of the landscape and are perceived as a negative aesthetic element that stands out from the surrounding relief of the landscape. Whether they are overgrown with invasive vegetation over time or they are after more or less successful remediation, these are still areas with limited options for further use. In addition to the adverse effect on the landscape, the presence of surface coal waste dumps is closely related to their impact on the environment [2,5,6]. From the point of view of their environmental burden, spoil heaps can be particularly problematic due to their thermal activity, and also the chemical composition of the deposited material which, as a result of the age of the spoil heap and its thermal activity, cannot be clearly determined or more precisely estimated.
In the past, a significant impact of carbon tailings stored in spoil heaps on the individual components of the environment was not assumed. Carboniferous tailings as such do not contain any contaminants, but under certain conditions these can be uncontrollably released from the tailings. The main risk factor that is closely associated with carboniferous tailings is the possibility of endogenous combustion [7]. The chemical reactivity of rocks deposited in spoil heaps is mainly determined by the amount of unstable minerals present (e.g., pyrite, carbonates, and feldspars). Pyrite weathers very easily. It occurs due to the infiltration of precipitation or the penetration of air volume into the spoil heap, resulting in the formation of sulphuric acid, or sulphates. This acidification process is more visible especially in places of endogenous combustion. The degree of thermal transformation of the waste affects the leachability of metals. Significantly more elements (Al, Co, Cr, Cu, Ni) are leached from the deeply thermally transformed samples (sinters) than from samples of thermally intact (unburnt) waste and slightly thermally transformed (burnt) waste. This is due to the presence of a large amount of glaze in sinter, and this glaze is subject to devitrification and the release of metals contained in it [8]. Changes in temperature also affect the chemical status of organic substances found in the coal waste dump. This leads to the formation of phenols and their derivatives, which can pass into water and subsequently cause its pollution [9,10,11]. The material deposited on spoil heaps is also characterized by different particle sizes [12]. If the material is porous, the heat spreads more easily and much faster. With fine-grained materials, air movement is slower and the temperature therefore increases gradually. The geotechnical condition of the spoil heap or other types of waste or sludge materials that might have been deposited on the spoil heaps in the past must also be taken into consideration. They can represent a secondary source of contamination of the rock environment [8,11,13].
Spontaneous combustion usually triggers the process of long-lasting subsurface fires. Due to the content of sulphide sulphur and other combustible substances (debris of conveyor belts, wooden parts of mine supports, etc.), carboniferous tailings represent a constant risk of combustion (e.g., by inappropriate intervention in old spoil heaps). That is why the remediation work also plays an important role, because it should lead to thermal phenomena inhibition on the spoil heap, and should be adapted to the local morphology, technology, composition and volume of the deposited waste material [7,11]. An increase in the temperature of the subsoil and the surface is a very significant negative factor related to thermal processes, and it leads to changes in the topography, soil cover, and plant cover, as well as the atmosphere [2,14]. If fires occur in the tailing dumps, the emission of pollutants into the air will increase significantly. As a result of the increased temperature and more intense evaporation, the material on the spoil heap sheds its moisture, and dry particles get into the air more easily. If the coal contained in the waste turns into ash during fire, its tendency to be carried away by the wind increases, especially during the operations related to fire extinguishing, removal, and transport of burnt material, or during remediation works. Since the waste material contains sulphur, SO2 and H2S are generated. This is evidenced by the yellow efflorescence on the surface of the spoil heap body (Figure 1). As a result of wind erosion, spoil heaps are therefore a secondary source of air-borne dust (including respirable PM10 particles) [15,16].

Figure 1.
Heřmanice spoil heap.
The formation of vegetation groups is always a positive phenomenon, because the decomposition of phytomass supports the formation of humus. In places with vegetation, endogenous combustion can turn into an open fire and thus confound the investments spent for recultivation. This was the case, for example, in thermally active Heřmanice or Hedvika spoil heaps (Ostrava, Moravian-Silesian Region, Czech Republic). It is therefore not an isolated case when already reclaimed and adapted areas of former spoil heaps start to spontaneously heat up [2]. It is very difficult to deal with hot spots and prevent endogenous combustion of old spoil heaps that contain large amounts of coal debris and were often placed in a loose, unconsolidated configuration that allows oxygen to easily interact with the waste. Regular monitoring makes their identification possible, but apparently, the only way to deal with them is to intensify the combustion by improving the access of air and thus allowing the tailings to burn through [17]. This is the reason why many entities decided to recultivate coal waste dumps only after they had been burned, extinguished, or partially excavated. Research has shown that in the case of burning coal waste dumps, the introduction of vegetation has no effect unless the object is sufficiently protected against fire. In such situations, spontaneous succession is the only solution [2].
The removal of damages caused by mining activities is not related to the Ostrava-Karvina Coalfield only. In a number of legislative and legal measures of other countries, there is an obligation to recultivate damaged areas on the surface. This is a global problem and a long-term process [18]. These anthropogenic geomorphological bodies are, on the one hand, a burden for the environment; but on the other hand, they can be an important a source of cheap and available aggregates. The current pressure to limit the use of primary raw materials, the extraction of which causes additional unpleasant impacts on the landscape and the environment, necessarily leads to the use of waste as secondary raw materials. In the Czech Republic, this problem was solved legislatively in 2001, because mining waste is not classified as waste, but as a product used in land reclamation or road construction.
In Europe, the two economic sectors producing the largest volume of waste are mining and quarrying (28.1%), and building industry and demolition (34.7%) [19]. In the Czech Republic, construction and demolition waste constitutes a significant part of the total amount of waste produced in terms of weight. In the years 2014–2020, the production of construction waste accounted for more than half of the total production of waste (see Table 1). On the contrary, with regard to the decline in mining activity, waste from mining and quarrying accounted for only 0.05% of the total amount of waste produced in 2020 [20].

Table 1.
Production of waste in the years 2014–2020 [20].
In compliance with the EU communication COM/2014/398 “Towards a circular economy: A zero waste program for Europe”, which introduces the main objectives and measures in the field of circular economy, the landfilling of recyclable waste will not be allowed from the year 2025, and it will be completely prohibited from the year 2050 [21]. As a corrective measure, it is recommended to increase the neutralization potential by liming, especially in locations with an increased concentration of pyrite, or with the occurrence of jarosite, which can be an important indicator in this regard. An increase in the neutralization potential can also slow down the kinetics of pyrite oxidation. However, a decrease in tailings acidity by surface application of lime can be very difficult and time-consuming given the limited movement of lime through the spoil heap body.
The aim of this article is to assess the effect of the admixture of construction and demolition waste (CDW) on the properties of tailings with an acidic character. According to the current practices of the application of CDW to thermal waste, it is used to fill up the depressions created, for example, by burning through hot spots, but this does not achieve the ultimate goal that would lead to a pH adjustment. One of the objectives was therefore to check whether a greater effect could be achieved by mixing CDW and unburnt tailings, and to check whether the percentage of unburnt tailings and CDW would have an effect on the final situation. In addition, unburnt mine waste could be used in line with the 3Rs (Reduce, Reuse, and Recycle) concept of the circular economy, thus removing old environmental burdens.
2. Materials and Methods
2.1. Description of the Mining Site
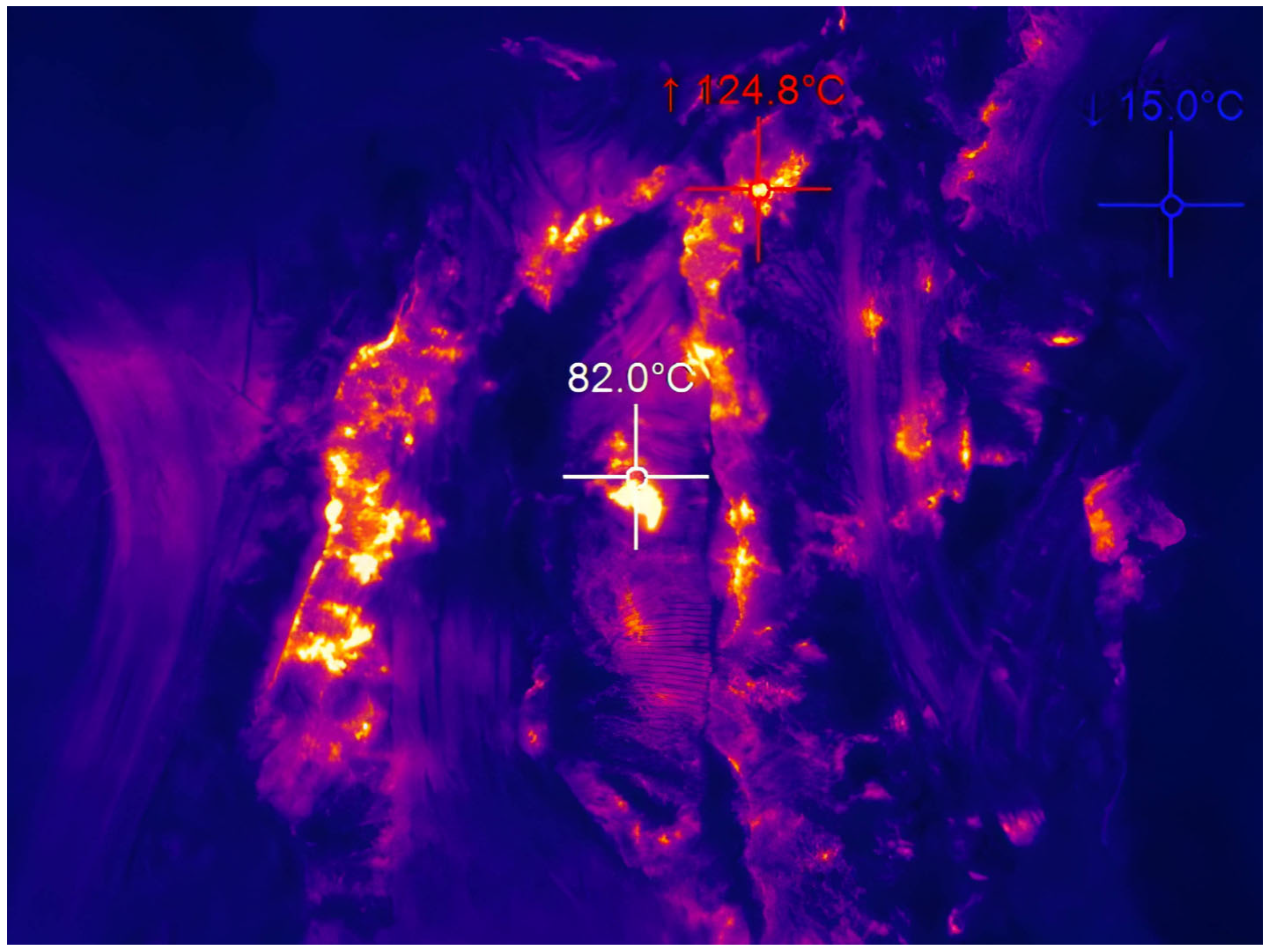
Tailings from the largest and most thermally active complex (Figure 2) in the entire Ostrava-Karvina Coalfield, the Heřmanice spoil heap (area of 881,993 m2, volume of 20,106 m3, repose height 20–30 m), have been chosen for the experiments. This is the youngest thermally active spoil heap of a terraced character, which was created from the mid-19th century, when tailings from the Ida Mine were deposited here. Intense thermal activity was recorded in the body of the spoil heap in 2004, when there were open fires at the roots of trees planted as part of remediation. In 2005, a grouting wall was built here to prevent these thermal processes, but the combustion process is still taking place here at this time. In 2009, the process of removal was started in order to build an air barrier between the rock and the burning material. Construction waste was also deposited here in an uncontrolled manner, but it was not incorporated into the tailings in any way.
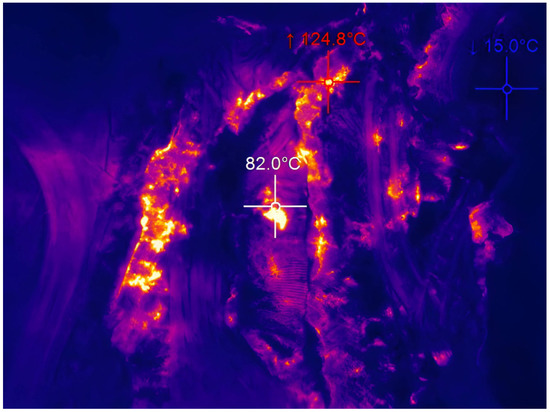
Figure 2.
Thermal activity in Heřmanice spoil heap.
2.2. Sampling and Pre-Analytical Steps
Sampling of tailings from the surface of the spoil heap body was carried out over its entire area (30 sampling points) where thermal activity is taking place. First, approx. 10–20 cm of the upper layer of the spoil heap, which was not part of the sample taken, was removed. Tailing samples weighing approx. 5 kg were taken from the depth of 20–50 cm. By careful mixing, the tailing samples from all the sampling points (see Figure 3) were homogenized into one complex sample, which was stored in a closable plastic container after cooling. The tailing samples were first dried in a laboratory at laboratory temperature (±23 °C) and then homogenized using a Retsch jaw crusher type BB200 WC (Haan, Germany). After homogenization, the tailings were sieved through a Retsch stainless steel sieve with the mesh size of 2 mm and subsequently dried to a constant weight in a vacuum dryer VO29 MEMMERT (Schwabach, Germany). The dried tailing samples were kept in a desiccator.

Figure 3.
Sampling points of Heřmanice spoil heap.
The samples of burnt tailings were taken from the place where the tailings are removed (points marked with a triangle) for the purpose of comparison of the chemical composition of burnt and unburnt tailings; see Figure 3. The procedure for treating burnt tailing samples is identical to the procedure for treating unburnt tailing samples, which is described above.
A sample of a mixture of construction and demolition waste was taken during the demolition of a civic amenity building, and it contained the remains of bricks, plaster, and tiles without admixture of heterogenous materials (remains of cables, paper, plastics). The samples were treated in the same way as the tailing samples.
A mixture of tailings and construction waste in different ratios was used to prepare samples for the determination of the physical-chemical parameters and sequential extraction analysis (SEA). The mixed samples contained tailings and a mixture of construction waste in the ratios of 9:1 (90% tailings + 10% construction mix), 7:3 (70% tailings + 30% construction mix) and 8:2 (80% tailings + 20% construction mix). A higher ratio of construction waste was not examined. The effort was to maintain a higher ratio of tailings, which is the main treated material.
2.3. Calculations and Instrumental Conditions
To recalculate the results, it was necessary to determine the dry matter. The determination was made using the gravimetric method. The calculation of dry matter and water content was carried out according to Equations (1) and (2).
Calculation of mass moisture content:
where:
- w
- —mass moisture content in %,
- m1
- —original sample weight in g,
- m2
- —sample weight after drying in g.
Calculation of dry matter:
where:
s = 100 − w,
- s
- —dry matter in %,
- w
- —mass moisture content in %.
Determination of the pH value in the aqueous leachate was carried out according to ČSN ISO 10390 (836221) Soil quality—Determination of pH.
Exchangeable acidity was determined according to ČSN EN ISO 14254 (836223) Soil quality—Determination of exchangeable acidity in calcium chloride leaching. The potential exchange reaction also includes adsorbed H+ protons and Al3+ and Fe3+ ions. An inoLab® pH 7110 laboratory pH meter from Xylem Analytics Germany Sales GmbH (Weilheim, Germany) was used to measure both parameters. When leaching protons from tailing samples using a neutral salt solution (CaCl2), it is not possible to displace all H+ protons from the sorption complex. Hydrolytically alkaline sodium acetate salts were therefore used to displace all bound protons.
Hydrolytic acidity Ha (mmol kg−1) was determined by titration using sodium acetate solution. Sodium ions displace hydrogen ions from the sorption complex, which form acetic acid in the tailing solution, the amount of which is determined by titration with a measured sodium hydroxide solution. The hydrolytic acidity was then calculated according to the equation (see Equation (3)):
where:
- Ha
- —hydrolytic acidity in mmol kg−1,
- a
- —consumption of NaOH during titration in mL,
- f
- —factor 0.1 M NaOH,
- c
- NaOH—concentration of NaOH,
- 1000
- —conversion to 1 kg of soil,
- K
- —correction to sodium acetate,
- g
- —weight of soil in g.
2.3.1. Sequential Extraction Analysis (SEA)
The reactivity or mobility of potentially toxic elements (PTEs) in soils, sediments, or other materials and their potential toxicity depends on the phase in which the risk element is contained, as well as the physical and chemical processes these phases undergo. A variety of sequential extraction types have been developed to determine PTE mobility. The number of individual steps in these types of sequential extractions is quite heterogeneous [22,23,24,25,26,27]. In our experiment, the sequential extraction analysis used was created by the European Commission in the program called Standards, Measurement and Testing Programme, formally called the BCR (Bureau Community of Reference). It is used in different modifications and consists of three steps [28,29,30,31,32,33,34,35]. After each step, i.e., after 16 h, the samples were centrifuged using an EBA 21 centrifuge from Schoeller Instruments (Prague, Czech Republic) to separate the solid and liquid phases. The centrifugation was carried out for 20 min at 3000× g rpm. The individual steps of the sequential BCR extraction analysis used are summarized in Table 2.

Table 2.
Overview of extraction agents and conditions for BCR analysis.
2.3.2. Analytical Methods
The F-AAS (Flame atomic absorption spectrometry) method was applied to determine the concentration of selected hazardous metals in individual fractions using an AAS contrAA® 700 atomic absorption spectrometer from Analytik Jena GmbH company (Jena, Germany).
The chemical composition of the samples was determined semi-quantitatively by X-ray fluorescence on the XEPOS (Spectro, Kleve, Germany) energy dispersion spectrometer. After trituration, the samples were placed in a plastic cuvette with a Mylar protective foil and then analyzed in a protective atmosphere (He).
The phase composition and microstructural properties were determined using X-ray powder diffraction (XRD) technique. XRD patterns were obtained using a Rigaku SmartLab diffractometer (Rigaku, Tokyo, Japan) with a D/teX Ultra 250 detector. The X-ray source was a Co tube (CoKα, λ1 = 0.178892 nm, λ2 = 0.179278 nm) operating at 40 kV and 40 mA. The powder samples were finely ground with agate mortar and pressed with a microscope glass in a rotating sample holder and measured in the reflection mode (Bragg-Brentano geometry) prior to the analysis. The samples were rotated (30 rpm) during the measurement to eliminate the preferred orientation effect. The XRD patterns were collected within the range of 2θ 5°–90° with a step size of 0.01° and a speed of 0.5° deg.min−1. The measured XRD patterns were evaluated using PDXL 2 software (version 2.4.2.0) and compared with the PDF-2 database, 2015 release (ICDD, Newton Square, Worcester, MA, USA).
3. Results and Discussion
Tailings as a secondary product of coal mining are no longer considered waste in the Czech Republic, according to Act No. 185/2001 Coll., on waste and on the amendment of certain other laws. The handling of tailings, their storage, and use are mainly covered by mining legislation (Act No. 89/2016 Coll. Act of the Federal Assembly on the Protection and Use of Mineral Resources, the so-called “Mining Act” as amended). An optimal proposal for landscape regeneration in the area affected by mining activity must be based primarily on information describing the impact of tailings from mining and coal processing on the environment.
3.1. Mineralogical-Petrographic Characteristics of Tailings
The mineralogical-petrographic characteristics of tailings are important for assessing their potential impact on the environment. The representation of different types of rocks in the tailings is determined by the layer unit in which coal was mined. The content of combustible substances in the spoil heap material affects both the petrographic composition and the granulometry. During the study of spoil heap material in the Ostrava-Karvina Coalfield (Czech Republic), it was proven that the petrographic composition of the tailings is practically identical within the individual spoil heaps. The main rock types on the spoil heaps are various types of aleuropelites (black and black-gray to gray siltstones to very fine-grained sandstones with root soil content), which come from the immediate vicinity of the coal seams. The decay of siltstone is relatively fast. Fine-grained, medium-grained to coarse-grained sandstones are also represented in the spoil heap material in large and variable quantities. These rocks are somewhat more resistant to weathering. In sandstones especially, the cement of their sand grains is corroded to form sand eluvia. The rate of disintegration of sandstones, therefore, depends on the nature of their cement. Sandstones with carbonate and clay cement are especially less resistant, while silicified sandstones are very stable. The most common type of carbonates is ankerite or a representative of the dolomite-ankerite isomorphic series. As a rock-forming mineral, ankerite occurs relatively rarely in the cement of some sandstones, while its occurrence is more common in seam parting. A significant part of the so-called pelosiderites is made up of ankerite rather than siderite. Calcite is very rare in Ostrava-Karvina Coal Area (OKCA) rocks. Small amounts of clay minerals (of the illite-smectite mixed structure type) may show swelling when in contact with water [36].
The mining material is characterized primarily by rocks and rock material (clay stones, mudstones, sandstones, etc.). There are also large amounts of silica (SiO2) in it, alumina (Al2O3), iron oxides (FexOy), potassium oxide (K2O), carbon (C) and calcium oxide (CaO), sodium oxide (Na2O), or titanium oxide (TiO2) [37,38]. The mineralogical composition of the tailings sample from the thermally active Heřmanice spoil heap is presented in Table 3. SiO2 is also the most abundant (43%), and the accompanying aluminosilicates are present in smaller amounts.

Table 3.
Mineralogical composition of tailings using XRD method in %.
3.2. Chemical Composition of Tailings
At the Heřmanice spoil heap, which is thermally active, both the tailings found in the upper part of the spoil heap body and the burnt tailings that are being removed have been analyzed. In unburnt tailings, which were in the centre of attention, the prevailing elements included Si (27%), Fe (25%), and Al (11%). It constituted the most decisive share of inorganic components. Considering the percentage presence of silicon, it can be assumed that acidification does not occur due to the loss of Si, but due to the lack of basic cations during the decomposition of aluminosilicates. The content of Si (48%) and Al (19%) in burnt tailings increased, while the content of Fe (15%) decreased.
In unburnt tailings from the surface of the Heřmanice spoil heap body, the total sulphur content was 3.5%. Raclavská et al. also states that the total sulphur content of the anhydrous sample in OKC coal is generally low, and that the average in the individual layers and areas are usually lower than 1%, and reach an average of 0.78% in working seams [36]. Pešek presents a much larger range of total sulphur values of 0.4–4.8% with a mean value of 2.1% [39]. In burnt tailings, the total sulphur content is reduced to 0.5% due to endogenous combustion. Pešek also states that similar to sulphur content, fluoride and chloride content is highly variable but generally low, and phosphorus, fluorides, and chlorides are bound to the occurrence of hydroxylapatite. However, the chloride content in the black coal of the Upper Silesian Basin is usually lower than 0.025% [39]. Unburnt tailings from the Heřmanice spoil heap contained 0.3% of P and 0.5% of chlorides. Their higher representation in tailings in the Heřmanice spoil heap may be related to secondary halite contamination. The content of elements such as Na (<0.01%), Ca (0.7%), Mg (0.2%) in the unburnt tailings is very low. Daniels, Stewart, and Zipper (2018) report that heavy metals such as copper, nickel, and zinc are often linked with pyrite and other sulphide minerals. Increased levels of heavy metals in the soil solution can be toxic to plant roots and microbes, and they can also pose a risk to water quality [40]. However, the content of heavy metals in the OKCA carboniferous rocks is very low and their representation does not exceed the volume of other industrial emissions from an ecological point of view. That is why it does not represent a serious environmental burden (see Table 4).

Table 4.
Elemental analysis of samples using XRF method in %.
Many older spoil heaps contain large amounts of coal fragments. These piles were often built in a loose, unconsolidated configuration that allowed oxygen to readily interact with the tailings. Due to the fact that the oxidation of pyrite is an exothermic reaction, spontaneous combustion occurs very often in older spoil heaps. Such thermally active tailing heaps then represent a local air quality problem and are virtually impossible to remediate unless the combustion is stopped. Modern spoil heaps generally have a lower coal content than older ones thanks to improved coal separation technologies, and they are compacted to reduce air and water intrusion. The requirement for a strong top layer of soil during the reclamation of spoil heaps should further limit the movement of oxygen into the spoil heap body. However, our experience with thermally active spoil heap Hedvika or Heřmanice indicates that significant oxidation of sulphur occurs in the spoil heaps even below 4 m of the top layer of soil. Daniels et al. had a similar experience [40].
3.3. Chemical Composition of Construction and Demolition Waste
In a circular economy, it is important to identify all possible ways of reusing resources, and construction and demolition waste is a rich source of a whole range of basic cations. The chemical composition of the used construction waste is shown in Table 5.

Table 5.
Chemical composition of construction and demolition waste.
The chemical composition of construction and demolition waste shows that the predominant elements are Si (45%) and Ca (27%). Since the content of heavy metals is also negligible and their representation does not exceed the volume of other industrial emissions from an ecological point of view, it therefore does not represent a serious environmental burden. Construction and demolition waste can therefore be used to prepare a suitable mixed material that could be used for the remediation of the immediate surroundings of thermally active spoil heaps. It can be used as a base layer on which soil would then be spread. In this way, a suitable base for remediation could be created. At the same time, a problematic spoil heap having a negative impact on its surroundings and the health of local residents would be removed as well.
3.4. Acidification of Tailings
Many of the environmental problems associated with rock material containing sulphide are related to the oxidation of pyrite and the occurrence of spontaneous acidification. A large part of the total sulphur contained in tailings is in the form of pyrite (FeS2) and other sulphides. They oxidize to sulphuric acid in the presence of water and oxygen. As a result of weathering, the pH values of the spoil heap body can drop dramatically to a very low pH value (2.0 to 3.5). Daniels et al. states that this highly acidified water can also have a pH value < 3.0. During its seeping into the deeper parts of the spoil heap body, it dissolves the surrounding mineral matrix, and is thus enriched with various metals (Al, Mn, etc.), cations and salts [40].
This chemical oxidation of pyrite (initiation reaction), where acidity is produced and thus Fe is released, can be in a simple way described as follows:
2 FeS2 + 7 O2 + H2O → 2 Fe2+ + 4 H+ + 4 SO42−
The oxidation of pyrite is catalyzed by acidophilic bacteria such as Acidithiobacillus ferrooxidans, which are present in coal seams and are capable of functioning even in environments with very low oxygen content (less than 1.0% partial pressure). This means that as long as acidic water penetrates through the spoil heap, pyrite oxidation will continue to occur even deep inside the body, regardless of efforts to restore vegetation and to stabilize the surface. This bio-chemical oxidation of Fe2+ to Fe3+, which is influenced by the bacteria of Acidithiobacillus ferrooxidans, whose metabolism starts to be fully manifested only at pH < 3.0, can be described in a simple way as follows:
The above presented reaction is a limiting factor in the oxidation of pyrite because the oxidation of Fe2+ to Fe3+ is slow under abiotic conditions.
The chemical oxidation of pyrite by bacterially produced Fe3+ ions, which remain in soluble form under acidic conditions and react chemically with the pyrite present to form sulphate and ferric ions, can be described as follows:
FeS2 + 14 Fe3+ + 8 H2O → 2 SO42− + 16 H+ + 15 Fe2+
At pH < 3.5, there is a chemical hydrolysis of Fe3+, with subsequent acidification of the environment, because under these conditions Fe3+ hydrolyzes to:
[Fe(OH)]2+ → [Fe(OH)2]+ → Fe(OH)3
Fe3+ + 3 H2O ↔ Fe(OH)3 + 3 H+
The above presented chemical reactions show that due to the acidic nature of the tailings, the leaching or dissolution of potentially hazardous metals can be much more intense if the tailings are improperly handled during spoil heap remediation. As a corrective measure, we therefore recommend increasing the neutralisation potential by mixing the tailings with DCW, especially in areas with higher pyrite concentrations or with the presence of jarosite, which can be an important indicator in this respect. Increasing the neutralisation potential may also lead to a slower kinetics of pyrite oxidation. Due to the total mass of pyrite in many tailing piles and the relatively slow rate of water movement through them, it can be expected that acid mine drainage (AMD) will be emitted for decades, if not longer [20]. This is the reason why any attempt to recultivate the surface of the spoil heap, e.g., by applying a layer of soil and subsequent forest recultivation is usually unsuccessful in the long term. The study of tailings acidification is also necessary, with regard to its massive use in the redevelopment and regeneration of the area affected by mining in mountainous landscapes.
Active acidity (pH/H2O), exchangeable acidity (pH/CaCl2), and hydrolytic acidity Ha were therefore monitored in all samples (Table 6).

Table 6.
Physical-chemical parameters of samples.
Active acidity (pH/H2O) is determined by the activity of H3O+ cations in the solution, the sources of which are dissociated mineral and organic acids, acid salts, and acidoids [41]. It has a direct physiological significance, as it fundamentally affects the bio-chemical processes taking place in the soil, such as the availability and intake of nutrients especially by plants, but also the activity of microorganisms. In an acidic environment, for example, the growth of plant roots can slow down due to the toxic effects of aluminium. From the values listed in Table 6, it can be seen that by mixing tailings with pH/H2O of 3.7 and CDW in a ratio of 9:1, the value of active acidity is significantly adjusted to a value of 6.1.
Exchangeable acidity was evaluated in all samples as well. It is caused by adsorbed H+ and Al3+ ions (or Fe ions), which are exchanged for basic ions by a neutral salt solution (international standard 0.01M CaCl2). Even this potential part of protons is released into the solution and can thus affect soil processes. The results clearly show that protons will most likely be bound to the sorption complex in tailings and CDW, because the exchangeable acidity value was lower by 0.3 in both tailings and CDW than in pH/H2O. In this case, it can be stated that even a small addition of CDW has a positive effect on the properties of the tailings. At the same time, it is evident that with the increasing addition of CDW, the exchange of adsorbed cations no longer occurs.
Based on the results of pH/H2O and pH/CaCl2 and Ha, all metals will be dissolved in the solution. Due to the very low limestone content, it cannot be expected that the pH will increase and the environment will turn alkaline. There will therefore be no deposition of metals due to the change in pH.
Taking into account the fact that even extraction of the material using a neutral salt solution (CaCl2) fails to displace the more tightly bound H+ ions in the sorption complex, the hydrolytic activity was therefore determined as well. It expresses the ability of the material to change the reaction of solutions of hydrolytically dissociable salts. Based on the obtained results, it is possible to confirm the hypothesis that a relatively large amount of H+ bound in the sorption complex will occur in pure tailings. If the tailings from the surface of the body were used in an untreated state to recultivate the surrounding terrain, this could have a negative effect on the physical, chemical, and biological properties of the soil as a result. The influence of pH is essential in the soil-forming process, because it affects weathering and substance flows, and the soil structure. It also has an effect on sorption, and thus on the availability and mobility of both nutrients and risk elements. Fe, Mn, B, Cu, Zn, and Al become the most available in an acidic environment [42,43]. If the pH value is too low, it is assumed that the potential plant growth will be inhibited by the phytotoxic effect of aluminium. In addition to that, the activity of microorganisms and zooedaphon is also slowed down, which can result in the deterioration of the soil structure [43].
3.5. Sequential Extraction Analysis
The mobility of elements is related to sorption processes on clay minerals, humus and iron, manganese and aluminium oxides. It also depends on the hydrothermal conditions, such as the temperature and humidity of the given locality [44]. Most authors [45,46,47,48] state that the uptake of metals by plants does not show a linear dependence on their total content in the environment, but above all on their availability. In order to understand the possible consequences for the environment, it is also necessary to know the form in which the metal is present in the environment. Since it is difficult to determine their exact form, methods that can determine to which fraction of the material the metal is bound are applied. It is a good compromise which can provide information about the risk of contamination [24,46,49,50,51,52,53].
Sequential extraction analysis (SEA) essentially simulates processes that normally occur in nature, such as acidification, reduction (e.g., in the subsoil), or oxidation (e.g., in the topsoil). Various extraction agents with increasing extraction strength are used for this purpose, in which solid matrices are leached. Although these processes do not inform about the release kinetics of potentially toxic elements (PTEs) from the solid matrix during natural processes, they can provide information about their total amount that is potentially available under specific environmental conditions [29,31,54,55,56,57].
A simplified three-step methodology established by the European Commission as part of the Standards, Measurement and Testing Program (BCR analysis) was applied in order to determine the bioavailability of metals present in tailings and their mixtures with CDW, which could be applied either with soil admixture or even separately to modify the terrain in the mountainous landscape. BCR analysis includes three steps, and therefore three basic fractions, into which metals are divided according to the form in which they are found. Based on the analysis of the overall composition of tailings, it has been found that it contains a higher amount of aluminium and iron. That is why the BCR analysis was also focused on these two metals.
3.5.1. Aluminium
Based on the study of acidity, it was proven that compared to burnt tailings (pH/H2O = 8.4, pH/CaCl2 = 8.9 and Ha = 1.4 mmol kg−1), unburned tailings show acidic properties (pH/H2O = 3.7, pH/CaCl2 = 3.6 a Ha = 205 mmol kg−1). In general, acidification helps to release aluminium from the parent rock minerals, so this potentially toxic aluminium can enter the soil solution, where it can become significantly phytotoxic. This problem could arise if unburnt tailings were covered with soil as part of reclamation. A high concentration of aluminium causes physiological problems to the root system, which can limit the growth of invasive plants in primary succession. In addition to the pH value, the ratio of Ca, Mg, and K cations to Al is also important. At a low ratio, aluminium settles at the exchange sites of cell membranes and thus prevents the active transport of ions. These processes, which cause the death of roots, poor intake of nutrients and overall weakening of the plant, are already present at a pH lower than 5.0 [58,59,60,61].
The results obtained by BCR analysis for aluminium are shown in Table 7, while the total aluminium content determined by XRF analysis in unburnt tailings was 111,600 mg Al kg−1 of dry matter. Aluminium toxicity is a potential factor limiting the growth of plants in acidic soils worldwide. Lindsay reported that the concentration of aluminium in soil ranges from 1 to 30% (10,000 to 300,000 mg Al kg−1) [62].

Table 7.
Results of BCR analysis for Al in mg kg−1 of dry matter.
During the first step of BCR analysis, the amount of released exchangeable metals and metals bound to carbonates can be assessed. The fraction bound to carbonates (soluble in water and acid) is very sensitive to changes in the pH value. The release of metals occurs at a pH value < 5.0, which means that the release of metals in the environment already takes place under weakly acidic conditions. This fraction is therefore the most dangerous for the environment. Carbonates and partly also oxides and hydroxides are dissolved in it [26,63,64,65]. The results of the BCR analysis clearly show that the increasing addition of CDW leads to stabilization of aluminium ions. Considering the acidic reaction of unburnt tailings (pH = 3.7), it can be assumed that aluminium present in the tailings will be adsorbed on the surface by weak electrostatic interactions, and will therefore be easily released into the environment by ion exchange.
Based on the second step of the BCR analysis (reducible fraction), it can be assessed whether Al present in the studied material is firmly bound to Fe/Mn oxides and hydroxides, which are very good metal sorbents. They can be released if the environment changes to an anoxic state (e.g., due to the activity of microorganisms). In strongly oxidizing conditions, this material degrades and the release of hazardous metal can occur [63,64,65,66,67,68]. The positive effect of CDW addition on the properties of unburnt tailings is visible in this step as well. Based on the results, it has been found that both in CDW and in unburnt tailings, aluminium will most likely be firmly bound to Fe oxides and hydroxides, considering its higher content in the sample (250,000 mg Fe kg−1).
Much stronger bonds already exist in the fraction bound to organic matter (including living organisms) and sulphides (oxidizable fraction). Considering the nature of the environment, Al is more likely to be bound to sulphides. The release of these metals can then occur in contact with oxygenated water [63,66,69]. Metals from this phase can be released in the environment, for example, during resuspension, which can be caused, for example, by spoil heap removal, but also during contact with richly oxygenated water.
The results of the BCR analysis have revealed that aluminium from the tailings will be released from both the reducible and from the oxidizable fractions, where it will be primarily bound to sulphides.
Aluminium is generally one of the problematic metals found in the environment. In tailings, it is stabilized in the form of feldspars, either K-feldspar (KAlSi3O8), Na-feldspar (NaAlSi3O8), or Ca-feldspar (CaAl2Si2O8). This explains the high content of aluminium in the sample (about 10%). It is a naturally occurring mineral in the Moravian-Silesian Region. This also supports the results in terms of Si content in the XRF analysis. Additional Si occurs in quartz, which is also abundant here. This fact was also confirmed by the XRF analysis.
Aluminium is generally very mobile in acidic environments. Thus, by creating a mixture of tailings and CDW, an increase in pH value can be achieved due to the higher calcium content in CDW (confirmed by XRF analysis). The released calcium ions (Ca2+) present in the CDW will displace the aluminium ions (Al3+) in the tailings, which will result in partial reduction in its availability and will therefore be stabilized. This can be simplified as follows:
CDW—Ca + Al3+ ↔ CDW—Al + Ca2+
Aluminium stabilisation can also take place by the formation of low-soluble aluminium compounds that are less toxic to plants. Their formation can be facilitated by the addition of various supplements, such as material rich in calcium or phosphate fertilisers. The stabilisation of aluminium using the calcium cations (Ca2+) present in CDW can also be mediated by their reaction with aluminium ions to form the less soluble compound Ca3Al2(OH)12, which is less soluble in acidic environments. The equation describing this stabilization may be as follows:
2 Al3+ + 3 Ca2+ + 12 OH− → Ca3Al2(OH)12
The addition of phosphate-based fertilizers can not only reduce the availability of aluminium in the reclaimed subsoil, but can also promote the growth of vegetation in the reclaimed area. The reactions are complex and can involve various interactions, including the precipitation and ion exchange processes. For example, when phosphate fertilisers are added, aluminium may combine with phosphate ions to form the low-soluble AlPO4. A simplified equation describing this stabilisation is as follows:
Al3+ + H2PO4− → AlPO4 + 2 H+
However, when applying phosphate fertilisers, a careful site-specific assessment/evaluation of the problem must always be carried out to ensure that, for example, surface water is not affected by the possible eutrophication.
3.5.2. Iron
According to its composition, iron in the tailings will occur mainly in the chalcophilic form (strong affinity to sulphur), which was confirmed by the BCR analysis (Table 8). In general, it can be said that the mobility of Fe is supported by reducing conditions and a low pH value. On the contrary, oxidizing conditions combined with high pH will support coagulation. High concentrations of iron and the associated toxic effects depend on the plant species and its stage of development. The accumulation of iron in leaves can cause damage to plant tissues and their death [47].

Table 8.
Results of BCR analysis for selected Fe in mg kg−1 of dry matter.
The results show that the lowest concentration of Fe (14 mg Fe kg−1 dry matter) was found in the exchangeable fraction (Fraction I). The forms of the exchangeable fraction are easily mobilized and the results lead to the conclusion that the iron in tailings will not be easily bioavailable. The total concentration of Fe in the tailings (250,000 mg kg−1) together with Si (267,000 mg kg−1) is among the most represented elements here, compared to the other elements. However, the Regional Screening Level (RSL) value for iron according to EPA for industrial areas (820,000 mg Fe kg−1) has not been exceeded [70].
On the basis of the results, it was found that the pH value of the mixture shows a basic character, which is why we assume, also on the basis of the Eh diagram, that Fe will occur mainly in oxides and hydroxides in CDW and in unburnt tailings. Furthermore, it can be seen that there is not much difference in the ratio in which the raw materials are mixed.
The relatively high concentration of Fe in the oxidizable fraction (2002 mg Fe kg−1) indicates that Fe is bound to sulphides in the tailings and is presumably caused by the high content of residual pyrite and sulphides in the dumped material. With the addition of 20% and 30% of CDW, Fe loses its affinity to sulphur and will probably be preferentially bound in the form of oxides and hydroxides in the mixture.
4. Conclusions
The use of a mine waste dump after deep coal mining causes a number of practical problems. The most important problem to be solved is thermal activity. The pace and dynamics of succession in burning coal waste dumps depends on the stage of the fire, topography, and character of the substrate. Coal waste varies in colour, from light gray to black. A large part of the incoming solar radiation is thus retained as heat, and in sunny weather, the temperature on the surface of the spoil heap often exceeds the air temperature several times, which is fatal for plants. Another problem related to the thermal activity of the spoil heaps is their burning temperature, which reaches up to 1300 °C in some hot spots, specifically at the Heřmanice spoil heap. The evaporated PTEs are mostly sorbed on the smallest particles of the generated ash during subsequent cooling (after entering the air). This results in larger particles being depleted of toxic metals. The remaining metals mainly enrich PMx due to sorption and condensation of vapours on fine PMx particles. Since the dust particle has the largest specific surface, the surface of the particles also has the highest concentration of toxic metals.
In the event that the body of the landfill contaminates its surroundings (PMx, AMD) and intensive thermal activity occurs, such as in the case of the thermally active Heřmanice spoil heap, it is most often recommended to completely remove it. If the spoil heap is completely removed, then the area can be considered as a perfectly normal foundation soil with regard to the location of the body and on the bedrock. Dynamically developing, more flexible, and advanced technologies make it possible to apply the 3R principle (reduction, reuse, and recycling) of the circular economy in practice to the mining industry as well. The excavated burnt tailings, together with a larger fraction of unburnt tailings, can be used as construction material for embankments, roads, railways, and other constructions. They can also be used as raw material for civil engineering, industry, and reclamation.
Forest reclamation and the so-called controlled succession are mainly used in the Ostrava-Karvina Coalfield. The land can be used, for example, as recreational areas, golf courses, hippodromes, city parks, construction sites, etc. In a circular economy, it is important to identify all possible ways of reuse of resources. Mixing alkaline construction waste with unburnt tailings from the surface of the spoil heap significantly affected its acidity, which has a positive effect on the increase of active and exchangeable acidity and the decrease of hydrolytic acidity. Construction and demolition waste (CDW) is also a rich source of a whole range of basic cations, which can be used to enrich the tailings. This can speed up and positively affect the reclamation of mountain landscapes. The excavated area can then be used, for example, for development in the form of residential units, shopping centres, manufacturing plants and many others. The area can also be used to build recreational centres, such as a tennis hall, football field, cultural facilities, and others.
Author Contributions
Conceptualization, E.P., T.D. and V.V.; methodology, E.P. and T.D.; validation, E.P., T.D. and V.V.; formal analysis, K.M., V.V. and T.D.; investigation, L.S., J.C., K.M. and L.B.; resources, J.C.; writing—original draft preparation, E.P., V.V., T.D. and L.S.; writing—review and editing, E.P. and T.D.; visualization, T.D.; supervision, E.P.; project administration, V.V. and J.C.; funding acquisition, J.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by: VSB-TUO, Faculty of Mining and Geology—grants number SP2022/57; VSB-TUO, Faculty of Mining and Geology—grants number SP2023/017. Project CZ.11.4.120/0.0/0.0/15_006/0000074 TERDUMP Cooperation VŠB-TUO/GIG Katowice on the survey of burning dumps on both sides of the common border.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hilson, G. Pollution Prevention and Cleaner Production in the Mining Industry: An Analysis of Current Issues. J. Clean. Prod. 2000, 8, 119–126. [Google Scholar] [CrossRef]
- Abramowicz, A.; Rahmonov, O.; Chybiorz, R. Environmental Management and Landscape Transformation on Self-Heating Coal-Waste Dumps in the Upper Silesian Coal Basin. Land 2021, 10, 23. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, H.; Yin, P.; Wu, L.; Li, G. Landscape Ecological Quality Assessment and Its Dynamic Change in Coal Mining Area: A Case Study of Peixian. Environ. Earth Sci. 2019, 78, 708. [Google Scholar] [CrossRef]
- de Fátima Ulbrich, K.; de Campos, C.E.M. Obtaining of Hematite from Industrial Steel Waste Using Dry-Milling and High Temperature. Clean. Eng. Technol. 2021, 5, 100327. [Google Scholar] [CrossRef]
- Bondarenko, V.; Kovalevs’ka, I.; Illiashov, M.; Pivnyak, G. (Eds.) Geomechanical Processes during Underground Mining: School of Underground Mining 2012; CRC Press: London, UK, 2012; ISBN 978-0-429-21674-9. [Google Scholar]
- Mi, J.; Yang, Y.; Zhang, S.; An, S.; Hou, H.; Hua, Y.; Chen, F. Tracking the Land Use/Land Cover Change in an Area with Underground Mining and Reforestation via Continuous Landsat Classification. Remote Sens. 2019, 11, 1719. [Google Scholar] [CrossRef]
- Jelínek, P.; Marschalko, M.; Lamich, D.; Yilmaz, I.; Zastěrová, P.; Bednárik, M.; Heviánková, S.; Kyncl, M.; Drusa, M.; Růčková, H. Monitoring and Analysis of Burning in Coal Tailing Dumps: A Case Study from the Czech Republic. Environ. Earth Sci. 2015, 73, 6601–6612. [Google Scholar] [CrossRef]
- Różański, Z. Fire Hazard in Coal Waste Dumps—Selected Aspects of the Environmental Impact. IOP Conf. Ser. Earth Environ. Sci. 2018, 174, 012013. [Google Scholar] [CrossRef]
- Establishing a Sustainable Mining Operation: An Overview-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0959652610003471 (accessed on 14 February 2023).
- Hendrychová, M.; Svobodova, K.; Kabrna, M. Mine Reclamation Planning and Management: Integrating Natural Habitats into Post-Mining Land Use. Resour. Policy 2020, 69, 101882. [Google Scholar] [CrossRef]
- Hendrychová, M. Reclamation Success in Post-Mining Landscapes in the Czech Republic: A Review of Pedological and Biological Studies. J. Landsc. Stud. 2008, 1, 63–78. [Google Scholar]
- Prakash, A.; Fielding, E.J.; Gens, R.; Van Genderen, J.L.; Evans, D.L. Data Fusion for Investigating Land Subsidence and Coal Fire Hazards in a Coal Mining Area. Int. J. Remote Sens. 2001, 22, 921–932. [Google Scholar] [CrossRef]
- Demirel, N.; Düzgün, Ş.; Emil, M.K. Landuse Change Detection in a Surface Coal Mine Area Using Multi-Temporal High-Resolution Satellite Images. Int. J. Min. Reclam. Environ. 2011, 25, 342–349. [Google Scholar] [CrossRef]
- Ciesielczuk, J.; Czylok, A.; Fabiańska, M.J.; Misz-Kennan, M. Plant Occurrence on Burning Coal Waste–A Case Study from the Katowice-Wełnowiec Dump, Poland. Environ. Socio-Econ. Stud. 2015, 3, 1–10. [Google Scholar] [CrossRef]
- Popovych, V.; Petlovanyi, M.; Henyk, Y.; Popovych, N.; Bosak, P. Efficiency of Vegetative Reclamation of Coal Spoil Heaps. Ecol. Eng. Environ. Technol. 2022, 23, 172–177. [Google Scholar] [CrossRef]
- Dulias, R. Landscape Planning in Areas of Sand Extraction in the Silesian Upland, Poland. Landsc. Urban Plan. 2010, 95, 91–104. [Google Scholar] [CrossRef]
- Stracher, G.B.; Prakash, A.; Sokol, E.V. (Eds.) Chapter 15—The Thermal History of Select Coal-Waste Dumps in the Upper Silesian Coal Basin, Poland. In Coal and Peat Fires: A Global Perspective; Elsevier: Boston, MA, USA, 2015; pp. 431–462. ISBN 978-0-444-59509-6. [Google Scholar]
- Zástěrová, P.; Marschalko, M.; Niemiec, D.; Durďák, J.; Bulko, R.; Vlček, J. Analysis of Possibilities of Reclamation Waste Dumps after Coal Mining. Procedia Earth Planet. Sci. 2015, 15, 656–662. [Google Scholar] [CrossRef]
- Home-Eurostat. Available online: https://ec.europa.eu/eurostat (accessed on 14 February 2023).
- Statistical Yearbook of the Czech Republic. Available online: https://www.czso.cz/csu/czso/statistical-yearbook-of-the-czech-republic-2022 (accessed on 14 February 2023).
- Communication From the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions towards a Circular Economy: A Zero Waste Programme for Europe; European Union: Brussels, Belgium, 2014.
- Mossop, K.F.; Davidson, C.M. Comparison of Original and Modified BCR Sequential Extraction Procedures for the Fractionation of Copper, Iron, Lead, Manganese and Zinc in Soils and Sediments. Anal. Chim. Acta 2003, 478, 111–118. [Google Scholar] [CrossRef]
- Kiratli, N.; Ergin, M. Partitioning of Heavy Metals in Surface Black Sea Sediments. Appl. Geochem. 1996, 11, 775–788. [Google Scholar] [CrossRef]
- Tessier, A.; Campbell, P.G.C.; Bisson, M. Sequential Extraction Procedure for the Speciation of Particulate Trace Metals. Anal. Chem. 1979, 51, 844–851. [Google Scholar] [CrossRef]
- Huang, S.-J.; Chang, C.-Y.; Mui, D.T.; Chang, F.-C.; Lee, M.-Y.; Wang, C.-F. Sequential Extraction for Evaluating the Leaching Behavior of Selected Elements in Municipal Solid Waste Incineration Fly Ash. J. Hazard. Mater. 2007, 149, 180–188. [Google Scholar] [CrossRef]
- Usero, J.; Gamero, M.; Morillo, J.; Gracia, I. Comparative Study of Three Sequential Extraction Procedures for Metals in Marine Sediments. Environ. Int. 1998, 24, 487–496. [Google Scholar] [CrossRef]
- Lã, O.R.; Barra, C.M.; do Amaral Sobrinho, N.M.B.; Mazur, N.; Velloso, A.C.X. Avaliação dos métodos de extração sequencial de Tessier, Keller e Miller na determinação de ferro nativo em três tipos de solos: Orgânico, brunizem e latossolo. Quím. Nova 2003, 26, 323–330. [Google Scholar] [CrossRef]
- Papassiopi, N.; Kontoyianni, A.; Vaxevanidou, K.; Xenidis, A. Assessment of Chromium Biostabilization in Contaminated Soils Using Standard Leaching and Sequential Extraction Techniques. Sci. Total Environ. 2009, 407, 925–936. [Google Scholar] [CrossRef] [PubMed]
- Nemati, K.; Bakar, N.K.A.; Abas, M.R. Investigation of Heavy Metals Mobility in Shrimp Aquaculture Sludge—Comparison of Two Sequential Extraction Procedures. Microchem. J. 2009, 91, 227–231. [Google Scholar] [CrossRef]
- Nemati, K.; Abu Bakar, N.K.; Sobhanzadeh, E.; Abas, M.R. A Modification of the BCR Sequential Extraction Procedure to Investigate the Potential Mobility of Copper and Zinc in Shrimp Aquaculture Sludge. Microchem. J. 2009, 92, 165–169. [Google Scholar] [CrossRef]
- Giacomino, A.; Abollino, O.; Malandrino, M.; Mentasti, E. The Role of Chemometrics in Single and Sequential Extraction Assays: A Review. Part II. Cluster Analysis, Multiple Linear Regression, Mixture Resolution, Experimental Design and Other Techniques. Anal. Chim. Acta 2011, 688, 122–139. [Google Scholar] [CrossRef]
- Gholami, L.; Rahimi, G. Chemical Fractionation of Copper and Zinc after Addition of Carrot Pulp Biochar and Thiourea-Modified Biochar to a Contaminated Soil. Environ. Technol. 2021, 42, 3523–3532. [Google Scholar] [CrossRef]
- De Silveira Pereira, W.V.; Teixeira, R.A.; de Souza, E.S.; de Moraes, A.L.F.; Campos, W.E.O.; do Amarante, C.B.; Martins, G.C.; Fernandes, A.R. Chemical Fractionation and Bioaccessibility of Potentially Toxic Elements in Area of Artisanal Gold Mining in the Amazon. J. Environ. Manag. 2020, 267, 110644. [Google Scholar] [CrossRef] [PubMed]
- Fractionation of Lead in Lignite Coal Samples of Thar Coalfield, Pakistan by Time-saving Single-step Based on BCR Sequential Extraction Scheme-Lashari-2020-Environmental Progress & Sustainable Energy-Wiley Online Library. Available online: https://aiche.onlinelibrary.wiley.com/doi/10.1002/ep.13439 (accessed on 14 February 2023).
- Zdeb, M.; Pawłowska, M.; Pacan, J. The Influence of Anaerobic Digestion on Selected Heavy Metals Fractionation in Sewage Sludge. J. Ecol. Eng. 2020, 21, 27–35. [Google Scholar] [CrossRef]
- Raclavská; Matýsek; Škrobánková. Problémové Parametry Při Posuzování Vyuřitelnosti Hlušin v OKR. Uhlí Rudy Geol. Průzkum 2003, 51, 20–26. [Google Scholar]
- Zloch, J.; Adamcová, D.; Šindelář, O.; Šourková, M.; Vaverková, M.D.; Zloch, J.; Adamcová, D.; Šindelář, O.; Šourková, M.; Vaverková, M.D. Testing of Phytotoxicity of Mining Waste to Determine the Direction of Future Development. AIMS Environ. Sci. 2020, 7, 324–334. [Google Scholar] [CrossRef]
- Łupieżowiec, M.; Rybak, J.; Różański, Z.; Dobrzycki, P.; Jędrzejczyk, W. Design and Construction of Foundations for Industrial Facilities in the Areas of Former Post-Mining Waste Dumps. Energies 2022, 15, 5766. [Google Scholar] [CrossRef]
- Pešek, J. Major and Minor Elements in the Hard Coal from the Czech Upper Paleozoic Basins; Czech Geological Survey: Prague, Czech Republic, 2010; ISBN 978-80-7075-741-3. [Google Scholar]
- Daniels, W.; Stewart, B.; Zipper, C. Reclamation of Coal Refuse Disposal Areas; Virginia Tech: Blacksburg, VA, USA, 1995. [Google Scholar]
- Onwuka, M.I.; Ozurumba, U.V.; Nkwocha, O.S. Changes in Soil PH and Exchangeable Acidity of Selected Parent Materials as Influenced by Amendments in South East of Nigeria. J. Geosci. Environ. Prot. 2016, 4, 80–88. [Google Scholar] [CrossRef]
- Soil Acidity. Available online: https://isbnsearch.org/isbn/9783642744440 (accessed on 21 February 2023).
- Handbook of Soil Acidity (Books in Soils, Plants & the Environment). Available online: https://isbnsearch.org/isbn/9780824708900 (accessed on 21 February 2023).
- The Meaning of Metal Toxicity in Soil-Plant Systems. In Toxic Metals in Soil Plant Systems; Wiley: Hoboken, NJ, USA, 1994; pp. 27–61.
- Wenzel, W.W.; Blum, W.E.H. Fluorine Speciation and Mobility in F-Contaminated Soils. Soil Sci. 1992, 153, 357–364. [Google Scholar] [CrossRef]
- Heavy Metals Testing in Soil Alloway. Available online: https://www.alloway.com/features/heavy-metals-testing-soil (accessed on 14 February 2023).
- ISBN 9781420093681-Trace Elements in Soils and Plants. Available online: https://isbnsearch.org/isbn/9781420093681 (accessed on 14 February 2023).
- Komárek, M.; Chrastný, V.; Ettler, V.; Tlustos, P. Evaluation of Extraction/Digestion Techniques Used to Determine Lead Isotopic Composition in Forest Soils. Anal. Bioanal. Chem. 2006, 385, 1109–1115. [Google Scholar] [CrossRef]
- Thomson, E.A.; Luoma, S.N.; Cain, D.J.; Johansson, C. The Effect of Sample Storage on the Extraction of Cu, Zn, Fe, Mn and Organic Material from Oxidized Estuarine Sediments. Water Air Soil Pollut. 1980, 14, 215–233. [Google Scholar] [CrossRef]
- Quevauviller, P.; Rauret, G.; Muntau, H.; Ure, A.M.; Rubio, R.; López-Sánchez, J.F.; Fiedler, H.D.; Griepink, B. Evaluation of a Sequential Extraction Procedure for the Determination of Extractable Trace Metal Contents in Sediments. Fresenius J. Anal. Chem. 1994, 349, 808–814. [Google Scholar] [CrossRef]
- Kersten, M.; Förstner, U. Effect of Sample Pretreatment on the Reliability of Solid Speciation Data of Heavy Metals—Implications Sesfor the Study of Early Diagenetic Processes. Mar. Chem. 1987, 22, 299–312. [Google Scholar] [CrossRef]
- Pueyo, M.; Rauret, G.; Lück, D.; Yli-Halla, M.; Muntau, H.; Quevauviller, P.; López-Sánchez, J.F. Certification of the Extractable Contents of Cd, Cr, Cu, Ni, Pb and Zn in a Freshwater Sediment Following a Collaboratively Tested and Optimised Three-Step Sequential Extraction Procedure. J. Environ. Monit. 2001, 3, 243–250. [Google Scholar] [CrossRef]
- Salomons, W.; Förstner, U. Trace Metal Analysis on Polluted Sediments. Environ. Technol. 1980, 1, 506–517. [Google Scholar] [CrossRef]
- Tokalioglu, S.; Kartal, S.; Birol, G. Application of a Three-Stage Sequential Extraction Procedure for the Determination of Extractable Metal Contents in Highway Soils. Turk. J. Chem. 2003, 27, 333–346. [Google Scholar]
- Leleyter, L.; Baraud, F. Évaluation de la mobilité des métaux dans les sédiments fluviaux du bassin de la Vire (Normandie, France) par extractions simples ou séquentielles. Comptes Rendus Geosci. 2005, 337, 571–579. [Google Scholar] [CrossRef]
- Reid, M.K.; Spencer, K.L.; Shotbolt, L. An Appraisal of Microwave-Assisted Tessier and BCR Sequential Extraction Methods for the Analysis of Metals in Sediments and Soils. J. Soils Sediments 2011, 11, 518–528. [Google Scholar] [CrossRef]
- Krasnodębska-Ostręga, B.; Pałdyna, J.; Kowalska, J.; Jedynak, Ł.; Golimowski, J. Fractionation Study in Bioleached Metallurgy Wastes Using Six-Step Sequential Extraction. J. Hazard. Mater. 2009, 167, 128–135. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1767. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Ahn, S.; Matsumoto, H. Inhibition of Growth and Development of Root Border Cells in Wheat by Al. Physiol. Plant. 2003, 117, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Rout, G.R.; Samantaray, S.; Das, P. Aluminium Toxicity in Plants: A Review. Agronomie 2001, 21, 3–21. [Google Scholar] [CrossRef]
- Rosseland, B.O.; Eldhuset, T.D.; Staurnes, M. Environmental Effects of Aluminium. Env. Geochem. Health 1990, 12, 17–27. [Google Scholar] [CrossRef]
- Chemical Equilibria in Soils. Available online: https://isbnsearch.org/isbn/9781930665118 (accessed on 21 February 2023).
- Gleyzes, C.; Tellier, S.; Astruc, M. Fractionation Studies of Trace Elements in Contaminated Soils and Sediments: A Review of Sequential Extraction Procedures. TrAC Trends Anal. Chem. 2002, 21, 451–467. [Google Scholar] [CrossRef]
- Fuentes, A.; Lloréns, M.; Sáez, J.; Soler, A.; Aguilar, M.I.; Ortuño, J.F.; Meseguer, V.F. Simple and Sequential Extractions of Heavy Metals from Different Sewage Sludges. Chemosphere 2004, 54, 1039–1047. [Google Scholar] [CrossRef]
- Rao, C.R.M.; Sahuquillo, A.; Lopez-Sanchez, J.F. Comparison of Single and Sequential Extraction Procedures for the Study of Rare Earth Elements Remobilisation in Different Types of Soils. Anal. Chim. Acta 2010, 662, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Pickering, W.F. Metal Ion Speciation—Soils and Sediments (A Review). Ore Geol. Rev. 1986, 1, 83–146. [Google Scholar] [CrossRef]
- Kheboian, C.; Bauer, C.F. Accuracy of Selective Extraction Procedures for Metal Speciation in Model Aquatic Sediments. Anal. Chem. 1987, 59, 1417–1423. [Google Scholar] [CrossRef]
- Van Herck, P.; Vandecasteele, C. Evaluation of the Use of a Sequential Extraction Procedure for the Characterization and Treatment of Metal Containing Solid Waste. Waste Manag. 2001, 21, 685–694. [Google Scholar] [CrossRef]
- CEP Consultants. Heavy Metals in the Environment: International Conference Heidelberg, September 1983; CEP Consultants: Melville, NY, USA, 1983; ISBN 978-0-905941-07-3. [Google Scholar]
- US EPA. Regional Screening Levels (RSLs). Available online: https://www.epa.gov/risk/regional-screening-levels-rsls (accessed on 21 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).