True Non-Contrast Phase versus Virtual-Non Contrast: “Lights and Shadows” of Dual Energy CT Angiography in Peripheral Arterial Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Study Design
2.2. CT Study Protocol
2.3. DE-CTA Image Reconstruction
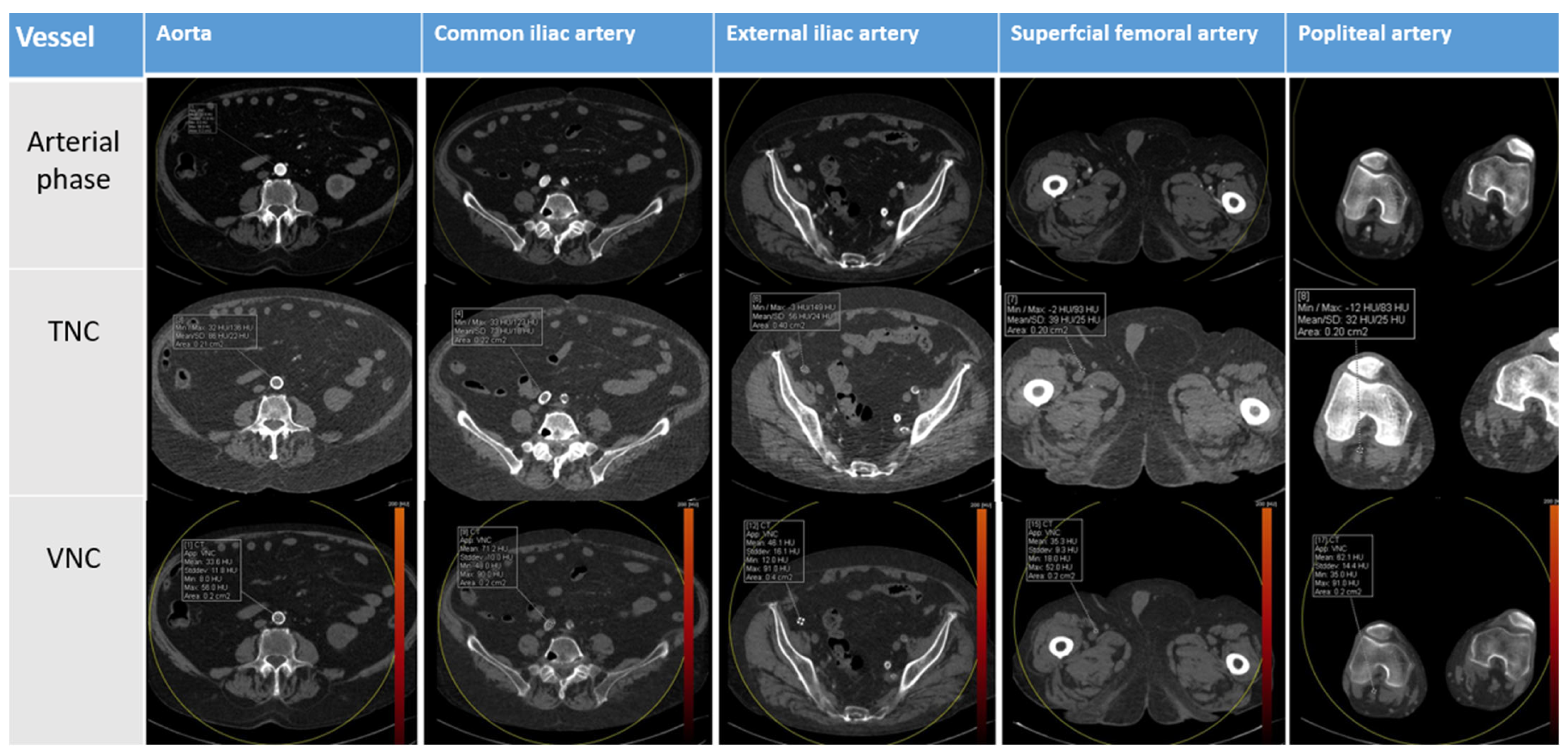
2.4. Image Analysis
2.5. Dose Radiation Calculation
2.6. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aday, A.W.; Matsushita, K. Epidemiology of Peripheral Artery Disease and Polyvascular Disease. Circ. Res. 2021, 128, 1818–1832. [Google Scholar] [CrossRef]
- Criqui, M.H.; Matsushita, K.; Aboyans, V.; Hess, C.N.; Hicks, C.W.; Kwan, T.W.; McDermott, M.M.; Misra, S.; Ujueta, F. Lower Extremity Peripheral Artery Disease: Contemporary Epidemiology, Management Gaps, and Future Directions: A Scientific Statement from the American Heart Association. Circulation 2021, 144, e171–e191. [Google Scholar] [CrossRef]
- Fowkes, F.G.; Rudan, D.; Rudan, I.; Aboyans, V.; Denenberg, J.O.; McDermott, M.M.; Norman, P.E.; Sampson, U.K.; Williams, L.J.; Mensah, G.A.; et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: A systematic review and analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Aboyans, V.; Ricco, J.B.; Bartelink, M.E.L.; Björck, M.; Brodmann, M.; Cohnert, T.; Collet, J.P.; Czerny, M.; De Carlo, M.; Debus, S.; et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteriesEndorsed by: The European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur. Heart J. 2018, 39, 763–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shwaiki, O.; Rashwan, B.; Fink, M.A.; Kirksey, L.; Gadani, S.; Karuppasamy, K.; Melzig, C.; Thompson, D.; D’Amico, G.; Rengier, F.; et al. Lower extremity CT angiography in peripheral arterial disease: From the established approach to evolving technical developments. Int. J. Cardiovasc. Imaging 2021, 37, 3101–3114. [Google Scholar] [CrossRef]
- Yadav, V.; Khanduri, S.; Yadav, P.; Pandey, S.; Tyagi, E.; Yadav, H.; Krishnam, A.; Hamza, M. Diagnostic Accuracy of Color Doppler and Calcium Scoring versus Dual-Energy Computed Tomography Angiography in the Assessment of Peripheral Arterial Diseases of Lower Limb. J. Clin. Imaging Sci. 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.C.; Levin, D.C.; Parker, L.; Rao, V.M. Have CT and MR angiography replaced catheter angiography in diagnosing peripheral arterial disease? J. Am. Coll. Radiol. 2015, 12, 909–914. [Google Scholar] [CrossRef]
- Met, R.; Bipat, S.; Legemate, D.A.; Reekers, J.A.; Koelemay, M.J. Diagnostic performance of computed tomography angiography in peripheral arterial disease: A systematic review and meta-analysis. JAMA 2009, 301, 415–424. [Google Scholar] [CrossRef]
- Napoli, A.; Anzidei, M.; Zaccagna, F.; Cavallo Marincola, B.; Zini, C.; Brachetti, G.; Cartocci, G.; Fanelli, F.; Catalano, C.; Passariello, R. Peripheral arterial occlusive disease: Diagnostic performance and effect on therapeutic management of 64-section CT angiography. Radiology 2011, 261, 976–986. [Google Scholar] [CrossRef]
- Itoga, N.K.; Kim, T.; Sailer, A.M.; Fleischmann, D.; Mell, M.W. Lower extremity computed tomography angiography can help predict technical success of endovascular revascularization in the superficial femoral and popliteal artery. J. Vasc. Surg. 2017, 66, 835–843.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyersohn, N.M.; Walker, T.G.; Oliveira, G.R. Advances in axial imaging of peripheral vascular disease. Curr. Cardiol. Rep. 2015, 17, 87. [Google Scholar] [CrossRef] [PubMed]
- Criqui, M.H.; Aboyans, V. Epidemiology of peripheral artery disease. Circ. Res. 2015, 116, 1509–1526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Fleisher, L.A.; Fowkes, F.G.; Hamburg, N.M.; Kinlay, S.; et al. AHA/ACC Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e686–e725. [Google Scholar] [CrossRef] [PubMed]
- Iezzi, R.; Santoro, M.; Marano, R.; Di Stasi, C.; Dattesi, R.; Kirchin, M.; Tinelli, G.; Snider, F.; Bonomo, L. Low-dose multidetector CT angiography in the evaluation of infrarenal aorta and peripheral arterial occlusive disease. Radiology 2012, 263, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Xu, L.; Yang, L.; Wang, R.; Hsieh, J.; Sun, Z.; Fan, Z.; Leipsic, J.A. Blooming Artifact Reduction in Coronary Artery Calcification by A New De-blooming Algorithm: Initial Study. Sci. Rep. 2018, 8, 6945. [Google Scholar] [CrossRef] [Green Version]
- De Santis, D.; De Cecco, C.N.; Schoepf, U.J.; Nance, J.W.; Yamada, R.T.; Thomas, B.A.; Otani, K.; Jacobs, B.E.; Turner, D.A.; Wichmann, J.L.; et al. Modified calcium subtraction in dual-energy CT angiography of the lower extremity runoff: Impact on diagnostic accuracy for stenosis detection. Eur. Radiol. 2019, 29, 4783–4793. [Google Scholar] [CrossRef]
- Schicchi, N.; Fogante, M.; Oliva, M.; Esposto Pirani, P.; Agliata, G.; Giuseppetti, G.M.; Giovagnoni, A. Radiation dose and image quality with new protocol in lower extremity computed tomography angiography. Radiol. Med. 2019, 124, 184–190. [Google Scholar] [CrossRef]
- Aschof, A.J.; Catalano, C.; Kirchin, M.A.; Krix, M.; Albrecht, T. Low radiation dose in computed tomography: The role of iodine. Br. J. Radiol. 2017, 90, 20170079. [Google Scholar] [CrossRef]
- Yang, L.; Sun, J.; Li, J.; Peng, Y. Dual-energy spectral CT imaging of pulmonary embolism with Mycoplasma pneumoniae pneumonia in children. Radiol. Med. 2022, 127, 154–161. [Google Scholar] [CrossRef]
- Foti, G.; Mantovani, W.; Faccioli, N.; Crivellari, G.; Romano, L.; Zorzi, C.; Carbognin, G. Identification of bone marrow edema of the knee: Diagnostic accuracy of dual-energy CT in comparison with MRI. Radiol. Med. 2021, 126, 405–413. [Google Scholar] [CrossRef]
- Foti, G.; Lombardo, F.; Guerriero, M.; Rodella, T.; Cicciò, C.; Faccioli, N.; Serra, G.; Manenti, G. Management of vertebral compression fractures: The role of dual-energy CT in clinical practice. Radiol. Med. 2022, 127, 627–636. [Google Scholar] [CrossRef]
- Agostini, A.; Borgheresi, A.; Carotti, M.; Ottaviani, L.; Badaloni, M.; Floridi, C.; Giovagnoni, A. Third-generation iterative reconstruction on a dual-source, high-pitch, low-dose chest CT protocol with tin filter for spectral shaping at 100 kV: A study on a small series of COVID-19 patients. Radiol. Med. 2021, 126, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Tagliati, C.; Lanza, C.; Pieroni, G.; Amici, L.; Carotti, M.; Giuseppetti, G.M.; Giovagnoni, A. Ultra-low-dose chest CT in adult patients with cystic fibrosis using a third-generation dual-source CT scanner. Radiol. Med. 2021, 126, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Agostini, A.; Borgheresi, A.; Mari, A.; Floridi, C.; Bruno, F.; Carotti, M.; Schicchi, N.; Barile, A.; Maggi, S.; Giovagnoni, A. Dual-energy CT: Theoretical principles and clinical applications. Med. Radiol. 2019, 124, 1281–1295. [Google Scholar] [CrossRef] [PubMed]
- Sanghavi, P.S.; Jankharia, B.G. Applications of dual energy CT in clinical practice: A pictorial essay. Indian J. Radiol. Imaging 2019, 29, 289–298. [Google Scholar] [CrossRef]
- Javadi, S.; Elsherif, S.; Bhosale, P.; Jensen, C.T.; Layman, R.R.; Jacobsen, M.C.; Le, O.; Jia, S.; Parikh, R.J.; Tamm, E.P. Quantitative attenuation accuracy of virtual non-enhanced imaging compared to that of true non-enhanced imaging on dual-source dual-energy CT. Abdom. Radiol. 2020, 45, 1100–1109. [Google Scholar] [CrossRef]
- Cicero, G.; Mazziotti, S.; Silipigni, S.; Blandino, A.; Cantisani, V.; Pergolizzi, S.; D’Angelo, T.; Stagno, A.; Maimone, S.; Squadrito, G.; et al. Dual-energy CT quantification of fractional extracellular space in cirrhotic patients: Comparison between early and delayed equilibrium phases and correlation with oesophageal varices. Radiol. Med. 2021, 126, 761–767. [Google Scholar] [CrossRef]
- Garnett, R. A comprehensive review of dual-energy and multi-spectral computed tomography. Clin. Imaging 2020, 67, 160–169. [Google Scholar] [CrossRef]
- Megibow, A.J.; Kambadakone, A.; Ananthakrishnan, L. Dual-Energy Computed Tomography: Image Acquisition, Processing, and Workflow. Radiol. Clin. North Am. 2018, 56, 507–520. [Google Scholar] [CrossRef]
- Huang, S.Y.; Nelson, R.C.; Miller, M.J.; Kim, C.Y.; Lawson, J.H.; Husarik, D.B.; Boll, D.T. Assessment of vascular contrast and depiction of stenoses in abdominopelvic and lower extremity vasculature: Comparison of dual-energy MDCT with digital subtraction angiography. Acad. Radiol. 2012, 19, 1149–1157. [Google Scholar] [CrossRef]
- Brockmann, C.; Jochum, S.; Sadick, M.; Huck, K.; Ziegler, P.; Fink, C.; Schoenberg, S.O.; Diehl, S.J. Dual-energy CT angiography in peripheral arterial occlusive disease. Cardiovasc. Interv. Radiol. 2009, 32, 630–637. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Park, S.H.; Park, S.; Hwang, J.H.; Kim, J.H.; Pak, S.Y.; Lee, K.; Schmidt, B. Imaging Findings of Peripheral Arterial Disease on Lower-Extremity CT Angiography Using a Virtual Monoenergetic Imaging Algorithm. J. Korean Soc. Radiol. 2022, 83, 1032–1045. [Google Scholar] [CrossRef]
- Kau, T.; Eicher, W.; Reiterer, C.; Niedermayer, M.; Rabitsch, E.; Senft, B.; Hausegger, K.A. Dual-energy CT angiography in peripheral arterial occlusive disease-accuracy of maximum intensity projections in clinical routine and subgroup analysis. Eur. Radiol. 2011, 21, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Klink, T.; Wilhelm, T.; Roth, C.; Heverhagen, J.T. Dual-Energy CTA in Patients with Symptomatic Peripheral Arterial Occlusive Disease: Study of Diagnostic Accuracy and Impeding Factors. Rofo 2017, 189, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Koo, B.J.; Won, J.H.; Choi, H.C.; Na, J.B.; Kim, J.E.; Park, M.J.; Jo, S.H.; Park, H.O.; Lee, C.E.; Kim, M.J.; et al. Automatic Plaque Removal Using Dual-Energy Computed Tomography Angiography: Diagnostic Accuracy and Utility in Patients with Peripheral Artery Disease. Medicina 2022, 58, 1435. [Google Scholar] [CrossRef]
- Almutairi, A.; Sun, Z.; Poovathumkadavi, A.; Assar, T. Dual Energy CT Angiography of Peripheral Arterial Disease: Feasibility of Using Lower Contrast Medium Volume. PLoS ONE 2015, 10, e0139275. [Google Scholar] [CrossRef]
- Tanaka, R.; Yoshioka, K.; Takagi, H.; Schuijf, J.D.; Arakita, K. Novel developments in non-invasive imaging of peripheral arterial disease with CT: Experience with state-of-the-art, ultra-high-resolution CT and subtraction imaging. Clin. Radiol. 2019, 74, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Cicero, G.; Ascenti, G.; Albrecht, M.H.; Blandino, A.; Cavallaro, M.; D’Angelo, T.; Carerj, M.L.; Vogl, T.J.; Mazziotti, S. Extra-abdominal dual-energy CT applications: A comprehensive overview. Radiol. Med. 2020, 125, 384–397. [Google Scholar] [CrossRef]
- Björck, M.; Earnshaw, J.J.; Acosta, S.; Bastos Gonçalves, F.; Cochennec, F.; Debus, E.S.; Hinchliffe, R.; Jongkind, V.; Koelemay, M.J.W.; Menyhei, G.; et al. Editor’s Choice—European Society for Vascular Surgery (ESVS) 2020 Clinical Practice Guidelines on the Management of Acute Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2020, 59, 173–218. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Park, H.J.; Kim, J.N.; Kim, M.S.; Hong, S.W.; Park, J.H.; Kang, C.H. Virtual non-contrast images from dual-energy CT angiography of the abdominal aorta and femoral arteries: Comparison with true non-contrast CT images. Br. J. Radiol. 2022, 95, 20220378. [Google Scholar] [CrossRef] [PubMed]
- Sommer, C.M.; Schwarzwaelder, C.B.; Stiller, W.; Schindera, S.T.; Stampfl, U.; Bellemann, N.; Holzschuh, M.; Schmidt, J.; Weitz, J.; Grenacher, L.; et al. Iodine removal in intravenous dual-energy CT-cholangiography: Is virtual non-enhanced imaging effective to replace true non-enhanced imaging? Eur. J. Radiol. 2012, 81, 692–699. [Google Scholar] [CrossRef]
- Toepker, M.; Moritz, T.; Krauss, B.; Weber, M.; Euller, G.; Mang, T.; Wolf, F.; Herold, C.J.; Ringl, H. Virtual non-contrast in second-generation, dual-energy computed tomography: Reliability of attenuation values. Eur. J. Radiol. 2012, 81, e398–e405. [Google Scholar] [CrossRef] [PubMed]
- Lehti, L.; Söderberg, M.; Höglund, P.; Nyman, U.; Gottsäter, A.; Wassélius, J. Reliability of virtual non-contrast computed tomography angiography: Comparing it with the real deal. Acta Radiol. Open 2018, 7, 205846011879011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.J.; Peng, J.; Wu, S.Y.; Wang, Z.J.; Wu, X.S.; Zhou, C.S.; Ji, X.M.; Lu, G.M. Liver virtual non-enhanced CT with dual-source, dual-energy CT: A preliminary study. Eur. Radiol. 2010, 20, 2257–2264. [Google Scholar] [CrossRef] [PubMed]
- Sauter, A.P.; Muenzel, D.; Dangelmaier, J.; Braren, R.; Pfeiffer, F.; Rummeny, E.J.; Noël, P.B.; Fingerle, A.A. Dual-layer spectral computed tomography: Virtual non-contrast in comparison to true non-contrast images. Eur. J. Radiol. 2018, 104, 108–114. [Google Scholar] [CrossRef]




| Demographic | n (Range) |
|---|---|
| 40 |
| 69 (41–93) |
| 77 (53–126) |
| 171 (158–193) |
| 25.7 (17–33.5) |
| Parameters | Single-Energy CT | DE-CT |
|---|---|---|
| Pitch | 0.6 | 0.4 |
| Slice collimation | 3 mm (acquisition 192 × 0.6 mm2) | 1 mm (acquisition 192 × 0.6 mm2) |
| Rotation time | 0.5 | 0.5 |
| Field of view (FOV) | 300 mm | 300 mm |
| Reconstruction slice thickness | 3 mm | 1 mm |
| Anatomical Region | Contrast Phase | Mean Attenuation (HU) | Standard Deviation (DS) | p |
|---|---|---|---|---|
| Aorta | TNC | 47.1 | 6.6 | <0.000 |
| VNC | 33.0 | 8.2 | ||
| AIC | TNC | 47.8 | 8.9 | <0.000 |
| VNC | 34.1 | 11.4 | ||
| AIE | TNC | 46.6 | 9.4 | <0.27 |
| VNC | 33.1 | 6.4 | ||
| AFS | TNC | 48.5 | 9.0 | <0.001 |
| VNC | 38.2 | 10.4 | ||
| AP | TNC | 49.4 | 7.8 | <0.000 |
| VNC | 40.7 | 9.6 |
| Parameters | TNC | VNC |
|---|---|---|
| CTDI vol | 3.7 (2.4–9.4) | 3.5 (1.1–6.6) |
| DLP (mGyxcm) | 546 (289–906) | 463.5 (150–871) |
| E (mSv) | 3.25 (1.72–5.39) | 2.76 (0.89–5.19) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Floridi, C.; Cacioppa, L.M.; Agliata, G.; Cellina, M.; Rossini, N.; Valeri, T.; Curzi, M.; Felicioli, A.; Bruno, A.; Rosati, M.; et al. True Non-Contrast Phase versus Virtual-Non Contrast: “Lights and Shadows” of Dual Energy CT Angiography in Peripheral Arterial Disease. Appl. Sci. 2023, 13, 7134. https://doi.org/10.3390/app13127134
Floridi C, Cacioppa LM, Agliata G, Cellina M, Rossini N, Valeri T, Curzi M, Felicioli A, Bruno A, Rosati M, et al. True Non-Contrast Phase versus Virtual-Non Contrast: “Lights and Shadows” of Dual Energy CT Angiography in Peripheral Arterial Disease. Applied Sciences. 2023; 13(12):7134. https://doi.org/10.3390/app13127134
Chicago/Turabian StyleFloridi, Chiara, Laura Maria Cacioppa, Giacomo Agliata, Michaela Cellina, Nicolo’ Rossini, Tommaso Valeri, Martina Curzi, Alessandro Felicioli, Alessandra Bruno, Marzia Rosati, and et al. 2023. "True Non-Contrast Phase versus Virtual-Non Contrast: “Lights and Shadows” of Dual Energy CT Angiography in Peripheral Arterial Disease" Applied Sciences 13, no. 12: 7134. https://doi.org/10.3390/app13127134
APA StyleFloridi, C., Cacioppa, L. M., Agliata, G., Cellina, M., Rossini, N., Valeri, T., Curzi, M., Felicioli, A., Bruno, A., Rosati, M., Candelari, R., & Giovagnoni, A. (2023). True Non-Contrast Phase versus Virtual-Non Contrast: “Lights and Shadows” of Dual Energy CT Angiography in Peripheral Arterial Disease. Applied Sciences, 13(12), 7134. https://doi.org/10.3390/app13127134






