Abstract
Waste generated in mushroom cultivation represents a valuable raw material with health benefits, the use of which contributes to a reduction in food waste and to the circular economy. Hydroethanolic extracts obtained by ultrasound-assisted extraction of four edible mushroom species (Agaricus bisporus var. Portobello, Boletus edulis, Lentinula edodes, and Pleurotus ostreatus) were characterized according to their antioxidant, antibacterial, and cosmeceutical potential. The extract with the best properties was incorporated into a cosmetic cream. Concerning the phenolic contents and antioxidant capacity, the extracts of A. bisporus and B. edulis stood out from the other two due to their higher levels. The compounds p-hydroxybenzoic and gallic acids were present in all mushrooms in the ranges of 0.010 to 2.554 µg/g DW and 0.032 to 0.112 µg/g DW, respectively. The extract of B. edulis inhibited all the bacterial isolates, with minimum inhibitory concentration values ranging from 5 to 20 mg/mL for Gram-positive and 10–20 mg/mL for Gram-negative strains. In the anti-hyaluronidase assay, A. bisporus extract (10 mg/mL) was the most effective, with 74.4 ± 7.5% enzyme inhibition, and was selected for incorporation into the cosmetic cream. The cream with A. bisporus extract showed significantly higher phenolic contents and antioxidant capacity than the cream without the extract. Overall, our results support the high value of mushroom reuse as a source of natural antioxidants in the cosmetic industry.
1. Introduction
According to the Food and Agriculture Organization (FAO), around one-third of the food produced for human consumption is lost or wasted along the food supply chain [1]. This large amount of waste represents a loss of valuable material that can be used as raw material for new products and applications, thus contributing to a circular economy [2,3]. The world production of edible mushrooms has been increasing year on year [4], representing an increase in the waste generated by this industry. Such wasted material includes mushrooms, caps, and stipes with shapes, sizes, or calibers that do not meet commercial standards (5–20% of the production weight), surplus production (≤5%), and spent mushroom substrate (>20% of the production weight) [5]. Nevertheless, this discarded material also offers enormous potential due to its richness in bioactive compounds [5].
The health benefits of mushrooms have been covered in several recent reviews [6,7,8,9]. Edible mushrooms are low in fat and cholesterol and high in fiber, making them a healthy food. In addition, mushrooms have been described to possess beneficial health effects such as gut microbiota modulation [10,11], reduction in brain oxidative stress [10], anti-obesogenic effects and liver steatosis prevention [12,13], anti-cancer [14,15], anti-inflammatory [16,17,18], and antimicrobial [19,20,21], among others [6,7,8]. Moreover, they have antioxidant, anti-hyaluronidase, anti-tyrosinase, anti-elastase, and anti-collagenase activity, making them suitable for use as cosmetic ingredients [22]. One of the most promising options to add value to food waste is the extraction of bioactive compounds, which aligns with the current market demands for health-promoting products [2]. Phenolic compounds extracted from mushroom waste can be used in the production of functional foods, dietary supplements, or nutraceuticals and used as natural antioxidants in the cosmetic industry [22,23].
The species Agaricus bisporus var. Portobello (brown button mushroom), Lentinula edodes (shiitake), and Pleurotus ostreatus (oyster mushroom) are some of the most cultivated and consumed mushrooms [24]. In addition, Boletus edulis (Porcini or King Bolete) is one of the most highly prized wild edible mushrooms, very appreciated for its flavor and texture. In the present work, we analyzed the phenolic contents and antioxidant capacity, the antibacterial properties, and the cosmeceutical potential of the hydroethanolic extracts of these mushroom species.
2. Materials and Methods
2.1. Mushroom Materials
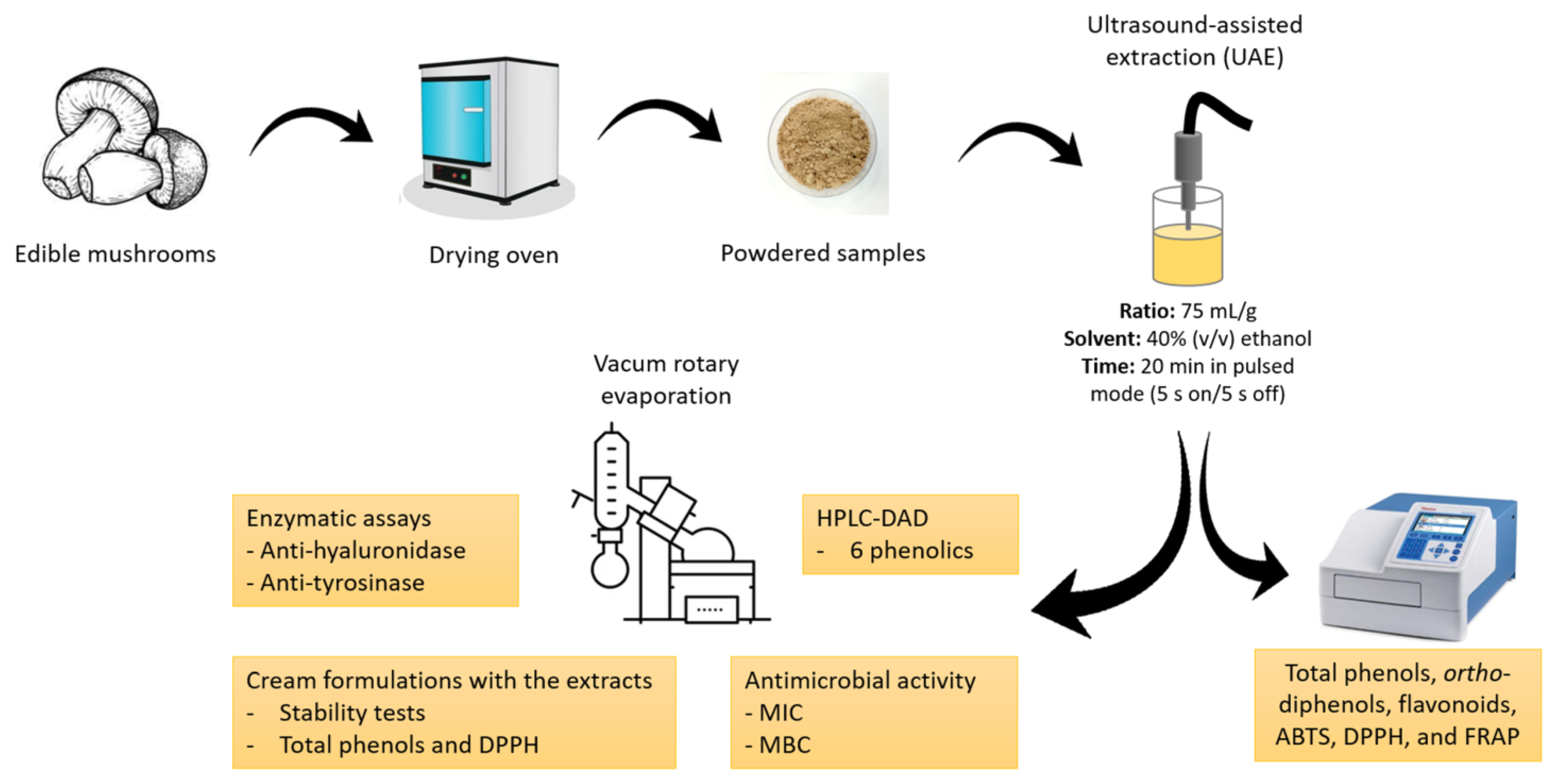
The mushrooms Agaricus bisporus var. Portobello, Lentinula edodes, and Pleurotus ostreatus were cultivated in greenhouses and obtained from the Portuguese mushroom producers Mogaricus Cogumelos (Mogadouro, Portugal), Aparência Primaveril (Meda, Portugal), and Floresta Viva (Amarante, Portugal), respectively. A. bisporus was produced in compost, and L. edodes and P. ostreatus were produced in lignocellulosic substrate based on wheat straw and sawdust. Boletus edulis was collected in a mixed forest in Sabugal, Guarda, Portugal. The mushrooms were dried at 40 °C in an oven (Termaks, Nordic Labtech AB, Gründau, Germany) and then ground to a fine powder. The samples were kept in the dark in hermetically sealed plastic bags until analysis (Figure 1).
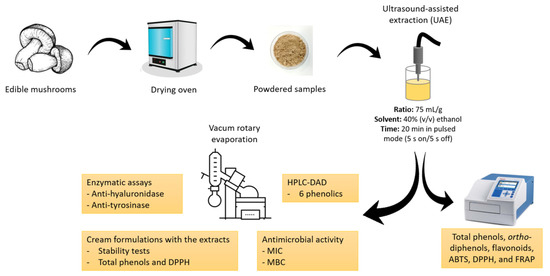
Figure 1.
Flow chart of the experimental design. ABTS, 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid); DPPH, 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl; FRAP, ferric-reducing antioxidant power; HPLC-DAD, high-performance liquid chromatography with diode array detector; MBC, minimum bactericidal concentration; MIC, minimum inhibitory concentration.
2.2. Mushroom Extracts
Mushroom extracts were obtained using ultrasound-assisted extraction, as previously described [25]. The solvent used was 40% (v/v) ethanol (Sigma Aldrich, St. Louise, MO, USA) according to previous analysis carried out by our group. This concentration proved to be better than the other tested regarding the extraction of phenolic compounds and antioxidant activity. Briefly, solid–liquid extractions were performed at a ratio of 1:75 (g:mL). The extraction was carried out for 20 min in a pulsed mode of 5 s on/5 s off cycles using an ultrasonic processor device (Hielscher UP400St, Berlin, Germany), with a sonotrode of 14 mm diameter, 400 Watts, 24 kHz, and adjustable amplitude (1:2.55). After completion of the extraction, the samples were centrifuged (4500× g for 20 min at 4 °C), and the supernatants were filtered (Whatman no. 4 filter paper), collected, and stored at −20 °C until analysis. All the experiments were performed in triplicate. Finally, the total extracted volume was concentrated in a vacuum rotary evaporator (IKA-RV 10, IKA, Staufen im Breisgau, Germany) at 38 °C to remove ethanol and stored at −20 °C before lyophilization to obtain the final extract.
2.3. Evaluation of Phenolic Composition
The total phenol, ortho-diphenol, and flavonoid contents were assessed using colorimetric and spectrophotometric methods based on the literature [26], with minor changes. The assays were adapted to microscale using 96-well microplates and a microplate reader (Multiskan GO Microplate Photometer, TermoFisher Scientific, Vantaa, Finland).
Total phenols. The content of phenolic compounds in the extracts was determined by mixing 20 μL of each sample extract, 100 μL of 10% (v/v) Folin–Ciocalteu reagent (Sigma Aldrich), and 80 μL of 7.5% (w/v) aqueous sodium carbonate (Sigma Aldrich) in a 96-well plate and then incubating at 42 °C for 30 min protected from the light. The absorbance was then measured at 750 nm. The calibration curve was generated using gallic acid (Sigma Aldrich) as a standard at concentrations ranging from 5 to 200 mg/L. The results were expressed as milligrams of gallic acid per gram of dry weight (mg GA/g DW).
Ortho-diphenols. The ortho-diphenol content was evaluated by mixing 160 μL of the diluted extracts with 40 μL of 5% (w/v) sodium molybdate solution (Sigma Aldrich) prepared with methanol/water (50:50, v/v). The plates were incubated at room temperature and protected from the light for 15 min. The calibration curve of this method was generated using caffeic acid (Sigma Aldrich) as a standard at concentrations ranging from 2.5 to 30 mg/L. The absorbance was read at 375 nm, and the results were expressed as milligrams of caffeic acid per gram of dry weight (mg CA/g DW).
Flavonoids. For the assessment of flavonoid content, 24 μL of the diluted extracts was added to 28 μL of 5% (w/v) sodium nitrite (Merck, Darmstadt, Germany). After a 5 min incubation at room temperature, 28 μL of a 10% (w/v) aluminum chloride (Merck) solution was added, and the mixture was left to react for 6 min. Then, 120 μL of 1 M sodium hydroxide (Merck) was added, and the mixture was shaken for 30 s. The calibration curve was generated using catechin (Sigma Aldrich) as a standard at concentrations ranging from 5 to 200 mg/L. The absorbance was read at 520 nm, and the results were expressed as milligrams of catechin per gram of dry weight (mg catechin/g DW).
2.4. Determination of Antiradical and Antioxidant Capacities
The antioxidant activity of sample extracts was determined using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS), 2,2-di(4-tert-octylphenyl)-1-picrylhydrazyl (DPPH), and ferric-reducing antioxidant power (FRAP) spectrophotometric methods, as reported by Yu et al. [27], with some modifications.
ABTS. For the preparation of ABTS + radical cations, an ABTS stock solution (7.0 mM) was prepared with ABTS salt (Sigma Aldrich). Then, 5 mL of the ABTS stock solution was mixed with 88 μL of a 148 mM solution of potassium persulfate (Sigma Aldrich) and diluted to a working solution with sodium acetate buffer (20 mM, pH 4.5), showing an absorbance of 0.70 ± 0.02 at 734 nm. Subsequently, 188 μL of ABTS working solution and 12 μL of sample dilutions or distilled water (blank) were mixed and reacted for 30 min at room temperature. The absorbance was read at 734 nm.
DPPH. A DPPH solution (8.87 mM) was prepared by diluting DPPH (Sigma Aldrich) in methanol (99.9%, v/v, Sigma Aldrich). Then, the DPPH solution was diluted to a working concentration with 70% (v/v) methanol to reach an absorbance of 1.000 at 520 nm. Then, 10 µL of sample dilutions or 70% methanol (v/v, blank) was added to 190 µL of the DPPH solution. The plate was allowed to rest in the dark, and the absorbance was read at 520 nm after 15 min of incubation.
For both assays, the inhibition percentage of the radicals was calculated using the following Equation (1):
FRAP. To measure the reducing capacity, a FRAP working solution was prepared by mixing 10-volume acetate buffer (300 mM, pH 3.6), 1-volume 2,4,6-tris(2-pyridyl)-s-triazine (TPTZ, Sigma Aldrich) (10 mM dissolved in hydrochloric acid), and 1-volume ferric chloride (20 mM in water, Sigma Aldrich). The solution was maintained at 37 °C for 10 min before use. Next, 20 μL of sample dilutions was added to each well of the microplate, followed by the addition of 280 μL of FRAP working solution. The reaction was incubated at 37 °C for 30 min, and the absorbance was read at 593 nm.
The three antioxidant assays were adapted to microscale using 96-well microplates and a microplate reader (Multiskan GO Microplate Photometer, TermoFisher Scientific, Vantaa, Finland), using Trolox (6-hydroxy-2,5,7,8-tetramethlychroman-2-carboxylic acid, Sigma Aldrich) as a standard at concentrations varying from 0.016 to 0.500 mM for ABTS, from 0.156 to 1.250 mM for DPPH, and from 0.039 to 1.250 mM for FRAP. All the results were expressed as micromole of Trolox per gram of dry weight (µmol Trolox/g DW).
2.5. Phenolic Compound Analysis Using High-Performance Liquid Chromatography with Diode Array Detector (HPLC-DAD)
The profile and content of polyphenols of each mushroom extract were analyzed using HPLC-DAD, as previously described [28], with minor modifications. Briefly, sample extracts (10 μL), in triplicate, were injected into a C18 column (250 × 4.6 mm, 5 μm particle size; ACE HPLC Columns, Advanced Chromatography Technologies Ltd., Aberdeen, Scotland, UK) with water with 0.1% of trifluoroacetic acid (TFA, Sigma Aldrich) (solvent A) and acetonitrile (Sigma Aldrich) with 0.1% TFA (solvent B) as eluent. The elution was performed at a flow rate of the solvent of 1 mL/min, with a gradient starting from 0% solvent B at 0 min, 0% solvent B at 5 min, 20% solvent B at 15 min, 50% solvent B at 30 min, 100% solvent B at 45 min, 100% solvent B at 50 min, 0% solvent B at 55 min, and 0% solvent B at 60 min. Chromatograms were recorded in a 200–600 nm range. The identification of individual polyphenols was assessed using the following parameters: UV spectra and UV maximum absorbance bands, peak retention time, external standards, and the literature. The external standards used were catechin, epigallocatechin gallate, gallocatechin gallate, gallic acid, p-hydroxybenzoic acid, and naringenin (Extrasynthese, Lyon Nord, Genay Cedex, France). Naringin (internal standard; Extrasynthese) was run simultaneously with the samples. All standards (1 mg/mL; 70% (v/v) methanol) were freshly prepared and injected into the HPLC system before the samples. The concentrations of the individual compounds were determined using the internal standard method and expressed as µg/g DW.
2.6. Antibacterial Activity
Clinical isolates were collected from patients hospitalized in the Hospital Center of Trás-os-Montes and Alto Douro (CHTMAD), Portugal. Ethical approval was granted by the Ethics Committee of CHTMAD. These strains belong to MJH and MJMC collections and are stored at −70 °C in aliquots of brain–heart infusion medium with 15% (v/v) glycerol. The antimicrobial activity of the mushroom extracts was assessed using Gram-positive and Gram-negative bacteria obtained from wound exudates. All the strains were identified by morphological and biochemical tests (Gram staining, morphological identification of colonies, conventional biochemical identification methods, and MicroScan WalkAway identification panels), followed by Kirby–Bauer antibiotic sensitivity assays with different antibiotics. Escherichia coli (E. coli; ATCC 25922) and Staphylococcus aureus (S. aureus; ATCC 25923) strains were obtained from the American Type Culture Collection.
Minimum inhibitory concentration (MIC). The MIC was evaluated using the microdilution assay, as reported by Taofiq et al. [29], with minor changes. Initially, each extract was dissolved in 50% dimethyl sulfoxide (DMSO, Sigma Aldrich) to reach a final concentration of 220 mg/mL. Then, 50 μL of the extract solution in 50% DMSO at 220 mg/mL was added to 450 μL of Mueller–Hinton Broth (MHB) medium (Oxoid, Basingstoke, United Kingdom), making a solution with a final concentration of 22 mg/mL at 5% DMSO. Subsequently, 190 μL of this extract solution was added to the first well of each column of the 96-well microplate (Orange Scientific, Braine-l’Alleud, Belgium) and submitted to successive dilutions over the remaining wells containing 90 μL of MHB medium, starting by withdrawing a volume of 100 μL from the well containing the highest concentration and adding it to the well just below and thus repeating this procedure sequentially. Afterwards, 100 μL of inoculum (1.5 × 108 CFU/mL) was diluted in 9.9 mL of MHB medium. Then, 10 μL of this solution was added to all the wells, achieving a final volume of 100 μL in each well and concentrations ranging from 20 to 0.156 mg/mL. A negative control was prepared with MHB 5% DMSO. The positive control used was gentamicin (Applied Chemical Laboratories, Basel, Switzerland). The microplates were left at 38 °C and incubated for 24 h in a heated incubator (MIR-162-PE, Panasonic, Japan). The MICs of each extract were determined by adding 2,3,5-triphenyltetrazolium chloride (TTC, Merck; 0.2 mg/mL, 40 μL) to each well and incubating the microplates at 38 °C for 2 h. In the presence of viable bacteria, the colorless TTC dye is reduced to formazan (red color). The lowest extract concentration that prevented the color change, i.e., that shows complete inhibition of microbial growth, is defined as the MIC [30].
Minimum bactericidal concentration (MBC). The assessment of MBC, the lowest concentration of mushroom extract that kills 99.9% of the bacterial populations tested, was carried out as previously described [21]. Briefly, the content from each well without changes in color determined from the previous assay was plated on Mueller–Hinton Agar (MHA) medium (Oxoid, Basingstoke, United Kingdom) and left at 38 °C for 24 h. After this subculturing, the MBC was defined considering the lowest concentration that yielded no growth.
2.7. Anti-Hyaluronidase Activity
The hyaluronidase inhibition was evaluated using a turbidimetric method adapted to the microscale, as reported in [31], with minor changes. In brief, 20 µL of the samples (extracts dissolved in distilled water at 0.1, 1, or 10 mg/mL) was added to 20 µL of enzyme diluent (20 mM sodium phosphate with 77 mM NaCl and 0.1 mg/mL of albumin (Sigma Aldrich); pH 7; 37 °C) and 20 µL of the Type I-S hyaluronidase from bovine testes (ref. H3506, Sigma Aldrich) (40–100 U/mL in the same enzyme diluent). The mixtures were left at 37 °C for 10 min. After the incubation, 20 µL of hyaluronic acid (ref. H7630, Sigma Aldrich) (0.5 mg/mL in 300 mM sodium phosphate buffer; pH 5.35; 37 °C) was added. The hyaluronic acid solution was heated to 90–95 °C until the hyaluronic acid dissolved completely, and then the solution was placed in a water bath and cooled to 37 °C. The mixtures were incubated at 37 °C for 45 min, after that, 100 µL of acid albumin solution (2 mg/mL in 79 mM acetic acid with 24 mM sodium acetate; pH 3.75; 25 °C) was added to precipitate undigested hyaluronic acid. The reaction mixtures were left at room temperature for 10 min, and then the turbidance was measured at 600 nm in a microplate reader (Multiskan GO 1510, Thermo Fisher Scientific, Vantaa, Finland). For the assay, 5 blank (B1 to B5) samples were prepared according to Paczkowska-Walendowska et al. [32]:
- -
- B1: 20 µL of distilled water + 20 µL of enzyme diluent + 20 µL of enzyme diluent + 20 µL of 300 mM sodium phosphate buffer + 100 µL of acid albumin solution;
- -
- B2: 20 µL of distilled water + 20 µL of enzyme diluent + 20 µL of enzyme diluent + 20 µL of hyaluronic acid solution + 100 µL of acid albumin solution;
- -
- B3: 20 µL of distilled water + 20 µL of enzyme diluent + 20 µL of hyaluronidase enzyme solution + 20 µL of hyaluronic acid + 100 µL of acid albumin solution.
For each sample, the following blanks were prepared:
- -
- B4: 20 µL of sample + 20 µL of enzyme diluent + 20 µL of hyaluronidase enzyme solution + 20 µL of 300 mM sodium phosphate buffer + 100 µL of acid albumin solution;
- -
- B5: 20 µL of sample + 20 µL of enzyme diluent + 20 µL of enzyme diluent + 20 µL of hyaluronic acid solution + 100 µL of acid albumin solution.
The percentage of hyaluronidase inhibition was calculated using the following Equation (2):
where AS—absorbance of the sample, AB1—absorbance of blank 1, AB3—absorbance of blank 3, AB4—absorbance of blank 4, AB5—absorbance of blank 5.
The half inhibitory concentration (IC50) was calculated by regression of a dose–response curve. Three independent experiments were carried out in triplicate.
2.8. Anti-Tyrosinase Activity
The anti-tyrosinase activity was determined according to a method formerly described [33], with slight changes. The ethanolic extracts of each mushroom were dissolved in 50% DMSO at a final concentration of 0.1, 1, or 10 mg/mL. The assay was executed in a 96-well microplate by adding 40 µL of mushroom tyrosinase enzyme (Sigma Aldrich) (1000 U/mL), 80 µL of phosphate buffer (50 mM, pH 6.5), and 40 µL of the extracts properly diluted (or control). For each sample (or control), a blank containing all the reaction mixture components except the tyrosinase enzyme (replaced by phosphate buffer) was included. In this assay, 50% DMSO instead of sample was used as a negative control and 1 mg/mL Kojic acid (Sigma Aldrich) (dissolved in distilled water) was used as a positive control. The microplates were incubated at 30 ± 1 °C for 10 min, and then 40 µL of L-tyrosine solution (0.2 mg/mL prepared in distilled water and slightly heated in a water bath until complete dissolution) was added. Then, the reaction was incubated in the oven at 30 ± 1 °C for 60 min, and the absorbances were read at 475 nm. The Equation (3) was used to calculate the percentage of tyrosinase inhibition:
where C1—absorbance of the control with enzyme, C2—absorbance of the control without enzyme, S1—absorbance of sample with enzyme, S2—absorbance of sample without enzyme.
The half inhibitory concentration (IC50) was calculated by regression of a dose–response curve. Three independent experiments were carried out in triplicate.
2.9. Cosmetic Cream Formulations with the Extracts
The extract with the best cosmeceutical properties was incorporated into a cream with natural ingredients prepared using a commercial kit (Kit Crema Facial Antiedad, purchased from Gran Velada, Zaragoza, Spain). Accordingly, the selected extract was added to the formulation at a percentage of 0.2%. As a control, a cream without extract (0%) was used and is hereinafter referred to as foundation (FD).
2.9.1. Stability Tests
Four batches containing two creams (with and without extract) were prepared and analyzed as follows:
Centrifuge Test. The cream stabilities were assessed as described by Rodrigues Ueoka and co-workers [34]. Briefly, to evaluate possible instabilities of the creams, such as phase separation, precipitation, sedimentation, and coalescence, a centrifugation method was carried out at 3000 rpm for 30 min (Hettich Benchtop centrifuge UNIVERSAL 320, Tuttlingen, Germany).
Thermal Stability Tests. An accelerated stability test to check physicochemical variations was also performed using heating and cooling cycles, as previously described [35], with some modifications. One batch containing two creams (with and without extract) was placed at 4 °C for 24 h and immediately put at 40 °C for another 24 h. This step was repeated four times, completing a period of 8 consecutive days. For the storage test, 3 batches containing 2 creams each (with and without extract) were kept at 4 °C, 25 °C, or 40 °C for 30 days. Prior to evaluation, all the samples were left to achieve room temperature.
pH Measurements. The determination of pH values was conducted using a VWR pHenomenal™ MU 6100 L meter (Darmstadt, Germany). The measurements were taken 24 h after the preparation of the cream, after the heating and cooling cycles and after the 30-day storage assay at different temperatures. For the measurement, the samples were previously diluted to 10% (w/v) in distilled water.
2.9.2. TPC and Antioxidant Activity of the Creams
The sample preparation for the determination of TPC and antioxidant activity using the DPPH assay was performed as reported by others [36], with slight changes. Shortly, 1 g of cream was diluted in 10 mL of 40% (v/v) ethanol and then centrifuged at 4500 rpm for 30 min and filtered. Then, the quantifications of TPC and antioxidant activity using the DPPH assay of the creams were evaluated as defined above for the extracts. The TPC was expressed as milligrams of GA per 1 g of cream, and the antioxidant activity by DPPH was expressed as µM of Trolox per 1 g of cream. The measurements were taken in triplicate, 24 h after the preparation of the cream.
2.10. Statistical Analysis
All the measurements were conducted in triplicate. Statistical analyses were performed using the GraphPad Prism software (San Diego, CA, USA) for Windows (version 7). The results of the samples are presented as mean ± standard deviation (SD) (n = 3). Differences between samples were evaluated with the non-parametric Mann–Whitney U test considering a significance level of p < 0.05.
3. Results and Discussion
3.1. Phenolic Contents and In Vitro Antioxidant Capacity
The TPC, ortho-diphenols, and flavonoids present in the ethanolic extracts of the studied species are shown in Table 1. Regarding the TPC contents, all extracts were significantly different from each other (p < 0.05). The extract of B. edulis had the highest TPC values followed by A. bisporus, while L. edodes and P. ostreatus extracts presented the lowest contents. Bach et al. [19] quantified the TPC levels of A. bisporus, both white and Portobello varieties, and L. edodes, obtaining similar values to ours. In a previous investigation, B. edulis also presented the highest contents of phenolics, followed by A. bisporus and then by P. ostreatus [18]; nevertheless, the TPC levels obtained were lower than ours. Garcia et al. [20] evaluated the TPC levels in the aqueous and methanolic extracts of two varieties of L. edodes, Donko and Koshin, obtaining lower TPC levels compared to our results, except in the aqueous extract of the var Koshin, which was similar. In another work of Garcia et al. [21], the TPC values of B. edulis aqueous and methanolic extracts were considerably higher than ours. Concerning the ortho-diphenol levels (Table 1), both B. edulis and L. edodes extracts showed the highest contents (p < 0.05), differing significantly from A. bisporus and P. ostreatus. In the work of Garcia et al. [20], the contents of ortho-diphenols found in the two varieties of L. edodes were considerably lower compared to ours, independently of the extraction solvent used to obtain the extract. In turn, Garcia et al. [21], showed that the ortho-diphenol values in B. edulis aqueous and methanolic extracts were much higher than ours. The use of different extraction approaches may be responsible in part for the differences between our results and those obtained in previous studies for both TPC and ortho-diphenols, which influence the concentrations of these two parameters. Regarding the flavonoid levels, A. bisporus extract had the highest contents (p < 0.05), while P. ostreatus displayed the lowest values. All extracts were significantly different from each other (p < 0.05). Palacios et al. [18] showed that B. edulis had the highest flavonoid content, followed by A. bisporus and then by P. ostreatus.

Table 1.
Phenolic composition and antioxidant activity of ethanolic extracts from four edible mushrooms.
To evaluate the capacity of the mushroom extracts to scavenge ABTS radical cation (ABTS+) and DPPH radical (DPPH·) and to reduce ferric (III) iron to ferrous (II) iron, in vitro antioxidant activity assays (Table 1) were performed. Globally, for the three assays, the extract of A. bisporus presented the best results (p < 0.05), followed by B. edulis, L. edodes, and then by P. ostreatus, which presented the lowest antioxidant capacity. For the ABTS and DPPH assays, all extracts showed significant differences between them (p < 0.05), while in the FRAP assay, A. bisporus and B. edulis had similar reducing powers. Bach et al. [19] also showed that A. bisporus has a higher antioxidant capacity compared to L. edodes. Furthermore, Reis et al. [37] demonstrated that A. bisporus methanolic extracts provided the highest DPPH values versus L. edodes and P. ostreatus. In another study, A. bisporus and P. ostreatus ethanolic extracts had the highest DPPH values and reducing power, while L. edodes exhibited the lowest antioxidant activity [29].
In general, the extracts of A. bisporus and B. edulis stand out from the other two mushroom species due to their high phenolic contents and antioxidant capacity, while P. ostreatus always had the lower levels. Despite this, all mushroom extracts showed a phenolic-rich composition and antioxidant capacity, making them suitable to be incorporated, for example, into functional foods [23,38]. In fact, the mushroom supplementation of bakery products, pasta, noodles, or rice porridge has been addressed in a few studies [38,39,40,41,42,43]. The addition of A. bisporus, L. edodes, and B. edulis powder was shown to enhance the TPC levels and the antioxidant capacity of mushroom-powder-supplemented pasta and wheat bread [41,42]. Moreover, the supplementation with these mushrooms reduced the rate of reducing sugars released during the in vitro digestion of cooked pasta or bread, possibly due to the increase in dietary fiber provided by the mushrooms [41,42]. The use of mushroom by-products can thus be an important way of enriching food products while contributing to waste reduction and a circular economy. In addition to the possible utilization of mushroom waste to produce functional foods, the antioxidant capacity and phenolic contents of these raw materials are also characteristics that make them good candidates for the design of cosmeceutical formulations, since phenolic compounds are potent antioxidant molecules able to scavenge skin reactive oxygen species (ROS), thus contributing to the anti-aging effects of cosmetics [44,45].
3.2. Phenolic Profile
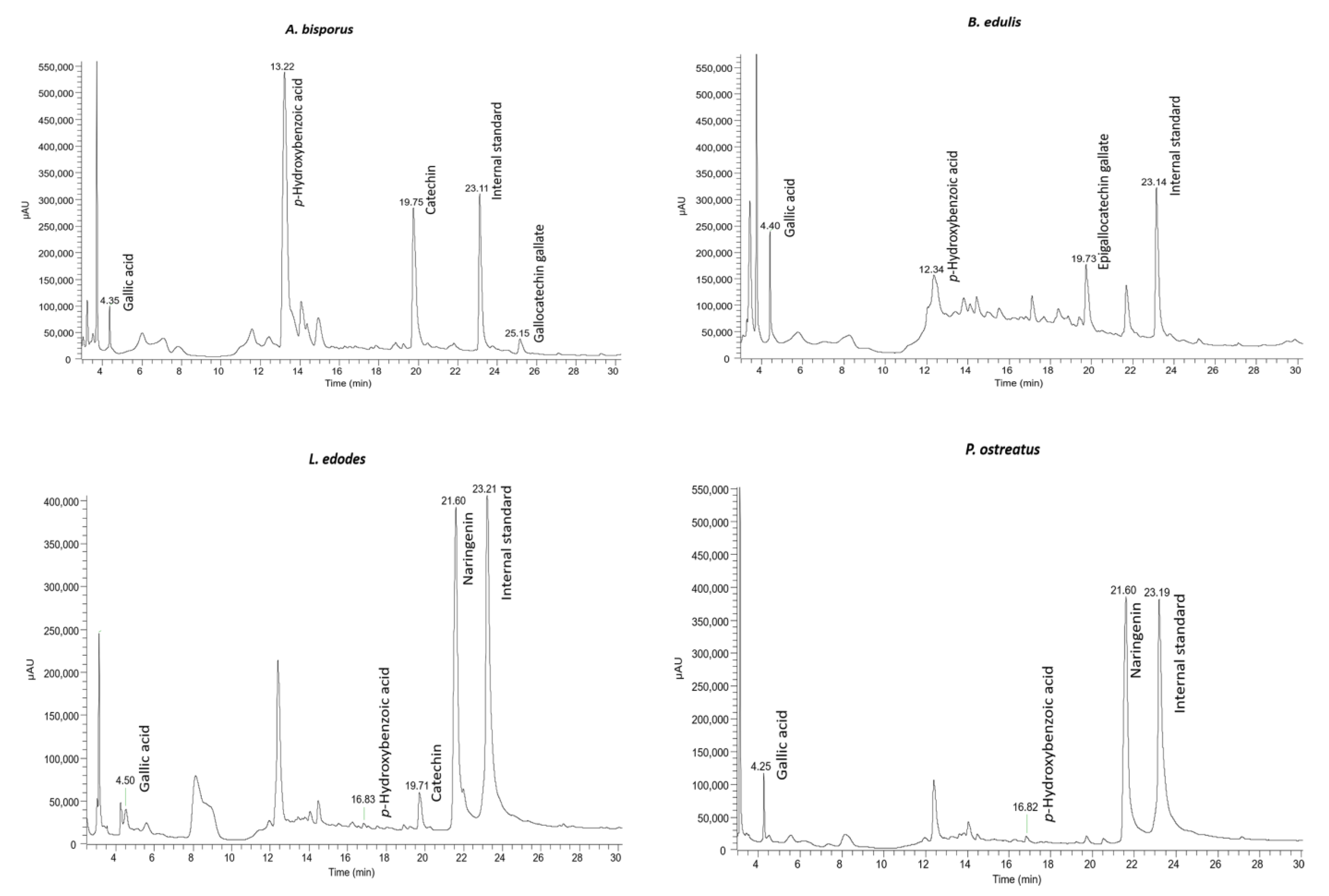
HPLC-DAD was carried out to identify and quantify the phenolic compounds present in the extracts of the four mushrooms in this study (Figure 2 and Table 2). From the obtained results, among six phenolic compounds identified, p-hydroxybenzoic and gallic acids were present in the four studied mushroom extracts in the ranges of 0.010 to 2.554 µg/g DW and 0.032 to 0.112 µg/g DW, respectively. Our results are in accordance with other works [18,19] that also identified these compounds in A. bisporus, B. edulis, L. edodes, and P. ostreatus. Other compounds, such as catechin (A. bisporus and L. edodes), epigallocatechin gallate (B. edulis), gallocatechin galate (A. bisporus), and naringenin (L. edodes and P. ostreatus) were identified in our samples. In other studies, catechin and gallocatechin were also identified in B. edulis [21,46] and P. ostreatus [18,46,47]. According to our findings, Gąsecka and co-workers [48] detected the phenolic compound naringenin in the extract from two different species of Pleurotus, namely P. eryngii and P. ostreatus.
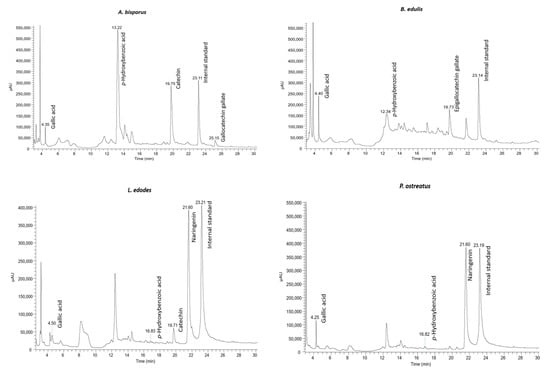
Figure 2.
HPLC-DAD chromatograms of A. bisporus, B. edulis, L. edodes, and P. ostreatus, recorded at 280 nm.

Table 2.
Phenolic compounds identified and quantified from four edible mushrooms, expressed as µg/g DW, and respective wavelengths of maximum absorption in the visible region (λmax) of the standards and samples.
In the literature, for the same mushroom species analyzed in this work, the phenolic profile may differ. These differences in the compounds identified, as well as in the quantities present in the samples, can be due to several factors. Such factors include cultivation techniques, climatic and stress conditions, and ripening stage, as well as different extraction methods and analysis techniques [47,49].
3.3. Antibacterial Activity
Nosocomial or hospital-acquired infections are mainly caused by ESKAPE pathogens, which include a list of six bacteria species: Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, Enterobacter spp., and Escherichia coli [50]. Most of those pathogens are multidrug-resistant and are, therefore, a significant problem for global public health with enormous economic costs. Accordingly, there is an emerging need to find antimicrobials to combat ESKAPE pathogens and minimize their adverse effects [51]. Previously, Garcia et al. [21] reported the importance of mushrooms as a source of valuable antibacterial compounds.
In our work, we assessed the effect of the four extracts from A. bisporus, B. edulis, L. edodes, and P. ostreatus against ESKAPE isolates. The MIC and MBC of each mushroom extract are shown in Table 3. The extract of B. edulis was the only one that inhibited the growth of all bacterial isolates tested, with MIC values ranging from 5 to 20 mg/mL. The MBC values of the B. edulis extract were similar to the MIC, except for MR S. aureus (MJMC 565-A), which was 10 mg/mL, and for E. faecium, which showed no bactericidal activity at the highest concentration tested. The extract of A. bisporus was able to inhibit the growth of all Gram-positive bacterial isolates, with MIC values ranging from 10 to 20 mg/mL. Concerning the Gram-negative isolates, the A. bisporus extract inhibited the growth of three of the five species under analysis, with MICs ranging from 10 to 20 mg/mL. For the extract of A. bisporus, the MBC and MIC values were similar, except for E. faecium, which had no bactericidal effect. The extracts of L. edodes and P. ostreatus had virtually the same effect on the bacterial isolates tested, except for MR S. aureus (MJMC 534-B) and A. baumannii, where P. ostreatus showed no growth inhibition. For the extract of L. edodes, the MBC values observed were different from the MICs in E. faecium and E. aerogenes, and in those bacterial isolates, the extract did not have a bactericidal activity. In turn, P. ostreatus extract does not have a bactericidal effect on any of the bacterial isolates tested, except for E. coli, in which the MBC was equal to the MIC (20 mg/mL).

Table 3.
Minimum inhibitory concentration (MIC; mg/mL) and minimum bactericidal concentration (MBC; mg/mL) for ethanolic extracts of four edible mushroom species.
According to our results, Fogarasi and co-workers [52] showed that among the methanolic extracts of A. bisporus, B. edulis, and P. ostreatus, the extract of B. edulis was the one that displayed the best activity against S. aureus, E. coli, and P. aeruginosa, with MIC values of 13.49, 13.49, and 28.34 mg/mL, respectively. Bach et al. [19] also evaluated the antimicrobial effect of ethanolic extracts from L. edodes and A. bisporus against S. aureus and E. coli. The authors found that L. edodes extract was the most efficient against the bacteria under analysis, with MIC values of 1.56 mg/mL and 100 mg/mL for S. aureus and E. coli, respectively, while A. bisporus obtained a MIC value of 200 mg/mL for both bacteria. In our investigation, L. edodes did not show such a great result against S. aureus, showing a MIC of 20 mg/mL for both MS and MR strains. However, we obtained a better result against E. coli with a MIC value of 20 mg/mL. In another study by Erdoğan Eliuz [53], the antimicrobial effect of ethanolic extracts of L. edodes and A. bisporus against K. pneumoniae, S. aureus, and A. baumannii was evaluated. The author demonstrated that the extracts of L. edodes were more effective, showing MIC values ranging from 5.9 to 6.01 mg/mL, while the extracts of A. bisporus displayed MICs ranging from 7.21 to 9.54 mg/mL [53]. According to Taofiq et al. [29], the ethanolic extracts of P. ostreatus and L. edodes were more efficient than those of A. bisporus in inhibiting the growth of MR and MS S. aureus, with MIC values of 2.5 mg/mL and 10 mg/mL, respectively. In this study, the extract of A. bisporus showed the same MIC for MS S. aureus but not for MR S. aureus (20 mg/mL). Regarding the effect of the extracts against P. aeruginosa, only B. edulis was able to inhibit its growth (MIC = 20 mg/mL); our results are in agreement with the findings of Taofiq et al. [29].
Several studies evaluate the antimicrobial effect of mushroom extracts [54], and the results vary considerably among them. There are many factors that could be responsible for this discrepancy, such as the mushroom origin, the extraction methodology, which includes per se many variables (ratio, solvent, temperature, and the type of assisted extraction, among others), and the nature of the bacteria, which can be from a culture collection or from clinical isolates with an antibiotic resistance profile. In our study, the edible mushroom B. edulis was the only one that inhibited the growth of all the bacterial isolates tested. Since mushrooms have bioactive compounds that, combined with conventional therapies, can have synergistic effects in cancer cell signaling pathways [55,56], it is possible that B. edulis could also enhance the effect of antibiotics or other agents used to treat microbial infections.
3.4. Cosmeceutical Properties of Mushroom Extracts
The pursuit of ingredients of natural origin with bioactive effects is a constant in the cosmetic industry. In this regard, we evaluated the capacity of each mushroom extract to inhibit the activity of skin-related enzymes, namely, hyaluronidase and tyrosinase, which have direct implications in skin aging, one of the main targets of the cosmetic industry.
The anti-hyaluronidase activity of mushroom extracts has been assessed to determine their potential as anti-aging and anti-inflammatory agents [57]. Table 4 shows the percentage of inhibition of the hyaluronidase enzyme of each mushroom species at three known extract concentrations (0.1, 1, and 10 mg DE/mL). Comparing the four species under analysis, the ethanolic extract of A. bisporus demonstrated the most significant capacity to inhibit the enzyme hyaluronidase at 1 and 10 mg DE/mL, with values reaching 43.0 ± 7.1% and 74.4 ± 7.5%, respectively, and an IC50 of 5.4 ± 0.4 mg DE/mL. Recently, Nitthikan et al. [58] also assessed the hyaluronidase inhibitory activity of brown A. bisporus extracts. The authors found that, at 1 mg/mL, the ethanolic and the aqueous extracts inhibited the hyaluronidase enzyme by 55.03 ± 0.25 and 55.94 ± 3.98%, respectively. At the same concentration, our results are only slightly lower. Nevertheless, at the higher concentration of the extract (10 mg DE/mL), the inhibition percentage reached was considerably higher. Thus, A. bisporus extract could be considered a good candidate as a skin anti-aging agent. In our study, the extracts of B. edulis, L. edodes, and P. ostreatus did not show anti-hyaluronidase activity. Furthermore, these same extracts did not show a dose response; hence, the IC50 values are not displayed. On the contrary, Abd Razak and co-workers [57] demonstrated that aqueous extracts of P. ostreatus, at 10 mg/mL, reached above 50% inhibition of hyaluronidase. In another study, carried out on a different species of Pleurotus [59], no activity against the hyaluronidase enzyme was observed.

Table 4.
Anti-hyaluronidase activity of the ethanolic extracts from four edible mushrooms (0.1–10.0 mg DE/mL) expressed in percentage (%) of inhibition and IC50 values expressed in mg DE/mL.
Our results suggest that extracts of A. bisporus could be incorporated into anti-aging natural formulations, since it displays excellent levels of hyaluronidase enzyme inhibition. Indeed, there are already commercial products that incorporate extracts of A. bisporus (https://incidecoder.com/ingredients/agaricus-bisporus-extract). For instance, the cosmetic “Fastscription™ Instant De-Puff Eye Gel” (https://wexlerdermatology.com) owned by Dr. Patricia Wexler (Wexler Dermatology) contains extracts of A. bisporus and other ingredients that improve the skin surface and prevent wrinkling [22]. Other applications for the use of A. bisporus extracts have been suggested. For example, the high vitamin D content and several minerals in A. bisporus extracts make them suitable to be incorporated into shampoos to resolve hair problems such as dandruff, oily hair, and hair loss [60].
The most common approach to achieving skin whitening is through the inhibition of tyrosinase, an enzyme which catalyzes the most important steps of mammalian melanogenesis [61,62]. Many factors can cause hyperpigmentation of the skin [63]; therefore, finding inhibitors of this process, i.e., natural inhibitors of the tyrosinase enzyme, is of great interest in the natural cosmetic domain.
Regarding the ability of our extracts to inhibit tyrosinase activity, Table 5 shows that at the tested concentrations, none of the four mushroom species reached a 50% inhibition of the enzyme. However, at the higher concentration tested, A. bisporus inhibited the activity of tyrosinase up to 39.9 ± 2.6%. Since the tyrosinase inhibition percentage increases as the dose of DE used increases, it is possible that a slightly higher concentration of the DE could result in an inhibition near 50%. In accordance, A. bisporus also showed the lowest IC50 value compared to the other mushroom extracts. To evaluate the cosmeceutical potential of ethanolic extracts from A. bisporus, P. ostreatus, and L. edodes, Taofiq et al. [29] reported that A. bisporus ethanolic extracts had the highest anti-tyrosinase activity of the three extracts, while L. edodes and P. ostreatus presented the lowest activities. Our results demonstrated that L. edodes extract displayed the lowest ability to inhibit tyrosinase, followed by B. edulis and P. ostreatus, which showed similar results. In agreement, L. edodes showed the highest IC50 value, followed by P. ostreatus and B. edulis. On the contrary, Yoon et al. [64] described that the methanolic extracts of L. edodes (0.125–1 mg/mL) showed anti-tyrosinase activity of 15.12–54.61%. In another study in methanolic extracts of P. ostreatus (0.125–1 mg/mL), the authors reported a significant inhibition of tyrosinase activity of up to 11.36–59.56% [65].

Table 5.
Anti-tyrosinase activity of the ethanolic extracts from four edible mushrooms (0.1–10.0 mg DE/mL) expressed in percentage (%) of inhibition and IC50 values expressed in mg DE/mL.
There are few publications that address the inhibition of the tyrosinase enzyme in these mushroom species, and the variation in the results obtained in the existing studies may be due to the numerous factors that influence the ability of the extracts to produce a specific effect. Although the extract of A. bisporus was, among the four, the one that presented a greater inhibition percentage at the higher concentration used, none of the mushroom extracts in this study, in the tested conditions, can be considered a natural whitening agent.
3.5. Incorporation of A. bisporus Extract into a Cosmetic Cream
The addition of an extract with antioxidant capacity to cosmetic creams may protect the skin from external and internal harmful factors, such as pollutants, ultraviolet rays, and stress, that, with time, are responsible for the formation of wrinkles and dark spots [57]. Moreover, extracts that are able to inhibit the hyaluronidase enzyme may be incorporated into cosmetic formulations to improve skin suppleness and reduce the formation of wrinkles, acting as anti-aging and anti-inflammatory agents [66]. In this regard, as the extract of A. bisporus showed the best antioxidant and anti-hyaluronidase capacity, it was selected to be incorporated into a cosmetic cream (hereinafter referred to as AB).
3.5.1. Stability Tests of a Cosmetic Cream Containing A. bisporus Extract
To ensure the quality and safety of the creams, stability studies, which consist of subjecting the samples to different conditions, were performed. Accordingly, the creams were assessed by testing at 4 °C, 25 °C, and 40 °C for 30 days and under four alternating heating and cooling cycles (one cycle corresponds to 24 h at 4 °C and then 24 h at 40 °C). All the potential alterations in the characteristics of the formulations between the pre- and post-assays were recorded in terms of visual color and texture, phase separation, and pH (Table 6). The results showed that both creams (FD and AB) were stable and maintained the initial characteristics after the thermal stability tests, with no phase separation or pH variations. Likewise, the color, homogeneity, and odor of the creams remained unchanged. Therefore, the formulations were considered stable.

Table 6.
Results of the stability tests of the creams without (FD) and with 0.2% A. bisporus extract (AB) under different conditions: 4 °C, 25 °C, and 40 °C for 30 days and four heating and cooling cycles.
3.5.2. Phenolic Contents and In Vitro Antioxidant Capacity of a Cosmetic Cream Containing A. bisporus Extract
The biological properties of the creams, including the total phenolic content and the antioxidant activity (by the DPPH assay), were evaluated. For this, the samples (FD and AB) were assessed 24 h after the production of the formulations (Table 7). The considerable difference between these values and those previously obtained for TPC and DPPH are due to the dilution of the extract in the cream. The results showed that both total phenol contents and antioxidant activity were significantly higher (p < 0.01) in the cream containing the extract of A. bisporus compared to the foundation. Thus, adding A. bisporus extract considerably improved the antioxidant potential of the cream, a very desirable property in the cosmetic industry that has been experiencing an increasing demand for natural antioxidants [67].

Table 7.
Total phenols and antioxidant activity (measured with DPPH) of the creams without (FD) and with 0.2% A. bisporus extract (AB).
4. Conclusions
The mushroom cultivation process contributes to food loss and waste generation. Therefore, it is important to value these residues and encourage their reuse, since due to their rich composition in bioactive compounds, they have the potential for application in several fields. In this study, we evaluated the antioxidant, antibacterial, and cosmeceutical potential of four common edible mushrooms (A. bisporus, B. edulis, L. edodes, and P. ostreatus), and the extract showing the best properties was chosen to be incorporated into a cosmetic cream. The extracts of A. bisporus and B. edulis stood out from the other two mushroom species in all the parameters assessed. Considering its excellent antioxidant activity and anti-aging effect, the extract of A. bisporus was incorporated into a cosmetic cream, which was compared to the same cream but without the extract of A. bisporus. The cream with A. bisporus extract showed a greater total phenol content and increased antioxidant activity compared to the cream without the extract, emphasizing the high value of the reuse of mushrooms as source of natural antioxidants in the cosmetic industry.
Author Contributions
Conceptualization, G.M., L.M.-C., M.J.S. and T.M.; methodology, A.A., G.M., L.M.-C., M.J.S. and T.M.; formal analysis: A.A., L.M.-C. and T.M.; investigation, G.M., L.M.-C. and T.M.; resources, A.A., G.M. and M.J.S.; data curation, L.M.-C. and T.M.; writing—original draft preparation, L.M.-C. and T.M.; writing—review and editing: A.A., G.M., L.M.-C., M.J.S. and T.M.; funding acquisition: G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Fungi4Health project (no. NORTE-01-0247-FEDER-070171), financed by the European Regional Development Fund (ERDF) through NORTE 2020 (North Regional Operational Program 2014/2020).
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Dataset will be provided upon reasonable request.
Acknowledgments
This work was supported by National Funds by FCT—Portuguese Foundation for Science and Technology, under the project UIDB/04033/2020.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. The State of Food and Agriculture 2019. Moving Forward on Food Loss and Waste Reduction; FAO: Rome, Italy, 2019. [Google Scholar]
- Esparza, I.; Jiménez-Moreno, N.; Bimbela, F.; Ancín-Azpilicueta, C.; Gandía, L.M. Fruit and vegetable waste management: Conventional and emerging approaches. J. Environ. Manag. 2020, 265, 110510. [Google Scholar] [CrossRef]
- Roy, P.; Mohanty, A.K.; Dick, P.; Misra, M. A Review on the Challenges and Choices for Food Waste Valorization: Environmental and Economic Impacts. ACS Environ. Au 2023, 3, 58–75. [Google Scholar] [CrossRef] [PubMed]
- FAO. Food and Agriculture Organization of the United Nations. FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 10 March 2023).
- Antunes, F.; Marçal, S.; Taofiq, O.; Morais, A.; Freitas, A.C.; Ferreira, I.; Pintado, M. Valorization of Mushroom By-Products as a Source of Value-Added Compounds and Potential Applications. Molecules 2020, 25, 2672. [Google Scholar] [CrossRef] [PubMed]
- Abdelshafy, A.M.; Belwal, T.; Liang, Z.; Wang, L.; Li, D.; Luo, Z.; Li, L. A comprehensive review on phenolic compounds from edible mushrooms: Occurrence, biological activity, application and future prospective. Crit. Rev. Food Sci. Nutr. 2022, 62, 6204–6224. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Mehra, R.; Guiné, R.P.F.; Lima, M.J.; Kumar, N.; Kaushik, R.; Ahmed, N.; Yadav, A.N.; Kumar, H. Edible Mushrooms: A Comprehensive Review on Bioactive Compounds with Health Benefits and Processing Aspects. Foods 2021, 10, 2996. [Google Scholar] [CrossRef]
- Singh, M.P.; Rai, S.N.; Dubey, S.K.; Pandey, A.T.; Tabassum, N.; Chaturvedi, V.K.; Singh, N.B. Biomolecules of mushroom: A recipe of human wellness. Crit. Rev. Biotechnol. 2022, 42, 913–930. [Google Scholar] [CrossRef]
- Venturella, G.; Ferraro, V.; Cirlincione, F.; Gargano, M.L. Medicinal Mushrooms: Bioactive Compounds, Use, and Clinical Trials. Int. J. Mol. Sci. 2021, 22, 634. [Google Scholar] [CrossRef]
- García-Sanmartín, J.; Bobadilla, M.; Mirpuri, E.; Grifoll, V.; Pérez-Clavijo, M.; Martínez, A. Agaricus Mushroom-Enriched Diets Modulate the Microbiota-Gut-Brain Axis and Reduce Brain Oxidative Stress in Mice. Antioxidants 2022, 11, 695. [Google Scholar] [CrossRef]
- Vamanu, E.; Dinu, L.D.; Pelinescu, D.R.; Gatea, F. Therapeutic Properties of Edible Mushrooms and Herbal Teas in Gut Microbiota Modulation. Microorganisms 2021, 9, 1262. [Google Scholar] [CrossRef]
- Iñiguez, M.; Pérez-Matute, P.; Villanueva-Millán, M.J.; Recio-Fernández, E.; Roncero-Ramos, I.; Pérez-Clavijo, M.; Oteo, J.-A. Agaricus bisporus supplementation reduces high-fat diet-induced body weight gain and fatty liver development. J. Physiol. Biochem. 2018, 74, 635–646. [Google Scholar] [CrossRef]
- Ekowati, N.; Yuniati, N.I.; Hernayanti, H.; Ratnaningtyas, N.I. Antidiabetic Potentials of Button Mushroom (Agaricus bisporus) on Alloxan-Induced Diabetic Rats. Biosaintifika J. Biol. Biol. Educ. 2018, 10, 655–662. [Google Scholar] [CrossRef]
- Park, H.-J. Current Uses of Mushrooms in Cancer Treatment and Their Anticancer Mechanisms. Int. J. Mol. Sci. 2022, 23, 10502. [Google Scholar] [CrossRef]
- Finimundy, T.C.; Scola, G.; Scariot, F.J.; Dillon, A.J.P.; Moura, S.; Echeverrigaray, S.; Henriques, J.P.; Roesch-Ely, M. Extrinsic and Intrinsic Apoptotic Responses Induced by Shiitake Culinary-Medicinal Mushroom Lentinus edodes (Agaricomycetes) Aqueous Extract against a Larynx Carcinoma Cell Line. Int. J. Med. Mushrooms 2018, 20, 31–46. [Google Scholar] [CrossRef]
- Muszyńska, B.; Grzywacz-Kisielewska, A.; Kała, K.; Gdula-Argasińska, J. Anti-inflammatory properties of edible mushrooms: A review. Food Chem. 2018, 243, 373–381. [Google Scholar] [CrossRef]
- Hu, Q.; Yuan, B.; Xiao, H.; Zhao, L.; Wu, X.; Rakariyatham, K.; Zhong, L.; Han, Y.; Muinde Kimatu, B.; Yang, W. Polyphenols-rich extract from Pleurotus eryngii with growth inhibitory of HCT116 colon cancer cells and anti-inflammatory function in RAW264.7 cells. Food Funct. 2018, 9, 1601–1611. [Google Scholar] [CrossRef]
- Mwangi, R.W.; Macharia, J.M.; Wagara, I.N.; Bence, R.L. The antioxidant potential of different edible and medicinal mushrooms. Biomed. Pharmacother. 2022, 147, 112621. [Google Scholar] [CrossRef]
- Bach, F.; Zielinski, A.A.F.; Helm, C.V.; Maciel, G.M.; Pedro, A.C.; Stafussa, A.P.; Ávila, S.; Haminiuk, C.W.I. Bio compounds of edible mushrooms: In vitro antioxidant and antimicrobial activities. LWT 2019, 107, 214–220. [Google Scholar] [CrossRef]
- Garcia, J.; Afonso, A.; Fernandes, C.; Nunes, F.M.; Marques, G.; Saavedra, M.J. Comparative antioxidant and antimicrobial properties of Lentinula edodes Donko and Koshin varieties against priority multidrug-resistant pathogens. S. Afr. J. Chem. Eng. 2021, 35, 98–106. [Google Scholar] [CrossRef]
- Garcia, J.; Rodrigues, F.; Castro, F.; Aires, A.; Marques, G.; Saavedra, M.J. Antimicrobial, Antibiofilm, and Antioxidant Properties of Boletus edulis and Neoboletus luridiformis Against Multidrug-Resistant ESKAPE Pathogens. Front. Nutr. 2022, 8, 773346. [Google Scholar] [CrossRef]
- Taofiq, O.; González-Paramás, A.M.; Martins, A.; Barreiro, M.F.; Ferreira, I.C.F.R. Mushrooms extracts and compounds in cosmetics, cosmeceuticals and nutricosmetics—A review. Ind. Crop. Prod. 2016, 90, 38–48. [Google Scholar] [CrossRef]
- Ho, L.-H.; Zulkifli, N.A.; Tan, T.-C. Edible Mushroom: Nutritional Properties, Potential Nutraceutical Values, and Its Utilisation in Food Product Development. In An Introduction to Mushroom; Passari, A.K., Sánchez, S., Eds.; IntechOpen: Rijeka, Croatia, 2020; pp. 1–19. [Google Scholar]
- Royse, D.J.; Baars, J.; Tan, Q. Current Overview of Mushroom Production in the World. In Edible and Medicinal Mushrooms; Diego, C.Z., Pardo-Giménez, A., Eds.; John Wiley & Sons, Ltd.: West Sussex, UK, 2017; pp. 5–13. [Google Scholar]
- Machado-Carvalho, L.; Martins, T.; Aires, A.; Marques, G. Optimization of Phenolic Compounds Extraction and Antioxidant Activity from Inonotus hispidus Using Ultrasound-Assisted Extraction Technology. Metabolites 2023, 13, 524. [Google Scholar] [CrossRef] [PubMed]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Dominguez-Perles, R.; Rodrigues, M.; Barros, A.I.R.N.A. Monitoring the antioxidant and antimicrobial power of grape (Vitis vinifera L.) stems phenolics over long-term storage. Ind. Crop. Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Yu, M.; Gouvinhas, I.; Barros, A. Variation of the Polyphenolic Composition and Antioxidant Capacity of Freshly Prepared Pomegranate Leaf Infusions over One-Day Storage. Antioxidants 2021, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Aires, A.; Carvalho, R. Kiwi fruit residues from industry processing: Study for a maximum phenolic recovery yield. J. Food Sci. Technol. 2020, 57, 4265–4276. [Google Scholar] [CrossRef] [PubMed]
- Taofiq, O.; Heleno, S.A.; Calhelha, R.C.; Alves, M.J.; Barros, L.; Barreiro, M.F.; González-Paramás, A.M.; Ferreira, I.C. Development of Mushroom-Based Cosmeceutical Formulations with Anti-Inflammatory, Anti-Tyrosinase, Antioxidant, and Antibacterial Properties. Molecules 2016, 21, 1372. [Google Scholar] [CrossRef]
- Veiga, A.; Toledo, M.; Rossa, L.S.; Mengarda, M.; Stofella, N.C.F.; Oliveira, L.J.; Gonçalves, A.G.; Murakami, F.S. Colorimetric microdilution assay: Validation of a standard method for determination of MIC, IC(50%), and IC(90%) of antimicrobial compounds. J. Microbiol. Methods 2019, 162, 50–61. [Google Scholar] [CrossRef]
- Łyko, L.; Olech, M.; Nowak, R. LC-ESI-MS/MS Characterization of Concentrated Polyphenolic Fractions from Rhododendron luteum and Their Anti-Inflammatory and Antioxidant Activities. Molecules 2022, 27, 827. [Google Scholar] [CrossRef]
- Paczkowska-Walendowska, M.; Cielecka-Piontek, J. Chitosan as a Functional Carrier for the Local Delivery Anti-Inflammatory Systems Containing Scutellariae baicalensis radix Extract. Pharmaceutics 2022, 14, 2148. [Google Scholar] [CrossRef]
- No, J.K.; Soung, D.Y.; Kim, Y.J.; Shim, K.H.; Jun, Y.S.; Rhee, S.H.; Yokozawa, T.; Chung, H.Y. Inhibition of tyrosinase by green tea components. Life Sci. 1999, 65, PL241–PL246. [Google Scholar] [CrossRef]
- Rodrigues Ueoka, A.; Pedriali Moraes, C.A. Development and Stability Evaluation of Liquid Crystal-Based Formulations Containing Glycolic Plant Extracts and Nano-Actives. Cosmetics 2018, 5, 25. [Google Scholar] [CrossRef]
- Salem, Y.; Rajha, H.N.; Franjieh, D.; Hoss, I.; Manca, M.L.; Manconi, M.; Castangia, I.; Perra, M.; Maroun, R.G.; Louka, N. Stability and Antioxidant Activity of Hydro-Glyceric Extracts Obtained from Different Grape Seed Varieties Incorporated in Cosmetic Creams. Antioxidants 2022, 11, 1348. [Google Scholar] [CrossRef]
- Adeel, S.; Habiba, M.; Kiran, S.; Iqbal, S.; Abrar, S.; Hassan, C.M. Utilization of Colored Extracts for the Formulation of Ecological Friendly Plant-Based Green Products. Sustainability 2022, 14, 11758. [Google Scholar] [CrossRef]
- Reis, F.S.; Martins, A.; Barros, L.; Ferreira, I.C. Antioxidant properties and phenolic profile of the most widely appreciated cultivated mushrooms: A comparative study between in vivo and in vitro samples. Food Chem. Toxicol. 2012, 50, 1201–1207. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, L.; Brennan, M.; Brennan, C. How does the addition of mushrooms and their dietary fibre affect starchy foods. J. Futur Foods 2022, 2, 18–24. [Google Scholar] [CrossRef]
- Aishah, M.S.; Wan Rosli, W.D. The effect of addition of oyster mushroom (Pleurotus sajor-caju) on nutrient composition and sensory acceptation of selected wheat- and rice-based products. Int. Food Res. J. 2013, 20, 183–188. [Google Scholar]
- Gadallah, M.G.E.; Ashoush, I.S. Value Addition on Nutritional and Sensory Properties of Biscuit Using Desert Truffle (Terfezia claveryi) Powder. Food Nutr. Sci. 2016, 7, 1171–1181. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Guan, W.; Zhang, J.; Yuan, L.; Brennan, C.S. Enhancing the Nutritional Properties of Bread by Incorporating Mushroom Bioactive Compounds: The Manipulation of the Pre-Dictive Glycaemic Response and the Phenolic Properties. Foods 2021, 10, 731. [Google Scholar] [CrossRef]
- Lu, X.; Brennan, M.A.; Serventi, L.; Liu, J.; Guan, W.; Brennan, C.S. Addition of mushroom powder to pasta enhances the antioxidant content and modulates the predictive glycaemic response of pasta. Food Chem. 2018, 264, 199–209. [Google Scholar] [CrossRef]
- Thomas, B.; Sudheer, K.P.; Saranya, S.; Kothakota, A.; Pandiselvam, R.; Joseph, M. Development of protein enriched cold extruded pasta products using hybrid dried processed mushroom powder and defatted flours: A study on nutraceutical, textural, colour and sensory attributes. LWT 2022, 170, 113991. [Google Scholar] [CrossRef]
- Addor, F.A.S. Antioxidants in dermatology. An. Bras. Dermatol. 2017, 92, 356–362. [Google Scholar] [CrossRef]
- Papaccio, F.; D′Arino, A.; Caputo, S.; Bellei, B. Focus on the Contribution of Oxidative Stress in Skin Aging. Antioxidants 2022, 11, 1121. [Google Scholar] [CrossRef] [PubMed]
- Fogarasi, M.; Socaci, S.A.; Dulf, F.V.; Diaconeasa, Z.M.; Fărcaș, A.C.; Tofană, M.; Semeniuc, C.A. Bioactive Compounds and Volatile Profiles of Five Transylvanian Wild Edible Mushrooms. Molecules 2018, 23, 3272. [Google Scholar] [CrossRef] [PubMed]
- Çayan, F.; Deveci, E.; Tel-Çayan, G.; Duru, M.E. Identification and quantification of phenolic acid compounds of twenty-six mushrooms by HPLC–DAD. J. Food Meas. Charact. 2020, 14, 1690–1698. [Google Scholar] [CrossRef]
- Gąsecka, M.; Mleczek, M.; Siwulski, M.; Niedzielski, P. Phenolic composition and antioxidant properties of Pleurotus ostreatus and Pleurotus eryngii enriched with selenium and zinc. Eur. Food Res. Technol. 2016, 242, 723–732. [Google Scholar] [CrossRef]
- Vaz, J.A.; Barros, L.; Martins, A.; Morais, J.S.; Vasconcelos, M.H.; Ferreira, I.C.F.R. Phenolic profile of seventeen Portuguese wild mushrooms. LWT 2011, 44, 343–346. [Google Scholar] [CrossRef]
- Ayobami, O.; Brinkwirth, S.; Eckmanns, T.; Markwart, R. Antibiotic resistance in hospital-acquired ESKAPE-E infections in low- and lower-middle-income countries: A systematic review and meta-analysis. Emerg. Microbes Infect. 2022, 11, 443–451. [Google Scholar] [CrossRef]
- Clericuzio, M.; Bivona, M.; Gamalero, E.; Bona, E.; Novello, G.; Massa, N.; Dovana, F.; Marengo, E.; Robotti, E. A Systematic Study of the Antibacterial Activity of Basidiomycota Crude Extracts. Antibiotics 2021, 10, 1424. [Google Scholar] [CrossRef]
- Fogarasi, M.; Diaconeasa, Z.M.; Pop, C.R.; Fogarasi, S.; Semeniuc, C.A.; Fărcaş, A.C.; Țibulcă, D.; Sălăgean, C.-D.; Tofană, M.; Socaci, S.A. Elemental Composition, Antioxidant and Antibacterial Properties of Some Wild Edible Mushrooms from Romania. Agronomy 2020, 10, 1972. [Google Scholar] [CrossRef]
- Erdoğan Eliuz, E.A. Antibacterial activity and antibacterial mechanism of ethanol extracts of Lentinula edodes (Shiitake) and Agaricus bisporus (button mushroom). Int. J. Environ. Health Res. 2022, 32, 1828–1841. [Google Scholar] [CrossRef]
- Moussa, A.Y.; Fayez, S.; Xiao, H.; Xu, B. New insights into antimicrobial and antibiofilm effects of edible mushrooms. Food Res. Int. 2022, 162, 111982. [Google Scholar] [CrossRef]
- Joseph, T.P.; Chanda, W.; Padhiar, A.A.; Batool, S.; LiQun, S.; Zhong, M.; Huang, M. A Preclinical Evaluation of the Antitumor Activities of Edible and Medicinal Mushrooms: A Molecular Insight. Integr. Cancer Ther. 2018, 17, 200–209. [Google Scholar] [CrossRef]
- Plácido, A.I.; Roque, F.; Morgado, M. The Promising Role of Mushrooms as a Therapeutic Adjuvant of Conventional Cancer Therapies. Biologics 2022, 2, 58–68. [Google Scholar] [CrossRef]
- Abd Razak, D.L.; Jamaluddin, A.; Abd Rashid, N.Y.; Sani, N.A.; Abdul Manan, M. Assessment of Cosmeceutical Potentials of Selected Mushroom Fruitbody Extracts Through Evaluation of Antioxidant, Anti-Hyaluronidase and Anti-Tyrosinase Activity. J 2020, 3, 329–342. [Google Scholar] [CrossRef]
- Nitthikan, N.; Leelapornpisid, P.; Naksuriya, O.; Intasai, N.; Kiattisin, K. Potential and Alternative Bioactive Compounds from Brown Agaricus bisporus Mushroom Extracts for Xerosis Treatment. Sci. Pharm. 2022, 90, 59. [Google Scholar] [CrossRef]
- Yahaya, Y.A.; Don, M.M. Evaluation of Trametes Lactinea Extracts on the Inhibition of Hyaluronidase, Lipoxygenase and Xanthine Oxidase Activities in vitro. J. Phys. Sci. 2012, 23, 1–15. [Google Scholar]
- Usman, M.; Murtaza, G.; Ditta, A. Nutritional, Medicinal, and Cosmetic Value of Bioactive Compounds in Button Mushroom (Agaricus bisporus): A Review. Appl. Sci. 2021, 11, 5943. [Google Scholar] [CrossRef]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz-Munoz, J.; Garcia-Molina, F.; Garcia-Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Panzella, L.; Napolitano, A. Natural and Bioinspired Phenolic Compounds as Tyrosinase Inhibitors for the Treatment of Skin Hyperpigmentation: Recent Advances. Cosmetics 2019, 6, 57. [Google Scholar] [CrossRef]
- Nautiyal, A.; Wairkar, S. Management of hyperpigmentation: Current treatments and emerging therapies. Pigment Cell Melanoma Res. 2021, 34, 1000–1014. [Google Scholar] [CrossRef]
- Yoon, K.N.; Alam, N.; Lee, J.S.; Lee, K.R.; Lee, T.S. Detection of phenolic compounds concentration and evaluation of antioxidant and anti-tyrosinase activity of various extract from Lentinus edodes. World Appl. Sci. J. 2011, 12, 1851–1859. [Google Scholar]
- Alam, N.; Yoon, K.N.; Lee, K.R.; Shin, P.G.; Cheong, J.C.; Yoo, Y.B.; Shim, J.M.; Lee, M.W.; Lee, U.Y.; Lee, T.S. Antioxidant Activities and Tyrosinase Inhibitory Effects of Different Extracts from Pleurotus ostreatus Fruiting Bodies. Mycobiology 2010, 38, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Hoang, H.T.; Moon, J.-Y.; Lee, Y.-C. Natural Antioxidants from Plant Extracts in Skincare Cosmetics: Recent Applications, Challenges and Perspectives. Cosmetics 2021, 8, 106. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).