Abstract
The use of inhibitors is one of the most efficient methods to protect metals against corrosion, which affects many sectors and generates a significant effect on the world economy. This paper presents a prospection using plant extracts as green corrosion inhibitors, aiming at the use of environmentally friendly input. For this, the authors used scientific articles and patents, with recovery of 335 articles and 42 patents related to the subject, as the source. Most technological solutions consist of extracts prepared from leaves of interest plant species, with tests carried out in acidic corrosive environments, with carbon steel (SAE1020) being the most researched material to be protected. Among the identified technologies, some point to corrosion inhibition greater than 80%. The scientific and patent literature points to the excellent performance of these compounds added to the other data collected in the present study, indicating that the exploration of this area is on the rise and very promising. Special highlight is given to the studies and development of green inhibitors in Brazil, considering the potentialities of its high vegetable biodiversity.
1. Introduction
Corrosion consists of the degradation of a material inserted into a given environment, which can be of a chemical or electrochemical nature, and associated or not with mechanical stresses. In metals it is related to its tendency to reverse the natural state of lower internal energy [1,2].
Metals and their alloys are the most susceptible materials to this degradation. Carbon steel, for example, is the most used steel in several areas and one of the most produced materials worldwide. In 2020, 1864.0 million tons were produced [3], but it is estimated that 20% were destined for the replacement of parts of equipment or installations that suffered corrosion [4].
Thus, to avoid the loss of equipment, machinery and structures, scientific research is crucial. In addition, it can extend the life of parts and mitigate the costs associated with inspections, repairs and replacements. The impact caused by this high rate of corrosion affects many sectors and generates a significant effect on a country’s economy.
There is an estimate that an industrialized country spends 3 to 5% of its gross domestic product (GDP) on problems associated with corrosion. In 2015, a work carried out by the International Zinc Association (IZA) in partnership with the University of São Paulo (USP) estimated that in Brazil this expenditure was 4% of GDP. This percentage is equivalent to more than USD 40 billion [5,6,7].
The measures commonly used, individually or in combination, to combat corrosion are methods based on process modification (structure design; surface conditions; by the application of cathodic protection), modification of the corrosive environment (deaeration of water or neutral solution; purification or reduction of air humidity; addition of corrosion inhibitors), in the modification of the metal (increase in purity; addition of alloying elements; heat treatment) and in protective coatings (coatings with reaction products, such as chemical or electrochemical treatment of the metal surface; organic coatings, such as paints, resins or polymers; inorganic coatings, such as enamels and cements; metallic coatings; temporary protectors) [2].
One of the most practical methods to protect metal materials is the use of corrosion inhibitors. They are a class of substances capable of slowing the corrosion of a metal when added in low concentration to the environment to which it will be exposed. Its mechanism of action is associated with the formation of a protective barrier adsorbed on its surface, which interacts with the reaction sites and decreases the oxidation and/or reduction reactions associated with corrosion [8,9,10], thus, the inhibitors protect metals by slowing down the corrosion processes in one of the following ways: (1) increasing the anodic or cathodic polarization behavior (Tafel curves); (2) reducing the movement or diffusion of ions to the metallic surface and (3) increasing the electrical resistance of the metallic surface [9]. They are commonly classified by their action as cathodic, anodic and mixed inhibitors [10].
The cathodic inhibitors, also known as precipitation inhibitors, work by suppressing cathodic reactions. In this way, the reaction in the cathode is delayed or prevented, limiting the diffusion of the reducing species to the surface. These inhibitors present, in their composition, metallic ions capable of producing a cathodic reaction due to alkalinity, producing insoluble compounds that selectively precipitate in cathodic regions, increasing the protection of the surface from the diffusion process of reducing species, that is, the diffusion of oxygen and electrons drivers in these regions. The anodic inhibitors, also called passivation inhibitors, are those that act by suppressing anodic reactions. In this way, the reaction at the anode is retarded or impeded, due to the formation of an insoluble film adsorbed on the metal. In general, these inhibitors react with the corrosion product, initially formed, resulting in a cohesive and insoluble film on the metal surface. Mixed inhibitors act by reducing both cathodic and anodic reactions. They are typically film-forming compounds that cause precipitate formation on the surface, indirectly protecting the anodic and cathodic regions, usually protecting the metal by adsorption mechanisms [2,11,12,13].
Some inhibitors, however, are potentially polluting and harmful to human health, and this motivated the search for new formulations. Studies on the use of natural products, called “green inhibitors” or “ecological inhibitors” of corrosion, have been recurrent in the last 20 years [14]. This class is characterized by biocompatible, biodegradable substances from renewable sources, which are easy to acquire, low cost and nontoxic [15,16,17]. Inhibitors of this nature are usually based on organic compounds, generally acting as mixed-type inhibitors, as they have heteroatoms (i.e., N, S, O), electronegative groups and conjugated double bonds, characteristics present in good corrosion inhibitors as the main centers of adsorption. These inhibitors adsorb and form a covalent bond on the metal surface. Organic inhibitors cover the entire surface area of the metal with a thick film composed of several monolayers and change the structure of the electrical double layer at the metal interface, decreasing the corrosion rate. They can also act as a barrier film that blocks anodic and cathodic active sites or slows the rate of diffusion of electroactive species to the metal surface [12,18,19,20].
Plant extracts stood out within this class of inhibitors. They are defined as a reservoir of naturally synthesized compounds with easy insulation processes. They have a clear advantage over the exhaustive synthesis of compounds and their toxic effects, besides being abundant in nature. Most times natural products can also be less expensive than synthetic ones. Their success as green corrosion inhibitors is attributed to the presence of complex and effective constituents in reducing corrosion rates, such as tannins, flavonoids, alkaloids, anthraquinones, polyphenols, organic acids and nitrogen-based compounds [21,22,23,24].
Considering the strategic importance of the use of corrosion inhibitors and the potential of natural products for this purpose, in the present work bibliographic and patent surveys were carried out on the applicability of plant extracts as green corrosion inhibitors.
2. Materials and Methods
The surveys were carried out on scientific articles and patent databases to evaluate the scientific and technological scenario of the use of plant extract derivatives as green corrosion inhibitors. The sources were accessed from August of 2021 to August of 2022. The studies were limited to the year 2020 because of the pandemic scenario caused by COVID-19 in recent years, where most universities and research centers needed to stop their activities, consequently affecting the number of publications in the years 2021 and 2022.
The Web of Science [25] and SciELO [26] databases (Brazilian Public Repository) were used in the search for scientific articles. The sources used in the search for patents were that of the Brazilian National Institute of Industrial Property (INPI) [27] and the Derwent Innovations Index of Thomson Reuters Scientific (Derwent) [28]; Questel Orbit Intelligence software [29] was also used.
The descriptors used in the searches are presented in Table 1. The terms were used in Portuguese for the PTO source and in English for the other. “Corrosion” and “inhibitor” are easily justified as being the master keyword for this search. “Environmental”, “green” and “eco-friendly” are the most desirable attributes for these inhibitors and are generally listed as differential or advantageous in patent claims. “Extract” is related to the obtention process of compounds. The mix of these keywords in Portuguese and English were used to scan the Brazilian and the international databases.

Table 1.
Combination of descriptors used in searches of the sources of articles (SciELO and Web of Science) and patents (INPI, Derwent and Orbit).
The searches in the Web of Science, SciELO and Derwent databases were performed with the “topic” filter; the searches of the sources of the Brazilian Office for Industrial Property (PTO) and Orbit were carried out with the “advanced search” option. For both cases, the descriptors were searched for in the titles and summaries of the documents.
3. Results and Discussion
The quantity of scientific articles and patents found by the combination of the descriptors used are presented in Table 2.

Table 2.
Quantitative numbers of articles and patents recovered with the combination of descriptors in Table 1 in the databases of Web of Science, SciELO, Derwent and INPI.
The results (Table 2) show a significant number of articles and patents related to corrosion inhibitors (A), with 25,407 articles in the Web of Science database and 43,100 patents in the Derwent source. These data reinforce the high relevance and importance of protecting materials against corrosion. However, as specific descriptors were inserted for green corrosion inhibitors (B) and for the use of plant extracts in this category (C), the numbers decreased. A total of 2263 and 333 articles were found in the Web of Science database and 989 and 22 patents in the Derwent database for the B and C groups of words, respectively. A detailed discussion for each group of descriptors is presented in the following sections.
3.1. Corrosion Inhibitors
The results found for group A descriptors (corros* and (inhib* or inib*)) were very productive for the international databases, with 25,407 articles from the Web of Science, and 43,100 and 36,454 patents from Derwent and Orbit, respectively. These figures reflect the concern of the academic and industrial scientific community to mitigate the effects of corrosion in several fields and places.
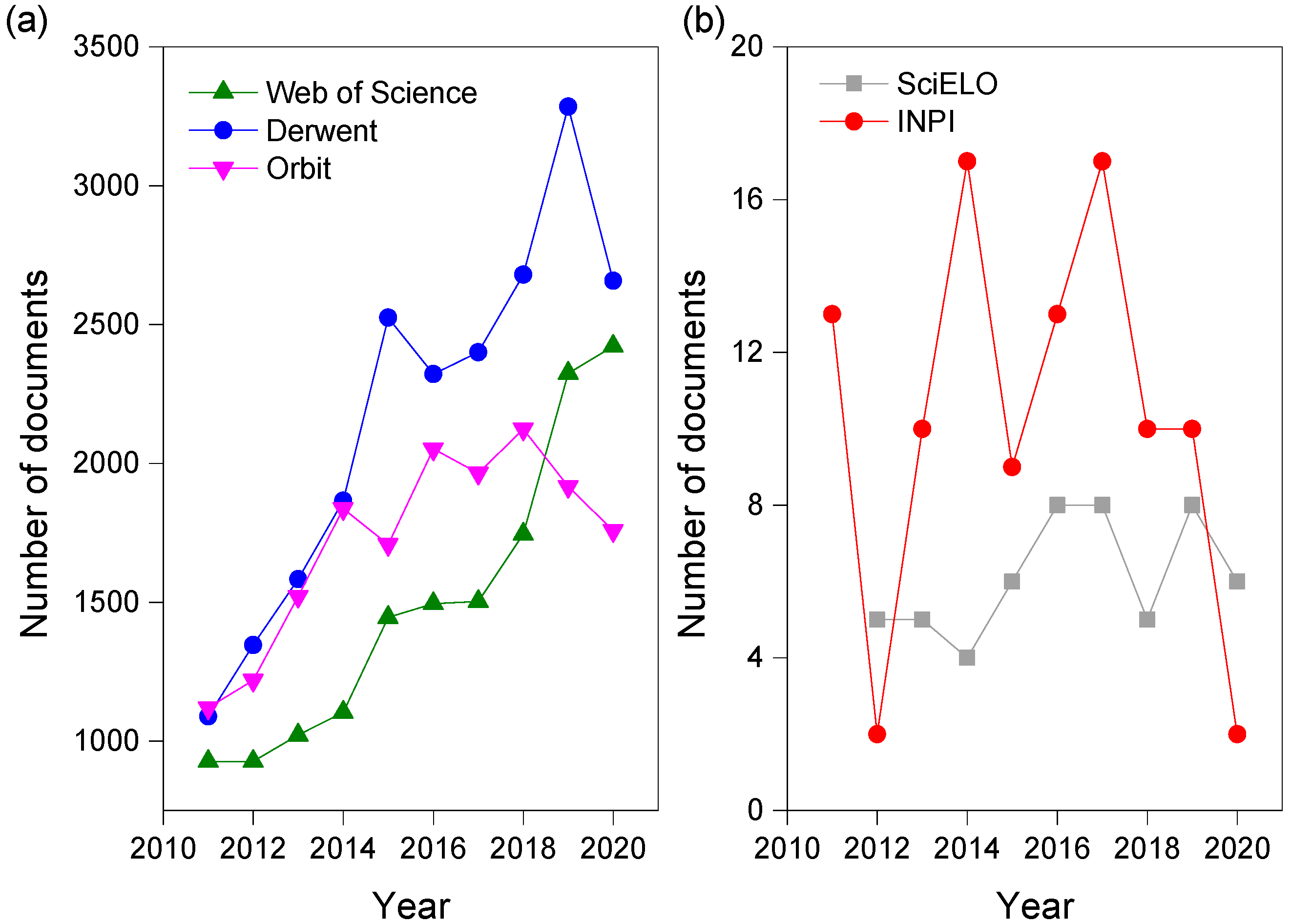
Figure 1a shows the distribution of these documents over the period evaluated (2011 to 2020). There is a very significant growth in the number of patents in 2016 at Orbit as well as in 2019 at Derwent, presenting 2051 and 3285 deposits, respectively. On the Web of Science, there is a continued growth in the number of published articles, although the number of patents remains higher. This fact may be directly related to the oil industry, for the development of these products internally or for the external production to be offered to this segment, as it is one of the industries that suffer most from economic losses caused by corrosion. For example, in 2014, China spent around USD 310 billion and it is estimated that around USD 1372 billion is spent annually due to problems related to corrosive processes in the oil sector [30,31].
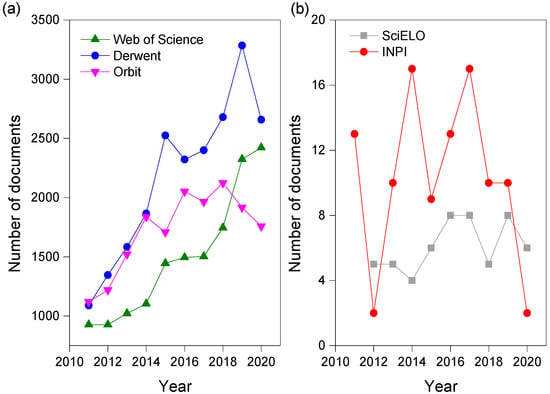
Figure 1.
Quantitative numbers of articles and patents recovered for group A descriptors over the last 10 years for (a) international searches and (b) specific searches for Brazil.
The largest holders of these patents are petrochemical industries. China Petroleum & Chemical Corporation is the largest depositor, with 568 and 596 records at Derwent and Orbit, respectively. Corrosion in this branch can generate direct and indirect costs associated with the loss of time, replacement of construction materials and continuous involvement of personnel in this management, in addition to environmental and safety consequences.
Figure 1b presents a comparison referring to the Brazilian scenario, with peaks in the PTO in 2014 and 2017, with 17 patent deposits each year. At SciELO, the peaks occurred in 2016, 2017 and 2019, with eight articles published each year. When comparing the two sources, the number of patents was only lower than years 2012 and 2020.
Finally, when comparing Figure 1a,b, the quantity of patents and articles is much lower in the specific searches for Brazil in relation to the international scenario. This demonstrates that the representativeness of the country is still discrete in corrosion inhibitors.
3.2. Green Corrosion Inhibitors
The results for group B descriptors (corros* AND (inhib* or inib*) AND (green OR environmental sustainable OR eco-friendly)) were less expressive when compared to those of group A, but indicate interest in the development of this class of inhibitors. A total of 2263 articles resulted from the Web of Science, about 9% of the number for group A (25,407), and 7 articles in SciELO. For Derwent, INPI and Orbit, 989, 10 and 632 patents were found, respectively.
The articles found in the SciELO database describe the use of various materials as green inhibitors, as listed in Table 3. Among the possible inhibitors evaluated there were: coffee powder [32], caffeine [33], Aniba canelilla [34], Hibisco-colibri [35], mango and orange peels [36], garlic [37] and castor bean [38]. All materials (Table 3) were analyzed to discover whether they inhibit carbon steel corrosion in acid medium and showed inhibition efficiencies above 80%. The exception was babassu coconut oil, with 56.45% efficiency [39].

Table 3.
Main information regarding the articles retrieved from the SciELO database for group B descriptors.
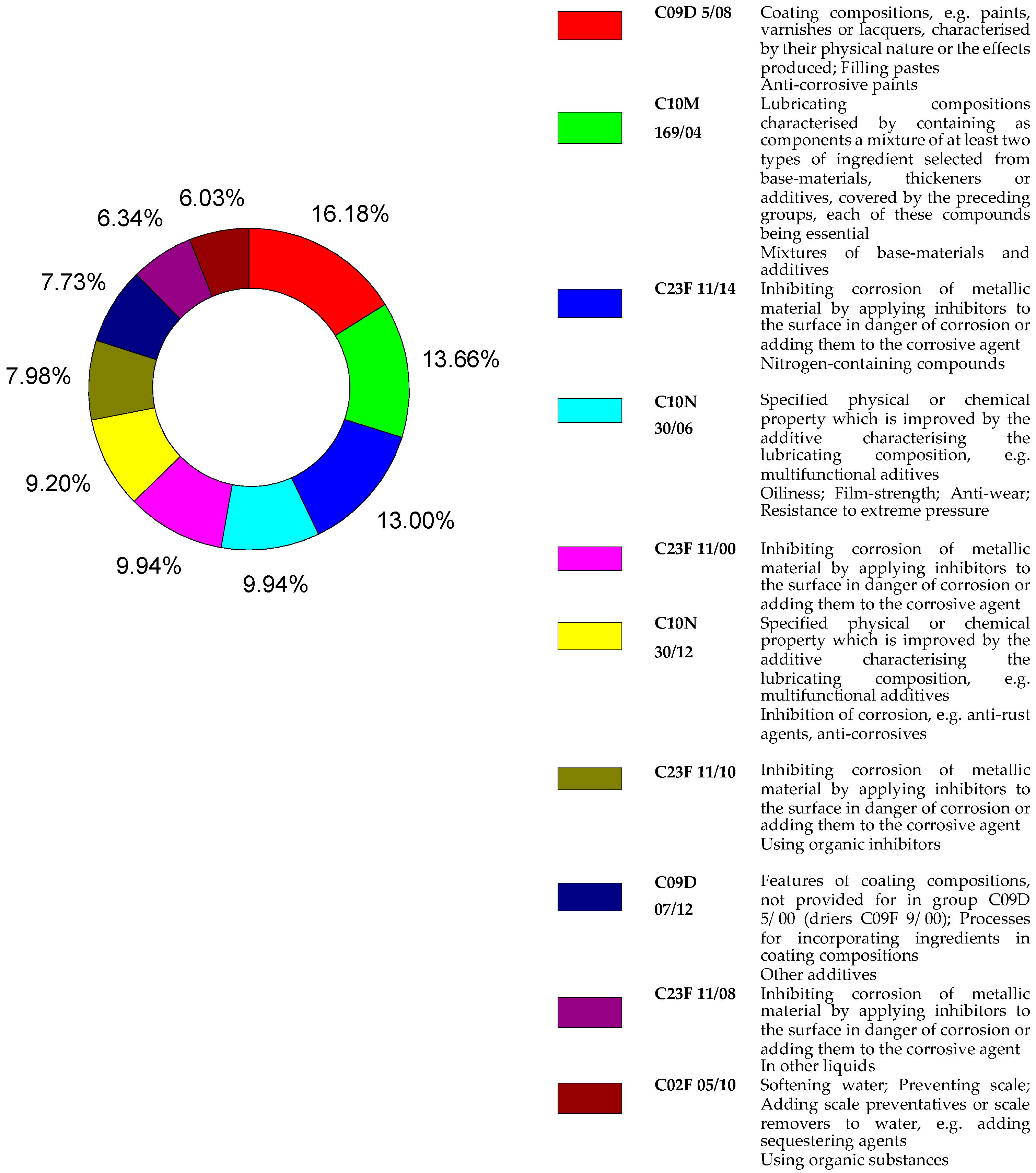
Figure 2 shows the percentages of the top 10 IPC (International Patent Classification) codes used for patents found in the Derwent database. Most of it related to code C09D 5/08, referring to: coating compositions (paints, varnishes or lacquers) characterized by their physical nature or effects produced; filling pastes and anti-corrosion paints. The other codes are related to section C, chemistry and metallurgy.
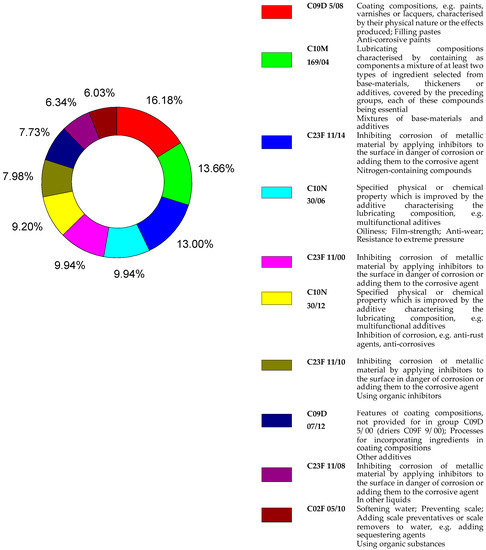
Figure 2.
Percentages of the top 10 IPC codes for patents recovered from the Derwent source with the B group of descriptors.
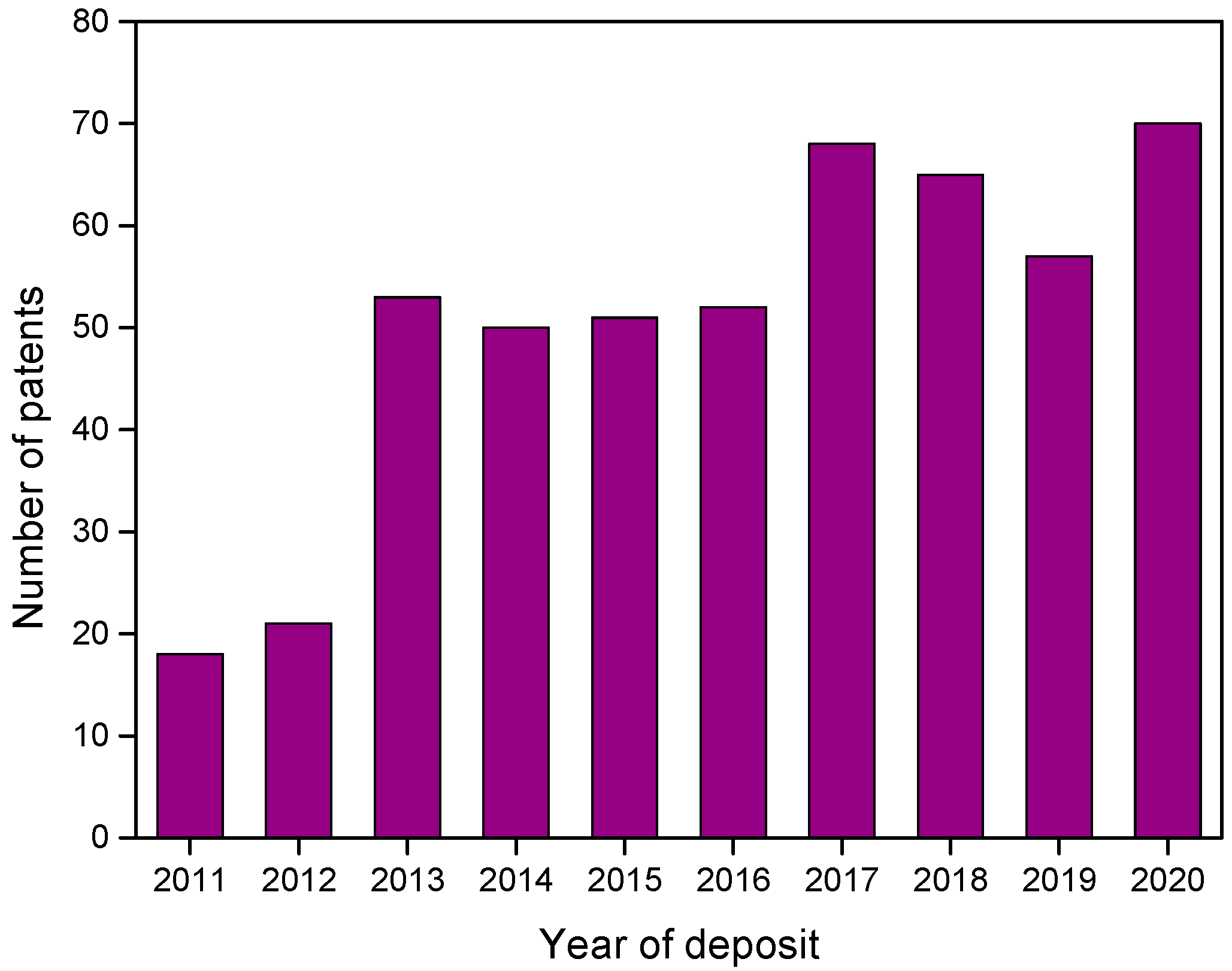
Figure 3 shows the distribution of patents over the last 10 years (2011 to 2020) found in Orbit software. There was a significant increase in deposits related to green corrosion inhibitors from 2013 onwards, about 140% more when compared to 2012.
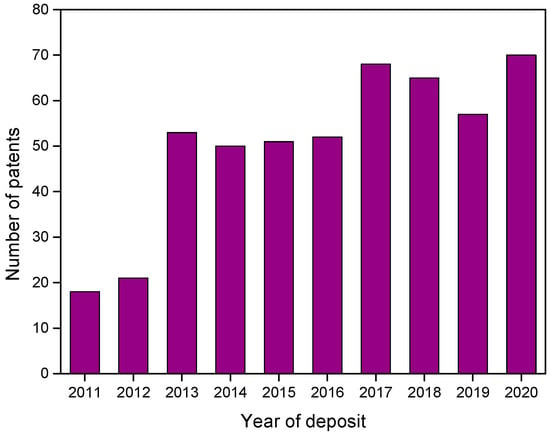
Figure 3.
Distribution over the last 10 years for patents recovered in Orbit with group B descriptors.
This increase in the number of deposits is related to a movement promoted by the United Nations (UN) in 2008. Through its general secretary, Mr. Ban Ki-Moon, who called for the World Intellectual Property Organization’s (WIPO) greater commitment and intervention in relation to the role of technology and industrial development on climate change issues. In addition, it called for mechanisms to enable greater harmonization for the concepts of “green” technologies existing in each WIPO country member. Thus, green patent programs were initiated worldwide [41].
Although this movement began in 2008, the implementation process in the countries along with their respective patent offices was gradual. It started in the United Kingdom (2009), followed by Australia (2009), South Korea (2009), Japan (2009), United States (2009), Israel (2009), Canada (2011), Brazil (2012), China (2012) and Taiwan (2014) [42].
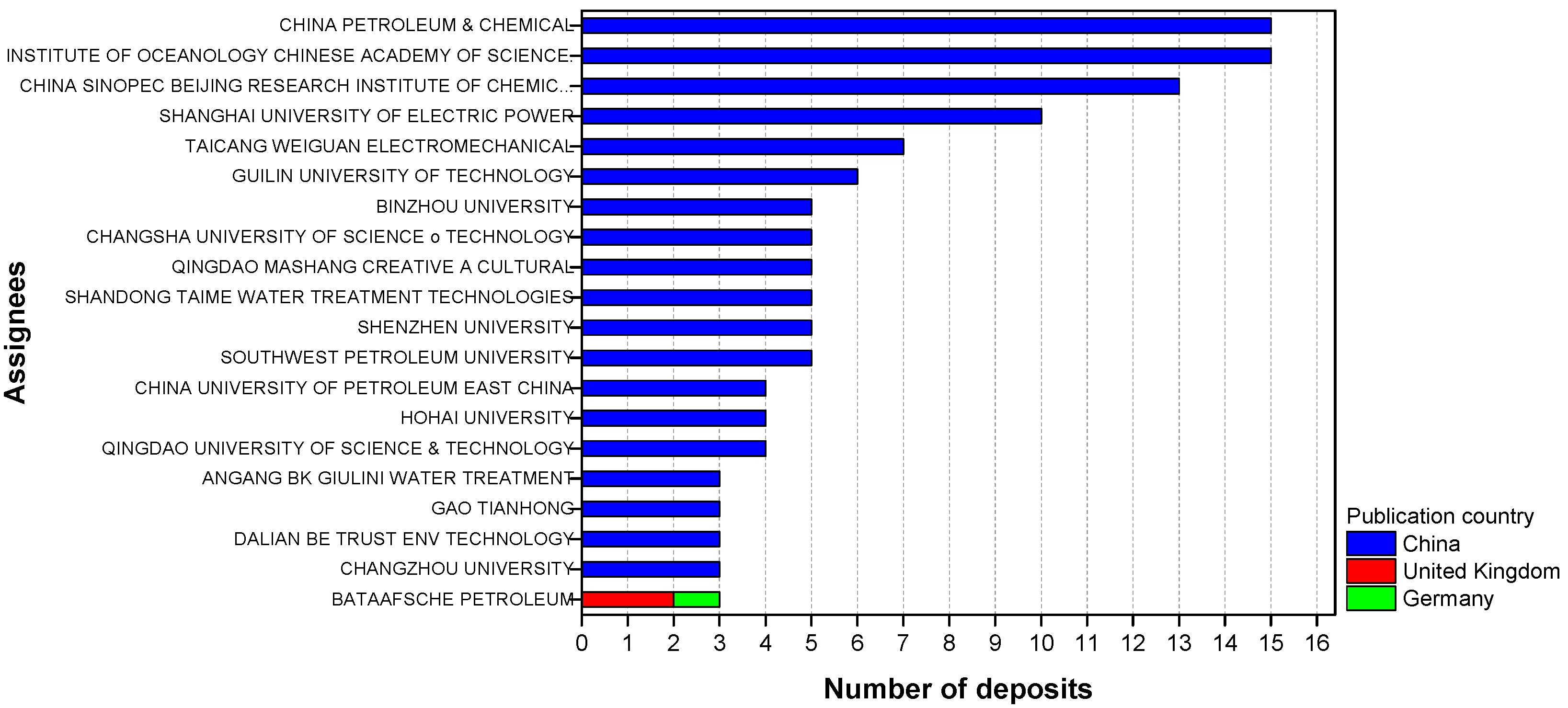
Furthermore, specifically for green corrosion inhibitors, China is the country with the highest number of deposits found in the searches and accounts for 19 of the largest players related to this area (Figure 4). Thus, by considering that its green patent program was only implemented in 2012, it is possible to justify that “jump” in the number of patents related to this type of inhibitor in 2013.
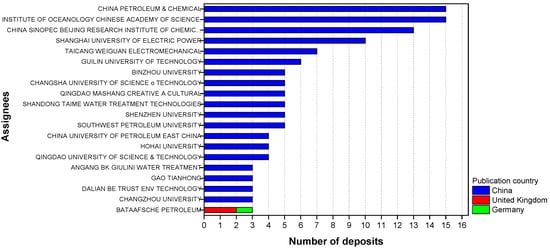
Figure 4.
The 20 largest players and their respective publishing countries recovered in Orbit with group B descriptors.
The patent filing documents found in the INPI source were analyzed. All were associated with new green corrosion inhibitors based on by-products of animal labor, oils and vegetable waste. Table 4 presents details of these documents.

Table 4.
Main information on the patents recovered from the PTO basis for group B descriptors.
All deposits have status “A2”, characterized by the request for publication insertion (PI) without search report. In addition, approximately 70% are associated with IPC code C23F 11, referring to the corrosion inhibition of metallic materials by applying inhibitors to surfaces subject to corrosion or by adding inhibitors to the corrosive agent.
3.3. Green Corrosion Inhibitors Derived from Plant Extracts
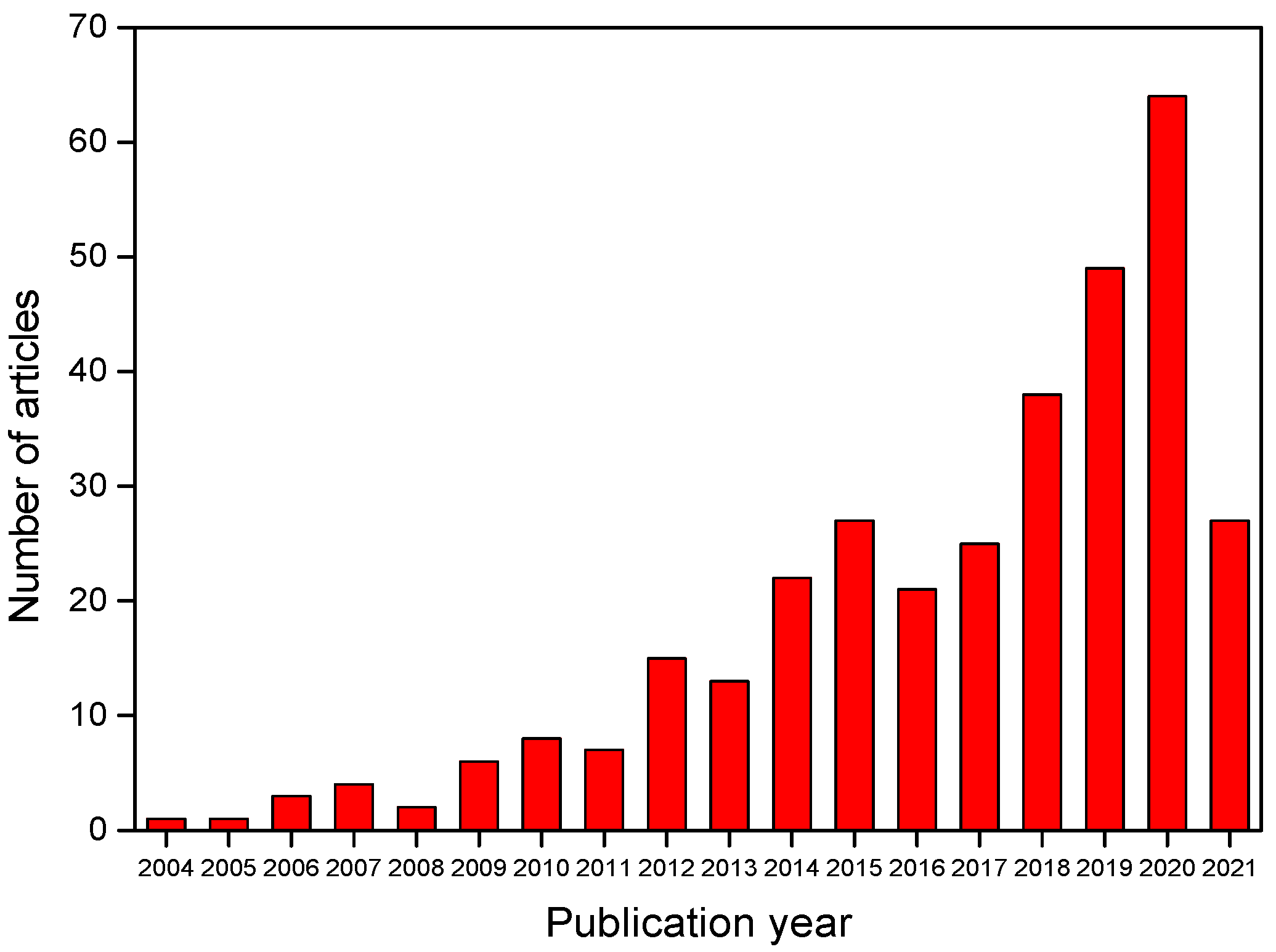
The combination of group C descriptors (corros* AND (inhib* or inib*) AND (green OR environmental sustainable OR eco-friendly) AND plant* AND extract) recovered 333 articles from the Web of Science database. Figure 5 shows its distribution over the years. Publications have grown significantly, especially in the last 10 years, indicating that interest in plant extract derivatives such as green corrosion inhibitors is on the rise.
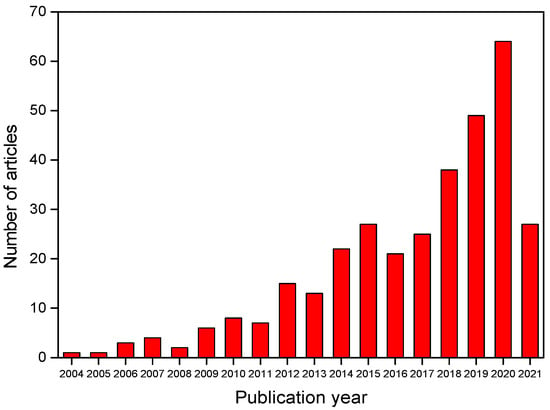
Figure 5.
Distribution over the years for articles retrieved from the Web of Science with group C descriptors.
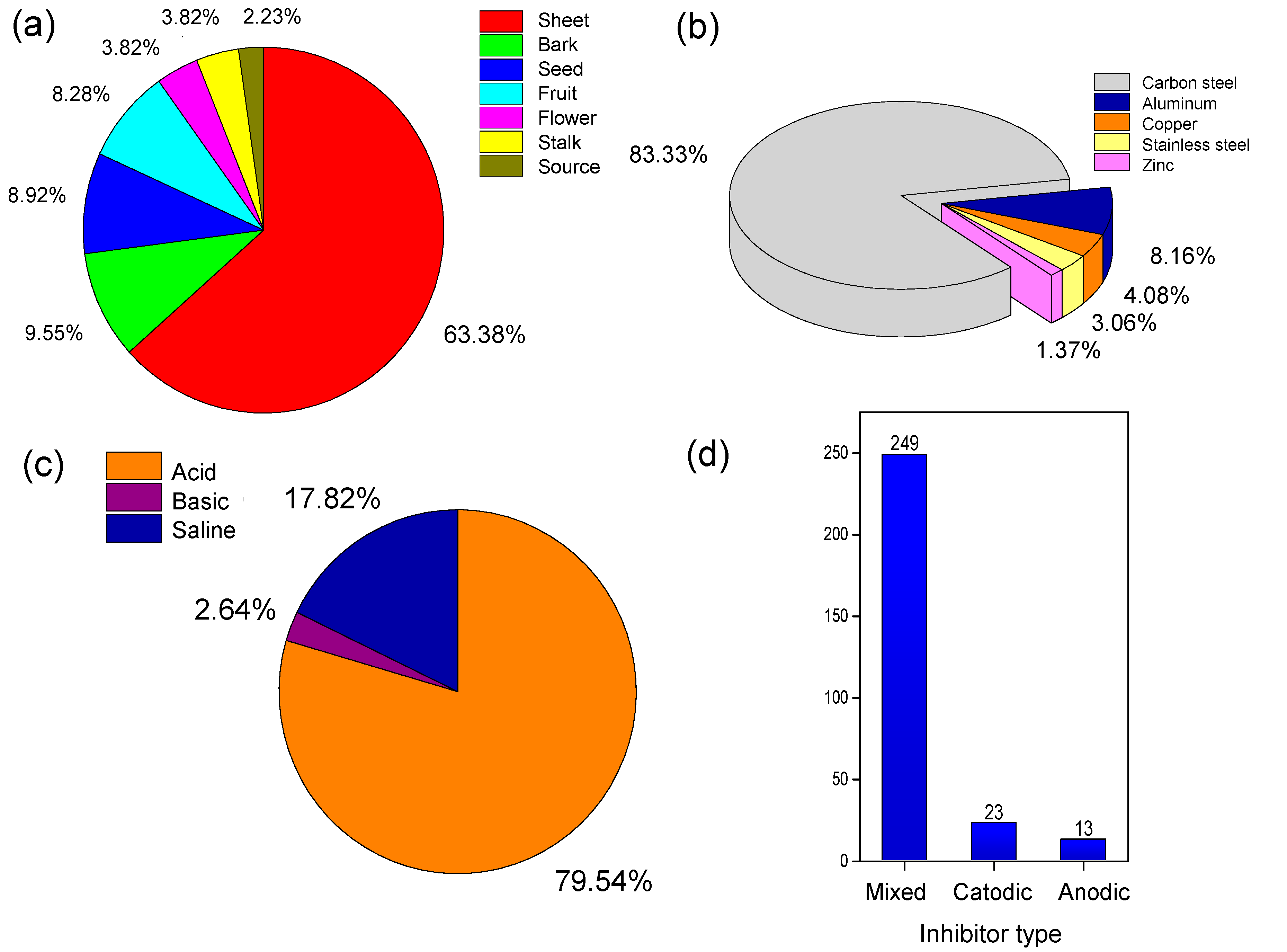
When analyzing the articles, it was found that 285 were directly related to the theme of interest. Then, a survey was conducted regarding the part of the plant used, the type of metal that should be protected, the aggressive/corrosive medium tested and the type of inhibitor that these studies reported. This data is shown in Figure 6.

Figure 6.
Information from the articles retrieved from the Web of Science with group C descriptors referring to (a) part of the plant used, (b) metallic material to be protected researched, (c) aggressive/corrosive medium tested and (d) type of action of the proposed inhibitor.
Plants are rich sources of organic matter and present variations in availability and types of compounds according to the parts analyzed. Most of the articles analyzed are concerned with the leaves (63.38%), as shown in Figure 6a. This choice is related to its bioavailability, easy extraction by low-cost methodologies and the fact that in most species it is the part where significant amounts of antioxidant compounds are found. This is mainly due to the fact that phytochemicals are produced mainly in the leaves, where they are synthesized in the presence of sunlight, water and CO2. Some of the most common phytochemicals that have a corrosion inhibiting effect are flavonoids, glycosides, alkaloids, saponins, phytosterols, tannins, anthraquinones, phenolic compounds, triterpenes and flobatanins [43,44,45]. These functional groups can donate electrons to empty d orbitals of iron atoms to form coordinated bonds and promote the adsorption of antioxidant molecules on the metal surface [46]. The complex chemical compositions of these plant extracts make it very difficult to attribute the inhibitory action to a particular component or group of components. Several authors propose that this inhibitory activity may occur due to the presence of flavonoids [47,48,49,50], which are especially important antioxidants due to their high redox potential, which allows them to act as reducing agents, hydrogen donors and singlet oxygen inhibitors. In addition, they have metal chelating potential. In this respect, several authors have suggested that their chelating activity is due to their binding to the metal ion (Mn+) at two sites in the molecule: on the orthodiphenolic group of one ring and on another ring of the molecule, forming mainly bidentate metal chelates [51,52,53]. Among the studies analyzed, some described the use of species: Endostemon tereticaulis [54], Tribulus terrestres [55], Ficus tikoua [56], Bagassa guianensis [57], Atriplex leucoclada [58] and Asparagus racemosus [59].
Wang et al. [56] used Ficus tikoua leaf extract (FTLE) to inhibit corrosion of carbon steel in HCl (1 M). The leaves were ground into powder. For extraction and processing, the leaf powder was added to boiling water. The filtrate was condensed and lyophilized to obtain FTLE powder. The extract was characterized by using Fourier transform infrared spectroscopy (FTIR), and the results revealed that the extract contained heteroatoms in functional groups or aromatic rings, universal characteristics of a traditional inhibitor. Electrochemical experiments showed that FTLE acted as a mixed-type inhibitor with an inhibition efficiency of up to 95.8% at 298 K, at a concentration of 200 mg·L−1. Surface analysis was performed by using scanning electron microscopy (SEM), demonstrating that samples submerged in this environment corroded seriously without the use of inhibitors. Metal and the acid medium as the corrosion inhibitor becomes adsorbed on the carbon steel surface, which effectively suppresses the dissolution reaction. The FTLE adsorption on the carbon steel surface followed the Langmuir isotherm.
Qiang et al. [20] evaluated the performance of Ginkgo leaf extract (GLE) in inhibiting corrosion of X70 steel in HCl (1 M). Ginkgo leaves were ground into powder and its extract was prepared by extraction with ethanol. The solution was concentrated in a rotary evaporator and then dried in an oven. The extract was characterized by FTIR, demonstrating that GLE contains oxygen and nitrogen atoms in functional groups and an aromatic ring, which corresponds to the general characteristics of traditional corrosion inhibitors. Electrochemical studies revealed an inhibition efficiency of over 90% in the presence of 200 mg·L−1 of GLE at all tested temperatures, acting as a mixed type inhibitor. The excellent inhibition capacity, which was attributed to the formation of films resulting from the adsorption of the inhibitor on the surface of X70 steel, was confirmed by using SEM and atomic force microscopy (AFM). The adsorption of GLE on the steel surface followed the Langmuir adsorption model.
The second most used part of the plants were bark (9.55%), interesting data, because the fruit peels are mostly considered residues, without an economic application [60]. This is added to the fact that they are already inhibitors from an organic source. Among the analyzed studies, some described the use of shells: coconut [61], rice [62], green beans [63], pomegranate [64] and watermelon [65].
Singh, Gupta and Gupta [66] evaluated the corrosion inhibition of carbon steel in H2SO4 solution (0.5 M) by lychee (Litchi chinensis) peel extract. The peels were ground into powder. For extraction and processing, the peel powder was added to water. The filtrate was evaporated and condensed in a water bath and dried in a desiccator to obtain a solid extract. The results show that the lychee bark extract acted as a mixed type inhibitor with an inhibition efficiency of 95.7% at 298 K at a concentration of 3.0 g·L−1; this behavior was confirmed by mass loss method and electrochemical tests. The corrosion inhibition is due to the adsorption of the extract on the metal surface, which is in accordance with the Langmuir adsorption isotherm. UV-Vis and FTIR spectroscopy studies demonstrated the presence of functional groups such as OH- and C=O, scanning electron microscopy (SEM) revealed that the metal surface showed roughness on the surface of the iron that was exposed only to the acid solution while there was a smooth surface on the surface of the metal that was exposed to acid solution with the extract, confirming that corrosion inhibition of steel occurs through adsorption of inhibitor molecules.
Rocha, Gomes and D’elia [36] investigated the aqueous extracts of mango (CME) and orange (COE) peels as inhibitors of corrosion on carbon steel in HCl solution (1 M). The electrochemical tests showed the action of the extracts as inhibitors of mixed type, with maximum efficiencies of 96% (CME) and 91% (COE), at a concentration of 600 mg·L−1. The complex chemical compositions of these extracts made it very difficult to attribute the inhibitory action to a particular constituent or group of constituents, but other studies point to these peels as rich sources of antioxidant compounds, such as polyphenols, carotenoids and vitamins C and E [47,67]. Phenolic compounds, particularly flavonoids, have been shown to possess significant antioxidant activity, which is mainly based on their structural characteristics (number and position of phenolic hydroxyls, other groups, conjugation) [47]. Thus, the inhibitory effect was promoted through the adsorption of the compounds present in the fruit peel extracts on the steel surface.
Regarding the type of metal to be protected against corrosion, carbon steel appears in 83.33% of the researches (Figure 6b). Being the most used steel in the world (in construction, chemical and oil industries, means of transport and communication, among others), its world production in 2020 was estimated at 1864.0 million tons [3]. This fact is due to its versatility and peculiar mechanical properties (promoted by the addition of carbon to the metal alloy), although the technology of these steels is well developed, representing an economic choice for many applications. However, these materials have some disadvantages, such as poor generalized corrosion performance, oxidation at low temperatures and rapid formation of FeO (or high oxides) at high temperatures [68].
Regarding the corrosive medium tested, 79.54% of the studies used acidic medium (Figure 6c), with more than 85% using hydrochloric acid (HCl). This result is justified since acidic solutions are used for various routine procedures in industries (chemical cleaning, decalcification and pickling), a fact that promotes corrosive acid attack [69]. Specifically, the use of HCl that is associated with the great damage that the oil industry suffers from corrosion. These costs are usually related to corrosive fouling, mud and dust, which cause failures in pipes and other industrial plant structures by metal corrosion. Moreover, the cleaning of these pipes is performed with the injection of HCl 15–28% solution to clear channels near the lumen, in the formation and increase in the oil flow [70,71,72].
Regarding the activity of the new inhibitors, most acted as mixed type (Figure 6d). This is related to the composition of extracts, mostly composed of heterocyclics such as flavonoids and alkaloids and other structures such as tannins, cellulose and polycyclic compounds. This composition can lead to the formation of a film on the metal surface and protect it by adsorption mechanism [73]. There is no absolute solution for incorporation of plant extracts and natural product inhibitors are well reviewed by Ong et al. [74]. Some inhibitors are incorporated into painting resin (water-based epoxy coatings, for example) directly or microencapsulated. Others can be used as waterborne processes as revealed by Quites et al. [75].
In the SciELO database, the combination of group C descriptors (corros* AND (inhib* or inib*) AND (green OR environmental sustainable OR eco-friendly) AND plant* AND extract) recovered two articles, already mentioned in Table 3 (Section 3.2). The first deals with the use of oil extracted from the fruit of babassu to inhibit corrosion in carbon steel 1020 in HCl 1 mol·L−1 solution. The extract showed good results, with 20% more efficiency in the comparison made with the commercial corrosion inhibitor Polydisperse HPAA (acid 2-Hidroxyphosphonocarboxylic), under the same conditions [39]. The second article used white tea extract in the inhibition of corrosion in carbon steel P110 in HCl 1 mol·L−1 solution. The inhibitor obtained efficiency of 90.2% [40].
In addition, no patent document was recovered with the combination of group C descriptors in the INPI source. However, some patents are described in Table 4 in which inhibitors are derived from plant extracts, such as: the use of cocoa almond bark (BR 10 2019 006077 8) of Phyllanthus amarus Schum. & Thonn leaves (BR 10 2014 031459 8), and Copaíba oil (BR 10 2014 022875 6), with efficiencies above 80%.
In the Orbit database, 20 patent documents were recovered for the combination of group C descriptors. Details of these documents are set out in the Table 5.

Table 5.
Key information on patents recovered in Orbit for the group C descriptors.
Patents reporting the use of corrosion inhibitors based on extracts such as leaves, seeds, fruit peels and roots, were found in the Web of Science and SciELO databases (group C). About 70% of these patents have been filed in the last four years; China is the country with the highest number of deposits, an expected result for the home country of 19, of the 20 largest players, in the area (Figure 4).
4. Conclusions
Corrosion affects the most varied sectors and directly impacts the economy of a country, especially industrialized and large oil and gas explorers. The need for means to reduce these impacts is evident and for this, science and technology are fundamental. The scientific and patent survey carried out in the present study recovered several documents about the use of derivatives of plant extracts as green corrosion inhibitors and allowed drawing an overview.
Most extracts are prepared from leaves, but the use of fruit peels, seeds, flowers and roots are also reported. Acid medium is the most tested corrosive environment and protection of carbon steel is the most researched. Excellent inhibition efficiencies were obtained at room temperature, with percentages above 80% for the corrosion rate. Results are associated with synergistic effect due to the presence of phytochemicals, known as nontoxic substances.
The work of Silva et al. (2014) [76] reported the various possibilities of using natural products from the Brazilian territory for “non-biological” applications, among them species containing polysaccharides, dyes and pigments, oils and other bioactive compounds. Researchers such as Rani (2012) [24], point out these potentially useful compounds as “green inhibitors”.
The excellent performance of these extracts as corrosion inhibitors, the information gathered in the present study and the fact that this applicability was investigated only in the early 2000s, indicate that the exploitation of this area is on the rise and is very promising.
5. Future Directions
The simplicity of many extraction procedures, combined with the bioavailability of plant sources and economic advantages, has triggered the exploitation of plant extracts as a new “trend” in corrosion studies. Within this context, some papers present a great advantage of evaluating extracts derived from natural residues, by-products or biomass residues. However, most of the authors do not manage to relate the inhibition effect to only a specific type of compound present in these extracts or even to relate it to a specific protection mechanism, due to its complex chemical composition. Thus, some authors use computational modeling to describe the protection mechanism, but they assume this action considering the majority compounds, especially flavonoids.
Attention should be paid to the fact that only some special bioactive compounds found in plant extracts can inhibit corrosion, although regular extracts are used to evidence hundreds or thousands of constituents. As a result, it is not clear which compound is responsible for the adhesion effects on corrosion barrier formation promoted by a plant extract. Separate isolation of bioactive compounds and evaluation tests of each compound may be a solution, provided that the operational costs are appropriate. Furthermore, evaluating whether compounds present in plant extracts can inhibit corrosion on their own (isolated) or in combination with others (synergistic) may be a promising field of study.
With the increasing demand for green technologies, the use of these extracts should be further investigated. The study presented here for papers and patents resulting from researchers and innovators from Brazil reiterates the potentialities of existing biodiversity in the Brazilian territory, sometimes not yet identified, and opens the opportunity for the discovery and production of industrial interest in various fields of work, including protective corrosion inputs that are environmentally biodiversity friendly and can add to knowledge to make the difference. Finally, the efficacy and performance of plant extracts should be carefully examined and validated before being used for commercial applications, although there are already patents for these products as this study shows.
Author Contributions
Conceptualization, W.R.d.S.M.; methodology, W.R.d.S.M. and J.T.; investigation, W.R.d.S.M.; writing—original draft preparation, W.R.d.S.M.; writing—review and editing, W.R.d.S.M., J.S.d.S., N.M.P.Q. and J.T.; supervision, C.L.d.P.e.S.Z., A.S.R. and J.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Brazil), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Brazil) and Fundação de Amparo à Pesquisa do Estado de Alagoas (FAPEAL/Brazil) for PhD fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Saviour, A.; Umoren, S.A.; Solomon, M.M.; Saji, V.S. Fundamentals of corrosion inhibition. In Polymeric Materials in Corrosion Inhibition, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 103–127. [Google Scholar] [CrossRef]
- Gentil, Corrosão, 7th ed.; Grupo Gen-LTC: Rio de Janeiro, Brazil, 2017; 584p.
- World Steel Association. Available online: https://worldsteel.org/media-centre/press-releases/2021/global-crude-steel-output-decreases-by-0-9-in-2020 (accessed on 18 August 2021).
- Reis, M.I.; da Silva, F.D.C.; Romeiro, G.A.; Rocha, A.A.; Ferreira, F. Deposição mineral em superfícies: Problemas e oportunidades na indústria do petróleo. ReVirtual Quim. 2011, 3, 2–13. [Google Scholar] [CrossRef]
- Sastri, V.S. Green Corrosion Inhibitors: Theory and Practice, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012; 304p. [Google Scholar] [CrossRef]
- Mazumder, M.A.J. Global impact of corrosion: Occurrence, cost and mitigation. Glob. J. Eng. Sci. 2020, 5, 1–4. [Google Scholar] [CrossRef]
- Chaubey, N.; Savita; Qurashi, A.; Chauhan, D.S.; Quraishi, M.A. Frontiers and advances in green and sustainable inhibitors for corrosion applications: A critical review. J. Mol. Liq. 2021, 321, 1–13. [Google Scholar] [CrossRef]
- Wei, H.; Heidarshenas, B.; Zhou, L.; Hussain, G.; Li, Q.; Ostrikov, K.K. Green inhibitors for steel corrosion in acidic environment: State of art. Mater. Today Sustain. 2020, 10, 100044. [Google Scholar] [CrossRef]
- Sherif, E.M. The role of corrosion inhibitors in protecting metallic structures against corrosion in harsh environments. In Role of Colloidal Systems in Environmental Protection; Fanun, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 509–526. [Google Scholar] [CrossRef]
- Fink, J.K. Corrosion Inhibitors. In Petroleum Engineer’s Guide to Oil Field Chemicals and Fluids, 1st ed.; Gulf Professional Publishing: Houston, TX, USA, 2021; pp. 217–252. [Google Scholar] [CrossRef]
- Agarwal, P.; Landolt, D. Effect of anions on the efficiency of aromatic carboxylic acid corrosion inhibitors in near neutral media: Experimental investigation and theoretical modeling. Corros. Sci. 1998, 40, 673–691. [Google Scholar] [CrossRef]
- Popov, B.N. Corrosion inhibitors. In Corrosion Engineering, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 581–597. [Google Scholar] [CrossRef]
- Dariva, C.G.; Galio, A.F. Corrosion Inhibitors–Principles, Mechanisms and Applications. In Developments in Corrosion Protection; Aliofkhazraei, M., Ed.; Intech.: London, UK, 2016; pp. 365–379. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M. Effect of halide ions on the corrosion inhibition efficiency of different organic species–A review. J. Ind. Eng. Chem. 2015, 21, 81–100. [Google Scholar] [CrossRef]
- Singh, P.; Srivastava, V.; Quraishi, M.A. Novel quinoline derivatives as green corrosion inhibitors for mild steel in acidic medium: Electrochemical, SEM, AFM, and XPS studies. J. Mol. Liq. 2016, 216, 164–173. [Google Scholar] [CrossRef]
- Kesavan, D.; Gopiraman, M.; Sulochana, N. Green inhibitors for corrosion of metals: A review. Chem. Sci. Rev. Lett. 2012, 1, 1–8. [Google Scholar]
- Felipe, M.B.M.; Maciel, M.A.M.; Medeiros, S.R.; Silva, D.R. Aspectos gerais sobre corrosão e inibidores vegetais. Rev. Virtual Quim. 2013, 5, 746–759. [Google Scholar] [CrossRef]
- Dean, S.W., Jr. Inhibitor types. Mater. Perform. 1981, 20, 47–51. [Google Scholar]
- Ishak, A.; Adams, F.V.; Madu, J.O.; Joseph, I.V.; Olubambi, P.A. Corrosion inhibition of mild steel in 1M hydrochloric acid using Haematostaphis barteri leaves extract. Procedia Manuf. 2019, 35, 1279–1285. [Google Scholar] [CrossRef]
- Qiang, Y.; Zhang, S.; Tan, B.; Chen, S. Evaluation of Ginkgo leaf extract as an eco-friendly corrosion inhibitor of X70 steel in HCl solution. Corros. Sci. 2018, 133, 6–16. [Google Scholar] [CrossRef]
- Bhawsar, J.; Jain, P.K.; Jain, P. Experimental and computational studies of Nicotiana tabacum leaves extract as green corrosion inhibitor for mild steel in acidic medium. Alex. Eng. J. 2015, 54, 769–775. [Google Scholar] [CrossRef]
- Khadraoui, A.; Khelifa, A.; Boutoumi, H.; Karzazi, Y.; Hammouti, B.; Al-Deyab, S.S. The oil from Mentha rotundifolia as green inhibitor of carbon steel corrosion in hydrochloric acid. Chem. Eng. Commun. 2016, 203, 270–277. [Google Scholar] [CrossRef]
- Bidi, M.A.; Azadi, M.; Rassouli, M. A new green inhibitor for lowering the corrosion rate of carbon steel in 1 M HCl solution: Hyalomma tick extract. Mat. Today Commun. 2020, 24, 100996. [Google Scholar] [CrossRef]
- Rani, B.E.; Basu, B.B.J. Green inhibitors for corrosion protection of metals and alloys: An overview. Int. J. Corros. 2012, 2012, 1–15. [Google Scholar] [CrossRef]
- Web of Science. Available online: https://www-webofscience.ez9.periodicos.capes.gov.br/wos/woscc/basic-search (accessed on 9 August 2021).
- Scientific Electronic Library Online (SCIELO). Available online: https://www.scielo.br/ (accessed on 11 August 2021).
- Instituto Nacional da Propriedade Industrial. Available online: https://www.gov.br/inpi/pt-br (accessed on 10 August 2021).
- Derwent Innovations Index. Available online: https://www-webofscience.ez9.periodicos.capes.gov.br/wos/diidw/basic-search (accessed on 10 August 2021).
- Questel Orbit. Available online: https://www.orbit.com (accessed on 11 August 2021).
- Hou, B.; Li, X.; Ma, X.; DU, C.; Zang, D.; Zheng, M.; Xu, W.; Lu, D.; Ma, F. The cost of corrosion in China. NPJ Mater. Degrad. 2017, 1, 1–10. [Google Scholar] [CrossRef]
- Perez, T.E. Corrosion in the oil and gas industry: An increasing challenge for materials. Jom 2013, 65, 1033–1042. [Google Scholar] [CrossRef]
- Costa, M.A.; Gois, J.S.D.; Toaldo, I.M.; Bauerfeldt, A.C.F.; Batista, D.B.; Bordignon-Luiz, M.T.; Lago, D.C.B.; Luna, A.S.; Senna, L.F.D. Optimization of espresso spent ground coffee waste extract preparation and the influence of its chemical composition as an eco-friendly corrosion inhibitor for carbon steel in acid medium. Mat. Res. 2020, 23, 1–12. [Google Scholar] [CrossRef]
- Souza, F.S.D.; Gonçalves, R.S.; Spinelli, A. Assessment of caffeine adsorption onto mild steel surface as an eco-friendly corrosion inhibitor. J. Braz. Chem. Soc. 2014, 25, 81–90. [Google Scholar] [CrossRef]
- Barros, I.B.D.; Custódio, D.L.; Andrade, M.C.D.; Veiga Junior, V.F.D.; Silva Neto, A.J.D.; Bastos, I.N. The Inhibitory Action of Aniba Canelilla (HBK) Mez. Extracts on the corrosion of carbon steel in hydrochloric acid medium. Mat. Res. 2018, 21, 1–8. [Google Scholar] [CrossRef]
- Valbon, A.; Ribeiro, B.F.; Soares, M.A.F.; Oliveira, M.C.; Neves, M.A.; Echevarria, A. Extrato de hibisco-colibri como inibidor verde de corrosão do aço-carbono em ácido sulfúrico. Quim. Nova 2019, 42, 797–802. [Google Scholar] [CrossRef]
- Rocha, J.C.; Gomes, J.A.C.P.; D’elia, E. Aqueous extracts of mango and orange peel as green inhibitors for carbon steel in hydrochloric acid solution. Mater. Res. 2014, 17, 1581–1587. [Google Scholar] [CrossRef]
- Barreto, L.S.; Tokumoto, M.S.; Guedes, I.C.; Melo, H.G.D.; Amado, F.D.R.; Capelossi, V.R. Evaluation of the anticorrosion performance of peel garlic extract as corrosion inhibitor for ASTM 1020 carbon steel in acidic solution. Matéria 2017, 22, e11852. [Google Scholar] [CrossRef]
- Santos, A.D.M.; Almeida, T.F.D.; Cotting, F.; Aoki, I.V.; Melo, H.G.D.; Capelossi, V.R. Evaluation of castor bark powder as a corrosion inhibitor for carbon steel in acidic media. Mat. Res. 2017, 20, 492–505. [Google Scholar] [CrossRef]
- Peres, J.; Conde, R.; Bezerra, C.; Costa, R.; Reis, G.; Souza, M.E.P.D.; Nascimento, C. Estudo da eficiência do Orbignya oleifera como inibidor verde de corrosão para aço com baixo teor de carbono comparado com inibidor comercial em solução HCl 1M. Matéria 2019, 24, 13. [Google Scholar] [CrossRef]
- Oliveira, T.M.D.; Cardoso, S.P. Avaliação do chá branco como potencial inibidor de corrosão. Matéria 2019, 24, 9. [Google Scholar] [CrossRef]
- Santos, N.; Oliveira, D.G. A patenteabilidade de tecnologias verdes como instrumento de desenvolvimento sustentável. Rev. Jurídica 2014, 4, 294–310. [Google Scholar]
- Reis, P.C.; Osawa, C.C.; Martinez, M.E.M.; Moreira, J.C.C.B.R.; Santos, D.A. Programa das Patentes Verdes no Brasil: Aliança Verde entre o Desenvolvimento Tecnológico, Crescimento Econômico e a Degradação Ambiental. In Proceedings of the XV Congreso Latino-Iberoamericano de Gestión Tecnológica, Medelín, Colombia, 21–31 October 2013. [Google Scholar]
- Degáspari, C.H.; Waszczynskyj, N. Propriedades antioxidantes de compostos fenólicos. Visão Acadêmica 2004, 5, 33–40. [Google Scholar] [CrossRef]
- Rocha, J.C.; Gomes, J.A.C.P.; D’Elia, E.; Cruz, A.P.G.; Cabral, L.M.C.; Torres, A.G.; Monteiro, M.V.C. Grape pomace extracts as green corrosion inhibitors for carbon steel in hydrochloric acid solutions. Int. J. Electrochem. Sci. 2012, 7, 11941–11956. [Google Scholar]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.A.; Ebenso, E.E. Challenges and advantages of using plant extract as inhibitors in modern corrosion inhibition systems: Recent advancements. J. Mol. Liq. 2021, 321, 114666. [Google Scholar] [CrossRef]
- Feng, L.; Yang, H.; Wang, F. Experimental and theoretical studies for corrosion inhibition of carbon steel by imidazoline derivative in 5% NaCl saturated Ca(OH)2 solution. Electrochim. Acta 2011, 58, 427–436. [Google Scholar] [CrossRef]
- Ajila, C.M.; Naidu, K.A.; Bhat, S.G.; Rao, U.J.S. Bioactive compounds and antioxidant potential of mango peel extract. Food Chem. 2007, 105, 982–988. [Google Scholar] [CrossRef]
- Liu, Q.; Song, Z.; Han, H.; Donkor, S.; Jiang, L.; Wang, W.; Chu, H. A novel green reinforcement corrosion inhibitor extracted from waste Platanus acerifolia leaves. Constr. Build. Mater. 2020, 260, 119695. [Google Scholar] [CrossRef]
- Vorobyova, V.; Skiba, M.; Chygyrynets, O.; Pylypenko, T.; Motronyuk, T.; Fateev, Y. Inhibition efficiency of apricot pomace extract as a “green” corrosion inhibitor. Mater. Today Proc. 2022, 50, 456–462. [Google Scholar] [CrossRef]
- Abdelaziz, S.; Benamira, M.; Messaadia, L.; Boughoues, Y.; Lahmar, H.; Boudjerda, A. Green corrosion inhibition of mild steel in HCl medium using leaves extract of Arbutus unedo L. plant: An experimental and computational approach. Colloids Surf. A Physicochem. Eng. Asp. 2021, 619, 126496. [Google Scholar] [CrossRef]
- Kejík, Z.; Kaplánek, R.; Masařík, M.; Babula, P.; Matkowski, A.; Filipenský, P.; Veselá, K.; Gburek, J.; Sýkora, D.; Martásek, P.; et al. Iron Complexes of Flavonoids-Antioxidant Capacity and Beyond. Int. J. Mol. Sci. 2021, 22, 646. [Google Scholar] [CrossRef]
- Faraj, L.; Khan, G.M. Application of natural product extracts as green corrosion inhibitors for metals and alloys in acid pickling processes-A. Int. J. Electrochem. Sci. 2015, 10, 6120–6134. [Google Scholar]
- Bertleff-Zieschang, N.; Rahim, M.A.; Ju, Y.; Braunger, J.A.; Suma, T.; Dai, Y.; Pan, S.; Cavalieri, F.; Caruso, F. Biofunctional metal–phenolic films from dietary flavonoids. Chem. Commun. 2017, 53, 1068–1071. [Google Scholar] [CrossRef]
- Abba, B.N.; Idouhli, R.; Ilagouma, A.T.; Abouelfida, A.; Khadiri, M.; Romane, A. Use of Endostemon tereticaulis (Pear.) M. Ashby and Hyptis spicigera Lam. Plant Extracts as Corrosion Green Inhibitors for Mild Steel in 1M HCl: Electrochemical and Surface Morphological Studies. Prot. Met. Phys. Chem. 2021, 57, 619–633. [Google Scholar] [CrossRef]
- Sivaraju, M.; Kannan, K. Eco-friendly inhibitor (Tribulus terrestris L) for mild steel corrosion in 1 N phosphoric acid. Asian J. Chem. 2010, 22, 233–244. [Google Scholar]
- Wang, Q.; Tan, B.; Bao, H.; Xie, Y.; Mou, Y.; Li, P.; Chen, D.; Shi, Y.; Li, X.; Yang, W. Evaluation of Ficus tikoua leaves extract as an eco-friendly corrosion inhibitor for carbon steel in HCl media. Bioelectrochemistry 2019, 128, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Lebrini, M.; Suedile, F.; Salvin, P.; Roos, C.; Zarrouk, A.; Jama, C.; Bentiss, F. Bagassa guianensis ethanol extract used as sustainable eco-friendly inhibitor for zinc corrosion in 3% NaCl: Electrochemical and XPS studies. Surf. Interfaces 2020, 20, 2–31. [Google Scholar] [CrossRef]
- Ahmed, R.K.; Zhang, S. Atriplex leucoclada extract: A promising eco-friendly anticorrosive agent for copper in aqueous media. J. Ind. Eng. Chem. 2021, 99, 334–343. [Google Scholar] [CrossRef]
- Bashir, S.; Singh, G.; Kumar, A. Shatavari (Asparagus Racemosus) as green corrosion inhibitor of aluminium in acidic medium. J. Mater. Environ. Sci. 2017, 8, 4284–4291. [Google Scholar] [CrossRef]
- Shahmoradi, A.R.; Ranjbarghanei, M.; Javidparvar, A.A.; Guo, L.; Berdimurodov, E.; Ramezanzadeh, B. Theoretical and surface/electrochemical investigations of walnut fruit green husk extract as effective inhibitor for mild-steel corrosion in 1M HCl electrolyte. J. Mol. Liq. 2021, 338, 13. [Google Scholar] [CrossRef]
- Umoren, S.A.; Eduok, U.M.; Israel, A.U.; Obot, I.B.; Solomon, M.M. Coconut coir dust extract: A novel eco-friendly corrosion inhibitor for Al in HCl solutions. Green Chem. Lett. Rev. 2012, 5, 303–313. [Google Scholar] [CrossRef]
- Nisar, N.; Bhat, J.A. Experimental investigation of Rice Husk Ash on compressive strength, carbonation and corrosion resistance of reinforced concrete. Aust. J. Civ. Eng. 2021, 19, 155–163. [Google Scholar] [CrossRef]
- Umoren, S.A.; Obot, I.B.; Akpabio, L.E.; Etuk, S.E. Adsorption and corrosive inhibitive properties of Vigna unguiculata in alkaline and acidic media. Pigment. Resin Technol. 2008, 37, 98–105. [Google Scholar] [CrossRef]
- Magni, M.; Postiglione, E.; Marzorati, S.; Verotta, L.; Trasatti, S.P. Green corrosion inhibitors from agri-food wastes: The case of Punica granatum extract and its constituent ellagic acid. A validation study. Processes 2020, 8, 272. [Google Scholar] [CrossRef]
- Odewunmi, N.A.; Umoren, S.A.; Gasem, Z.M. Watermelon waste products as green corrosion inhibitors for mild steel in HCl solution. J. Environ. Chem. Eng. 2015, 3, 286–296. [Google Scholar] [CrossRef]
- Singh, M. Ramananda; GUPTA, Prachi; GUPTA, Kaushal. The litchi (Litchi Chinensis) peels extract as a potential green inhibitor in prevention of corrosion of mild steel in 0.5 M H2SO4 solution. Arab. J. Chem. 2019, 12, 1035–1041. [Google Scholar] [CrossRef]
- Ribeiro, S.M.R.; Barbosa, L.C.A.; Queiroz, J.H.; Knödler, M.; Schieber, A. Phenolic compounds and antioxidant capacity of Brazilian mango (Mangifera indica L.) varieties. Food Chem. 2008, 110, 620–626. [Google Scholar] [CrossRef]
- Gomes, A.C.T. Análise da Corrosão e da Erosão-Corrosão do Aço Carbono em Meio com NaHCO3 e CO2. Master’s Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2005. [Google Scholar]
- Machnikova, E.; Whitmire, K.H.; Hackerman, N. Corrosion inhibition of carbon steel in hydrochloric acid by furan derivatives. Electrochim. Acta 2008, 53, 6024–6032. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; Alnajjar, A.O. Enhanced the protection capacity of poly (o-toluidine) by synergism with zinc or lanthanum additives at C-steel/HCl interface: A combined DFT, molecular dynamic simulations and experimental methods. J. Mol. Liq. 2020, 303, 112641. [Google Scholar] [CrossRef]
- Mahdavian, M.; Tehrani-Bagha, A.R.; Alibakhshi, E.; Ashhari, S.; Palimi, M.J.; Farashi, S.; Javadian, S.; Ektefa, F. Corrosion of mild steel in hydrochloric acid solution in the presence of two cationic gemini surfactants with and without hydroxyl substituted spacers. Corros. Sci. 2018, 137, 62–75. [Google Scholar] [CrossRef]
- Jawich, M.W.; Oweimreen, G.A.; Ali, S.A. Heptadecyl-tailed mono-and bis-imidazolines: A study of the newly synthesized compounds on the inhibition of mild steel corrosion in a carbon dioxide-saturated saline medium. Corros. Sci. 2012, 65, 104–112. [Google Scholar] [CrossRef]
- Rocha, J.C.D.; Gomes, J.A.D.C.P. Inibidores de corrosão naturais-Proposta de obtenção de produtos ecológicos de baixo custo a partir de resíduos industriais. Matéria 2018, 22 (Suppl. 1), 10. [Google Scholar] [CrossRef]
- Ong, G.; Kasi, R.; Subramaniam, R. A review on plant extracts as natural additives in coating applications. Prog. Org. Coat. 2021, 151, 106091. [Google Scholar] [CrossRef]
- Quites, D.; Leiza, J.R.; Mantione, D.; Somers, A.; Forsyth, M.; Paulis, M. Incorporation of a Coumarate Based Corrosion Inhibitor in Waterborne Polymeric Binders for Corrosion Protection Applications. Macromol. Mater. Eng. 2021, 307, 2100772. [Google Scholar] [CrossRef]
- Silva, V.C.; Rodrigues, C.M. Natural products: An extraordinary source of value-added compounds from diverse biomasses in Brazil. Chem. Biol. Technol. Agric. 2014, 1, 1–6. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).