Recent Trends and Progress in Corrosion Inhibitors and Electrochemical Evaluation
Abstract
:1. Introduction
2. Mechanism of Corrosion Inhibition
3. Types of Corrosion Inhibitors
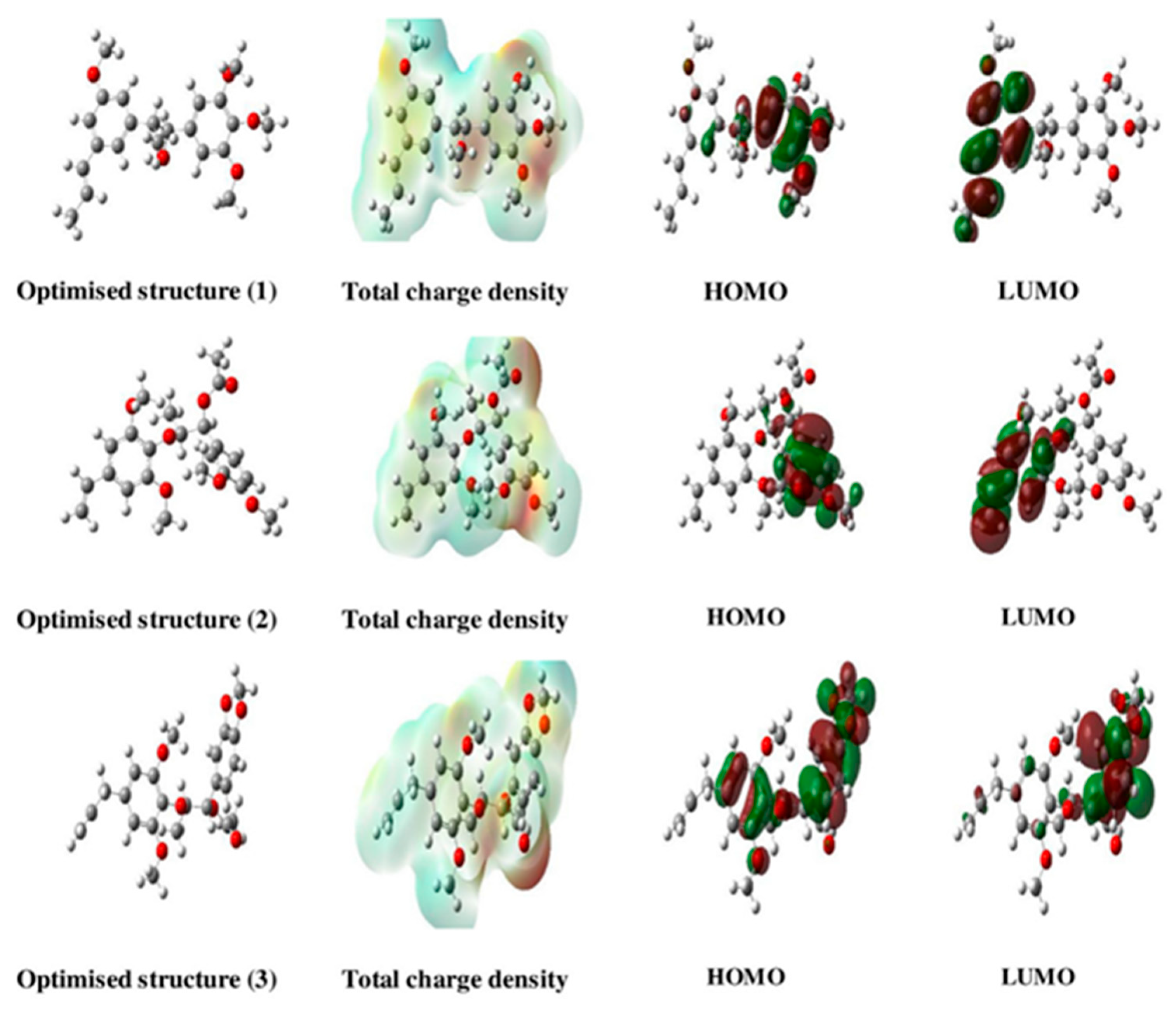
3.1. Organic Corrosion Inhibitors
3.2. Inorganic Corrosion Inhibitors
3.2.1. Anodic Corrosion Inhibitors
3.2.2. Cathodic Inhibitors
3.3. Green Corrosion Inhibitors
3.4. Composite-Based Corrosion Inhibitors
3.4.1. Biopolymer-Based Corrosion Inhibitors
3.4.2. Carbon-Based Corrosion Inhibitors
3.4.3. Silane-Based Composite Coatings
3.4.4. Sol-Gel-Based Composites
3.4.5. Micro-Arc Oxidation Coatings
3.4.6. Layered Double Hydroxides
3.4.7. Metal–Organic Frameworks
4. Encapsulation Technique for the Effective Corrosion Inhibition
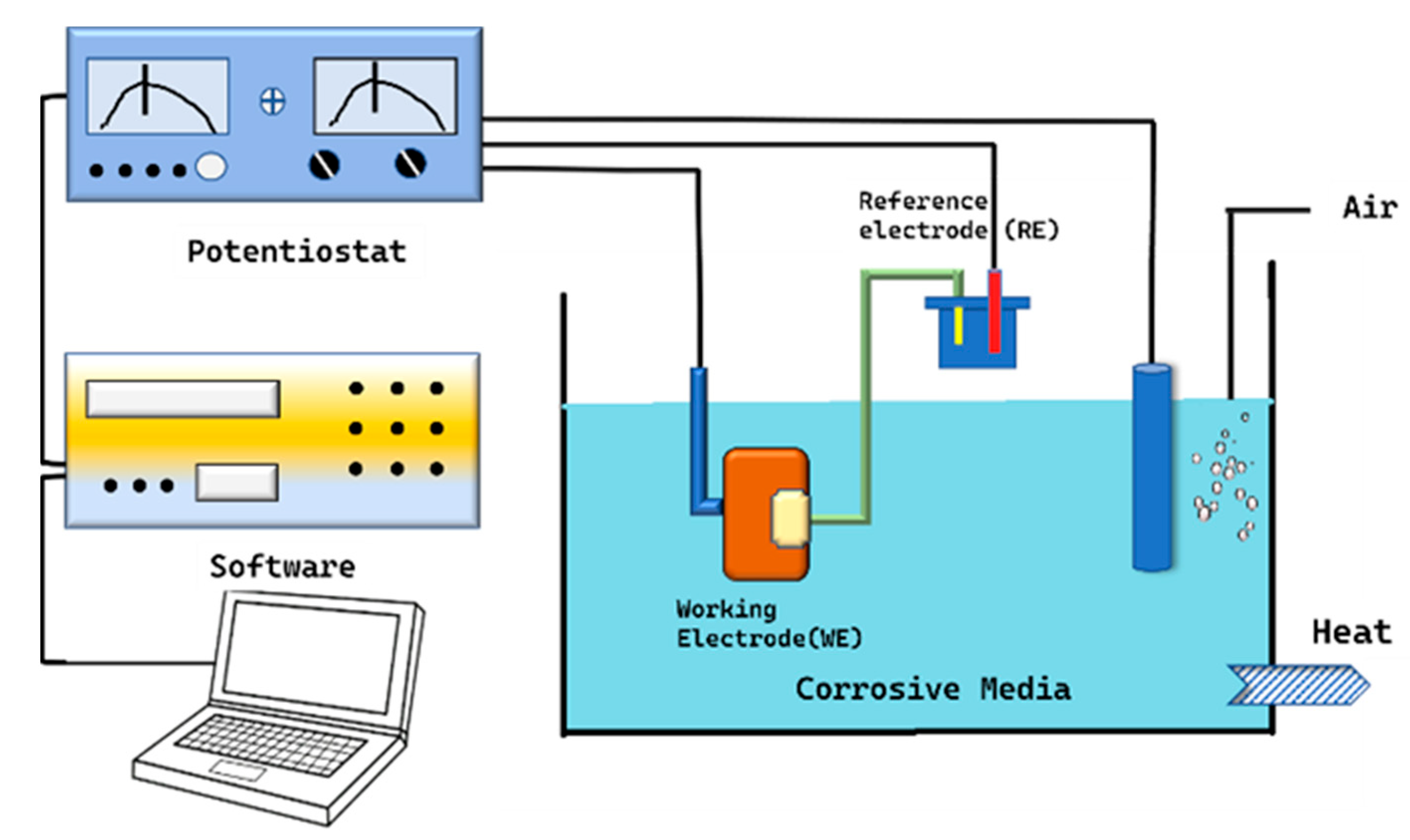
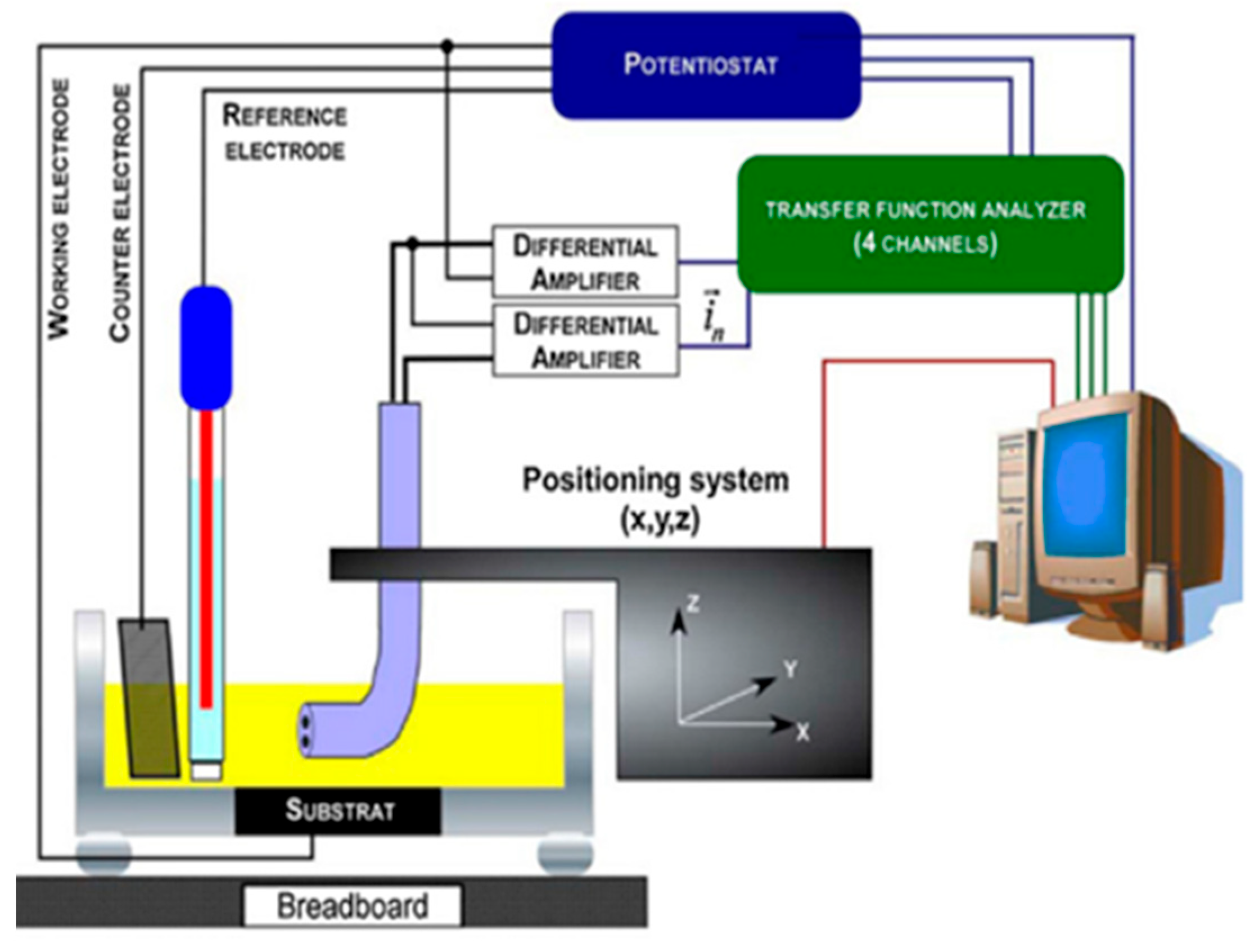
5. Corrosion Monitoring Techniques
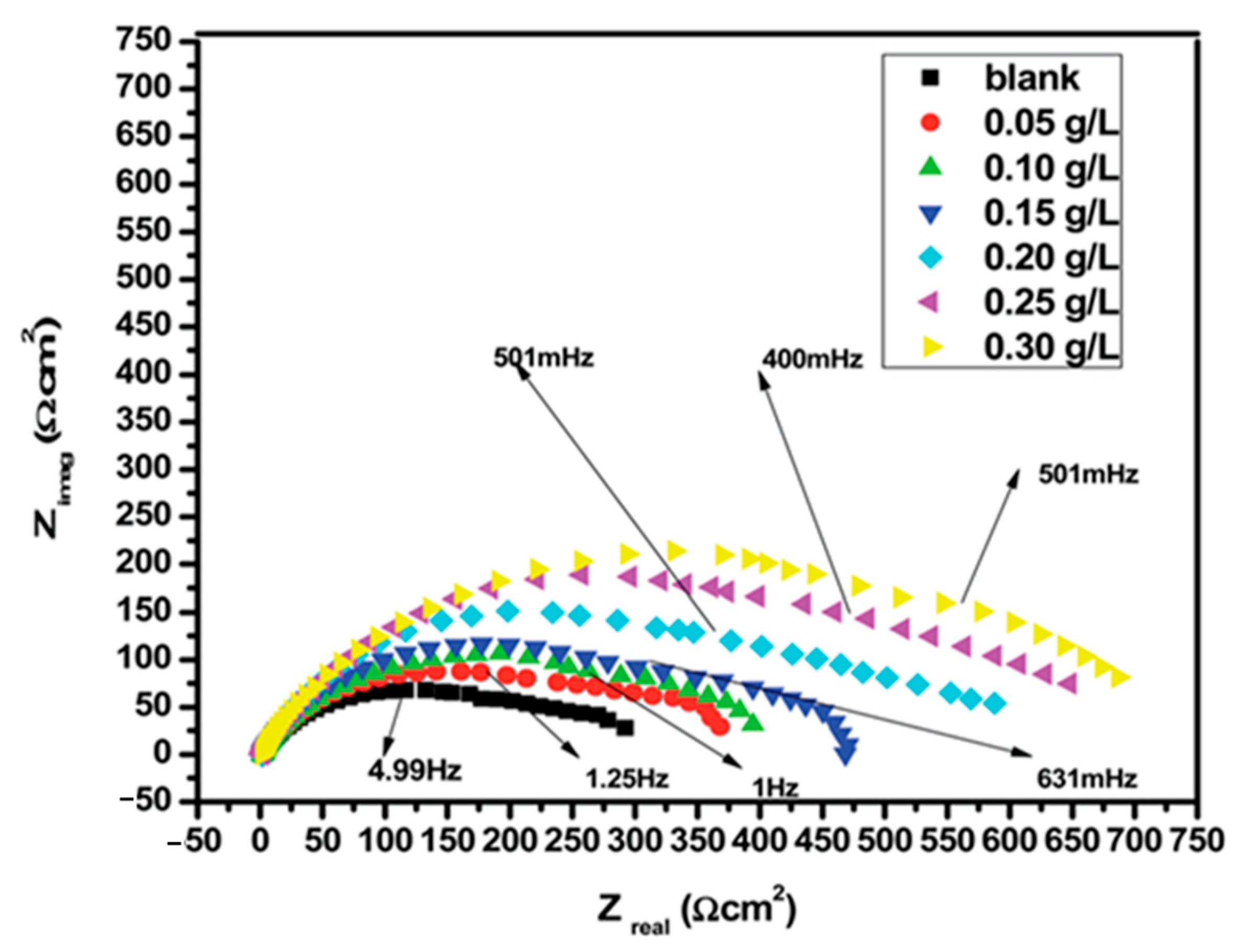
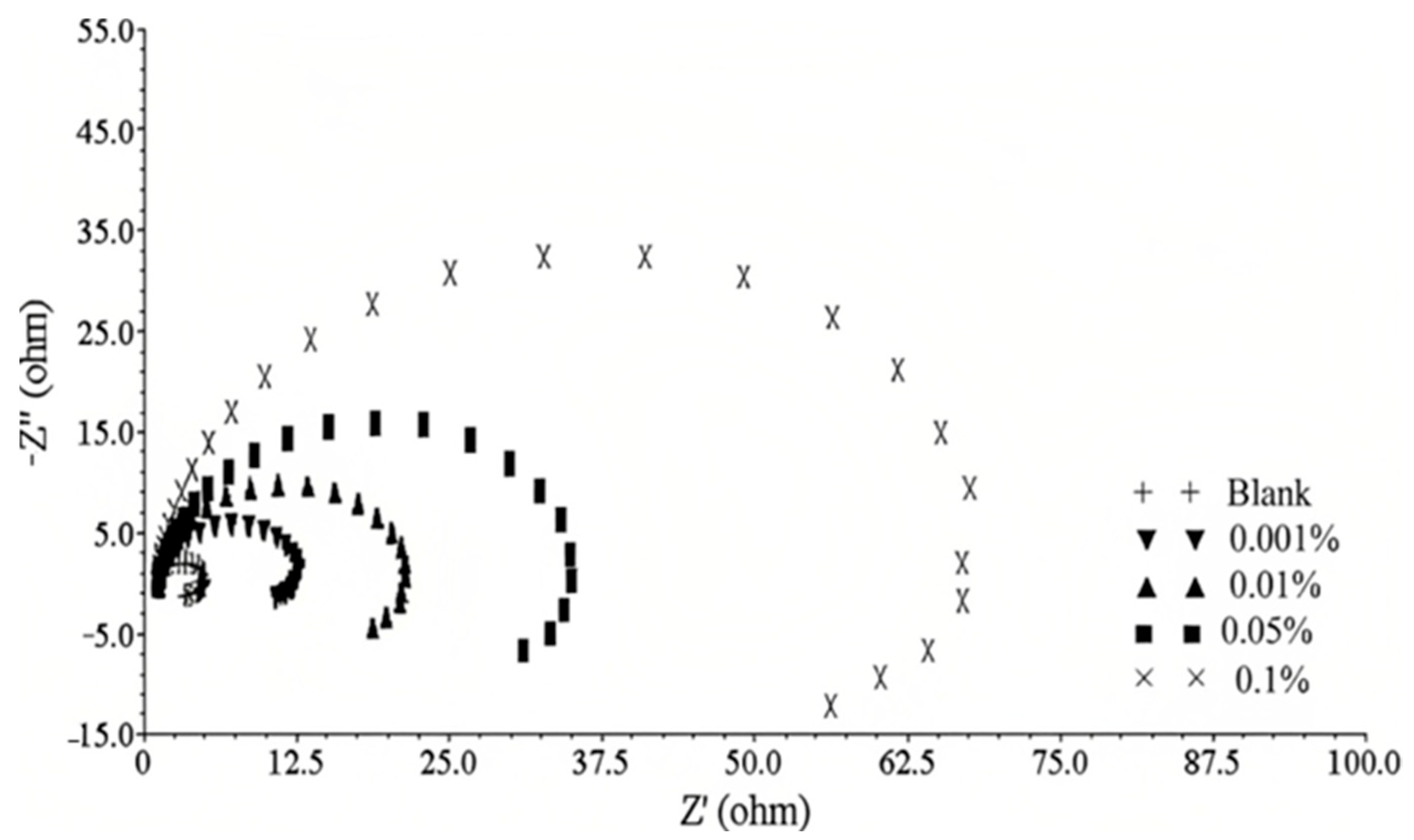
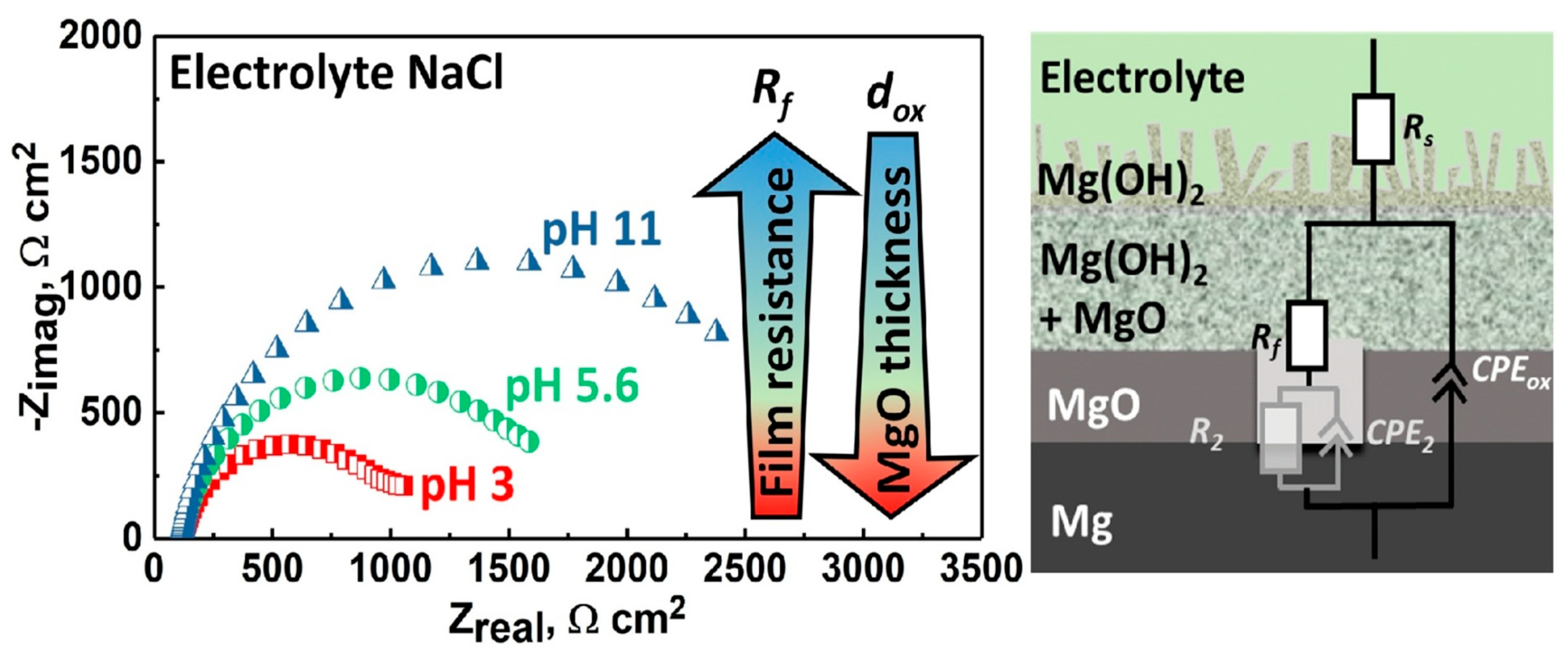
5.1. Electrochemical Impedance Method
5.2. Self-Healing Study Using A Scanning Vibrating Electrode Technique
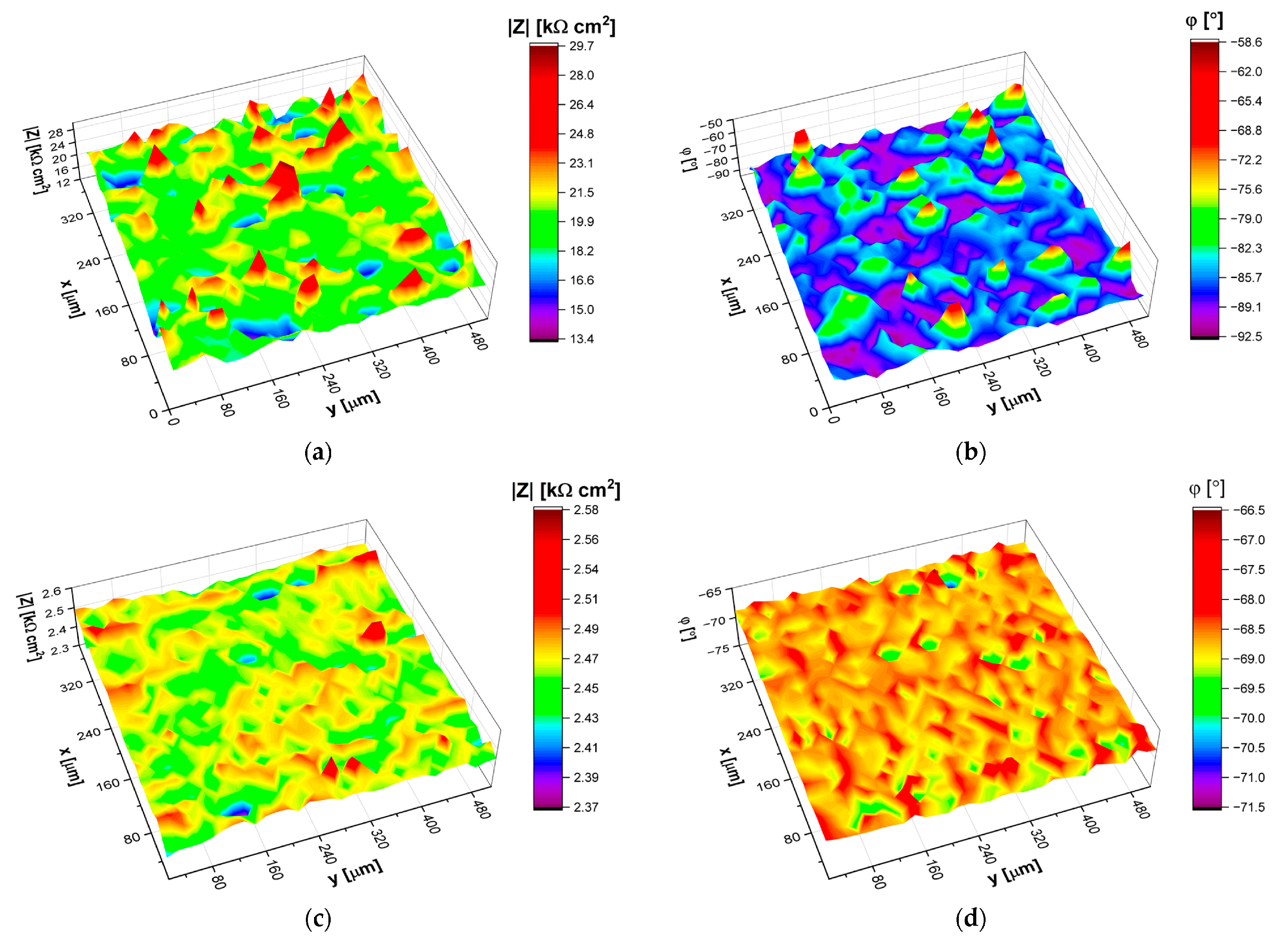
5.3. Localized Electrochemical Impedance Spectroscopy
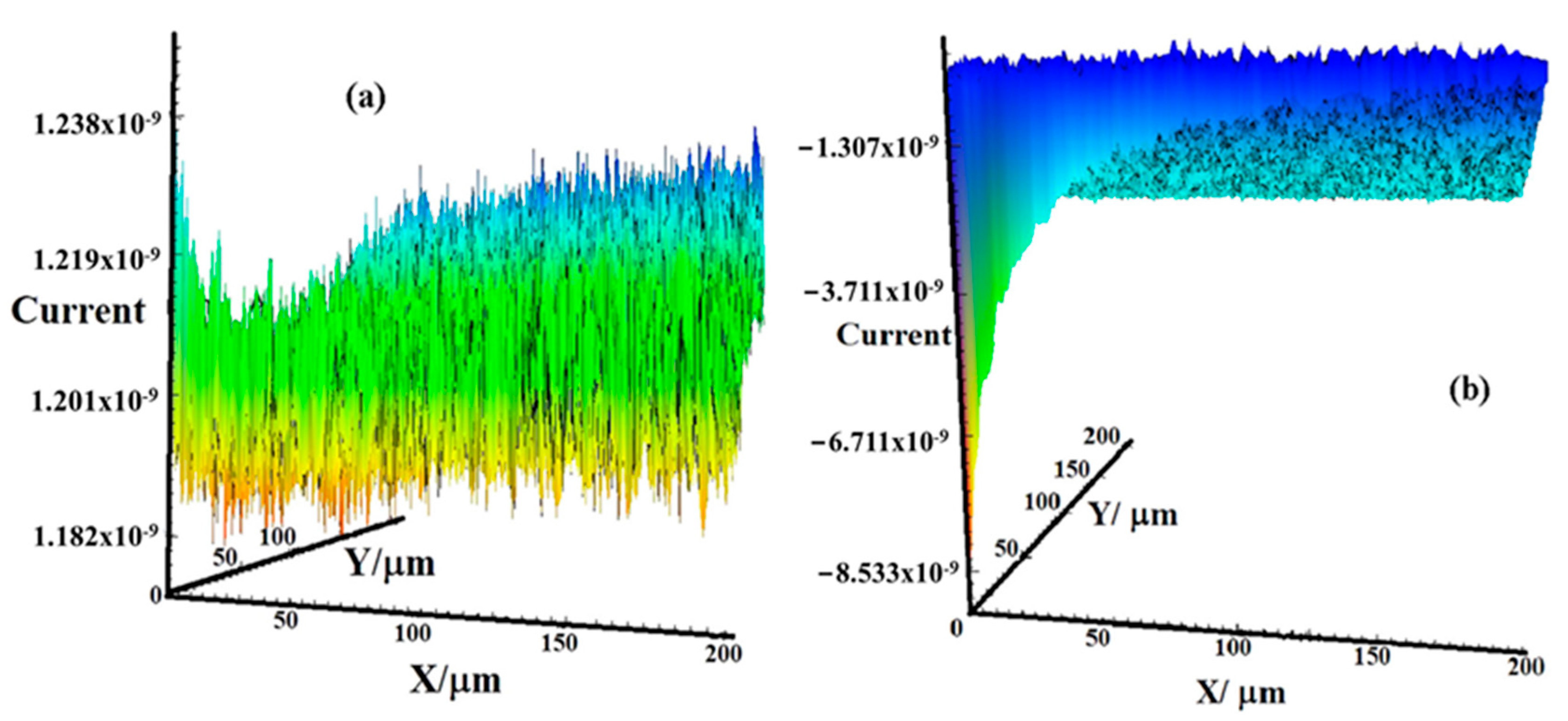
5.4. Scanning Electrochemical Microscopy
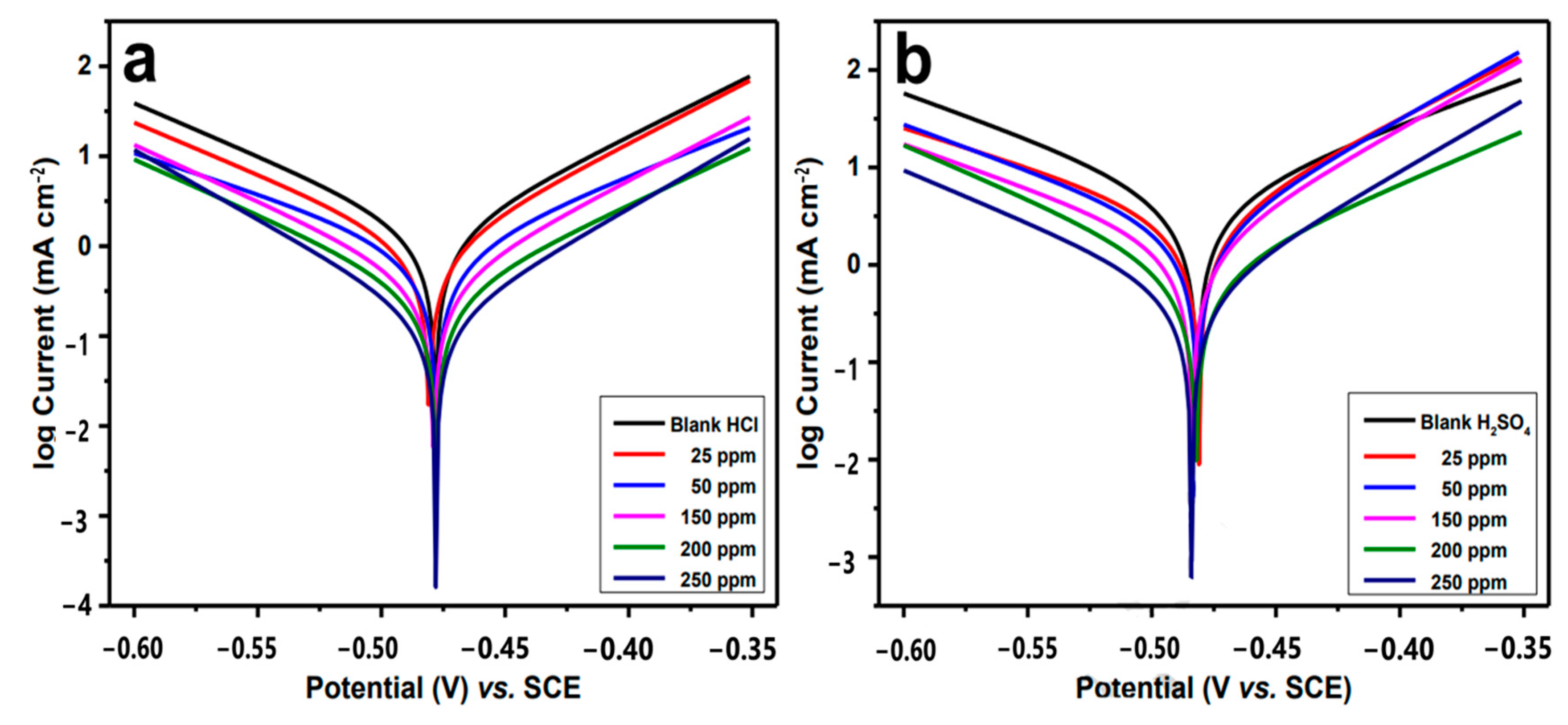
5.5. Tafel Polarization Techniques
6. Recent Trends and Developments in Corrosion Inhibitors
7. Summary and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Corrosion inhibitor | (CIs) |
| Corrosion layer | (CL) |
| Organic corrosion inhibitors | (OCI) |
| Mild steel | (MS) |
| Micro-arc oxidation | (MAO) |
| Plasma electrolytic oxidation | (PEO) |
| Layered double hydroxides | (LDHs) |
| Metal–organic frameworks | (MOFs) |
| Benzotriazole | (BTA) |
| Electrical resistance | (ER) |
| Linear polarization resistance | (LPR) |
| Potential monitoring | (PM) |
| Zero resistance ammeter | (ZRA) |
| Electrochemical noise | (EN) |
| Harmonic impedance spectroscopy | (HIS) |
| Electrochemical impedance spectroscopy | (EIS) |
| Constant phase element | (CPE) |
| Scanning vibrating electrode technique | (SVET) |
| Mesoporous silica nanoparticles | (MSNs) |
| Localized electrochemical impedance spectroscopy | (LEIS) |
| Scanning electrochemical microscopy | (SECM) |
| Microelectrode | (ME) |
| Scanning probe microscope | (SPM) |
| Density functional theory | (DFT) |
References
- Chen, Y.I. (Ed.) Nanotubes and Nanosheets: Functionalization and Applications of Boron Nitride and Other Nanomaterials; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9781466598102. [Google Scholar]
- Wang, W.; Wang, H.; Zhao, J.; Wang, X.; Xiong, C.; Song, L.; Ding, R.; Han, P.; Li, W. Self-Healing Performance and Corrosion Resistance of Graphene Oxide–mesoporous Silicon Layer–nanosphere Structure Coating under Marine Alternating Hydrostatic Pressure. Chem. Eng. J. 2019, 361, 792–804. [Google Scholar] [CrossRef]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A.; Hussain, C.M. Recent Developments in Sustainable Corrosion Inhibitors: Design, Performance and Industrial Scale Applications. Mater. Adv. 2021, 2, 3806–3850. [Google Scholar] [CrossRef]
- Aralekallu, S.; Palanna, M.; Hadimani, S.; Prabhu, C.P.K.; Sajjan, V.A.; Thotiyl, M.O.; Sannegowda, L.K. Biologically Inspired Catalyst for Electrochemical Reduction of Hazardous Hexavalent Chromium. Dalton Trans. 2020, 49, 15061–15071. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.; Venumuddala, V.R. Emerging Technologies and the Indian IT Sector; CRC Press: Boca Raton, FL, USA, 2023; ISBN 9781000853759. [Google Scholar]
- Toghan, A.; Gouda, M.; Shalabi, K.; El-Lateef, H.M.A. Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for SS316 Alloy during Acid Pickling Process: Experimental and Computational Methods. Polymers 2021, 13, 2275. [Google Scholar] [CrossRef] [PubMed]
- Sastri, V.S. 6-Types of Corrosion Inhibitor for Managing Corrosion in Underground Pipelines. In Underground Pipeline Corrosion; Orazem, M.E., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 166–211. ISBN 9780857095091. [Google Scholar]
- Amin, H.M.A.; Galal, A. Corrosion Protection of Metals and Alloys Using Graphene and Biopolymer Based Nanocomposites; CRC Press: Boca Raton, FL, USA, 2021; ISBN 9781351694643. [Google Scholar]
- Li, Q.; Xia, X.; Pei, Z.; Cheng, X.; Zhang, D.; Xiao, K.; Wu, J.; Li, X. Long-Term Corrosion Monitoring of Carbon Steels and Environmental Correlation Analysis via the Random Forest Method. Npj Mater. Degrad. 2022, 6, 1. [Google Scholar] [CrossRef]
- Liu, J.; Liao, C.; Jin, H.; Jiang, Z.; Zhou, H. Numerical and Experimental Research on the Effect of Rainfall on the Transporting Behavior of Chloride Ions in Concrete. Constr. Build. Mater. 2021, 302, 124160. [Google Scholar] [CrossRef]
- Jin, H.; Liu, J.; Jiang, Z.; Zhou, H.; Liu, J. Influence of the Rainfall Intensity on the Chloride Ion Distribution in Concrete with Different Levels of Initial Water Saturation. Constr. Build. Mater. 2021, 281, 122561. [Google Scholar] [CrossRef]
- Igwemezie, V.; Mehmanparast, A. Waveform and Frequency Effects on Corrosion-Fatigue Crack Growth Behaviour in Modern Marine Steels. Int. J. Fatigue 2020, 134, 105484. [Google Scholar] [CrossRef]
- Khan, M.A.A.; Irfan, O.M.; Djavanroodi, F.; Asad, M. Development of Sustainable Inhibitors for Corrosion Control. Sustain. Sci. Pract. Policy 2022, 14, 9502. [Google Scholar] [CrossRef]
- Xie, J.; Zhang, J.; You, Z.; Liu, S.; Guan, K.; Wu, R.; Wang, J.; Feng, J. Towards Developing Mg Alloys with Simultaneously Improved Strength and Corrosion Resistance via RE Alloying. J. Magnes. Alloys 2021, 9, 41–56. [Google Scholar] [CrossRef]
- Thompson, A.A.; Wood, J.L.; Palombo, E.A.; Green, W.K.; Wade, S.A. From Laboratory Tests to Field Trials: A Review of Cathodic Protection and Microbially Influenced Corrosion. Biofouling 2022, 38, 298–320. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Wang, C.; Zhao, B.; Zhou, Y.; Cheng, H. An Interfacial Coating with High Corrosion Resistance Based on Halloysite Nanotubes for Anode Protection of Zinc-Ion Batteries. J. Colloid Interface Sci. 2021, 602, 859–867. [Google Scholar] [CrossRef]
- Honarvar Nazari, M.; Zhang, Y.; Mahmoodi, A.; Xu, G.; Yu, J.; Wu, J.; Shi, X. Nanocomposite Organic Coatings for Corrosion Protection of Metals: A Review of Recent Advances. Prog. Org. Coat. 2022, 162, 106573. [Google Scholar] [CrossRef]
- Chukwuike, V.I.; Echem, O.G.; Prabhakaran, S.; AnandKumar, S.; Barik, R.C. Laser Shock Peening (LSP): Electrochemical and Hydrodynamic Investigation of Corrosion Protection Pre-Treatment for a Copper Surface in 3.5% NaCl Medium. Corros. Sci. 2021, 179, 109156. [Google Scholar] [CrossRef]
- Tang, Z. A Review of Corrosion Inhibitors for Rust Preventative Fluids. Curr. Opin. Solid State Mater. Sci. 2019, 23, 100759. [Google Scholar] [CrossRef]
- Ghosal, K.; Nayak, C. Recent Advances in Chemical Recycling of Polyethylene Terephthalate Waste into Value Added Products for Sustainable Coating Solutions—Hope vs. Hype. Mater. Adv. 2022, 3, 1974–1992. [Google Scholar] [CrossRef]
- Mazumder, M.A.J.; Quraishi, M.A.; Al-Ahmed, A. (Eds.) Polymeric Corrosion Inhibitors for Greening the Chemical and Petrochemical Industry; Wiley-VCH: Weinheim, Germany, 2022. [Google Scholar]
- Verma, C. Handbook of Science & Engineering of Green Corrosion Inhibitors: Modern Theory, Fundamentals & Practical Applications; Elsevier: Amsterdam, The Netherlands, 2021; ISBN 9780323907132. [Google Scholar]
- Singh, P.; Makowska-Janusik, M.; Slovensky, P.; Quraishi, M.A. Nicotinonitriles as Green Corrosion Inhibitors for Mild Steel in Hydrochloric Acid: Electrochemical, Computational and Surface Morphological Studies. J. Mol. Liq. 2016, 220, 71–81. [Google Scholar] [CrossRef]
- Abdel-Karim, A.M.; El-Shamy, A.M. A Review on Green Corrosion Inhibitors for Protection of Archeological Metal Artifacts. J. Bio- Tribo-Corros. 2022, 8, 35. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Berdimuradov, K.; Eliboev, I.; Azimov, L.; Rajabov, Y.; Mamatov, J.; Kholikov, A.; Akbarov, K.; Borikhonov, B. Chapter 3 Covalent and Noncovalent Surface Functionalization of Nanomaterials (for Enhanced Solubility, Dispersibility and Corrosion Prevention Potential). In Corrosion Prevention Nanoscience; De Gruyter: Berlin, Germany, 2023; pp. 37–52. ISBN 9783111071756. [Google Scholar]
- Dubey, V.; Dubey, N.; Domanska, M.M.; Jayasimhadri, M.; Dhoble, S.J. Rare-Earth-Activated Phosphors: Chemistry and Applications; Elsevier: Amsterdam, The Netherlands, 2022; ISBN 9780323886055. [Google Scholar]
- Ryl, J. Special Issue: Recent Advances in Corrosion Science. Materials 2020, 13, 1927. [Google Scholar] [CrossRef]
- Alrefaee, S.H.; Rhee, K.Y.; Verma, C.; Quraishi, M.A.; Ebenso, E.E. Challenges and Advantages of Using Plant Extract as Inhibitors in Modern Corrosion Inhibition Systems: Recent Advancements. J. Mol. Liq. 2021, 321, 114666. [Google Scholar] [CrossRef]
- Fazal, B.R.; Becker, T.; Kinsella, B.; Lepkova, K. A Review of Plant Extracts as Green Corrosion Inhibitors for CO2 Corrosion of Carbon Steel. npj Mater. Degrad. 2022, 6, 1–14. [Google Scholar] [CrossRef]
- De Ketelaere, E.; Moed, D.; Vanoppen, M.; Verliefde, A.R.D.; Verbeken, K.; Depover, T. Sodium Silicate Corrosion Inhibition Behaviour for Carbon Steel in a Dynamic Salt Water Environment. Corros. Sci. 2023, 217, 111119. [Google Scholar] [CrossRef]
- Rocca, E.; Faiz, H.; Dillmann, P.; Neff, D.; Mirambet, F. Electrochemical Behavior of Thick Rust Layers on Steel Artefact: Mechanism of Corrosion Inhibition. Electrochim. Acta 2019, 316, 219–227. [Google Scholar] [CrossRef]
- Zhang, W. Initiation and Propagation of Localized Corrosion of Mild Steel in Marginally Sour Environments. Doctoral Dissertation, Ohio University, Athens, OH, USA, 2020. [Google Scholar]
- Singh, H.; Sharma, S. Understanding the Hydration Thermodynamics of Cationic Quaternary Ammonium and Charge-Neutral Amine Surfactants. J. Phys. Chem. B 2022, 126, 9810–9820. [Google Scholar] [CrossRef]
- Desimone, M.P.; Grundmeier, G.; Gordillo, G.; Simison, S.N. Amphiphilic Amido-Amine as an Effective Corrosion Inhibitor for Mild Steel Exposed to CO2 Saturated Solution: Polarization, EIS and PM-IRRAS Studies. Electrochim. Acta 2011, 56, 2990–2998. [Google Scholar] [CrossRef]
- Singh, A.; Ansari, K.R.; Alanazi, A.K.; Quraishi, M.A.; Ali, I.H.; Lin, Y. Probing Inhibition Effect of Novel Biopolymer Based Composite for the Inhibition of P110 Steel Corrosion in 15% HCl under Dynamic Condition. Sustain. Chem. Pharm. 2022, 26, 100599. [Google Scholar] [CrossRef]
- Shahryari, Z.; Gheisari, K.; Yeganeh, M.; Ramezanzadeh, B. Designing a Dual Barrier-Self-Healable Functional Epoxy Nano-Composite Using 2D-Carbon Based Nano-Flakes Functionalized with Active Corrosion Inhibitors. J. Mater. Res. Technol. 2023, 22, 2746–2767. [Google Scholar] [CrossRef]
- Sriram, S.; Singh, R.K.; Kumar, A. Silica and Silane Based Polymer Composite Coating on Glass Slide by Dip-Coating Method. Surf. Interfaces 2020, 19, 100472. [Google Scholar] [CrossRef]
- Hamidon, T.S.; Ishak, N.A.; Hussin, M.H. Enhanced Corrosion Inhibition of Low Carbon Steel in Aqueous Sodium Chloride Employing Sol–gel-Based Hybrid Silanol Coatings. J. Sol-Gel Sci. Technol. 2021, 97, 556–571. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, L.; Yao, W.; Wu, J.; Yuan, Y.; Xie, Z.; Jiang, B.; Pan, F. Synergistic Effect of Graphene Oxide/Ternary Mg-Al-La Layered Double Hydroxide for Dual Self-Healing Corrosion Protection of Micro-Arc Oxide Coating of Magnesium Alloy. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130339. [Google Scholar] [CrossRef]
- Brycki, B.E.; Kowalczyk, I.H.; Szulc, A.; Kaczerewska, O.; Pakiet, M. Organic Corrosion Inhibitors. In Corrosion Inhibitors, Principles and Recent Applications; InTech: Nappanee, Indiana, 2018; ISBN 9789535139171. [Google Scholar]
- Kadhim, A.; Al-Amiery, A.A.; Alazawi, R.; Al-Ghezi, M.K.S.; Abass, R.H. Corrosion Inhibitors. A Review. Int. J. Corros. Scale Inhib. 2021, 10, 54–67. [Google Scholar]
- Fawzy, A.; Toghan, A. Inhibition Evaluation of Chromotrope Dyes for the Corrosion of Mild Steel in an Acidic Environment: Thermodynamic and Kinetic Aspects. ACS Omega 2021, 6, 4051–4061. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Yu, S.; Zhu, G.; Li, Q.; Liu, E.; Xiong, W.; Wang, B.; Yang, X. Corrosion and Wear Resistance of Micro-Arc Oxidation Coating on Glass Microsphere Reinforced Mg Alloy Composite. J. Mater. Sci. 2021, 56, 15379–15396. [Google Scholar] [CrossRef]
- Borisova, D.; Möhwald, H.; Shchukin, D.G. Influence of Embedded Nanocontainers on the Efficiency of Active Anticorrosive Coatings for Aluminum Alloys Part II: Influence of Nanocontainer Position. ACS Appl. Mater. Interfaces 2013, 5, 80–87. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, E. Introduction to Corrosion Science; Springer Science & Business Media: Berlin, Germany, 2010; ISBN 9781441904546. [Google Scholar]
- Verma, C.; Ebenso, E.E.; Quraishi, M.A. Molecular Structural Aspects of Organic Corrosion Inhibitors: Influence of –CN and –NO2 Substituents on Designing of Potential Corrosion Inhibitors for Aqueous Media. J. Mol. Liq. 2020, 316, 113874. [Google Scholar] [CrossRef]
- Wang, Z.; Gong, Y.; Jing, C.; Huang, H.; Li, H.; Zhang, S.; Gao, F. Synthesis of Dibenzotriazole Derivatives Bearing Alkylene Linkers as Corrosion Inhibitors for Copper in Sodium Chloride Solution: A New Thought for the Design of Organic Inhibitors. Corros. Sci. 2016, 113, 64–77. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A Review on Applications of Chitosan-Based Schiff Bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Verma, C.; Quraishi, M.A.; Alfantazi, A.; Rhee, K.Y. Corrosion Inhibition Potential of Chitosan Based Schiff Bases: Design, Performance and Applications. Int. J. Biol. Macromol. 2021, 184, 135–143. [Google Scholar] [CrossRef]
- Kim, C.; Goldsberry, R.; Karayan, A.I.; Milla, J.; Goehring, L.; Hassan, M.; Castaneda, H. Electrochemical Evaluation of Epoxy-Coated-Rebar Containing pH-Responsive Nanocapsules in Simulated Carbonated Concrete Pore Solution. Prog. Org. Coat. 2021, 161, 106549. [Google Scholar] [CrossRef]
- Kim, C.; Karayan, A.I.; Milla, J.; Hassan, M.; Castaneda, H. Smart Coating Embedded with pH-Responsive Nanocapsules Containing a Corrosion Inhibiting Agent. ACS Appl. Mater. Interfaces 2020, 12, 6451–6459. [Google Scholar] [CrossRef]
- Verma, D.K.; Sahu, R.; Berdimurodov, E.; Verma, C.; Quraishi, M.A.; Jain, V.K.; Berdimuradov, K. Isatin Is a New Core in the Developing of Corrosion Inhibitors: A Comprehensive Review. J. Mol. Struct. 2023, 1294, 136313. [Google Scholar] [CrossRef]
- Ali Asaad, M.; Bothi Raja, P.; Fahim Huseien, G.; Fediuk, R.; Ismail, M.; Alyousef, R. Self-Healing Epoxy Coating Doped with Elaesis Guineensis/silver Nanoparticles: A Robust Corrosion Inhibitor. Constr. Build. Mater. 2021, 312, 125396. [Google Scholar] [CrossRef]
- Verma, C.; Hussain, C.M.; Ebenso, E.E. Organic Corrosion Inhibitors: Synthesis, Characterization, Mechanism, and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2021; ISBN 9781119794486. [Google Scholar]
- Abrishami, S.; Naderi, R.; Ramezanzadeh, B. Fabrication and Characterization of Zinc acetylacetonate/Urtica Dioica Leaves Extract Complex as an Effective Organic/inorganic Hybrid Corrosion Inhibitive Pigment for Mild Steel Protection in Chloride Solution. Appl. Surf. Sci. 2018, 457, 487–496. [Google Scholar] [CrossRef]
- Serdaroğlu, G.; Kaya, S. Organic and inorganic corrosion inhibitors: A comparison. In Organic Corrosion Inhibitors: Synthesis, Characterization, Mechanism, and Applications; Wiley: Hoboken, NJ, USA, 2021; pp. 59–73. [Google Scholar]
- Harb, M.B.; Abubshait, S.; Etteyeb, N.; Kamoun, M.; Dhouib, A. Olive Leaf Extract as a Green Corrosion Inhibitor of Reinforced Concrete Contaminated with Seawater. Arab. J. Chem. 2020, 13, 4846–4856. [Google Scholar] [CrossRef]
- Heikal, M.; Ali, A.I.; Ibrahim, B.; Toghan, A. Electrochemical and Physico-Mechanical Characterizations of Fly Ash-Composite Cement. Constr. Build. Mater. 2020, 243, 118309. [Google Scholar] [CrossRef]
- Yee, Y.P.; Saud, S.N.; Hamzah, E. Pomelo Peel Extract as Corrosion Inhibitor for Steel in Simulated Seawater and Acidic Mediums. J. Mater. Eng. Perform. 2020, 29, 2202–2215. [Google Scholar] [CrossRef]
- Sun, Z.; Singh, A.; Xu, X.; Chen, S.; Liu, W.; Lin, Y. Inhibition Effect of Pomelo Peel Extract for N80 Steel in 3.5% NaCl Saturated with CO2 Solution. Res. Chem. Intermed. 2017, 43, 6719–6736. [Google Scholar] [CrossRef]
- Hauert, R.; Patscheider, J. From Alloying to Nanocomposites—Improved Performance of Hard Coatings. Adv. Eng. Mater. 2000, 2, 247–259. [Google Scholar] [CrossRef]
- Giuliani, C.; Pascucci, M.; Riccucci, C.; Messina, E.; Salzano de Luna, M.; Lavorgna, M.; Ingo, G.M.; Di Carlo, G. Chitosan-Based Coatings for Corrosion Protection of Copper-Based Alloys: A Promising More Sustainable Approach for Cultural Heritage Applications. Prog. Org. Coat. 2018, 122, 138–146. [Google Scholar] [CrossRef]
- Fayomi, O.S.I.; Akande, I.G.; Popoola, A.P.I. Corrosion Protection Effect of Chitosan on the Performance Characteristics of A6063 Alloy. J. Bio- Tribo-Corros. 2018, 4, 73. [Google Scholar] [CrossRef]
- Rbaa, M.; Benhiba, F.; Hssisou, R.; Lakhrissi, Y.; Lakhrissi, B.; Touhami, M.E.; Warad, I.; Zarrouk, A. Green Synthesis of Novel Carbohydrate Polymer Chitosan Oligosaccharide Grafted on D-Glucose Derivative as Bio-Based Corrosion Inhibitor. J. Mol. Liq. 2021, 322, 114549. [Google Scholar] [CrossRef]
- Zeng, Y.; Cao, H.; Jia, W.; Min, Y.; Xu, Q. An Eco-Friendly Nitrogen Doped Carbon Coating Derived from Chitosan Macromolecule with Enhanced Corrosion Inhibition on Aluminum Alloy. Surf. Coat. Technol. 2022, 445, 128709. [Google Scholar] [CrossRef]
- Umoren, P.S.; Kavaz, D.; Umoren, S.A. Corrosion Inhibition Evaluation of Chitosan–CuO Nanocomposite for Carbon Steel in 5% HCl Solution and Effect of KI Addition. Sustain. Sci. Pract. Policy 2022, 14, 7981. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Verma, D.K.; Kholikov, A.; Akbarov, K.; Guo, L. The Recent Development of Carbon Dots as Powerful Green Corrosion Inhibitors: A Prospective Review. J. Mol. Liq. 2022, 349, 118124. [Google Scholar] [CrossRef]
- Cen, H.; Cao, J.; Chen, Z. Functionalized Carbon Nanotubes as a Novel Inhibitor to Enhance the Anticorrosion Performance of Carbon Steel in CO2-Saturated NaCl Solution. Corros. Sci. 2020, 177, 109011. [Google Scholar] [CrossRef]
- Liu, S.; Jing, Y.; Zhang, T.; Zhang, J.; Xu, F.; Song, Q.; Ye, Q.; Liu, S.; Liu, W. Excellent Tribological and Anti-Corrosion Performances Enabled by Novel Hollow Graphite Carbon Nanosphere with Controlled Release of Corrosion Inhibitor. Chem. Eng. J. 2021, 412, 128648. [Google Scholar] [CrossRef]
- Sankara Narayanan, T.S.; Park, I.-S.; Lee, M.-H. Surface Modification of Magnesium and Its Alloys for Biomedical Applications: Modification and Coating Techniques; Elsevier: Amsterdam, The Netherlands, 2015; ISBN 9781782420835. [Google Scholar]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. NATO Adv. Sci. Inst. Ser. E Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Qin, G.; Zhang, Y.; Yang, E.; Peng, Z.; Wei, W.; Xie, X.; Li, W. Effect of Post-Sealing Treatment with Different Concentrations of NaH2PO4 on Corrosion Resistance of MAO Coating on 6063 Aluminum Alloy. Surf. Coat. Technol. 2022, 443, 128604. [Google Scholar] [CrossRef]
- Nikbakht, A.; Dehghanian, C.; Parichehr, R. Silane Coatings Modified with Hydroxyapatite Nanoparticles to Enhance the Biocompatibility and Corrosion Resistance of a Magnesium Alloy. RSC Adv. 2021, 11, 26127–26144. [Google Scholar] [CrossRef]
- Zeng, D.; Liu, Z.; Bai, S.; Zhao, J.; Wang, J. Corrosion Resistance of Bis-Silane-Modified Epoxy Coatings on an Al-Zn-Mg-Cu Alloy. J. Mater. Eng. Perform. 2020, 29, 5282–5290. [Google Scholar] [CrossRef]
- Jeyaram, R.; Elango, A.; Siva, T.; Ayeshamariam, A.; Kaviyarasu, K. Corrosion Protection of Silane Based Coatings on Mild Steel in an Aggressive Chloride Ion Environment. Surf. Interfaces 2020, 18, 100423. [Google Scholar] [CrossRef]
- Balaji, J.; Roh, S.-H.; Edison, T.N.J.I.; Jung, H.-Y.; Sethuraman, M.G. Sol-Gel Based Hybrid Silane Coatings for Enhanced Corrosion Protection of Copper in Aqueous Sodium Chloride. J. Mol. Liq. 2020, 302, 112551. [Google Scholar] [CrossRef]
- Flamini, D.O.; Trueba, M.; Trasatti, S.P. Aniline-Based Silane as a Primer for Corrosion Inhibition of Aluminium. Prog. Org. Coat. 2012, 74, 302–310. [Google Scholar] [CrossRef]
- Leszek, A.; Dobrzanski, G.E. Magnesium and Its Alloys: Technology and Applications, 1st ed.; Metals and alloys, 2020 Series; Dobrzański, L.A., Totten, G.E., Bamberger, M., Eds.; CRC Press: Boca Raton, FL, USA; Taylor & Francis Group: Abingdon, UK, 2019; ISBN 9781351045476. [Google Scholar]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Effective PEO/Silane Pretreatment of Epoxy Coating Applied on AZ31B Mg Alloy for Corrosion Protection. Corros. Sci. 2020, 169, 108608. [Google Scholar] [CrossRef]
- Kaseem, M.; Hussain, T.; Rehman, Z.U.; Ko, Y.G. Stabilization of AZ31 Mg Alloy in Sea Water via Dual Incorporation of MgO and WO3 during Micro-Arc Oxidation. J. Alloys Compd. 2021, 853, 157036. [Google Scholar]
- Cecchetto, L.; Denoyelle, A.; Delabouglise, D.; Petit, J.-P. A Silane Pre-Treatment for Improving Corrosion Resistance Performances of Emeraldine Base-Coated Aluminium Samples in Neutral Environment. Appl. Surf. Sci. 2008, 254, 1736–1743. [Google Scholar] [CrossRef]
- Matin, A.; Merah, N.; Ibrahim, A. Superhydrophobic and Self-Cleaning Surfaces Prepared from a Commercial Silane Using a Single-Step Drop-Coating Method. Prog. Org. Coat. 2016, 99, 322–329. [Google Scholar] [CrossRef]
- Figueira, R.B.; Fontinha, I.R.; Silva, C.J.R.; Pereira, E.V. Hybrid Sol-Gel Coatings: Smart and Green Materials for Corrosion Mitigation. Coat. World 2016, 6, 12. [Google Scholar] [CrossRef]
- Sfameni, S.; Del Tedesco, A.; Rando, G.; Truant, F.; Visco, A.; Plutino, M.R. Waterborne Eco-Sustainable Sol-Gel Coatings Based on Phytic Acid Intercalated Graphene Oxide for Corrosion Protection of Metallic Surfaces. Int. J. Mol. Sci. 2022, 23, 12021. [Google Scholar] [CrossRef]
- Samiee, R.; Ramezanzadeh, B.; Mahdavian, M.; Alibakhshi, E. Assessment of the Smart Self-Healing Corrosion Protection Properties of a Water-Base Hybrid Organo-Silane Film Combined with Non-Toxic Organic/inorganic Environmentally Friendly Corrosion Inhibitors on Mild Steel. J. Clean. Prod. 2019, 220, 340–356. [Google Scholar] [CrossRef]
- López, A.J.; Ureña, A.; López; Rams, J. Protection against Corrosion of Aluminium-SiC Composites by Sol–gel Silica Coatings. Surf. Coat. Technol. 2008, 202, 3755–3763. [Google Scholar] [CrossRef]
- Yan, W.; Ong, W.K.; Wu, L.Y.; Wijesinghe, S.L. Investigation of Using Sol-Gel Technology for Corrosion Protection Coating Systems Incorporating Colours and Inhibitors. Coat. World 2019, 9, 52. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Fan, J.; Kang, W.; Hu, W.; Yang, X. Novel Composite Films Prepared by Sol–gel Technology for the Corrosion Protection of AZ91D Magnesium Alloy. Prog. Org. Coat. 2009, 66, 328–335. [Google Scholar] [CrossRef]
- Komarneni, S.; Abothu, I.R.; Prasada Rao, A.V. Sol-Gel Processing of Some Electroceramic Powders. J. Sol-Gel Sci. Technol. 1999, 15, 263–270. [Google Scholar] [CrossRef]
- Ielo, I.; Giacobello, F.; Castellano, A.; Sfameni, S.; Rando, G.; Plutino, M.R. Development of Antibacterial and Antifouling Innovative and Eco-Sustainable Sol-Gel Based Materials: From Marine Areas Protection to Healthcare Applications. Gels 2021, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Wasekar, N.P.; Jyothirmayi, A.; Rama Krishna, L.; Sundararajan, G. Effect of Micro Arc Oxidation Coatings on Corrosion Resistance of 6061-Al Alloy. J. Mater. Eng. Perform. 2008, 17, 708–713. [Google Scholar] [CrossRef]
- Cai, J.; Cao, F.; Chang, L.; Zheng, J.; Zhang, J.; Cao, C. The Preparation and Corrosion Behaviors of MAO Coating on AZ91D with Rare Earth Conversion Precursor Film. Appl. Surf. Sci. 2011, 257, 3804–3811. [Google Scholar] [CrossRef]
- Rúa, J.M.; Zuleta, A.A.; Ramírez, J.; Fernández-Morales, P. Micro-Arc Oxidation Coating on Porous Magnesium Foam and Its Potential Biomedical Applications. Surf. Coat. Technol. 2019, 360, 213–221. [Google Scholar] [CrossRef]
- Jia, Z.; Xiu, P.; Li, M.; Xu, X.; Shi, Y.; Cheng, Y.; Wei, S.; Zheng, Y.; Xi, T.; Cai, H.; et al. Bioinspired Anchoring AgNPs onto Micro-Nanoporous TiO2 Orthopedic Coatings: Trap-Killing of Bacteria, Surface-Regulated Osteoblast Functions and Host Responses. Biomaterials 2016, 75, 203–222. [Google Scholar] [CrossRef]
- Chen, Q.; Li, W.; Ling, K.; Yang, R. Effect of Na2WO4 Addition on Formation Mechanism and Microstructure of Micro-Arc Oxidation Coating on Al-Ti Double-Layer Composite Plate. Mater. Des. 2020, 190, 108558. [Google Scholar] [CrossRef]
- Wang, R.; Xu, H.; Yao, Z.; Li, C.; Jiang, Z. Adhesion and Corrosion Resistance of Micro-Arc Oxidation/Polyurethane Composite Coating on Aluminum Alloy Surface. NATO Adv. Sci. Inst. Ser. E Appl. Sci. 2020, 10, 6779. [Google Scholar] [CrossRef]
- Wang, X.-M.; Zhang, F.-Q. Bioactive Submicron-Pore Design of Microarc Oxidation Coating on Ti6Al4V Alloy Prepared by Selective Laser Melting Method. Surf. Coat. Technol. 2022, 444, 128696. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, X.; Geng, Z.; Yin, Y.; Hang, R.; Huang, X.; Yao, X.; Tang, B. Preparation, Antibacterial Effects and Corrosion Resistant of Porous Cu–TiO2 Coatings. Appl. Surf. Sci. 2014, 308, 43–49. [Google Scholar] [CrossRef]
- Zhou, Y.; Yan, D.; Gu, Q.; Zhu, S.; Wang, L.; Peng, H.; Zhao, Y. Implanting Cation Vacancies in Ni-Fe LDHs for Efficient Oxygen Evolution Reactions of Lithium-Oxygen Batteries. Appl. Catal. B 2021, 285, 119792. [Google Scholar] [CrossRef]
- Zhao, S.; Tsen, W.-C.; Gong, C. 3D Nanoflower-like Layered Double Hydroxide Modified Quaternized Chitosan/polyvinyl Alcohol Composite Anion Conductive Membranes for Fuel Cells. Carbohydr. Polym. 2021, 256, 117439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zong, Q.; Zhang, Q.; Yang, H.; Du, W.; Wang, Q.; Zhan, J.; Wang, H. Ultra-Long Lifespan Asymmetrical Hybrid Supercapacitor Device Based on Hierarchical NiCoP@C@LDHs Electrode. Electrochim. Acta 2020, 334, 135589. [Google Scholar] [CrossRef]
- Xu, Z.; Wu, Y.; Zhang, Z.; Wang, Y.; Hu, J.; Ma, Y.; Zhang, Z.; Huang, H.; Wei, J.; Yu, Q.; et al. A Review on the Research Progress of LDHs as Corrosion Inhibitors for Reinforced Concrete. J. Build. Eng. 2023, 70, 106303. [Google Scholar] [CrossRef]
- Zhang, J.; Zhao, J.; Wang, J.; Tabish, M.; Zhang, J. Enhancing the Corrosion Resistance of Waterborne Epoxy Coating by Fumarate Intercalated LDHs Prepared by High Gravity Technology. Prog. Org. Coat. 2023, 174, 107271. [Google Scholar] [CrossRef]
- Czerwinski, F. Magnesium Alloys: Properties in Solid and Liquid States; BoD—Books on Demand: Paris, France, 2014; ISBN 9789535117285. [Google Scholar]
- Chen, Z.; Ye, H. Sequestration and Release of Nitrite and Nitrate in Alkali-Activated Slag: A Route toward Smart Corrosion Control. Cem. Concr. Res. 2021, 143, 106398. [Google Scholar] [CrossRef]
- Ma, L.; Qiang, Y.; Zhao, W. Designing Novel Organic Inhibitor Loaded MgAl-LDHs Nanocontainer for Enhanced Corrosion Resistance. Chem. Eng. J. 2021, 408, 127367. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, S.; Liu, Y.; Huangfu, H.; Guo, X.; Zhang, J. Enhancement of Corrosion Resistance of AZ31B Magnesium Alloy by Preparing MgAl-LDHs Coatings Modified with Imidazolium Based Dicationic Ionic Liquids. Surf. Coat. Technol. 2022, 440, 128504. [Google Scholar] [CrossRef]
- Ramezanzadeh, M.; Ramezanzadeh, B.; Mahdavian, M. Graphene Skeletal Nanotemplate Coordinated with pH-Responsive Porous Double-Ligand Metal-Organic Frameworks (DL-MOFs) through Ligand Exchange Theory for High-Performance Smart Coatings. Chem. Eng. J. 2023, 461, 141869. [Google Scholar] [CrossRef]
- Zafari, S.; Niknam Shahrak, M.; Ghahramaninezhad, M. New MOF-Based Corrosion Inhibitor for Carbon Steel in Acidic Media. Met. Mater. Int. 2020, 26, 25–38. [Google Scholar] [CrossRef]
- Cao, K.; Yu, Z.; Yin, D.; Chen, L.; Jiang, Y.; Zhu, L. Fabrication of BTA-MOF-TEOS-GO Nanocomposite to Endow Coating Systems with Active Inhibition and Durable Anticorrosion Performances. Prog. Org. Coat. 2020, 143, 105629. [Google Scholar] [CrossRef]
- Cao, K.; Yu, Z.; Yin, D. Preparation of Ce-MOF@TEOS to Enhance the Anti-Corrosion Properties of Epoxy Coatings. Prog. Org. Coat. 2019, 135, 613–621. [Google Scholar] [CrossRef]
- Wei, W.; Liu, Z.; Wei, R.; Han, G.-C.; Liang, C. Synthesis of MOFs/GO Composite for Corrosion Resistance Application on Carbon Steel. RSC Adv. 2020, 10, 29923–29934. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Pan, W.; Luo, J.; Liu, R. Synthesis of Inhibitor-Loaded Polyaniline Microcapsules with Dual Anti-Corrosion Functions for Protection of Carbon Steel. Electrochim. Acta 2020, 364, 137299. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Berdimuradov, K.; Bahodir, K.; Kholikov, A.; Akbarov, K.; Dagdag, O.; Rbaa, M.; El Ibrahimi, B.; Kumar Verma, D.; Haldhar, R.; et al. Chapter 1 Recent Trends and Developments in Carbon Dots. In Carbon Dots in Biology; De Gruyter: Berlin, Germany, 2023; pp. 1–14. ISBN 9783110799958. [Google Scholar]
- Gavrilović-Wohlmuther, A.; Laskos, A.; Kny, E. Chapter 12—Corrosion Inhibitor–loaded Smart Nanocontainers. In Corrosion Protection at the Nanoscale; Rajendran, S., Nguyen, T.A., Kakooei, S., Yeganeh, M., Li, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–223. ISBN 9780128193594. [Google Scholar]
- Kim, J.H.; Geem, Z.W. (Eds.) Harmony Search Algorithm: Proceedings of the 2nd International Conference on Harmony Search Algorithm (ICHSA2015); Springer: Berlin/Heidelberg, Germany, 2015; ISBN 9783662479261. [Google Scholar]
- Haddadi, S.A.; Ramazani, S.A.A.; Mahdavian, M.; Taheri, P.; Mol, J.M.C.; Gonzalez-Garcia, Y. Self-Healing Epoxy Nanocomposite Coatings Based on Dual-Encapsulation of Nano-Carbon Hollow Spheres with Film-Forming Resin and Curing Agent. Compos. Part B 2019, 175, 107087. [Google Scholar] [CrossRef]
- Ghazi, A.; Ghasemi, E.; Mahdavian, M.; Ramezanzadeh, B.; Rostami, M. The Application of Benzimidazole and Zinc Cations Intercalated Sodium Montmorillonite as Smart Ion Exchange Inhibiting Pigments in the Epoxy Ester Coating. Corros. Sci. 2015, 94, 207–217. [Google Scholar] [CrossRef]
- Htet, T.T.; Zeng, D. Study on the Corrosion and Scale Inhibition Mechanism of the Thiourea-Modified Polyepoxysuccinic Acid (CNS-PESA). J. Chem. Chem. Eng. 2022, 2022, 7773199. [Google Scholar] [CrossRef]
- Shirazi, Z.; Golikand, A.N.; Keshavarz, M.H. A New Nanocomposite Based on Poly (o-Anthranilic Acid), Graphene Oxide and Functionalized Carbon Nanotube as an Efficient Corrosion Inhibitor for Stainless Steel in Severe Environmental Corrosion. Compos. Commun. 2020, 22, 100467. [Google Scholar] [CrossRef]
- Li, C.; Zhao, X.; Meng, C.; Zhang, T.; Sun, S.; Hu, S. Application of Hollow Mesoporous Organosilica Nanoparticles as pH and Redox Double Stimuli-Responsive Nanocontainer in the Controlled Release of Corrosion Inhibitor Molecules. Prog. Org. Coat. 2021, 159, 106437. [Google Scholar] [CrossRef]
- Khatoon, H.; Iqbal, S.; Ahmad, S. Influence of Carbon Nanodots Encapsulated Polycarbazole Hybrid on the Corrosion Inhibition Performance of Polyurethane Nanocomposite Coatings. New J. Chem. 2019, 43, 10278–10290. [Google Scholar] [CrossRef]
- Hao, Y.; Sun, W.; Jiang, L.; Cui, J.; Zhang, Y.; Song, L.; Zhang, Y. Self-Healing Effect of Epoxy Coating Containing Mesoporous Polyaniline Hollow Spheres Loaded with Benzotriazole. Prog. Org. Coat. 2021, 159, 106445. [Google Scholar] [CrossRef]
- Gu, B.E.; Huang, C.Y.; Shen, T.H.; Lee, Y.L. Effects of Multiwall Carbon Nanotube Addition on the Corrosion Resistance and Underwater Acoustic Absorption Properties of Polyurethane Coatings. Prog. Org. Coat. 2018, 121, 226–235. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Kardar, P.; Arabi, A.M.; Amini, R.; Pasbakhsh, P. Acid-Modification and Praseodymium Loading of Halloysite Nanotubes as a Corrosion Inhibitor. Appl. Clay Sci. 2020, 184, 105355. [Google Scholar] [CrossRef]
- Popova, K.; Prošek, T. Corrosion Monitoring in Atmospheric Conditions: A Review. Metals 2022, 12, 171. [Google Scholar] [CrossRef]
- Li, S.; Kim, Y.-G.; Jung, S.; Song, H.-S.; Lee, S.-M. Application of Steel Thin Film Electrical Resistance Sensor for in Situ Corrosion Monitoring. Sens. Actuators B Chem. 2007, 120, 368–377. [Google Scholar] [CrossRef]
- Han, M.; Wang, Y.; Yao, J.; Liu, C.; Chew, J.W.; Wang, Y.; Dong, Y.; Han, L. Electrically Conductive Hydrophobic Membrane Cathode for Membrane Distillation with Super Anti-Oil-Fouling Capability: Performance and Mechanism. Desalination 2021, 516, 115199. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Eliboyev, I.; Berdimuradov, K.; Kholikov, A.; Akbarov, K.; Dagdag, O.; Rbaa, M.; El Ibrahimi, B.; Verma, D.K.; Haldhar, R.; et al. Chapter 3-Electrochemical Impedance (EIS) and Noise Analyses for Corrosion Measurements. In Electrochemical and Analytical Techniques for Sustainable Corrosion Monitoring; Aslam, J., Verma, C., Mustansar Hussain, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 39–58. ISBN 9780443157837. [Google Scholar]
- Wang, S.; Zhang, J.; Gharbi, O.; Vivier, V.; Gao, M.; Orazem, M.E. Electrochemical Impedance Spectroscopy. Nat. Rev. Methods Primers 2021, 1, 1–21. [Google Scholar] [CrossRef]
- Siva, T.; Kumari, S.S.; Sathiyanarayanan, S. Dendrimer like Mesoporous Silica Nano Container (DMSN) Based Smart Self Healing Coating for Corrosion Protection Performance. Prog. Org. Coat. 2021, 154, 106201. [Google Scholar]
- Manasa, S.; Jyothirmayi, A.; Siva, T.; Sathiyanarayanan, S.; Gobi, K.V.; Subasri, R. Effect of Inhibitor Loading into Nanocontainer Additives of Self-Healing Corrosion Protection Coatings on Aluminum Alloy A356. 0. J. Alloys Compd. 2017, 726, 969–977. [Google Scholar] [CrossRef]
- Gadow, H.S.; Fakeeh, M. Green Inhibitor of Carbon Steel Corrosion in 1 M Hydrochloric Acid: Seed Extract (experimental and Theoretical Studies). RSC Adv. 2022, 12, 8953–8986. [Google Scholar] [CrossRef] [PubMed]
- Siva, T.; Ramadoss, A.; Sathiyanarayanan, S. Emerging Action of Corrosion Prevention Based on Sustained Self-Healing Coatings. Surf. Interfaces 2021, 26, 101440. [Google Scholar] [CrossRef]
- Abdulmajid, A.; Hamidon, T.S.; Hussin, M.H. Characterization and Corrosion Inhibition Studies of Protective Sol–gel Films Modified with Tannin Extracts on Low Carbon Steel. J. Solgel Sci. Technol. 2022, 104, 287–299. [Google Scholar] [CrossRef]
- Patel, A.S.; Panchal, V.A.; Mudaliar, G.V.; Shah, N.K. Impedance Spectroscopic Study of Corrosion Inhibition of Al-Pure by Organic Schiff Base in Hydrochloric Acid. J. Saudi Chem. Soc. 2013, 17, 53–59. [Google Scholar] [CrossRef]
- Wang, L.; Snihirova, D.; Deng, M.; Wang, C.; Vaghefinazari, B.; Wiese, G.; Langridge, M.; Höche, D.; Lamaka, S.V.; Zheludkevich, M.L. Insight into Physical Interpretation of High Frequency Time Constant in Electrochemical Impedance Spectra of Mg. Corros. Sci. 2021, 187, 109501. [Google Scholar] [CrossRef]
- Raijmakers, L.H.J.; Danilov, D.L.; van Lammeren, J.P.M.; Lammers, M.J.G.; Notten, P.H.L. Sensorless Battery Temperature Measurements Based on Electrochemical Impedance Spectroscopy. J. Power Sources 2014, 247, 539–544. [Google Scholar] [CrossRef]
- Brett, C.M.A. Electrochemical Impedance Spectroscopy in the Characterisation and Application of Modified Electrodes for Electrochemical Sensors and Biosensors. Molecules 2022, 27, 1497. [Google Scholar] [CrossRef]
- Al-Amiery, A.A.; Mohamad, A.B.; Kadhum, A.A.H.; Shaker, L.M.; Isahak, W.N.R.W.; Takriff, M.S. Experimental and Theoretical Study on the Corrosion Inhibition of Mild Steel by Nonanedioic Acid Derivative in Hydrochloric Acid Solution. Sci. Rep. 2022, 12, 4705. [Google Scholar] [CrossRef]
- Syrett, J.A.; Remzi Becer, C.; Haddleton, D.M. Self-Healing and Self-Mendable Polymers. Polym. Chem. 2010, 1, 978–987. [Google Scholar] [CrossRef]
- Perrin, F.X.; Ziarelli, F.; Dupuis, A. Relation between the Corrosion Resistance and the Chemical Structure of Hybrid Sol-Gel Coatings with Interlinked Inorganic-Organic Network. Prog. Org. Coat. 2020, 141, 105532. [Google Scholar] [CrossRef]
- Xia, N.N.; Xiong, X.M.; Wang, J.; Rong, M.Z.; Zhang, M.Q. A Seawater Triggered Dynamic Coordinate Bond and Its Application for Underwater Self-Healing and Reclaiming of Lipophilic Polymer. Chem. Sci. 2016, 7, 2736–2742. [Google Scholar] [CrossRef]
- Huynh, T.-P.; Khatib, M.; Haick, H. Self-healable Materials for Underwater Applications. Adv. Mater. Technol. 2019, 4, 1900081. [Google Scholar] [CrossRef]
- Huang, Y.; Liu, T.; Ma, L.; Wang, J.; Zhang, D.; Li, X. Saline-Responsive Triple-Action Self-Healing Coating for Intelligent Corrosion Control. Mater. Des. 2022, 214, 110381. [Google Scholar] [CrossRef]
- Thébault, F.; Vuillemin, B.; Oltra, R.; Ogle, K.; Allely, C. Investigation of Self-Healing Mechanism on Galvanized Steels Cut Edges by Coupling SVET and Numerical Modeling. Electrochim. Acta 2008, 53, 5226–5234. [Google Scholar] [CrossRef]
- Leiva-García, R.; Sánchez-Tovar, R.; Escrivà-Cerdán, C.; García-Antón, J. Role of Modern Localised Electrochemical Techniques to Evaluate the Corrosion on Heterogeneous Surfaces. In Modern Electrochemical Methods in Nano, Surface and Corrosion Science; IntechOpen: Rijeka, Croatia, 2014; ISBN 9789535115861. [Google Scholar]
- Łosiewicz, B.; Popczyk, M.; Smołka, A.; Szklarska, M.; Osak, P.; Budniok, A. Localized Electrochemical Impedance Spectroscopy for Studying the Corrosion Processes in a Nanoscale. Diffus. Defect Data Pt. B 2015, 228, 383–393. [Google Scholar]
- Huang, V.M.; Wu, S.L.; Orazem, M.E.; Pébère, N.; Tribollet, B.; Vivier, V. Local Electrochemical Impedance Spectroscopy: A Review and Some Recent Developments. Electrochim. Acta 2011, 56, 8048–8057. [Google Scholar] [CrossRef]
- Thierry, D.; Nazarov, A.; Persson, D. Mechanical and Chemical Coupling in Tribocorrosion: In Situ and Ex Situ Characterization Techniques. In Mechanical and Electro-Chemical Interactions under Tribocorrosion; Elsevier: Amsterdam, The Netherlands, 2021; pp. 29–66. [Google Scholar]
- Baboian, R. Electrochemical Techniques for Corrosion Engineering; National Association of Corrosion Engineers: Houston, TX, USA, 1986; ISBN 9780915567232. [Google Scholar]
- Łosiewicz, B.; Stróż, A.; Kubisztal, J.; Osak, P.; Zubko, M. EIS and LEIS Study on In Vitro Corrosion Resistance of Anodic Oxide Nanotubes on Ti–13Zr–13Nb Alloy in Saline Solution. Coat. World 2023, 13, 875. [Google Scholar] [CrossRef]
- Sinebryukhov, S.L.; Gnedenkov, A.S.; Mashtalyar, D.V.; Gnedenkov, S.V. PEO-Coating/substrate Interface Investigation by Localised Electrochemical Impedance Spectroscopy. Surf. Coat. Technol. 2010, 205, 1697–1701. [Google Scholar] [CrossRef]
- Bard, A.J.; Fan, F.R.F.; Kwak, J.; Lev, O. Scanning Electrochemical Microscopy. Introduction and Principles. Anal. Chem. 1989, 61, 132–138. [Google Scholar] [CrossRef]
- Kumatani, A.; Matsue, T. Recent Advances in Scanning Electrochemical Microscopic Analysis and Visualization on Lithium-Ion Battery Electrodes. Curr. Opin. Electrochem. 2020, 22, 228–233. [Google Scholar] [CrossRef]
- Santana, J.J.; Izquierdo, J.; Souto, R.M. Uses of Scanning Electrochemical Microscopy (SECM) for the Characterization with Spatial and Chemical Resolution of Thin Surface Layers and Coating Systems Applied on Metals: A Review. Coat. World 2022, 12, 637. [Google Scholar] [CrossRef]
- Niu, L.; Yin, Y.; Guo, W.; Lu, M.; Qin, R.; Chen, S. Application of Scanning Electrochemical Microscope in the Study of Corrosion of Metals. J. Mater. Sci. 2009, 44, 4511–4521. [Google Scholar] [CrossRef]
- Singh, A.; Samih Mohamed, H.; Singh, S.; Yu, H.; Lin, Y. Corrosion Inhibition Using Guar Gum Grafted 2-Acrylamido-2-Methylpropanesulfonic Acid (GG-AMPS) in Tubular Steel Joints. Constr. Build. Mater. 2020, 258, 119728. [Google Scholar] [CrossRef]
- Izquierdo, J.; Nagy, L.; Varga, Á.; Santana, J.J.; Nagy, G.; Souto, R.M. Spatially Resolved Measurement of Electrochemical Activity and pH Distributions in Corrosion Processes by Scanning Electrochemical Microscopy Using Antimony Microelectrode Tips. Electrochim. Acta 2011, 56, 8846–8850. [Google Scholar] [CrossRef]
- da Silva, R.M.P.; Izquierdo, J.; Milagre, M.X.; SIBetancor-Abreu, A.M.; Costa, I.; Souto, R.M. Use of Amperometric and Potentiometric Probes in Scanning Electrochemical Microscopy for the Spatially-Resolved Monitoring of Severe Localized Corrosion Sites on Aluminum Alloy 2098-T351. Sensors 2021, 21, 1132. [Google Scholar] [CrossRef] [PubMed]
- Martín-Yerga, D.; Kang, M.; Unwin, P.R. Scanning Electrochemical Cell Microscopy in a Glovebox: Structure-activity Correlations in the Early Stages of Solid-electrolyte Interphase Formation on Graphite. ChemElectroChem 2021, 8, 4240–4251. [Google Scholar] [CrossRef]
- Dinolfo, P.H.; Hupp, J.T. Supramolecular Coordination Chemistry and Functional Microporous Molecular Materials. Chem. Mater. 2001, 13, 3113–3125. [Google Scholar] [CrossRef]
- Papavinasam, S. 3-Electrochemical Polarization Techniques for Corrosion Monitoring. In Techniques for Corrosion Monitoring, 2nd ed.; Yang, L., Ed.; Woodhead Publishing: Sawston, UK, 2021; pp. 45–77. ISBN 9780081030035. [Google Scholar]
- Eškinja, M.; Moshtaghi, M.; Hönig, S.; Zehethofer, G.; Mori, G. Investigation of the Effects of Temperature and Exposure Time on the Corrosion Behavior of a Ferritic Steel in CO2 Environment Using the Optimized Linear Polarization Resistance Method. Results Mater. 2022, 14, 100282. [Google Scholar] [CrossRef]
- Diki, N.Y.S.; Coulibaly, N.H.; Kassi, K.F.; Trokourey, A. Mild Steel Corrosion Inhibition by 7-(ethylthiobenzimidazolyl) Theophylline. J. Electrochem. Sci. Eng. 2021, 11, 97–106. [Google Scholar] [CrossRef]
- Cui, S.; Wang, J.; Mi, L.; Chen, K.; Ai, W.; Zhai, L.; Guan, X.; Zheng, Y.; Wang, D. A New Synergetic System Based on Triboelectric Nanogenerator and Corrosion Inhibitor for Enhanced Anticorrosion Performance. Nano Energy 2022, 91, 106696. [Google Scholar] [CrossRef]
- Rathod, M.R.; Rajappa, S.K.; Kittur, A.A. Garcinia Livingstonei Leaves Extract Influenced as a Mild Steel Efficient Green Corrosion Inhibitor in 1 M HCl Solution. Mater. Today Proc. 2022, 54, 786–796. [Google Scholar] [CrossRef]
- Edison, T.N.J.I.; Atchudan, R.; Pugazhendhi, A.; Lee, Y.R.; Sethuraman, M.G. Corrosion Inhibition Performance of Spermidine on Mild Steel in Acid Media. J. Mol. Liq. 2018, 264, 483–489. [Google Scholar] [CrossRef]
- Verma, C.; Lgaz, H.; Verma, D.K.; Ebenso, E.E.; Bahadur, I.; Quraishi, M.A. Molecular Dynamics and Monte Carlo Simulations as Powerful Tools for Study of Interfacial Adsorption Behavior of Corrosion Inhibitors in Aqueous Phase: A Review. J. Mol. Liq. 2018, 260, 99–120. [Google Scholar] [CrossRef]
- Alamiery, A. Corrosion Inhibition Effect of 2-N-Phenylamino-5-(3-Phenyl-3-Oxo-1-Propyl)-1,3,4-Oxadiazole on Mild Steel in 1 M Hydrochloric Acid Medium: Insight from Gravimetric and DFT Investigations. Mater. Sci. Energy Technol. 2021, 4, 398–406. [Google Scholar] [CrossRef]
- Damej, M.; Hsissou, R.; Berisha, A.; Azgaou, K.; Sadiku, M.; Benmessaoud, M.; Labjar, N.; El hajjaji, S. New Epoxy Resin as a Corrosion Inhibitor for the Protection of Carbon Steel C38 in 1M HCl. Experimental and Theoretical Studies (DFT, MC, and MD). J. Mol. Struct. 2022, 1254, 132425. [Google Scholar] [CrossRef]
- Abd El-Lateef, H.M.; El-Beltagi, H.S.; Mohamed Mohamed, M.E.; Kandeel, M.; Bakir, E.; Toghan, A.; Shalabi, K.; Tantawy, A.H.; Khalaf, M.M. Novel Natural Surfactant-Based Fatty Acids and Their Corrosion-Inhibitive Characteristics for Carbon Steel-Induced Sweet Corrosion: Detailed Practical and Computational Explorations. Front. Mater. 2022, 9, 843438. [Google Scholar] [CrossRef]
- Haldhar, R.; Kim, S.-C.; Prasad, D.; Bedair, M.A.; Bahadur, I.; Kaya, S.; Dagdag, O.; Guo, L. Papaver Somniferum as an Efficient Corrosion Inhibitor for Iron Alloy in Acidic Condition: DFT, MC Simulation, LCMS and Electrochemical Studies. J. Mol. Struct. 2021, 1242, 130822. [Google Scholar] [CrossRef]
- Lee, W.; Ji, R.; Gösele, U.; Nielsch, K. Fast Fabrication of Long-Range Ordered Porous Alumina Membranes by Hard Anodization. Nat. Mater. 2006, 5, 741–747. [Google Scholar] [CrossRef]
- Izatt, S.R.; Bruening, R.L.; Izatt, N.E. Metal Separations and Recovery in the Mining Industry. JOM 2012, 64, 1279–1284. [Google Scholar] [CrossRef]
- Lee, J.; Shin, Y.; Boo, C.; Hong, S. Performance, Limitation, and Opportunities of Acid-Resistant Nanofiltration Membranes for Industrial Wastewater Treatment. J. Memb. Sci. 2023, 666, 121142. [Google Scholar] [CrossRef]
- Mohamed, R.R.; Fekry, A. Antimicrobial and Anticorrosive Activity of Adsorbents Based on Chitosan Schiff’s Base. Int J Electrochem. Sci. 2011, 6, 2488–2508. [Google Scholar] [CrossRef]
- Christopher, G.; Kulandainathan, M.A.; Harichandran, G. Biopolymers Nanocomposite for Material Protection: Enhancement of Corrosion Protection Using Waterborne Polyurethane Nanocomposite Coatings. Prog. Org. Coat. 2016, 99, 91–102. [Google Scholar] [CrossRef]
- Zhang, W.; Li, C.; Wang, W.; Li, B.; Liu, X.; Liu, Y.; Guo, H.; Chen, S.; Feng, Y. Laminarin and Sodium Molybdate as Efficient Sustainable Inhibitor for Q235 Steel in Sodium Chloride Solution. Colloids Surf. A Physicochem. Eng. Asp. 2022, 637, 128199. [Google Scholar] [CrossRef]
- Szőke, Á.F.; Szabó, G.S.; Hórvölgyi, Z.; Albert, E.; Gaina, L.; Muresan, L.M. Eco-Friendly Indigo Carmine-Loaded Chitosan Coatings for Improved Anti-Corrosion Protection of Zinc Substrates. Carbohydr. Polym. 2019, 215, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.K.; Joshi, P.G.; Srivastava, V.; Quraishi, M.A. Chitosan: A Macromolecule as Green Corrosion Inhibitor for Mild Steel in Sulfamic Acid Useful for Sugar Industry. Int. J. Biol. Macromol. 2018, 106, 704–711. [Google Scholar] [CrossRef]
- Zheng, Y.; Gao, Y.; Li, H.; Yan, M.; Zhao, J.; Liu, Z. Chitosan-Acrylic Acid-Polysuccinimide Terpolymer as Environmentally Friendly Scale and Corrosion Inhibitor in Artificial Seawater. Desalination 2021, 520, 115367. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M.A. Chelation Capability of Chitosan and Chitosan Derivatives: Recent Developments in Sustainable Corrosion Inhibition and Metal Decontamination Applications. Curr. Res. Green Sustain. Chem. 2021, 4, 100184. [Google Scholar] [CrossRef]
- Zhao, Q.; Guo, J.; Cui, G.; Han, T.; Wu, Y. Chitosan Derivatives as Green Corrosion Inhibitors for P110 Steel in a Carbon Dioxide Environment. Colloids Surf. B Biointerfaces 2020, 194, 111150. [Google Scholar] [CrossRef]
- Ansari, K.R.; Chauhan, D.S.; Quraishi, M.A.; Mazumder, M.A.J.; Singh, A. Chitosan Schiff Base: An Environmentally Benign Biological Macromolecule as a New Corrosion Inhibitor for Oil & Gas Industries. Int. J. Biol. Macromol. 2020, 144, 305–315. [Google Scholar]





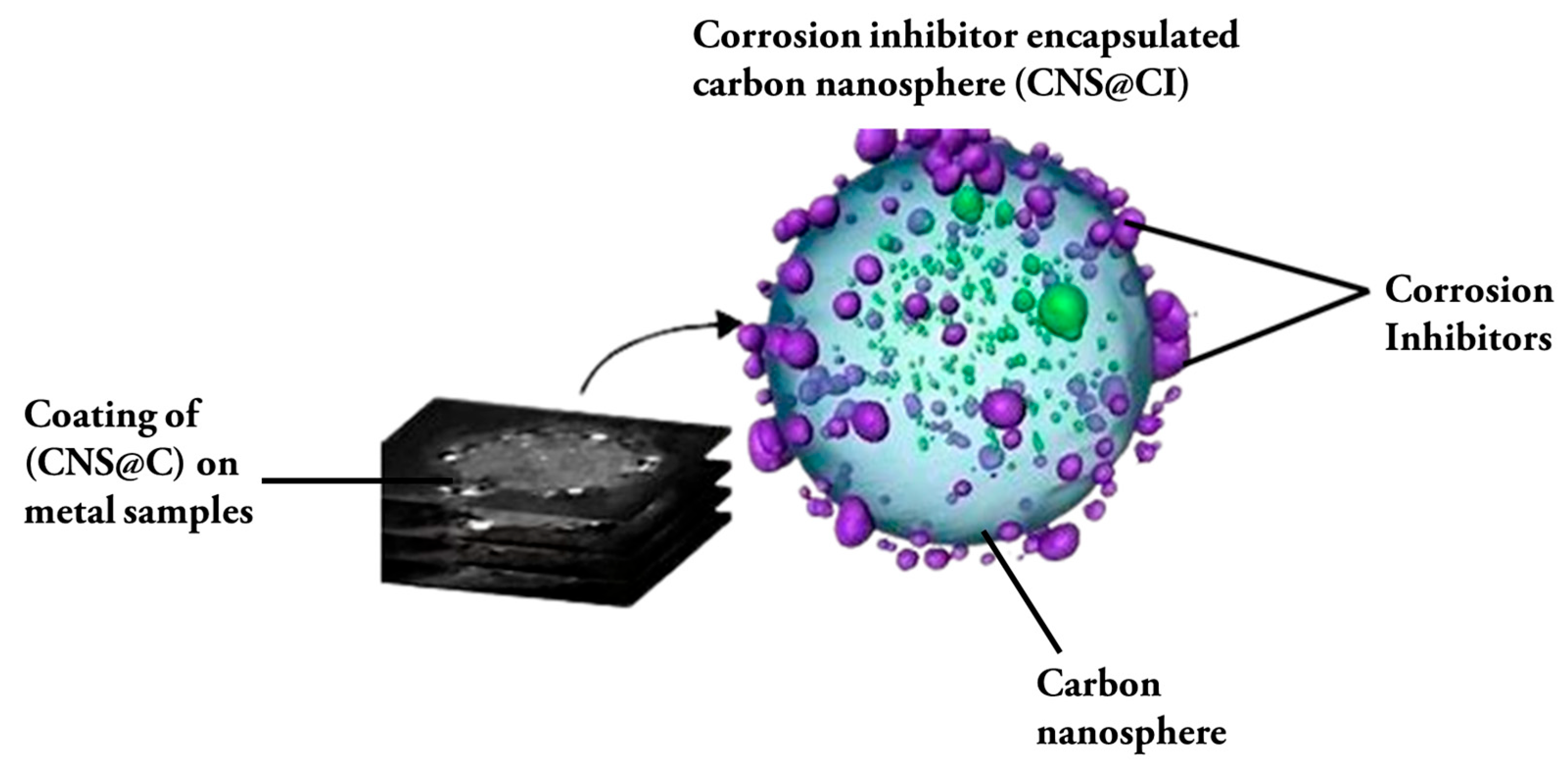

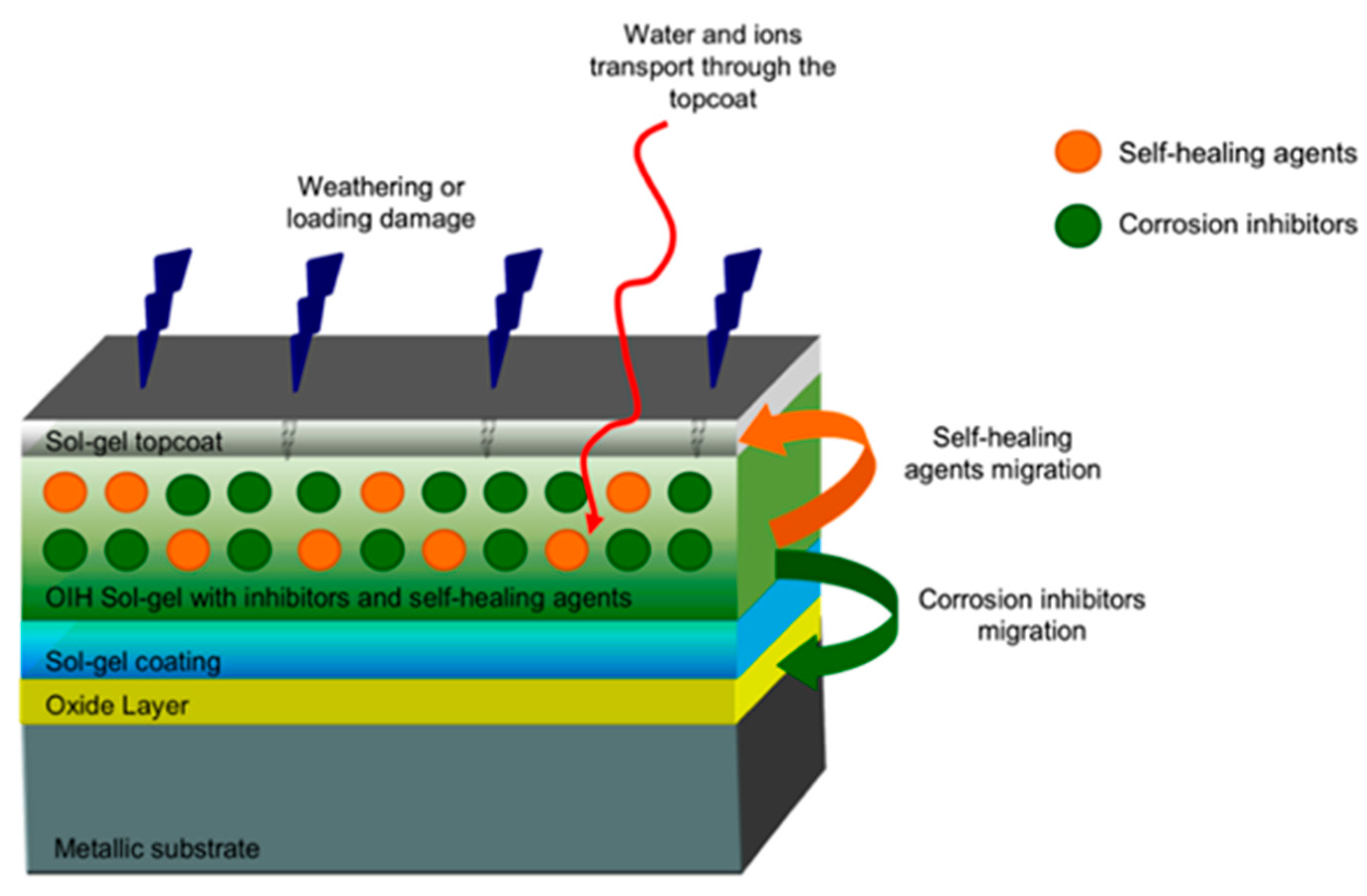
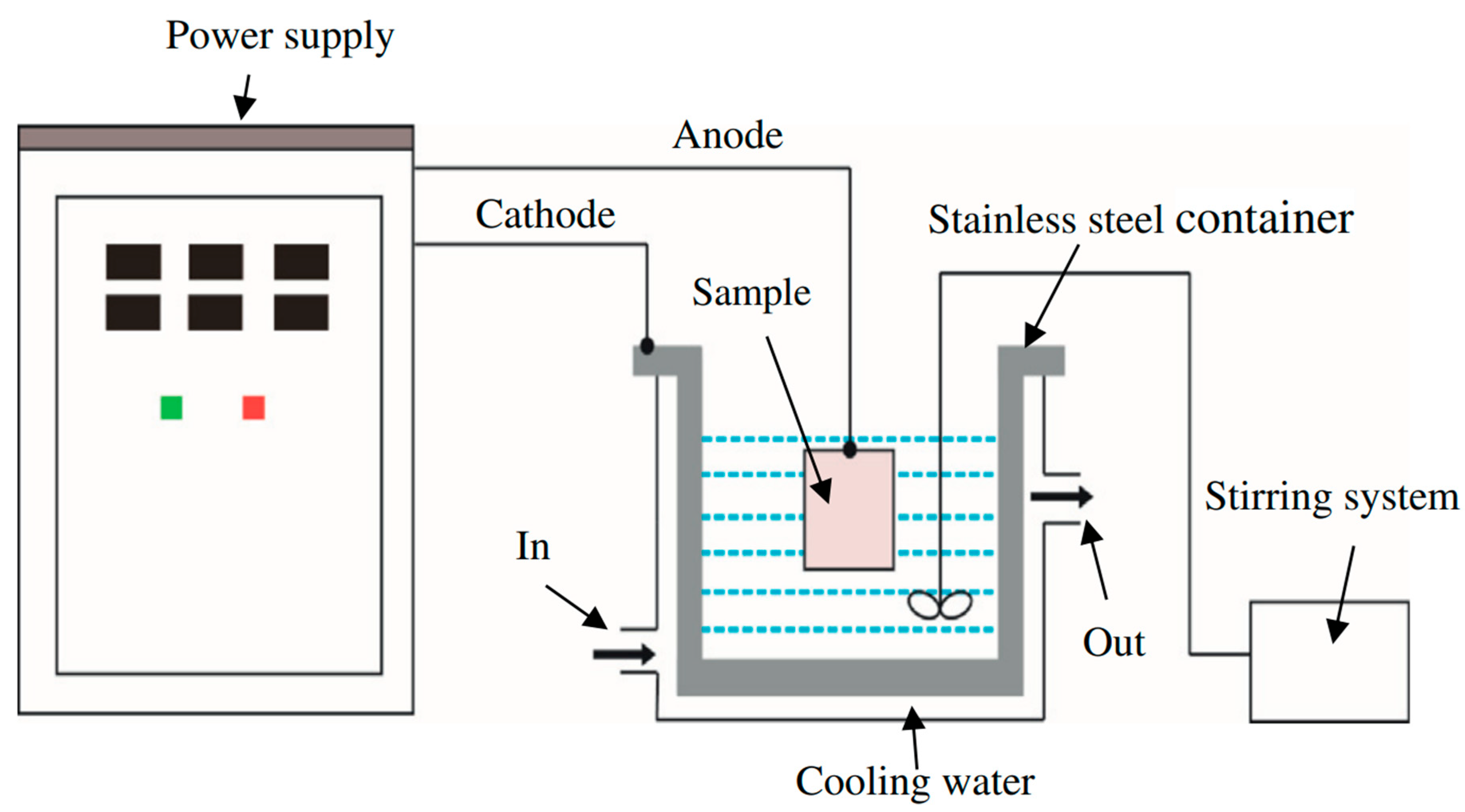

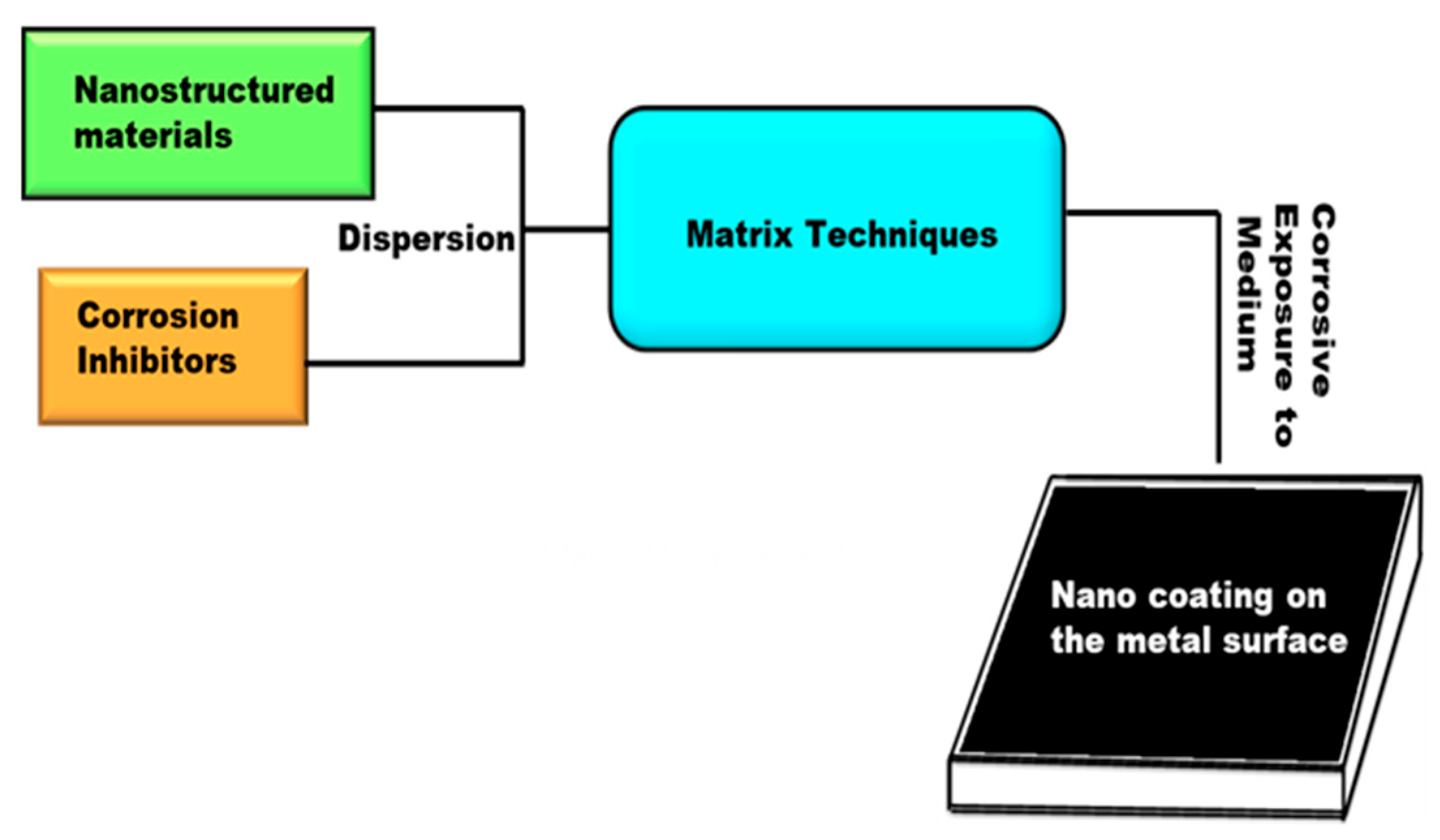


| Nanostructured | Encapsulated CI | Metal Sample | Corrosive Media | Reference |
|---|---|---|---|---|
| Carbon hollow spheres | 2-mercaptobenzi midazole | MS | 5 wt.% HCl | [56] |
| Polyaniline microcapsule | 2-mercaptoben zothiazole | Carbon steel | 3.5 wt.% NaCl | [113] |
| Mesoporous polyaniline (mPANI) hollow nanospheres | 1H-benzotriazole | MS | 3.5 wt.% NaCl | [117] |
| f-MWCNT | Poly (o-anthranilic acid) and GO | Stainless steel 316 | 2 M HCl | [120] |
| Hollow mesoporous organosilica | 2-mercap tobenzimidazole | Copper alloy | 3.5 wt.% NaCl | [121] |
| Carbon nanodots | Polycarbazole | Plain CS specimen | 5 wt.% NaCl | [122] |
| Mesoporous polyaniline hollow spheres | Benzotriazole | Carbon steel | 3.5 wt.% NaCl | [123] |
| Polyaniline (PANI) microcapsule | 2-mercaptobe nzothiazole | MS | 3.5 wt.% NaCl | [124] |
| Acid-modified halloysite nanotubes | Praseodymium ions (Pr) | AZ31 magnesium alloy | 3.5% NaCl | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bijapur, K.; Molahalli, V.; Shetty, A.; Toghan, A.; De Padova, P.; Hegde, G. Recent Trends and Progress in Corrosion Inhibitors and Electrochemical Evaluation. Appl. Sci. 2023, 13, 10107. https://doi.org/10.3390/app131810107
Bijapur K, Molahalli V, Shetty A, Toghan A, De Padova P, Hegde G. Recent Trends and Progress in Corrosion Inhibitors and Electrochemical Evaluation. Applied Sciences. 2023; 13(18):10107. https://doi.org/10.3390/app131810107
Chicago/Turabian StyleBijapur, Kiran, Vandana Molahalli, Apoorva Shetty, Arafat Toghan, Paola De Padova, and Gurumurthy Hegde. 2023. "Recent Trends and Progress in Corrosion Inhibitors and Electrochemical Evaluation" Applied Sciences 13, no. 18: 10107. https://doi.org/10.3390/app131810107
APA StyleBijapur, K., Molahalli, V., Shetty, A., Toghan, A., De Padova, P., & Hegde, G. (2023). Recent Trends and Progress in Corrosion Inhibitors and Electrochemical Evaluation. Applied Sciences, 13(18), 10107. https://doi.org/10.3390/app131810107










