Biomechanical Performance of a Novel Implant Design in Simulated Extraction Sites and Sinuslift Procedures
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamborena, I.; Sasaki, Y.; Blatz, M.B. Predictable immediate implant placement and restoration in the esthetic zone. J. Esthet. Restor. Dent. 2021, 33, 158–172. [Google Scholar] [CrossRef]
- Ekhlasmandkermani, M.; Amid, R.; Kadkhodazadeh, M.; Hajizadeh, F.; Abed, P.F.; Kheiri, L.; Kheiri, A. Sinus floor elevation and simultaneous implant placement in fresh extraction sockets: A systematic review of clinical data. J. Korean Assoc. Oral Maxillofac. Surg. 2021, 47, 411–426. [Google Scholar] [CrossRef] [PubMed]
- Christiaens, V.; Pitman, J.; Glibert, M.; Hommez, G.; Atashkadeh, M.; De Bruyn, H. Rationale for a reverse tapered body shift implant for immediate placement. Int. J. Oral Maxillofac. Surg. 2020, 49, 1630–1636. [Google Scholar] [CrossRef]
- Canellas, J.V.D.S.; Medeiros, P.J.D.; Figueredo, C.M.D.S.; Fischer, R.G.; Ritto, F.G. Which is the best choice after tooth extraction, immediate implant placement or delayed placement with alveolar ridge preservation? A systematic review and meta-analysis. J. Craniomaxillofac. Surg. 2019, 47, 1793–1802. [Google Scholar] [CrossRef]
- Garcia-Sanchez, R.; Dopico, J.; Kalemaj, Z.; Buti, J.; Pardo Zamora, G.; Mardas, N. Comparison of clinical outcomes of immediate versus delayed placement of dental implants: A systematic review and meta-analysis. Clin. Oral. Implants. Res. 2022, 33, 231–277. [Google Scholar] [CrossRef]
- Maddalone, M.; Mirabelli, L.; Venino, P.M.; Karanxha, L.; Porcaro, G.; Del Fabbro, M. Long-term stability of autologous bone graft of intraoral origin after lateral sinus floor elevation with simultaneous implant placement. Clin. Implant. Dent. Relat. Res. 2018, 20, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Rues, S.; Schmitter, M.; Kappel, S.; Sonntag, R.; Kretzer, J.P.; Nadorf, J. Effect of bone quality and quantity on the primary stability of dental implants in a simulated bicortical placement. Clin. Oral Investig. 2021, 25, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Kuo, P.J.; Lin, C.Y.; Hung, T.F.; Chiu, H.C.; Kuo, H.Y. A novel application of dynamic guided navigation system in immediate implant placement. J. Dent. Sci. 2022, 17, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pareek, R.; Rajawat, G.S.; Kadam, A.; Al Abdulsalam, M.; Al Abdulathim, A. Comparison of Bone Healing in Immediate Implant Placement versus Delayed Implant Placement. J. Pharm. Bioallied. Sci. 2021, 13, S1309–S1314. [Google Scholar]
- Weigl, P.; Strangio, A. The impact of immediately placed and restored single-tooth implants on hard and soft tissues in the anterior maxilla. Eur. J. Oral Implantol. 2016, 9, S89–S106. [Google Scholar]
- Rodrigues, D.M.; Petersen, R.L.; Montez, C.; de Moraes, J.R.; Ferreira, V.; Barboza, E.P. The relationship between tomographic sagittal root position of maxillary anterior teeth and the bone housing. J. Prosthet. Dent. 2022, ahead of print. [Google Scholar] [CrossRef]
- Botermans, A.; Lidén, A.; de Carvalho Machado, V.; Chrcanovic, B.R. Immediate Implant Placement in the Maxillary Aesthetic Zone: A Cone Beam Computed Tomography Study. J Clin. Med. 2021, 10, 5853. [Google Scholar] [CrossRef] [PubMed]
- Heimes, D.; Schiegnitz, E.; Kuchen, R.; Kämmerer, P.W.; Al-Nawas, B. Buccal Bone Thickness in Anterior and Posterior Teeth-A Systematic Review. Healthcare 2021, 9, 1663. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.M.; Shi, J.Y.; Qiao, S.C.; Zhang, X.; Lai, H.C.; Zhang, X.M. Accuracy and primary stability of tapered or straight implants placed into fresh extraction socket using dynamic navigation: A randomized controlled clinical trial. Clin. Oral Investig 2021, ahead of print. [Google Scholar] [CrossRef]
- Sanchez-Perez, A.; Nicolas-Silvente, A.I.; Sanchez-Matas, C.; Molina-García, S.; Navarro-Cuellar, C.; Romanos, G.E. Primary stability and PES/WES evaluation for immediate implants in the aesthetic zone: A pilot clinical double-blind randomized study. Sci. Rep. 2021, 11, 20024. [Google Scholar] [CrossRef]
- Yang, B.; Irastorza-Landa, A.; Heuberger, P.; Ploeg, H.L. Effect of insertion factors on dental implant insertion torque/energy-experimental results. J. Mech. Behav. Biomed. Mater. 2020, 112, 103995. [Google Scholar] [CrossRef]
- Noaman, A.T.; Bede, S.Y. The Effect of Bone Density Measured by Cone Beam Computed Tomography and Implant Dimensions on the Stability of Dental Implants. J. Craniofac. Surg. 2021, 33, e553–e557. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; López-Jarana, P.; Lemos, B.F.; Gil, F.J.; Falcão, C.; Ríos-Santos, J.V.; Ríos-Carrasco, B. Relevant Design Aspects to Improve the Stability of Titanium Dental Implants. Materials 2020, 13, 1910. [Google Scholar] [CrossRef]
- Chu, S.J.; Levin, B.P.; Egbert, N.; Saito, H.; Nevins, M. Use of a Novel Implant with an Inverted Body-Shift and Prosthetic Angle Correction Design for Immediate Tooth Replacement in the Esthetic Zone: A Clinical Case Series. Int. J. Periodontics Restor. Dent. 2021, 41, 195–204. [Google Scholar] [CrossRef]
- Hamzani, Y.; Yassien, E.; Moskovich, L.; Becker, T.; Chaushu, G.; Haj Yahya, B. Potential Circumferential Bone Engagement following Tooth Extraction in the Posterior Mandible: Computed Tomography Assessment. Medicina 2021, 57, 874. [Google Scholar] [CrossRef] [PubMed]
- Levin, B.P.; Chu, S.J.; Saito, H.; Nevins, M.; Levin, J.P. A Novel Implant Design for Immediate Extraction Sites: Determining Primary Stability. Int. J. Periodontics Restor. Dent. 2021, 41, 357–364. [Google Scholar] [CrossRef]
- Rowan, M.; Lee, D.; Pi-Anfruns, J.; Shiffler, P.; Aghaloo, T.; Moy, P.K. Mechanical versus biological stability of immediate and delayed implant placement using resonance frequency analysis. J. Oral Maxillofac. Surg. 2015, 73, 253–257. [Google Scholar] [CrossRef]
- Levin, B.P. The Correlation Between Immediate Implant Insertion Torque and Implant Stability Quotient. Int. J. Periodontics Restor. Dent. 2016, 36, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Bavetta, G.; Bavetta, G.; Randazzo, V.; Cavataio, A.; Paderni, C.; Grassia, V.; Dipalma, G.; Gargiulo Isacco, C.; Scarano, A.; De Vito, D.; et al. A Retrospective Study on Insertion Torque and Implant Stability Quotient (ISQ) as Stability Parameters for Immediate Loading of Implants in Fresh Extraction Sockets. Biomed. Res. Int. 2019, 2019, 9720419. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.J.; Saito, H.; Levin, B.P.; Baumgarten, H.; Egbert, N.; Wills, M.J.; Del Castillo, R.A.; Tarnow, D.P.; Nevins, M. Outcomes of a 1-Year Prospective Single-Arm Cohort Study Using a Novel Macro-Hybrid Implant Design in Extraction Sockets: Part 1. Int. J. Periodontics Restor. Dent. 2021, 41, 499–508. [Google Scholar] [CrossRef]
- Saito, H.; Chu, S.J.; Tarnow, D.P. The effect of implant macrogeometry in immediate tooth replacement therapy: A case series. J. Esthet. Restor. Dent. 2021, ahead of print. [Google Scholar] [CrossRef]
- Kwon, J.J.; Hwang, J.; Kim, Y.D.; Shin, S.H.; Cho, B.H.; Lee, J.Y. Automatic three-dimensional analysis of bone volume and quality change after maxillary sinus augmentation. Clin. Implant Dent. Relat. Res. 2019, 21, 1148–1155. [Google Scholar] [CrossRef]
- Andrés-García, R.; Ríos-Santos, J.V.; Herrero-Climent, M.; Bullón, P.; Fernández-Farhall, J.; Gómez-Menchero, A.; Fernández-Palacín, A.; Ríos-Carrasco, B. Sinus Floor Elevation via an Osteotome Technique without Biomaterials. Int. J. Environ. Res. Public Health 2021, 18, 1103. [Google Scholar] [CrossRef]
- Park, Y.H.; Jung, U.W.; Kim, C.S.; Choi, S.H.; Cho, K.S.; Lee, J.S. Resonance Frequency Analysis of Tapered Implants Placed at Maxillary Posterior Sites After Lateral Sinus Augmentation: A 1.5-year Follow-Up Prospective Study. Implant Dent. 2019, 28, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Kim, C.H.; Kim, J.H. Immediate implant placement following tooth extraction with simultaneous lateral sinus augmentation: A retrospective clinical study after at least 1 year of loading. Int. J. Implant Dent. 2021, 7, 96. [Google Scholar] [CrossRef]
- Bhandari, S.; Thomas, R.; Kumar, T.; Shah, R.; Mehta, D.S. Maxillary Sinus Augmentation Using Hydraulic Pressure by Lateral Approach and Simultaneous Implant Placement: Clinicoradiographic Study. Implant Dent. 2019, 28, 514–519. [Google Scholar] [CrossRef]
- De Oliveira Nicolau Mantovani, A.K.; de Mattias Sartori, I.A.; Azevedo-Alanis, L.R.; Tiossi, R.; Fontão, F.N.G.K. Influence of cortical bone anchorage on the primary stability of dental implants. Oral Maxillofac. Surg. 2018, 22, 297–301. [Google Scholar] [CrossRef] [PubMed]
- Irinakis, T.; Wiebe, C. Clinical evaluation of the NobelActive implant system: A case series of 107 consecutively placed implants and a review of the implant features. J. Oral. Implantol. 2009, 35, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Klär, V.; Grobecker-Karl, T.; Karl, M. Biomechanical rationale for a novel implant design reducing stress on buccal bone. Appl. Sci. 2023, 13, 666. [Google Scholar] [CrossRef]
- Dard, M.; Kuehne, S.; Obrecht, M.; Grandin, M.; Helfenstein, J.; Pippenger, B.E. Integrative Performance Analysis of a Novel Bone Level Tapered Implant. Adv. Dent. Res. 2016, 28, 28–33. [Google Scholar] [CrossRef]
- Wang, R.; Eppell, S.J.; Nguyen, C.; Morris, N. Relative Contribution of Trabecular and Cortical Bone to Primary Implant Stability: An In Vitro Model Study. J. Oral Implantol. 2016, 42, 145–152. [Google Scholar] [CrossRef]
- Steiner, C.; Karl, M.; Grobecker-Karl, T. Insertion and loading characteristics of three different bone-level implants. Int. J. Oral Maxillofac. Implants. 2020, 35, 560–565. [Google Scholar] [CrossRef]
- Karl, M.; Irastorza-Landa, A. Does implant design affect primary stability in extraction sites? Quintessence Int. 2017, 48, 219–224. [Google Scholar]
- Steiner, C.; Karl, M.; Grobecker-Karl, T. Wedge shaped vs. round implants: Bone strain during the insertion process. J. Oral Implantol. 2022, 48, 557–561. [Google Scholar] [CrossRef]
- Klär, V.; Karl, M.; Grobecker-Karl, T. Bone damage during dental implant insertion: A pilot study combining strain gauge and histologic analysis. Appl. Sci. 2022, 12, 291. [Google Scholar] [CrossRef]
- Velikov, S.; Susin, C.; Heuberger, P.; Irastorza-Landa, A. A New Site Preparation Protocol That Supports Bone Quality Evaluation and Provides Predictable Implant Insertion Torque. J. Clin. Med. 2020, 9, 494. [Google Scholar] [CrossRef]
- Dantas, T.A.; Carneiro Neto, J.P.; Alves, J.L.; Vaz, P.C.S.; Silva, F.S. In silico evaluation of the stress fields on the cortical bone surrounding dental implants: Comparing root-analogue and screwed implants. J. Mech. Behav. Biomed. Mater. 2020, 104, 103667. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, M.C.; Huang, C.H.; Hsu, M.L. Influences of screw design features on initial stability in immediate implant placement and restoration. Clin. Biomech. 2021, 89, 105453. [Google Scholar] [CrossRef]
- Ueno, D.; Nakamura, K.; Kojima, K.; Toyoshima, T.; Tanaka, H.; Ueda, K.; Koyano, K.; Kodama, T. A stepwise under-prepared osteotomy technique improves primary stability in shallow-placed implants: A preliminary study for simultaneous vertical ridge augmentation. Odontology 2018, 106, 187–193. [Google Scholar] [CrossRef]
- Sierra-Rebolledo, A.; Allais-Leon, M.; Maurette-O’Brien, P.; Gay-Escoda, C. Primary Apical Stability of Tapered Implants Through Reduction of Final Drilling Dimensions in Different Bone Density Models: A Biomechanical Study. Implant. Dent. 2016, 25, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef]
- Elias, C.N.; Rocha, F.A.; Nascimento, A.L.; Coelho, P.G. Influence of implant shape, surface morphology, surgical technique and bone quality on the primary stability of dental implants. J. Mech. Behav. Biomed. Mater. 2012, 16, 169–180. [Google Scholar] [CrossRef]
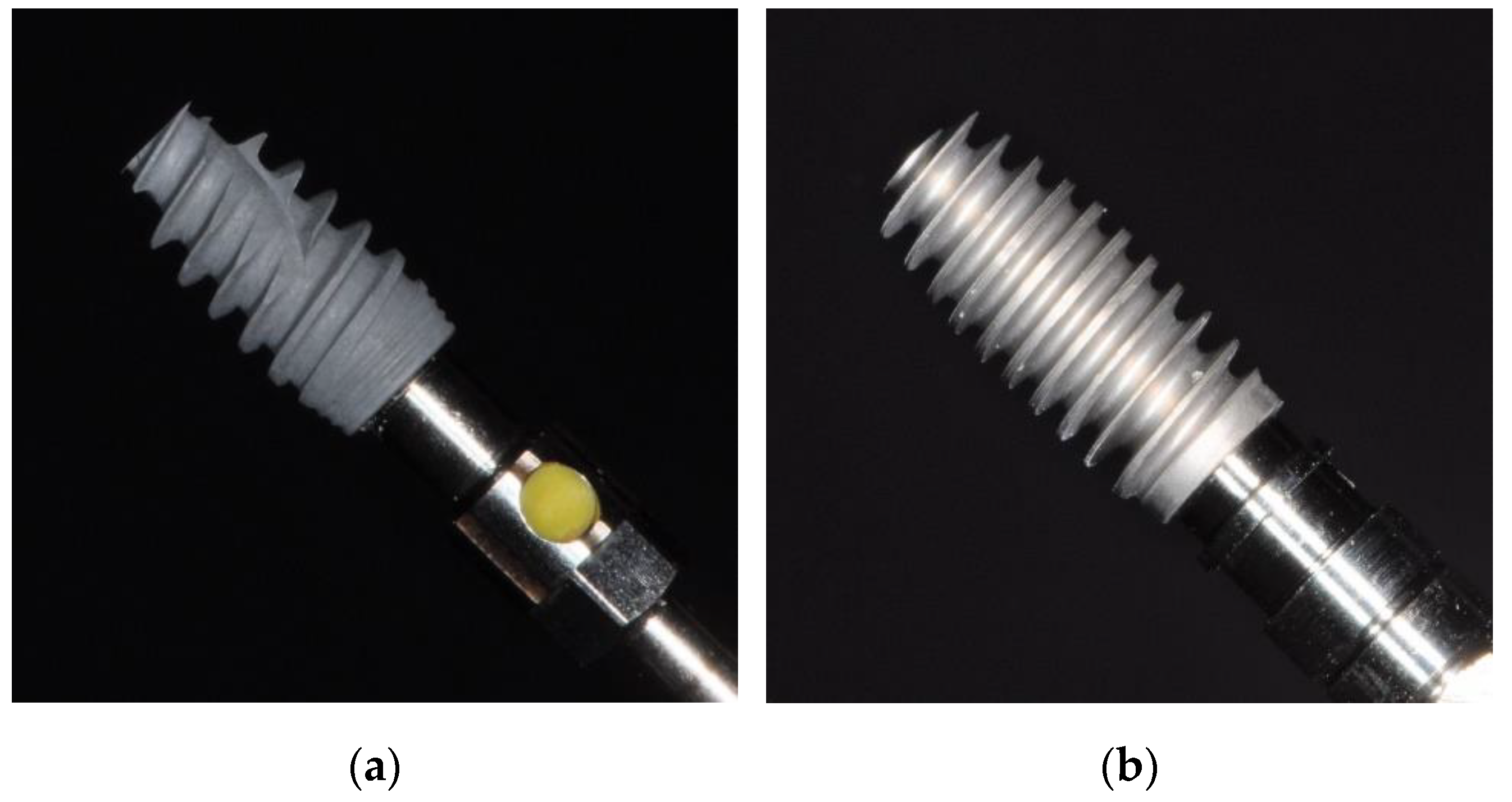
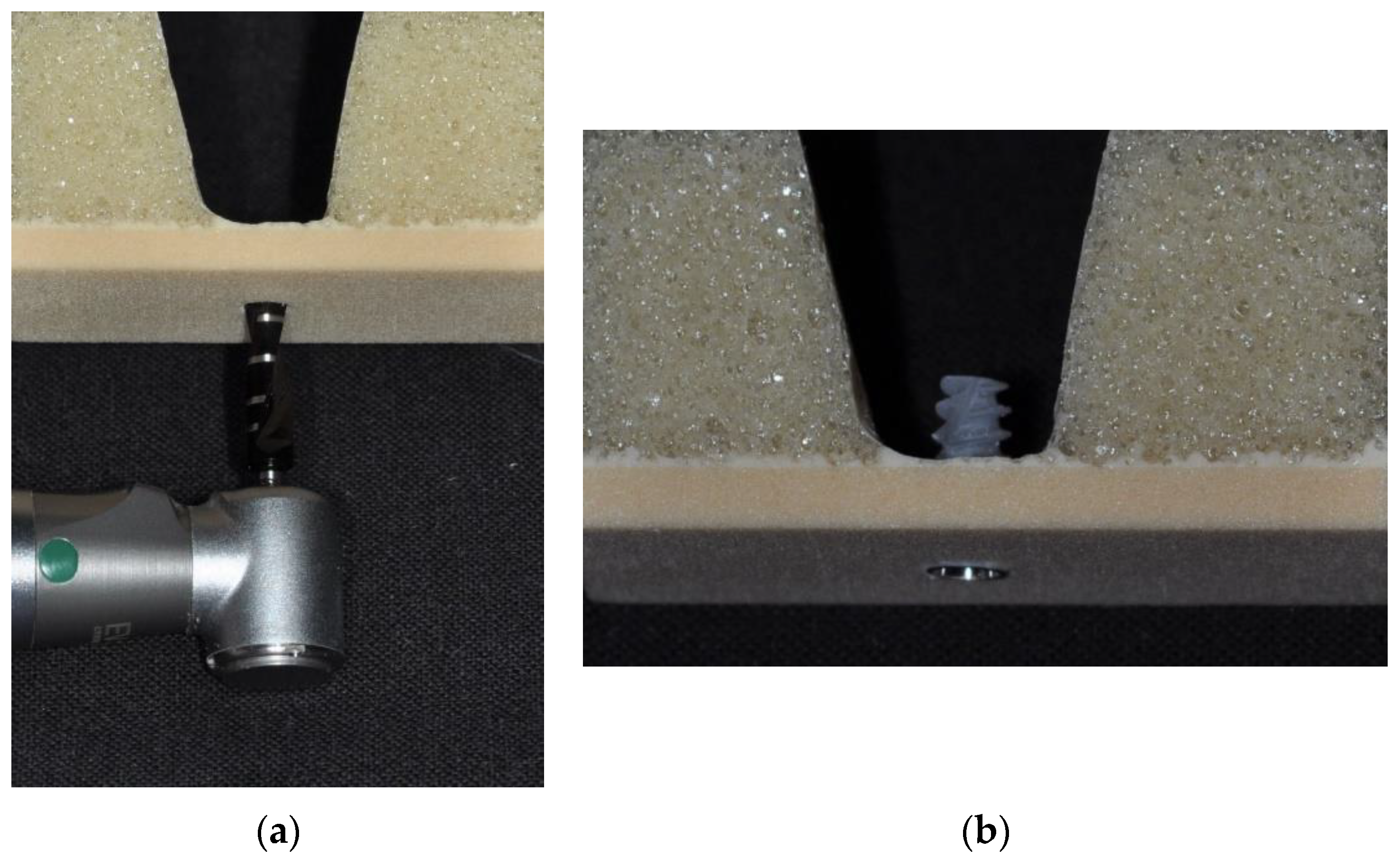
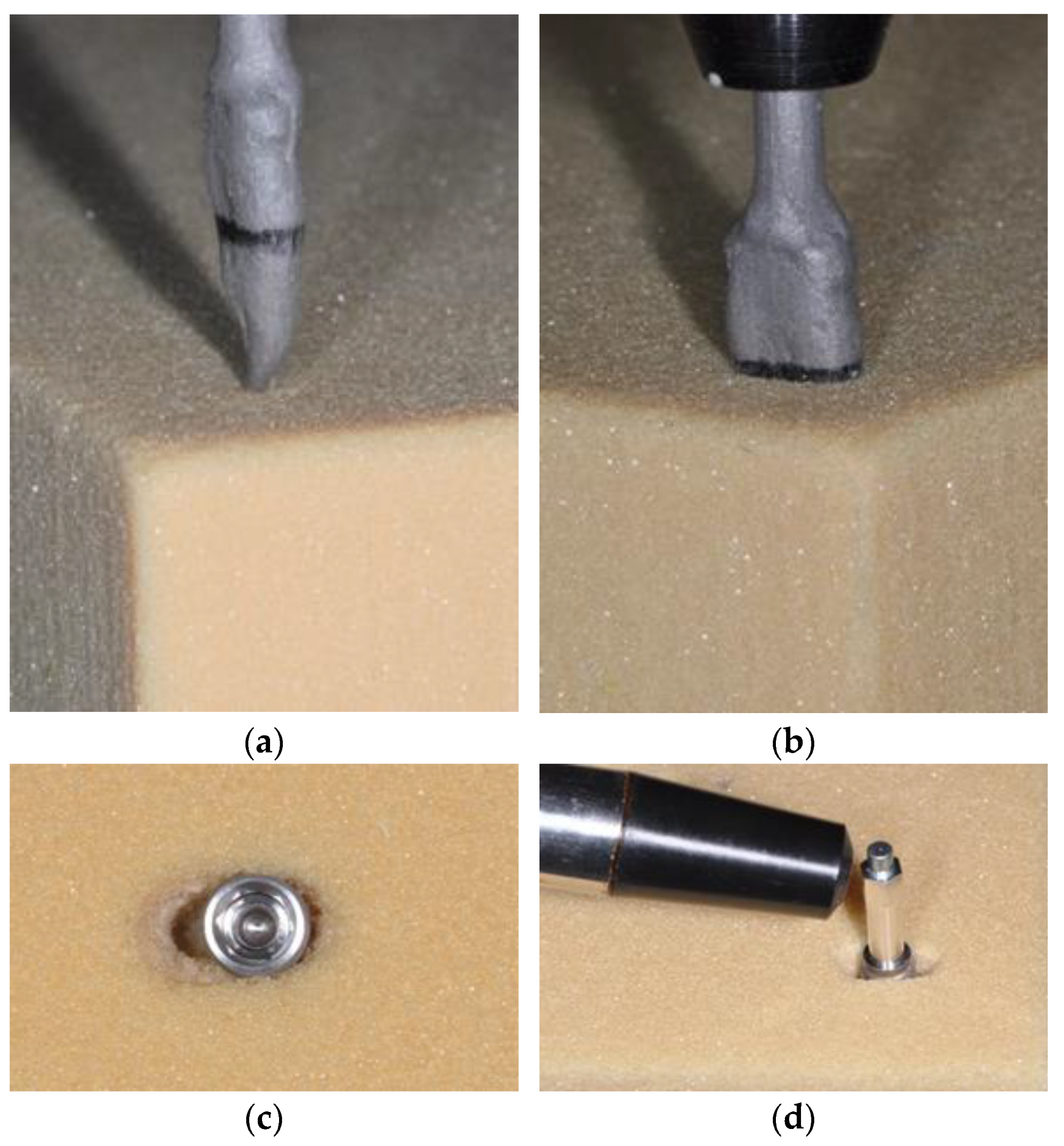
| Sinuslift | Extraction Site | |
|---|---|---|
| Implants | NobelActive Internal RP 4.3 × 10 mm REF 34131 LOT 13125039 | NobelActive Internal RP 5.0 × 10 mm REF 34137 LOT 12168347 |
| AlfaGate Novel Design 4.3 × 10 mm | AlfaGate Novel Design 5.0 × 10 mm | |
| Drill sequence | 2.0 2.4/2.8 3.2/3.6 | Preformed Socket Depth: 10 mm 2.4/2.8 |
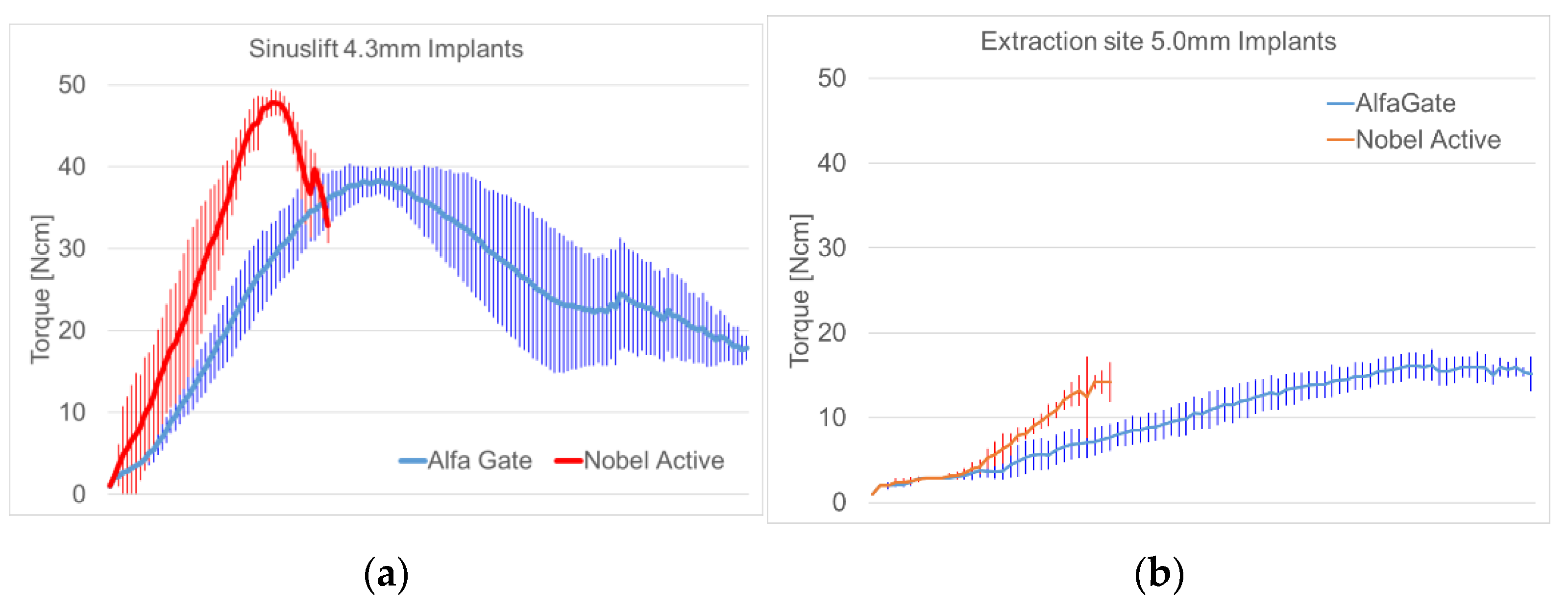
| Parameter | NobelActive | Shapiro-Wilk-Test (p-Value) | AlfaGate | Shapiro-Wilk-Test (p-Value) | |||
|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||||
| Sinuslift | Maximum insertion torque [Ncm] | 48.49 | 1.965 | 0.0298 | 40.42 | 1.460 | 0.1877 |
| Osstell [ISQ] | 40.65 | 5.874 | 0.8498 | 44.70 | 3.164 | 0.6327 | |
| Extraction site | Maximum insertion torque [Ncm] | 14.29 | 1.244 | 0.4605 | 16.43 | 1.578 | 0.2829 |
| Osstell [ISQ] | 41.80 | 3.225 | 0.9252 | 47.80 | 2.541 | 0.5593 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klär, V.; Zimmerer, R.; Schulz, A.; Lethaus, B. Biomechanical Performance of a Novel Implant Design in Simulated Extraction Sites and Sinuslift Procedures. Appl. Sci. 2023, 13, 7541. https://doi.org/10.3390/app13137541
Klär V, Zimmerer R, Schulz A, Lethaus B. Biomechanical Performance of a Novel Implant Design in Simulated Extraction Sites and Sinuslift Procedures. Applied Sciences. 2023; 13(13):7541. https://doi.org/10.3390/app13137541
Chicago/Turabian StyleKlär, Virgilia, Rüdiger Zimmerer, Annika Schulz, and Bernd Lethaus. 2023. "Biomechanical Performance of a Novel Implant Design in Simulated Extraction Sites and Sinuslift Procedures" Applied Sciences 13, no. 13: 7541. https://doi.org/10.3390/app13137541
APA StyleKlär, V., Zimmerer, R., Schulz, A., & Lethaus, B. (2023). Biomechanical Performance of a Novel Implant Design in Simulated Extraction Sites and Sinuslift Procedures. Applied Sciences, 13(13), 7541. https://doi.org/10.3390/app13137541






