Biochar Improved Sugarcane Growth and Physiology under Salinity Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental Procedure
2.3. Data Collection
2.4. Plant Growth and Physiological Responses
2.4.1. Growth Parameters
2.4.2. Physiological Parameters
2.5. Data Analysis and Statistics
3. Results
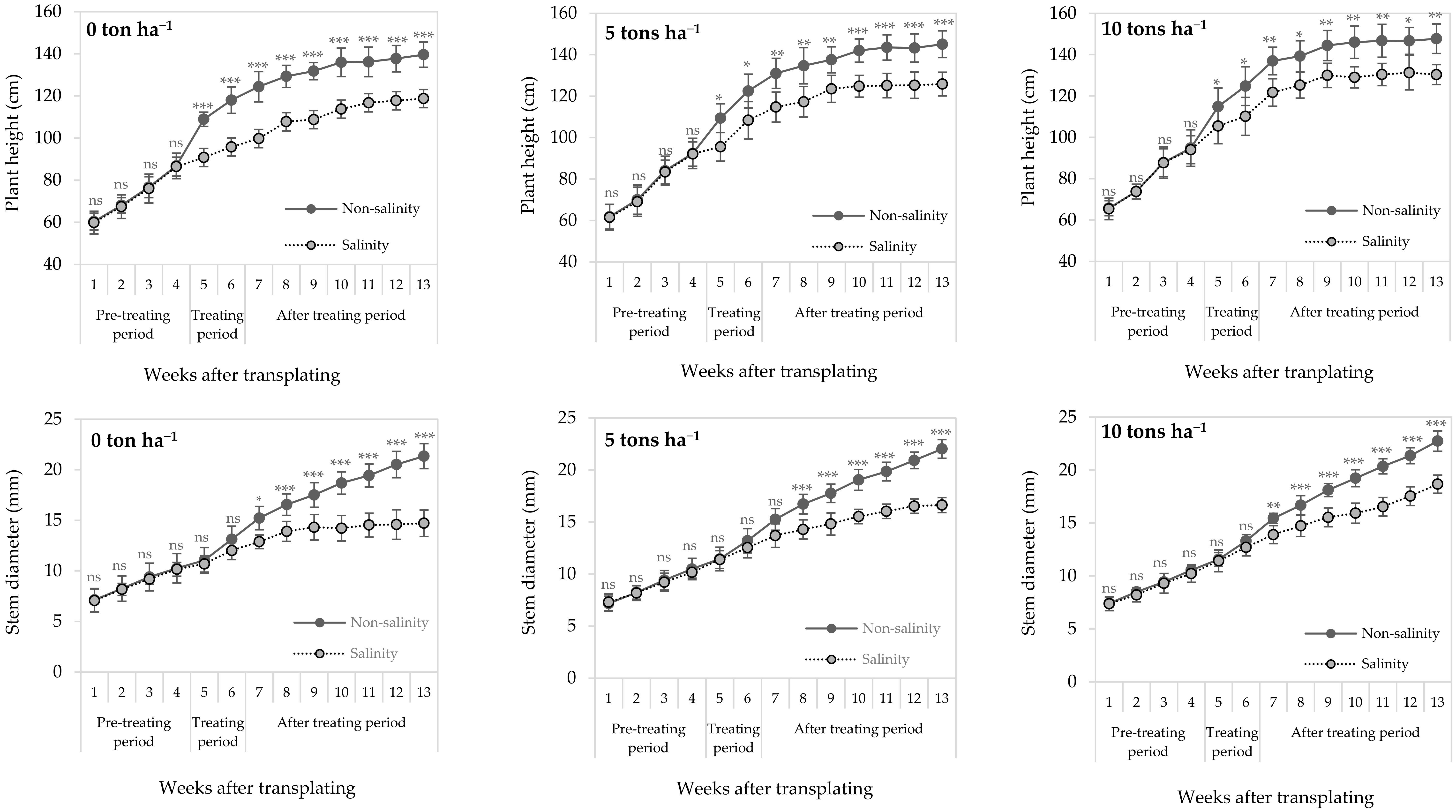
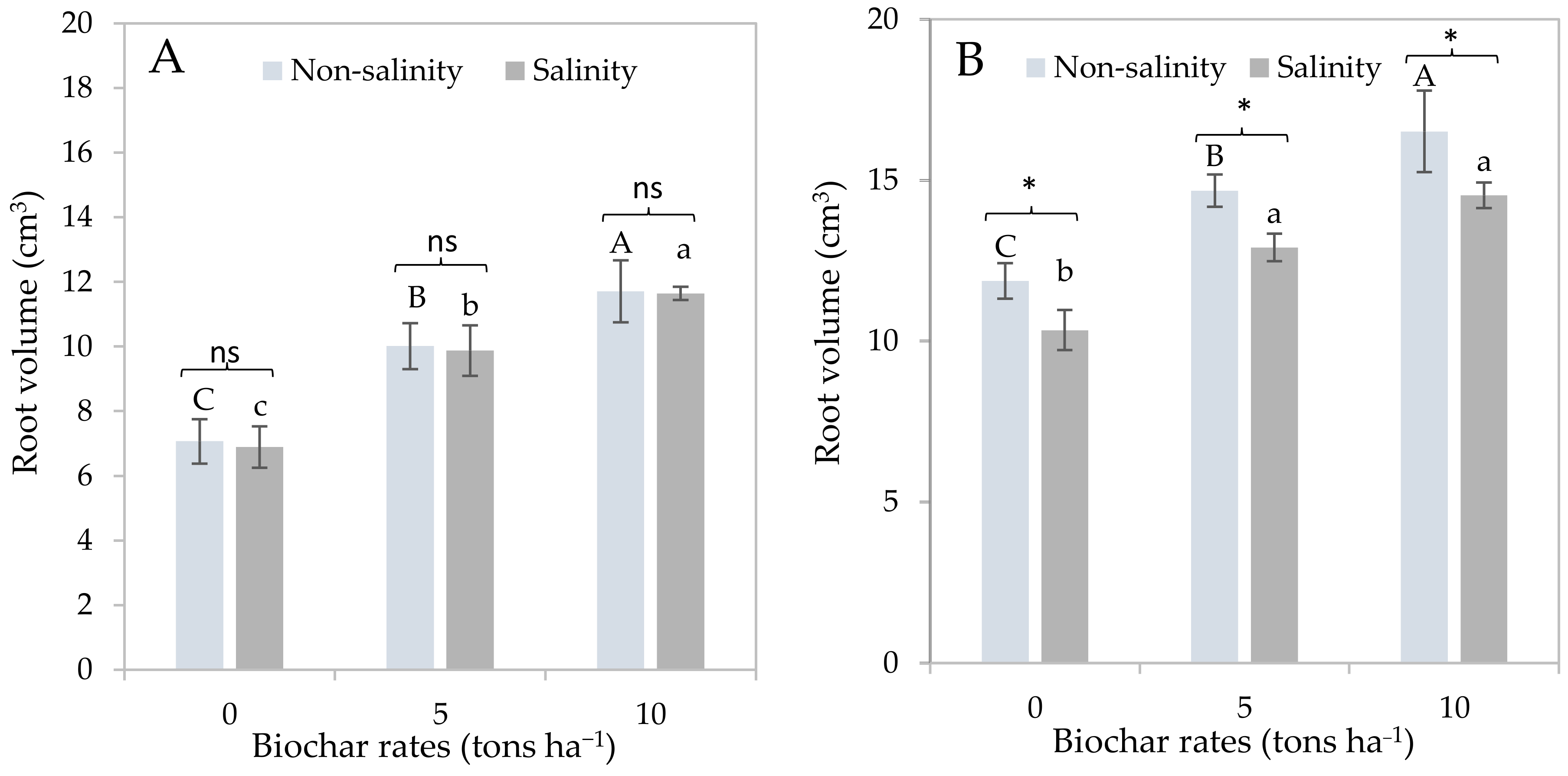
3.1. Effects of Salinity and Biochar Application on Sugarcane Growth Responses
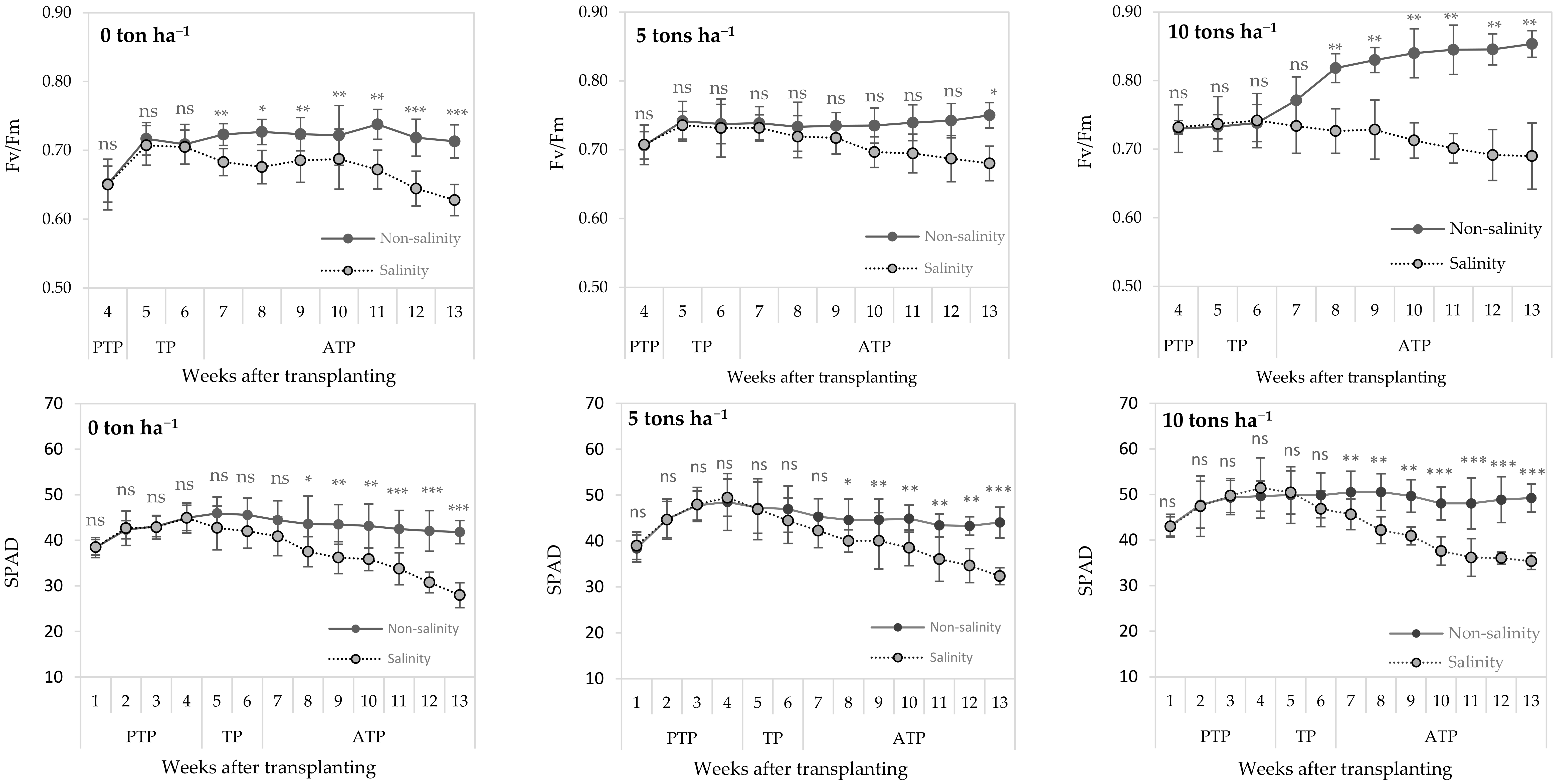
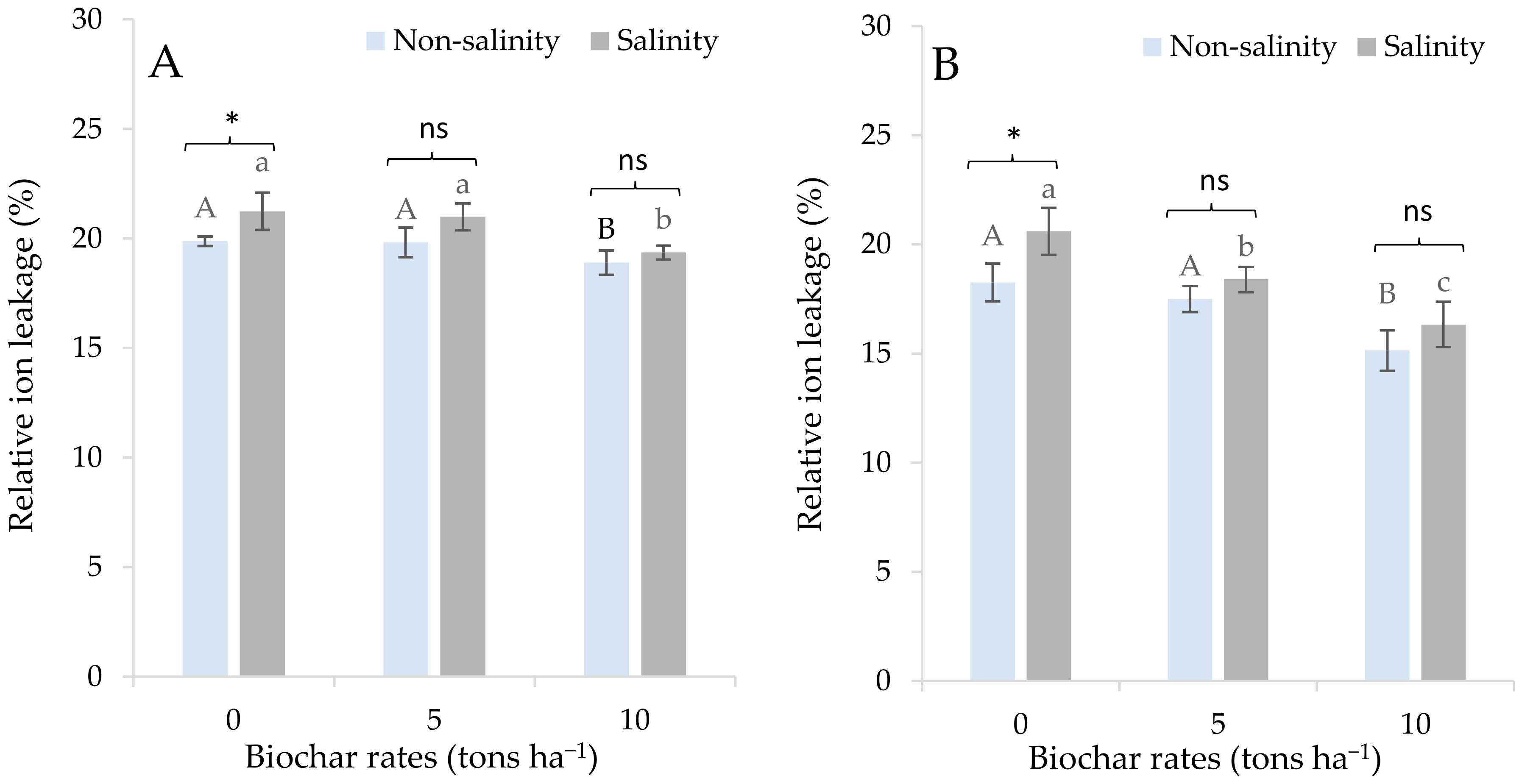
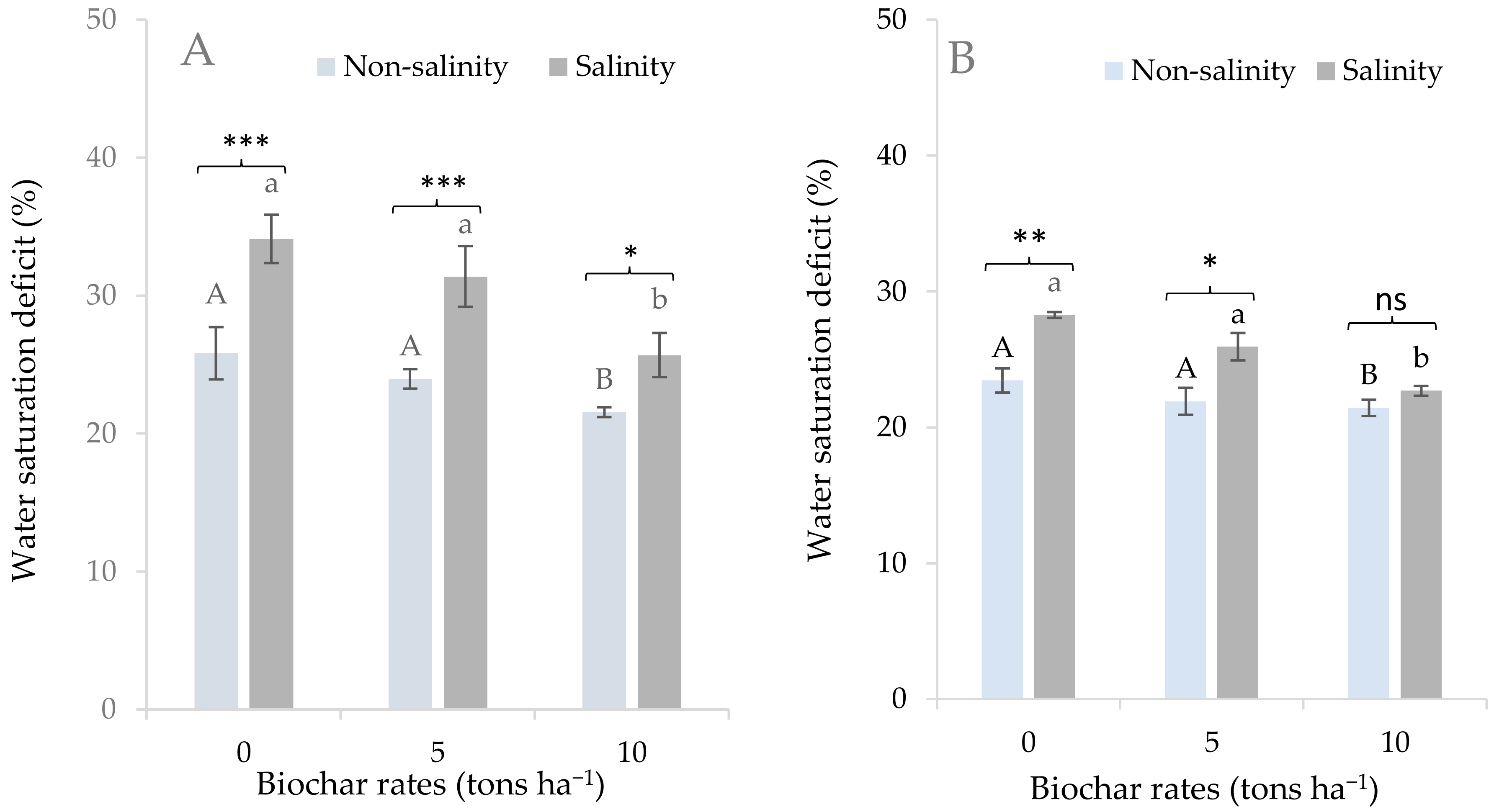
3.2. Effects of Salinity and Biochar Application on Physiological Responses of Sugarcane
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hang, R.; Wang, Y.; Hussain, S.; Yang, S.; Li, R.; Liu, S.; Chen, Y.; Wei, H.; Dai, Q.; Hou, H. Study on the effect of salt stress on yield and grain quality among different rice varieties. Front. Plant Sci. 2022, 13, 918460. [Google Scholar] [CrossRef]
- Takeda, S.; Matsuoka, M. Genetic approaches to crop improvement: Responding to environmental and population changes. Nat. Rev. Genet. 2008, 9, 444–457. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Sharma, P.K. Soil salinity and food security in India. Front. Sustain. Food Syst. 2020, 4, 533781. [Google Scholar] [CrossRef]
- Saifullah; Dahlawi, S.; Naeem, A.; Rengel, Z.; Naidu, R. Biochar application for the remediation of salt-affected soils: Challenges and opportunities. Sci. Total Envion. 2018, 625, 320–335. [Google Scholar] [CrossRef]
- Gupta, B.; Huang, B. Mechanism of salinity tolerance in plants: Physiological, biochemical, and molecular characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Demiral, T.; Turkan, I. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 2006, 56, 72–79. [Google Scholar] [CrossRef]
- Maggio, A.; Raimondi, G.; Martino, A.; Pascale, S.D. Salt stress response in tomato beyond the salinity tolerance threshold. Environ. Exp. Bot. 2007, 59, 276–282. [Google Scholar] [CrossRef]
- Netondo, G.W.; Onyango, J.C.; Beck, E. Sorghum and salinity: II. Gas exchange and chlorophyll fluorescence of sorghum under salt stress. Crop Sci. 2004, 44, 806–811. [Google Scholar] [CrossRef]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1; 4 and HKT1; 5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef]
- Patade, V.Y.; Suprasanna, P.; Bapat, V.A. Effects of salt stress in relation to osmotic adjustment on sugarcane (Saccharum officinarum L.) callus cultures. Plant Growth Regul. 2008, 55, 169–173. [Google Scholar] [CrossRef]
- Simões, W.L.; Calgaro, M.; Coelho, D.S.; Santos, D.B.D.; Souza, M.A.D. Growth of sugar cane varieties under salinity. Rev. Ceres 2016, 63, 265–271. [Google Scholar] [CrossRef]
- Zhao, D.; Zhu, K.; Momotaz, A.; Gao, X. Sugarcane plant growth and physiological responses to soil salinity during tillering and stalk elongation. Agriculture 2020, 10, 608. [Google Scholar] [CrossRef]
- Yunita, R.; Hartati, S.R.; Suhesti, S.; Syafaruddin. Response of bululawang sugarcane variety to salt stress. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Chiyoda, Tokyo, 2020. [Google Scholar] [CrossRef]
- Watanabe, K.; Takaragawa, H.; Ueno, M.; Kawamitsu, Y. Changes in agronomic and physiological traits of sugarcane grown with saline irrigation water. Agronomy 2020, 10, 722. [Google Scholar] [CrossRef]
- Zhao, D.; Li, Y.R. Climate change and sugarcane production: Potential impact and mitigation strategies. Int. J. Agron. 2015, 2015, 547386. [Google Scholar] [CrossRef]
- Joseph, S.D.; Camps-Arbestain, M.; Lin, Y.; Munroe, P.; Chia, C.H.; Hook, J.; Van Zwieten, L.; Kimber, S.; Cowie, A.; Singh, B.P.; et al. An investigation into the reactions of biochar in soil. Aust. J. Soil. Res. 2010, 48, 501–515. [Google Scholar] [CrossRef]
- Tomczyk, A.; Sokolowska, Z.; Boguta, P. Biochar physicochemical properties: Pyrolysis temperature and feedstock kind effects. Rev. Environ. Sci. Biotechnol. 2020, 19, 191–215. [Google Scholar] [CrossRef]
- Ippolito, J.A.; Cui, L.; Kammann, C.; Wrage-Mönnig, N.; Estavillo, J.M.; Fuertes-Mendizabal, T.; Cayuela, M.L.; Sigua, G.; Novak, J.; Spokas, K.; et al. Feedstock choice, pyrolysis temperature and type influence biochar characteristics: A comprehensive meta-data analysis review. Biochar 2020, 2, 421–438. [Google Scholar] [CrossRef]
- Qiu, M.; Sun, K.; Jin, J.; Gao, B.; Yan, Y.; Han, L.; Wu, F.; Xing, B. Properties of the plant-and manure-derived biochars and their sorption of dibutyl phthalate and phenanthrene. Sci. Rep. 2014, 4, 5295. [Google Scholar] [CrossRef]
- Awan, S.; Ippolito, J.A.; Ullman, J.L.; Ansari, K.; Cui, L.; Siyal, A.A. Biochars reduce irrigation water sodium adsorption ratio. Biochar 2021, 3, 77–87. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Andersen, M.N.; Liu, F. Residual effects of biochar on improving growth, physiology and yield of wheat under salt stress. Agric. Water Manag. 2015, 158, 61–68. [Google Scholar] [CrossRef]
- Chintala, R.; Mollinedo, J.; Schumacher, T.E.; Malo, D.D.; Julson, J.L. Effect of biochar on chemical properties of acidic soil. Arch. Agron. Soil Sci. 2014, 60, 393–404. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Li, G.; Andersen, M.N.; Liu, F. Biochar enhances yield and quality of tomato under reduced irrigation. Agric. Water Manag. 2014, 138, 37–44. [Google Scholar] [CrossRef]
- Elshaikh, N.A.; Zhipeng, L.; Dongli, S.; Timm, L.C. Increasing the okra salt threshold value with biochar amendments. J. Plant Interact. 2017, 3, 51–63. [Google Scholar] [CrossRef]
- Sappor, D.K.; Osei, B.A.; Ahmed, M.R. Reclaiming sodium affected soil: The potential of organic amendments. Int. J. Plant Soil Sci. 2017, 16, 1–11. [Google Scholar] [CrossRef]
- Usman, A.R.A.; AL-Wabel, M.I.; OK, Y.S.; Al-Harbi, A.; Wahb-Allah, M.; El-Naggar, A.H.; Ahmad, M.; Al-Faraj, A.; Al-Omran, A. Conocarpus biochar induces changes in soil nutrient availability and tomato growth under saline irrigation. Pedosphere 2016, 26, 27–38. [Google Scholar] [CrossRef]
- Lashari, M.S.; Liu, Y.M.; Li, L.Q.; Pan, W.N.; Fu, J.Y.; Pan, G.X.; Zheng, J.F.; Zheng, J.W.; Zhang, X.H.; Yu, X.Y. Effects of amendment of biochar–manure compost in conjunction with pyroligneous solution on soil quality and wheat yield of a salt-stressed cropland from central China Great Plain. Field Crops Res. 2013, 144, 113–118. [Google Scholar] [CrossRef]
- VSSA. Annual Report of Sugar Production of Season 2020–2021 and Production Plan for Season 2021–2022; Vietnam Sugarcane and Sugar Association: Ho Chi Minh City, Vietnam, 2021. [Google Scholar]
- MARD. Reviewing and Adjusting the Sugarcane Development Plan to 2020, with a Vision to 2030; The Final Report of the Ministerial Project; Ministry of Agriculture and Rural Development of Vietnam: Hanoi, Vietnam, 2016; p. 305.
- Van Tan, L.; Thanh, T. The effects of salinity on changes in characteristics of soils collected in a saline region of the Mekong Delta, Vietnam. Open Chem. 2021, 19, 471–480. [Google Scholar] [CrossRef]
- Chen, Y.; Shinogi, Y.; Taira, M. Influence of biochar use on sugarcane growth, soil parameters, and groundwater quality. Aust. J. Soil Res. 2010, 48, 526–530. [Google Scholar] [CrossRef]
- Liao, F.; Yang, L.; Li, Q.; Xue, J.; Li, Y.; Huang, D.; Yang, L. Effect of biochar on growth, photosynthetic characteristics and nutrient distribution in sugarcane. Sugar Tech. 2019, 21, 289–295. [Google Scholar] [CrossRef]
- Rajapaksha, A.U.; Ok, Y.S.; El-Naggar, A.; Kim, H.; Song, F.; Kang, S.; Tsang, Y.F. Dissolved organic matter characterization of biochars produced from different feedstock materials. J. Environ. Manag. 2019, 233, 393–399. [Google Scholar] [CrossRef]
- Burdett, A.N. A non-destructive method for measuring the volume of intact plant parts. Can. J. For. Res. 1979, 9, 120–122. [Google Scholar] [CrossRef]
- Hoang, T.L.H.; Jang, D.C.; Nguyen, Q.T.; Na, W.H.; Kim, I.S.; Vu, N.T. Biochar-improved growth and physiology of Ehretia asperula under water-deficit condition. Appl. Sci. 2021, 11, 10685. [Google Scholar] [CrossRef]
- Slavík, B. Relationship between the osmotic potential of cell sap and the water saturation deficit during the wilting of leaf tissue. Biol. Plant. 1963, 5, 258–264. [Google Scholar] [CrossRef]
- Pitman, M.G.; Lauchi, A. Global impact of salinity and agricultural ecosystems. In Salinity: Environment-Plants–Molecules; Läuchli, A., Lüttge, U., Eds.; Springer: Amsterdam, The Netherlands, 2002; pp. 3–20. [Google Scholar]
- Ghoulam, C.; Foursy, A.; Fares, K. Effects of salt stress on growth, inorganic ions and proline accumulation in relation to osmotic adjustment in five sugar beet cultivars. Environ. Exp. Bot. 2002, 47, 39–50. [Google Scholar] [CrossRef]
- Niu, X.; Bressan, R.A.; Hasegwa, P.M.; Pardo, J.M. Ion homeostasis in NaCl stress environments. Plant Physiol. 1995, 109, 735–742. [Google Scholar] [CrossRef]
- Jimenez, M.S.; Gonzalez-Rodriguez, A.M.; Morales, D.; Cid, M.C.; Socorro, A.R.; Caballero, M. Evaluation of chlorophyll fluorescence as a tool for salt stress detection in roses. Photosynthetica 1997, 33, 291–301. [Google Scholar] [CrossRef]
- Shin, Y.K.; Bhandari, S.R.; Lee, J.G. Monitoring of salinity, temperature, and drought stress in grafted watermelon seedlings using chlorophyll fluorescence. Front. Plant Sci. 2021, 12, 2913. [Google Scholar] [CrossRef]
- Seemann, J.R.; Critchley, C. Effects of salt stress on the growth, ion content, stomatal behaviour and photosynthetic capacity of a salt-sensitive species, Phaseolus vulgaris L. Planta 1985, 164, 151–162. [Google Scholar] [CrossRef]
- Dubey, R.S. Photosynthesis in plants under stressful conditions. In Handbook of Photosynthesis; Pessarakli, M., Ed.; Marcel Dekker: New York, NY, USA, 1997; pp. 859–875. [Google Scholar]
- Jamil, M.; Rehman, S.; Lee, K.J.; Kim, J.M.; Kim, H.S.; Rha, E.S. Salinity reduced growth PS II photochemistry and chlorophyll content in radish. Sci. Agric. 2007, 64, 1–10. [Google Scholar] [CrossRef]
- Ganivea, R.A.; Allahverdiyev, S.R.; Guseinova, N.B.; Kavakli, H.I.; Nafisi, S. Effect of salt stress and synthetic hormone polystimuline K on the photosynthetic activity of cotton (Gossypium hirsutum). Turk. J. Bot. 1998, 22, 217–221. [Google Scholar]
- Reddy, M.P.; Vora, A.B. Changes in pigment composition, hill reaction activity and saccharides metabolism in bajra (Pennisetum typhoides S&H) leaves under NaCl salinity. Photosynthetica 1986, 20, 50–55. [Google Scholar]
- Bonanomi, G.; Ippolito, F.; Cesarano, G.; Nanni, B.; Lombardi, N.; Rita, A.; Saracino, A.; Scala, F. Biochar as plant growth promoter: Better off alone or mixed with organic amendments? Front. Plant Sci. 2017, 8, 1570. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liao, F.; Huang, M.; Yang, L.T.; Li, Y.R. Biochar improves sugarcane seedling root and soil properties under a pot experiment. Sugar Tech. 2015, 17, 36–40. [Google Scholar] [CrossRef]
- Tafti, N.; Wang, J.; Gaston, L.; Park, J.H.; Wang, M.; Pensky, S. Agronomic and environmental performance of biochar amendment in alluvial soils under subtropical sugarcane production. Agrosyst. Geosci. Environ. 2021, 4, e20209. [Google Scholar] [CrossRef]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef]
- Bui, K.T.; Naruse, T.; Yoshida, H.; Toda, Y.; Omori, Y.; Tsuda, M.; Kaga, A.; Yamasaki, Y.; Tsujimoto, H.; Ichihashi, Y.; et al. Effects of irrigation on root growth and development of soybean: A 3-year sandy field experiment. Front. Plant Sci. 2022, 13, 5012. [Google Scholar] [CrossRef]
- Abiven, S.; Hund, A.; Martinsen, V.; Cornelissen, G. Biochar amendment increases maize root surface areas and branching: A shovelomics study in Zambia. Plant Soil 2015, 395, 45–55. [Google Scholar] [CrossRef]
- Liu, B.; Li, H.; Li, H.; Zhang, A.; Rengel, Z. Long-term biochar application promotes rice productivity by regulating root dynamic development and reducing nitrogen leaching. Glob. Chang. Biol. Bioenergy 2021, 13, 257–268. [Google Scholar] [CrossRef]
- Feng, L.; Xu, W.; Tang, G.; Gu, M.; Geng, Z. Biochar induced improvement in root system architecture enhances nutrient assimilation by cotton plant seedlings. BMC Plant Biol. 2021, 21, 269. [Google Scholar] [CrossRef]
- Chang, Y.; Rossi, L.; Zotarelli, L.; Gao, B.; Shahid, M.A.; Sarkhosh, A. Biochar improves soil physical characteristics and strengthens root architecture in Muscadine grape (Vitis rotundifolia L.). Chem. Biol. Technol. Agric. 2021, 8, 7. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Solomon, D.; Kinyangi, J.; Grossman, J.; O’Neill, B.; Skjemstad, J.O.; Thies, J.; Luizão, F.J.; Petersen, J.; et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006, 70, 1719–1730. [Google Scholar] [CrossRef]
- Wang, S.J.; Zheng, J.L.; Wang, Y.J.; Yang, Q.F.; Chen, T.T.; Chen, Y.L.; Chi, D.C.; Xia, G.M.; Siddique, K.H.M.; Wang, T.L. Photosynthesis, chlorophyll fluorescence, and yield of peanut in response to biochar application. Front. Plant Sci. 2021, 12, 650432. [Google Scholar] [CrossRef]
| Parameters | Values |
|---|---|
| Total N (%) | 0.09 |
| Total P (%) | 0.18 |
| Total K (%) | 1.33 |
| Exchangeable N (mg/100 g) | 4.25 |
| Exchangeable P (mg/100 g) | 50.05 |
| Exchangeable K (mg/100 g) | 11.75 |
| Organic matter (%) | 1.65 |
| pH | 6.35 |
| Salinity Treatment | Biochar Application Rates (tons ha−1) | Final Salinity Treatment Day | 4 Weeks after the Final Salinity Treatment Day | ||||
|---|---|---|---|---|---|---|---|
| Stem (g plant−1) | Leaves (g plant−1) | Root (g plant−1) | Stem (g plant−1) | Leaves (g plant−1) | Root (g plant−1) | ||
| Non-salinity | 0 | 3.76 b Z | 5.39 c | 1.60 b | 6.14 b | 7.93 b | 2.20 c |
| 5 | 3.86 b | 6.36 b | 1.75 b | 7.82 a | 8.41 a | 2.70 b | |
| 10 | 4.51 a | 7.53 a | 2.42 a | 8.43 a | 8.99 a | 2.98 a | |
| Salinity | 0 | 3.16 c | 4.74 c | 1.41 c | 4.38 c | 6.66 c | 1.64 d |
| 5 | 3.76 b | 6.21 b | 1.68 b | 5.35 bc | 7.35 bc | 2.17 c | |
| 10 | 4.26 a | 7.37 a | 2.37 a | 6.23 b | 7.47 b | 2.27 c | |
| CV% Y | 5.4 | 6.5 | 6.1 | 8.4 | 5.4 | 3.0 | |
| LSD X 0.05 T W B V | 0.38 | 0.75 | 0.21 | 0.97 | 0.76 | 0.13 | |
| Average of treating condition | Non-salinity | 4.04 A U | 6.43 A | 1.92 A | 7.46 A | 8.44 A | 2.63 A |
| Salinity | 3.73 B | 6.11 A | 1.82 A | 5.32 B | 7.16 B | 2.03 B | |
| LSD 0.05 T | 0.22 | 0.43 | 0.12 | 0.56 | 0.44 | 0.52 | |
| Average of biochar rates | 0 | 3.46 C | 5.07 C | 1.51 C | 5.26 C | 7.29 B | 1.92 B |
| 5 | 3.81 B | 6.29 B | 1.72 B | 6.58 B | 7.88 A | 2.44 A | |
| 10 | 4.39 A | 7.45 A | 2.40 A | 7.33 A | 8.23 A | 2.63 A | |
| LSD 0.05 B | 0.26 | 0.53 | 0.15 | 0.69 | 0.54 | 0.69 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, N.-T.; Bui, T.-K.; Vu, T.-T.-H.; Nguyen, T.-H.; Le, T.-T.-C.; Tran, A.-T.; Vu, N.-L.; Tran, V.-Q.; Tong, V.-G.; Nguyen, X.-T.; et al. Biochar Improved Sugarcane Growth and Physiology under Salinity Stress. Appl. Sci. 2023, 13, 7708. https://doi.org/10.3390/app13137708
Vu N-T, Bui T-K, Vu T-T-H, Nguyen T-H, Le T-T-C, Tran A-T, Vu N-L, Tran V-Q, Tong V-G, Nguyen X-T, et al. Biochar Improved Sugarcane Growth and Physiology under Salinity Stress. Applied Sciences. 2023; 13(13):7708. https://doi.org/10.3390/app13137708
Chicago/Turabian StyleVu, Ngoc-Thang, The-Khuynh Bui, Thi-Thuy-Hang Vu, Thu-Huyen Nguyen, Thi-Tuyet-Cham Le, Anh-Tuan Tran, Ngoc-Lan Vu, Van-Quang Tran, Van-Giang Tong, Xuan-Truong Nguyen, and et al. 2023. "Biochar Improved Sugarcane Growth and Physiology under Salinity Stress" Applied Sciences 13, no. 13: 7708. https://doi.org/10.3390/app13137708
APA StyleVu, N.-T., Bui, T.-K., Vu, T.-T.-H., Nguyen, T.-H., Le, T.-T.-C., Tran, A.-T., Vu, N.-L., Tran, V.-Q., Tong, V.-G., Nguyen, X.-T., Yang, H.-C., Lee, S.-J., Kim, Y.-H., Bae, Y.-H., Hyeon, S.-J., Dinh, T.-H., & Jang, D.-C. (2023). Biochar Improved Sugarcane Growth and Physiology under Salinity Stress. Applied Sciences, 13(13), 7708. https://doi.org/10.3390/app13137708








