Abstract
The objective of this research was to isolate and identify lactic acid bacteria living in the digestive tract of the queen scallop Aequipecten opercularis and further test it as a probiotic culture on the growth of the bivalve during one month in captivity. Classic microbiological techniques (API and MALDI TOF) were used and four different strains of Lactiplantibacillus and one Lactococcus were identified and further analyzed, namely: Lactiplantibacillus plantarum I, Lactiplantibacillus plantarum 1, Lactiplantibacillus plantarum 2, Lactococcus lactis, and Lactiplantibacillus brevis. The isolated cultures were further tested for the highest antimicrobial activity towards the most common marine pathogens and survival at different temperatures and pH levels. The strain Lactiplantibacillus plantarum I had the best results, with the highest antimicrobial activity (77–85% pathogen growth inhibition) and the best safety standards showing no antibiotic resistance, and no ability to synthesize biogenic amine and degrade red blood cells. Considering all the abovementioned characteristics, the strain Lpb. plantarum I was further tested on the growth and health status of the bivalve Aequipecten opercularis during a month of cultivation in captivity. The results showed that the incorporation of Lpb. plantarum I led to a decrease in the quantity of test microorganisms in the bivalve and an increase in both the growth rate weight and growth rate length of the queen scallop A. opercularis. Furthermore, the scallops fed with the algae culture + Lpb. plantarum I gained significantly higher meat yield (33.15 ± 2.63%) compared with the control scallops fed only with the algae culture (29.66 ± 2.87%). To conclude, the results from this research indicate that including Lpb. plantarum I as a dietary supplement could enhance growth performance and serve as a feasible approach to reduce pathogen levels while cultivating A. opercularis in captivity.
1. Introduction
In many coastal areas across the world, aquaculture represents the most rapidly expanding food sector. As per the Scientific, Technical and Economic Committee for Fisheries, aquaculture generated a turnover of EUR 4.1 billion from 1.2 million tons of produce in Europe during 2018 [1]. Croatian production volume increased from 13.92 tons (2012) to 24.05 tons in 2020 (73% increase) and from EUR 78 million to EUR 181 million in 2023 (142% increase). Fisheries play a significant role in the Republic of Croatia’s agricultural product exports, contributing approximately 7% of the total export value. The Croatian fisheries sector faces several key challenges, including the need to enhance safety and working conditions, as well as improve shellfish quality and enhance catch monitoring [2].
In Croatia, the Mediterranean mussel (Mytilus galloprovincialis) and the European flat oyster (Ostrea edulis) are the predominant shellfish species, holding great importance for the country’s shellfish industry [3,4]. Scallops play a significant role in supplying the shellfish market and support both capture fisheries and aquaculture efforts [5]. The Mediterranean scallop Pecten jacobaeus (L.), also known as “capesanta” in the local region, constitutes a considerable share of the market; however, overfishing has become a concern in the northern Adriatic Sea [6]. In contrast, the queen scallop Aequipecten opercularis (L.), locally referred to as “canestrelle,” is typically caught as bycatch during commercial fishing. For this reason, A. opercularis represents a potentially interesting species for the development of shellfish aquaculture and for filling the market demand, by replacing the vulnerable and overfished species with alternative ones in the Mediterranean [5].
Since shellfish and other organisms that filter seawater are in direct contact with the marine environment, they are vulnerable to diseases [7,8]. High stock density, improper feeding, seawater pollution, and pesticides containing agricultural drainage water affect the risk of bacterial, fungal, and viral diseases in marine shellfish [9]. In this century, the shellfish aquaculture industry has experienced significant and rapid development. Newly synthesized chemical compounds and antibiotics have had a negative impact on the environment, subsequently producing mutagenic microbial strains that adversely affect shellfish health, thus antibiotics are now discouraged for the prevention of disease outbreaks [10]. Conversely, probiotics, which refer to live microorganisms that can impart health benefits to the host when administered in the appropriate dosage, have found extensive application in aquaculture [11]. The gastrointestinal tract represents the primary site for probiotic isolation and action. The advancements in targeted delivery systems have facilitated the precise localization of probiotics to their intended destinations, enabling them to exert favorable effects [12]. Multiple studies have been carried out on cultured animals, substantiating the influence of diverse probiotics on the abundance of beneficial bacteria and the reduction in pathogenic burden [13,14]. Employing probiotics has been demonstrated to be an effective approach in enhancing shellfish health and productivity [15,16]. Various genera of bacteria are used as probiotics in aquaculture, including Aeromonas, Alteromonas, Arthrobacter, Bifidobacterium, Bacillus, Clostridium, Enterococcus, Lactobacillus, Paenibacillus, Phaeobacter, Pseudoalteromonas, Pseudomonas, Rhodosporidium, Roseobacter, Streptomyces, and Vibrio [14]. Lactic acid bacteria (LAB) have become increasingly used as probiotics in aquaculture over the past decade [17,18,19]. Most LAB are generally recognized as safe (GRAS) by the US Food and Drug Administration (FDA), i.e., they are listed as having a Qualified Presumption of Safety (QPS) by the European Food Safety Authority (EFSA). LABs have been shown to exhibit strong antagonistic activity against both related and unrelated microorganisms, including marine pathogens from genera such as Staphylococcus, Vibrio, Pseudomonas, and Escherichia coli. This antagonistic effect is mainly due to a reduction in the pH, microbiological competition for nutrients, and the production of a range of inhibitory substances, such as lactic and acetic acids, acetoin, diacetyl, hydrogen peroxide, and bacteriocins [20]. Furthermore, live stocks can be exposed to multiple toxins from seawater, such as heavy metals, mycotoxins, and pesticides, among others, which may exert combined effects. In such instances, LAB play a crucial role through lactic acid fermentation. Probiotic LAB strains effectively eliminate toxins, while enhancing the nutritional value of the seafood [21].
Lactic acid bacteria (LAB), including some Lactobacillus spp. (Lactiplantibacillus plantarum, Lactiplantibacillus acidophilus, Lactiplantibacillus thermophillus, Lactobacillus delbrueckii subsp. bulgaricus, Lactiplantibacillus casei, etc.), are commonly used as probiotics in fish and shellfish aquaculture nutrition [15,18,19,22,23,24,25]. Recent studies conducted with Lpb. plantarum demonstrate encouraging outcomes in enhancing the survival [26,27,28,29], growth, and physiological condition of shellfish [30]. While the exact functional role of Lpb. plantarum in bivalves has not been established, numerous publications suggest their potential involvement in the nutrition or immunity of the host organisms [22,23,31].
The isolation of lactic acid bacteria from the marine environment holds the potential to significantly enhance their efficiency and adaptation. These highly beneficial strains are commonly derived from diverse sources, including water, soil, fish exudates, commercial products, and even the gastrointestinal tracts of the target animals [32]. For lactic acid bacteria to be effective, they must fulfill several fundamental criteria to ensure their survival in the specific habitat conditions where they are employed. Additionally, they need to exhibit desirable characteristics that are beneficial for aquaculture. The relationship between the host and the microbe is an aspect that requires more attention, as the properties of isolated strains are specific to their origin [33]. Therefore, the objectives of this study were: (1) to isolate and identify lactic acid bacteria from A. opercularis; (2) conduct supplemented feeding of queen scallop to investigate how the addition of lactic acid bacteria affects its health status; and (3) observe the influence of lactic acid bacteria on the growth, commercial quality, and biological indices of queen scallop.
2. Materials and Methods
2.1. Origin of Scallops
A sampling of live Aequipecten opercularis Linnaeus, 1758, commonly known as queen scallops, was conducted by trawling 2 nautical miles southeast of the Albanež shoal in the E2 fishing zone (44°43′58.49″ N, 13°56′48.94″ E), from a depth of 49 m, using a fishing vessel and bottom-trawling net. The scallops (n = 160) were put in a tank with fresh seawater and were immediately transported to a flow-through tank (volume 1900 L) in the experimental facility, on the premises of Aquarium Pula, for acclimatization. Water flow in the tank was kept at 200 L/h. The scallops were fed daily to satiation with a live algae culture mix of Tetraselmis sp. (Chlorophyta) (5 × 105 cells/mL), Nannochloropsis sp. (Eustigmatophyceae) (30 × 105 cells/mL), and Phaeodactylum sp. (Bacillariophyta) (12 × 105 cells/mL).
2.2. Isolation and Identification of Lactic Acid Bacteria
Lactic acid bacteria (LAB) were obtained from the digestive system (intestine) of live queen scallops. The intestine was aseptically removed, 10 g of sample was diluted in 90 mL of sterile water and homogenized. Then, 1 mL of homogenized sample was added to de Man, Rogosa and Sharpe (MRS) broth (Biolife, Milan, Italy) and incubated overnight at 37 °C. Turbidity-indicating tubes were, subsequently, inoculated onto MRS agar plates (Biolife). These plates were then incubated for 24–72 h at 37 °C in an aerobic environment. The plates displaying colonies with a white and creamy appearance (indicative of lactic acid bacteria) were singled out for further analysis. To ensure purity, individual colonies were purified through a series of transfers on MRS agar (Biolife). The isolated strains were identified using the analytical profile index (API) biochemical test API 50 CHL (bioMérieux, Marcy-l’Étoile, France). Identification was also conducted on a Microflex LT™ matrix-assisted laser desorption/ionization time-of-flight mass spectrometer (MALDI-TOF MS; Bruker Daltonik, Bremen, Germany) at the “Ruđer Bošković” Institute, Zagreb, following their internal procedure. To preserve the cultures, they were stored in a 50% glycerol solution (Gram-Mol, Zagreb, Croatia) at –80 °C. Prior to characterization, the strains were revived in MRS broth (Biolife) and incubated at 37 °C without agitation.
2.3. Characterisation of LAB Isolates
2.3.1. Preparation of Bacterial Cell Suspension
To characterize the LAB isolates, overnight bacterial cultures were centrifuged at 10,000 rpm/15 min. The cells were washed with sterile sodium chloride solution (0.5%), centrifuged again, and resuspended in sterile sodium chloride solution (0.5%). During this suspension, survival analyzes were performed at different ranges of temperature and pH, as well as testing of the antimicrobial activity. The initial concentration of the cells was ~1010 CFU/mL.
2.3.2. Survival of LAB at Different Temperatures
Isolated strains of LAB were inoculated into MRS broth and grown at different temperatures (4 °C, 13 °C, 28 °C, 37 °C, 45 °C) for 24 h. The microbial growth was determined for all temperatures using traditional plate counting on MRS agar (Biolife), and the results were expressed as the colony forming units per mL (CFU/mL) [34].
2.3.3. Survival of LAB at Different pH Values
The cell suspension was prepared, as described above, and inoculated into MRS broth with a pre-adjusted pH value (2, 4, 6), and the samples were incubated overnight at 37 °C. Bacterial growth was determined as described above.
2.3.4. Antimicrobial Activity
The antimicrobial effectiveness of the LAB isolates were assessed against several pathogens, including Escherichia coli ATCC 25922, Staphylococcus aureus ATCC 25923, Pseudomonas aeruginosa ATCC 27853, Listeria monocytogenes ATCC 23074, and Vibrio sp., using the turbidimetric techniques outlined by Frece et al. [35]. Test microorganisms were grown in nutrient broth (Biolife). The LAB supernatant (240 μL) was added to microtiter plate wells with 10 μL of the test microorganism, and the antibacterial activity of the LAB culture was monitored over 24 h at 37 °C using a microtiter plate reader (Sunrise, Tecan, Männedorf, Switzerland) and the absorbance was measured spectrophotometrically at 620 nm. Blanks were prepared without the addition of microorganisms. The degree of inhibition was calculated using the expression: degree of inhibition (%) = (1 − A1/A0)⋅100 [35]. The pathogens were part of the collection of microorganisms from the Laboratory for General Microbiology and Food Microbiology, Department of Biochemical Engineering, Faculty of Food Technology and Biotechnology, University of Zagreb, Croatia. To eliminate the effects of lactic acid, the cell-free supernatant of the LAB was adjusted to pH = 6.5 with sterile 1 M NaOH (Gram-Mol).
2.3.5. Safety Aspects
Testing the Sensitivity of Bacterial Isolates to Antibiotics
To test the sensitivity of the bacterial culture an overnight culture of 100 µL was added to a test tube containing 12 mL of MRS agar nutrient medium that had been dissolved and cooled to 50 °C. The inoculated medium was poured into Petri dishes, and filter discs containing 12 different antibiotics were placed on top of the solidified nutrient medium using sterile tweezers. The antibiotics tested were kanamycin, methicillin, neomycin, erythromycin, tobramycin, ampicillin, streptomycin, gentamicin, chloramphenicol, rifampicin, vancomycin, and tetracycline. The Petri dishes were then incubated overnight at 37 °C, after which the diameter of the inhibition zones, including the disc diameter, was measured [35].
Determination of Biogenic Amines Production
In order to check whether the bacterial isolates produced histidine decarboxylase or tyrosine decarboxylase, the strains were previously grown on MRS agar and then inoculated on DA-H and DA-T plates with histidine, i.e., tyrosine. The media used in the experiment were prepared following the protocol outlined by Joosten and Northolt [36]. Anaerobic incubation was performed for a period of 5 days at 37 °C. A positive result was purple, i.e., transparent zones around grown colonies.
Hemolytic Activity
The bacterial isolates were added to Columbia agar plates with 5% of defibrinated sheep blood (Biolife) and then incubated at 30 °C for a duration of 24–48 h. Transparent zones around grown colonies were a sign that blood hemolysis had occurred [37].
2.4. Preparation of Wet Biomass for Supplementation and Feeding
Lpb. plantarum I was cultivated overnight in MRS broth (Biolife) at a temperature of 37 °C. Subsequently, the bacterial cells were collected through aseptic centrifugation (6000 rpm/10 min), followed by two washes with physiological saline. Finally, the cells were resuspended in sterile physiological saline. The total viable count (TVC) was determined using the standard dilution method on MRS agar (Biolife), and the plates were then incubated at 37 °C for 24–48 h. A final count of 109 viable bacterial cells of Lpb. plantarum I per gram of wet biomass was obtained [34].
2.5. Cultivation of Scallops with the Addition of L. plantarum I
After acclimatization, two groups of 30 individuals were placed in two identical 190 L flow-through tanks (control and experimental). Both tanks were aerated with a single aeration stone and the shellfish were kept in the tanks for a duration of 30 days. The temperature, pH, and dissolved oxygen were measured daily with a HI98193 multiparameter probe (Hanna Instruments, Zagreb, Croatia). The shells were fed daily to satiation with a live algae culture mix of Tetraselmis sp., Nannochloropsis sp., and Phaeodactylum sp. In the experimental tank, the scallops were supplemented daily with Lpb. plantarum I, which was kept in circulation with the aeration stone. The amount of food provided in both the control and experimental tanks was modified to the same final density. Additionally, the concentration of the algae was adjusted depending on the number of scallops present in the tank or the supplementation of Lpb. plantarum I. During feeding (9AM–3 PM/6 h), the water flow was kept closed. To remove excess food and feces, the bottom of the aquarium was siphoned. Microbiological analysis was conducted on 10 scallops chosen at random, both at the beginning and at the end of the experiment. These scallops were placed on a damp cloth, over ice and transported in a refrigerated cooler to the Laboratory for General Microbiology and Food Microbiology at the Department of Biochemical Engineering, Faculty of Food Technology and Biotechnology, University of Zagreb, Croatia, for analysis.
2.6. Microbiological Analysis
The quantification of the total aerobic mesophilic bacteria was conducted by incubating the samples on nutrient agar (Merck, Darmstadt, Germany) at a temperature of 37 °C for 48 h, in accordance with the HRN ISO 4833 method, thus the number of Enterobacteriaceae were determined after incubation on violet red bile glucose (VRBG) agar (Biolife) at 37 °C for 48 h (ISO 21528-1:2017). The coagulase-positive staphylococci were counted after incubation on Baird-Parker (BP) agar (Biolife) at 37 °C for 24 h (ISO 6888-1:2021) and the sulphite-reducing clostridia using sulphite iron agar (Merck) at 37 °C for 72 h (ISO/CD 15213-2). Bacteria from the Vibrio genera were detected using TCBS Kobayashi agar (Biolife) at 37 °C for 48 h (ISO 21872-1:2017). The number of LAB was determined using MRS agar (Biolife) incubated at 37 °C for 48 h. The microbial growth was assessed through the conventional plate counting method, and the results are reported as colony-forming units per gram of shellfish meat (CFU/g).
2.7. Growth and Scallops Indices
For individual monitoring, every scallop was assigned a unique plastic number. Throughout the month-long experiment, precise measurements were taken for the initial and final weights of the queen scallops, recorded to the nearest 0.01 g, to determine their total weight. Additionally, the total shell length, height, and thickness of the scallops were measured using a stainless-steel caliper with an accuracy of 0.1 mm. The GR and SGR of the shell length and wet weight were determined for thirty queen scallops per tank, by calculating the change in length and weight over the experimental period using the following equations: growth rate length (GRl) = (Lf − Li)/t; growth rate weight (GRw) = (Wf − Wi)/t; specific growth rate length (SGRl) = ln (ln(Lf) − ln(Li))/t × 100; and specific growth rate weight SGRw = ln (ln(Wf) − ln(Wi))/t × 100, where Lf is the final average shell length, Wf is the wet weight at the end of the experiment, Li is the initial average shell length, Wi is the wet weight at the beginning of the experiment, ln denotes the natural logarithm, and t is the experimental time in days.
For the determination of the condition index (CI), meat yield (MY), gonadosomatic index (GSI), and muscle index (MI), twenty scallop specimens were randomly selected from each tank (control and experimental). The soft tissues were dissected, and the gonads and muscles were separated. The CI and MY were determined as [meat wet weight (g)/shell wet weight (g)] × 100 and [meat wet weight (g)/total wet weight (g)] × 100, respectively, following the methods of Okumus and Stirling [38] and Orban [39]. The GSI and MI were calculated as [gonad wet weight (g)/adductor weight (g)] × 100 and [muscle wet weight (g)/wet body weight (g)] × 100, respectively, following the methods of Okumus and Stirling [38] and Jenkins [40].
2.8. Statistical Analysis
Statistical analysis of all growth performance and indices data was conducted using Statistica software version 9.0 (StatSoft Inc., Tulsa, OK, USA). The normality of the growth length and weight data for each group was first examined using the Kolmogorov–Smirnov test and, then, validated by conducting the Levene test to check for homogeneity of variances. The Tukey HSD test was used to determine whether significant differences existed between the control and LAB-treated scallops at a 95% confidence limit. In addition, the commercial quality and somatic indices between the control and LAB-treated scallops were evaluated with a non-parametric Mann–Whitney test, given a sample size of n = 20. All data were presented as means ± standard deviation.
3. Results and Discussion
3.1. Identification and Characterization of Lactic Acid Bacteria
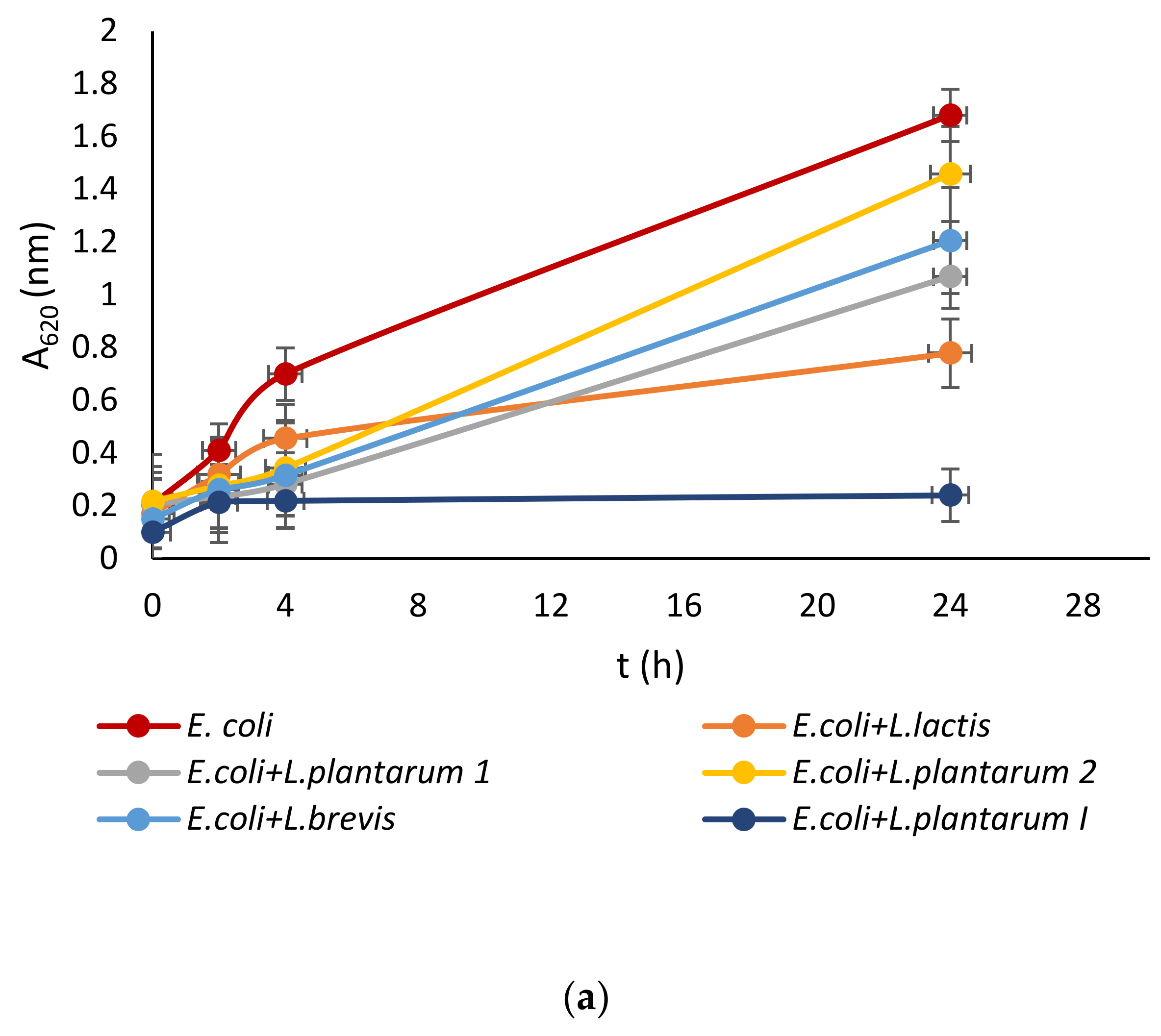
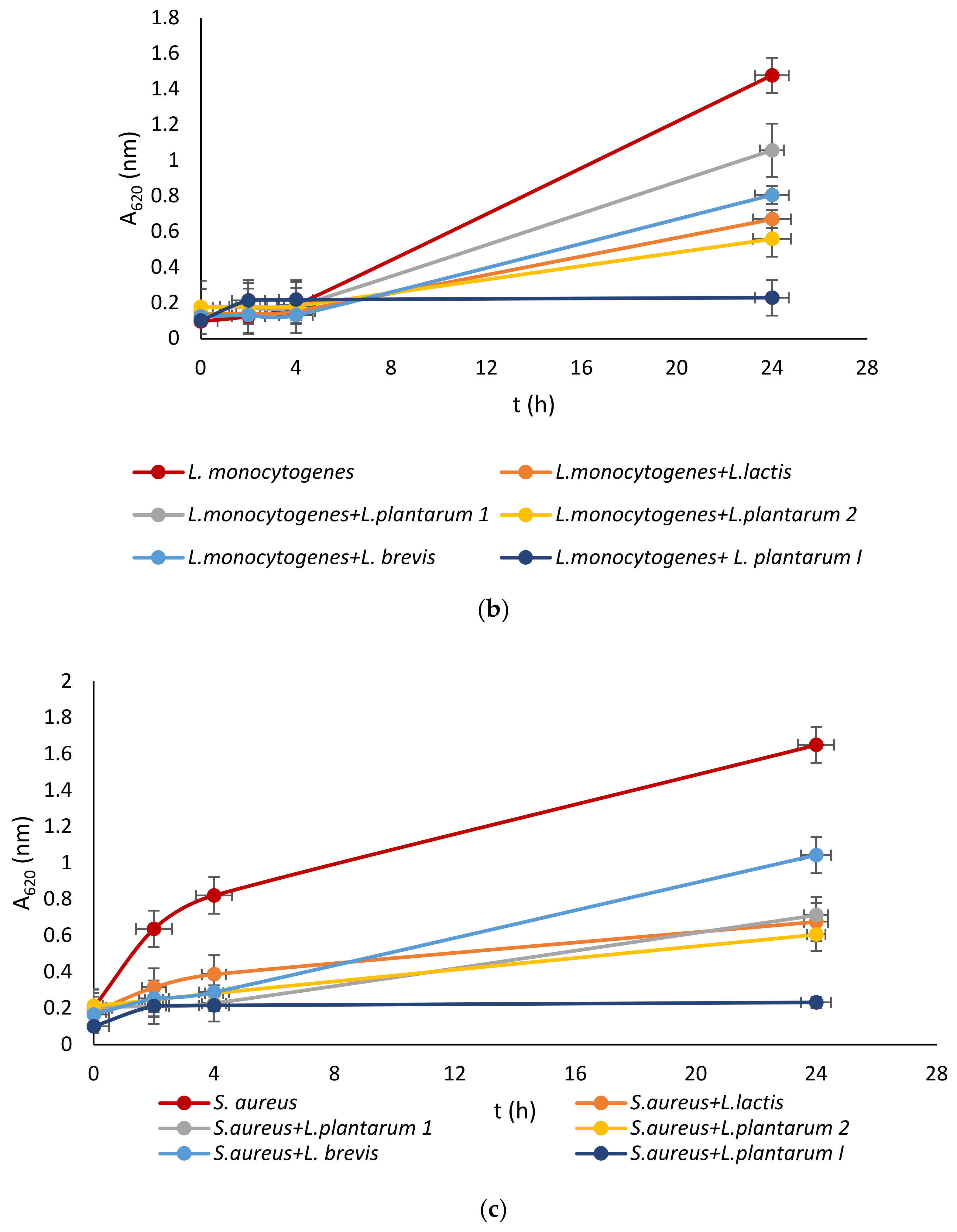
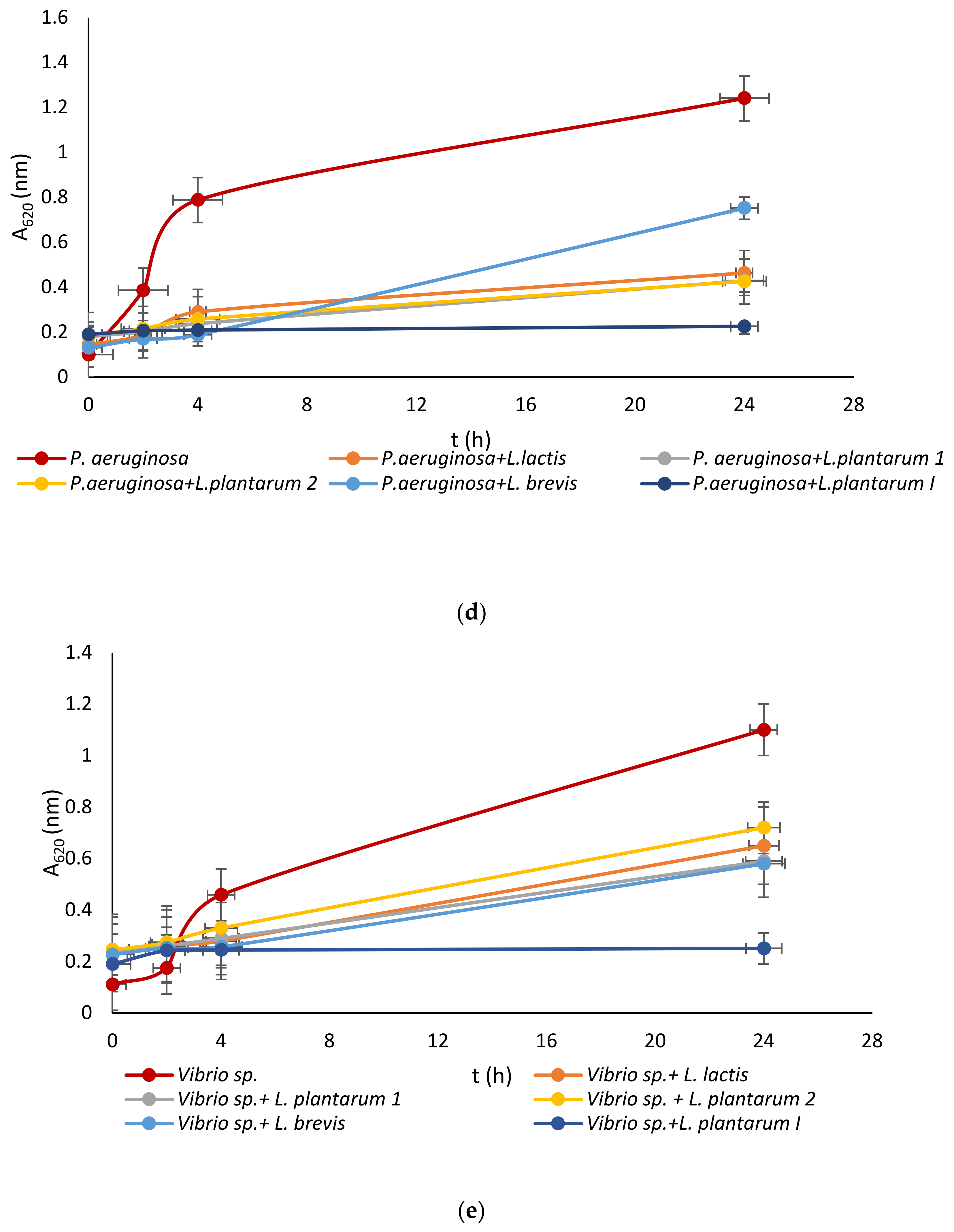
Lactic acid bacteria were isolated from the queen scallop (Aequipecten opercularis) digestive tract and the five isolated strains were identified as Lactiplantibacillus plantarum I, Lpb. plantarum 1, Lpb. plantarum 2, Lactococcus lactis, and Lactiplantibacillus brevis, with a high MALDI-TOF score in the range 2–3 indicating a very reliable identification. The strains were also previously tested with the API biochemical method and the results showed a high percentage of identification in the range of 95–99%. The strains were evaluated for their antimicrobial activity against common marine pathogens, and the results demonstrated effective inhibitory activity against all the tested microorganisms (Figure 1). The best antimicrobial activity was detected for the Lpb. plantarum I strain, which inhibited all pathogens in a range of 77–85%. The highest survival in the test microorganisms was detected after treatment with the Lpb. brevis supernatant and was 52–72%. Among all the test microorganisms, bacteria E. coli had the lowest percentage of inhibition with a mean value of 47%. These results are in agreement with previous research, where LAB isolated from marine shellfish showed good antimicrobial activity against marine pathogens [33,40]. For comparison, in the research by Reda et al. [41] when determining the antimicrobial activity of nine LAB isolates from the digestive tract of Nile tilapia (Oreochromis niloticus) against the most common fish pathogens, only one isolated strain, Lysinibacillus sp. 38HT, successfully inhibited the growth of all pathogens, while the most resistant pathogen proved to be S. aureus inhibited by only three LAB isolates.
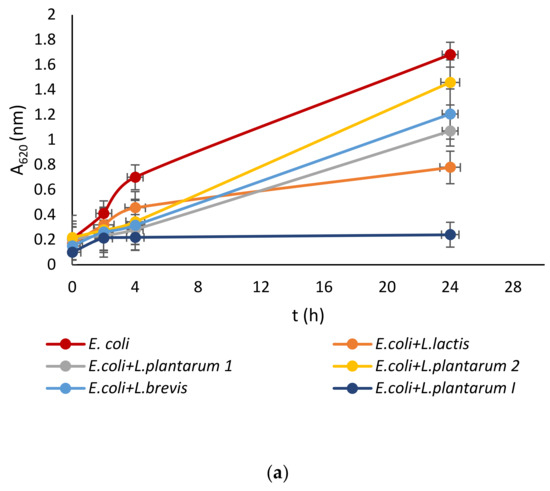
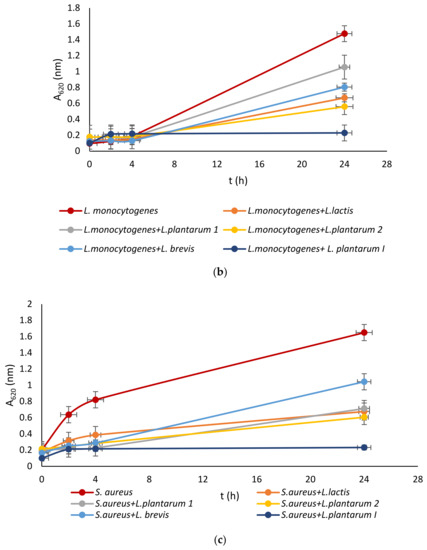
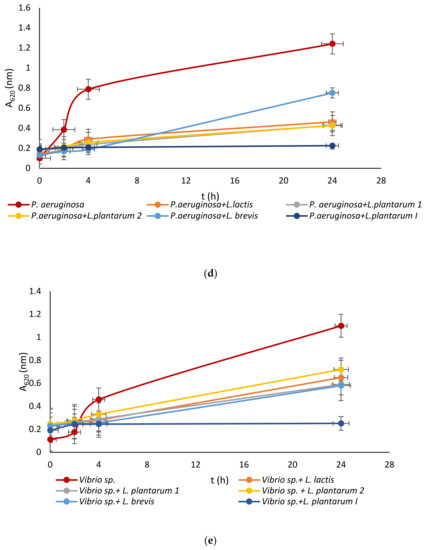
Figure 1.
Effect of LAB isolate supernatant on growth of: (a) E. coli, (b) L. monocytogenes, (c) S. aureus, (d) P. aeruginosa, and (e) Vibrio sp.
LAB not only exhibit an inhibitory effect through the production of organic acids, such as lactic acid, which leads to a decrease in pH, but they also generate various antimicrobial compounds. These compounds include hydrogen peroxide, CO2, diacetyl, acetaldehyde, D-isomers of amino acids, reuterin, and bacteriocins [42]. Since the supernatant of tested LAB was neutralized to avoid the inhibitory effect of acid, good activity toward pathogen growth had to be further examined.
Further testing of the five isolates included the determination of the optimal growth temperature and pH, as well as safety parameters. The optimal temperature for all five strains was 28 °C with a high number of viable cells ranging from 108–109 CFU/mL, which is a minimal reduction compared to the initial number of 1010 CFU/mL. The Lpb. Brevis, L. lactis, and Lpb. plantarum I showed good survival at lower temperatures (105–107 CFU/mL), as well as at a higher temperature of 45 °C with the number of viable cells ranging from 104–108 CFU/mL. The good survival rate of LAB at low and higher temperatures contributes to a much better industrial application of the strains. The optimal pH value for all strains was 6, which is in accordance with the results by von Wright and Axelson [43], who state that maximum bacterial growth is achieved at pH 5–6 with possible exceptions depending on the strain. Further, LAB isolated from marine animals are usually tolerant to acidic conditions, growing optimally at a neutral or slightly acidic pH [44].
As far as safety criteria are concerned, all isolates showed sensitivity to the tested antibiotics, as well as the inability to synthesize histidine and tyramine, and the growth test on blood agar showed that they do not have the ability to degrade red blood cells. It is of utmost importance that isolates with future animal or human use are safe for the host, and previous research into the safety aspects of marine LAB supports the results of this work [33,45,46].
3.2. Microbial Analysis of Shellfish with the Addition of Lpb. plantarum I
Since the strain Lpb. plantarum I showed the best characterization results it was used as a supplement during the feeding of the queen scallops. After one month of supplemented feeding, in a tank with added LAB, a decrease in the number of all the tested microorganisms was detected compared to the control sample. The highest reduction was observed in the number of Enterobacteriaceae that were present in the control tank at a concentration of 102 CFU/g, while with the addition of LAB this genera was no longer detected. Bacteria of the genus Vibrio were detected in a control sample (101 CFU/g) as expected, since this genus belongs to the natural microflora of marine organisms [20]. However, after one month of supplemented feeding with Lpb. plantarum I this genus was no longer detected.
The number of aerobic mesophilic bacteria remained the same both in the control and experimental tank and ranged from 102 to 103 CFU/g, which was expected as a consequence of the naturally present microflora. Considering that the shellfish were caught in colder months, this number is slightly lower compared to the concentration at higher sea temperatures.
3.3. Growth and Shellfish Indices with the Addition of Lpb. plantarum I
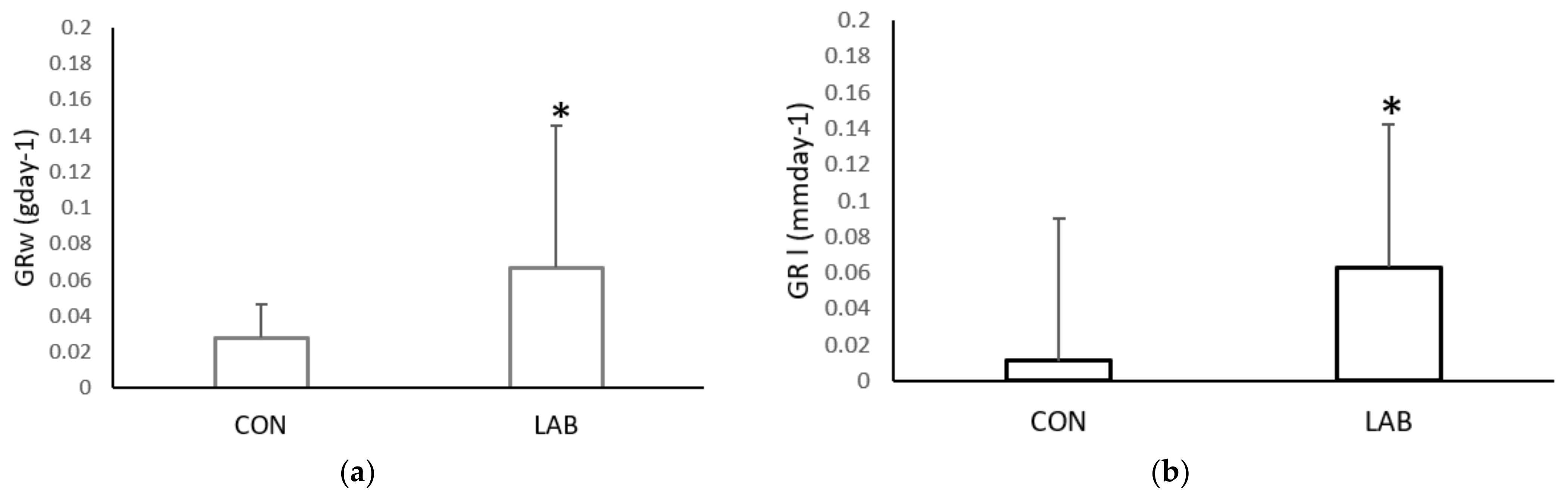
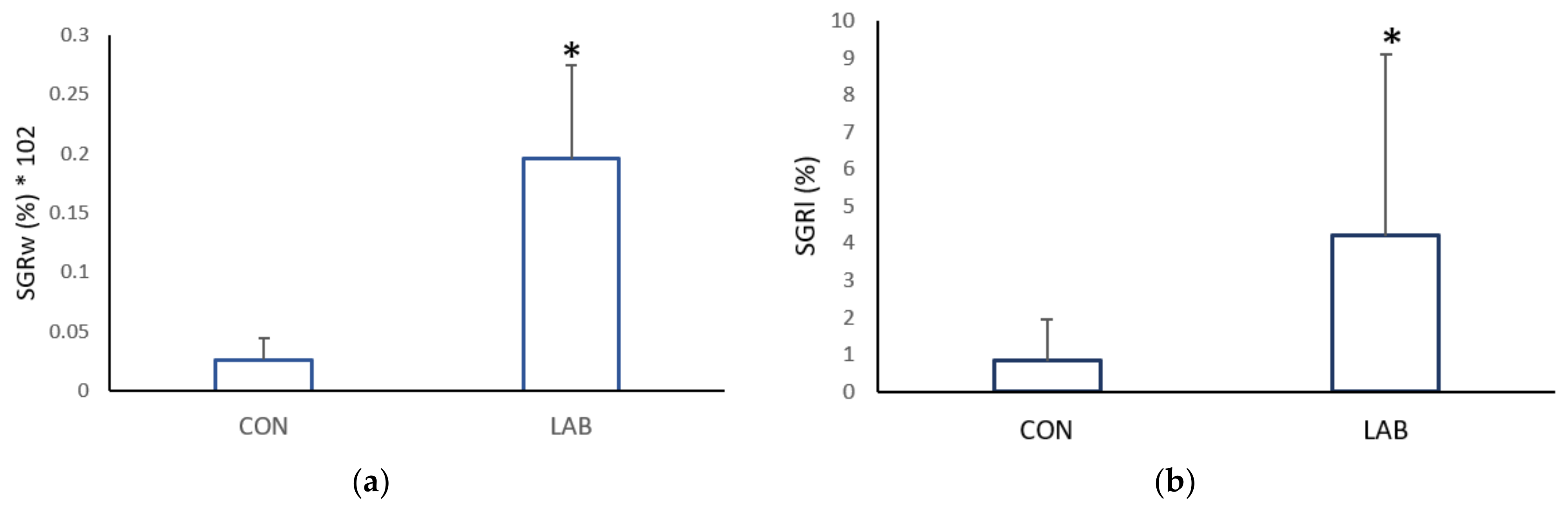
The growth rate in the weight and shell length of Aequipecten opercularis was significantly higher after adding Lpb. plantarum I (Figure 2). After one month, the scallops in the experimental tank fed with Lpb. plantarum I grew more in weight (0.07 ± 0.06 g day−1) and in length (0.08 ± 0.06 mm day−1) than the scallops in the control tank fed only with a live algae mix culture (GRw 0.02 ± 0.01%; GRl 0.01 ± 0.01%). Compared with the control scallops, the specific growth rate weight and specific growth rate length were lower (0.02 ± 0.001 mm day−1; 0.82 ± 1.12 g day−1) than in the scallops treated with Lpb. plantarum I (0.20 ± 0.006 mm day−1; 4.21 ± 4.90 g day−1) (Figure 3). Previous studies have reported positive effects of various LAB on the growth performance of both fish and shellfish. In a study conducted by Savin-Amador et al. [29], it was observed that Litopenaeus vannamei larvae fed with a diet supplemented with Lactiplantibacillus plantarum 69Cr, Lactiplantibacillus fermentum 101Cc, and Lactiplantibacillus casei, exhibited improved growth performance and survivability. Similarly, the inclusion of the probiotic Lactiplantibacillus acidophilus (FLa) in the diet of Siberian sturgeon (Acipenser baerii) resulted in enhanced growth indices, biochemical content, and blood composition [18]. Other studies have also reported higher growth performance in Pagrus major [23], S. aurata [21], and C. caprio [25], when fed diets fortified with LAB probiotics.
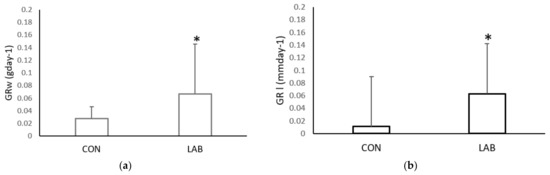
Figure 2.
Growth rate: (a) weight (GRw) and (b) length (GRl), of the control (CON) A. opercularis and those exposed to LAB isolates (LAB), over four weeks. * p < 0.001.
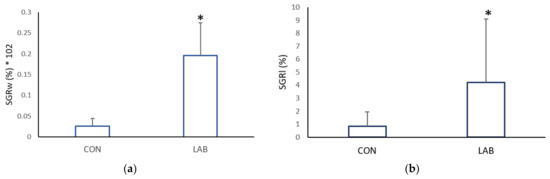
Figure 3.
Specific growth rate: (a) weight (GRw) and (b) length (GRl), of the control (CON) A. opercularis and those exposed to LAB isolates (LAB), over four weeks. * p < 0.001.
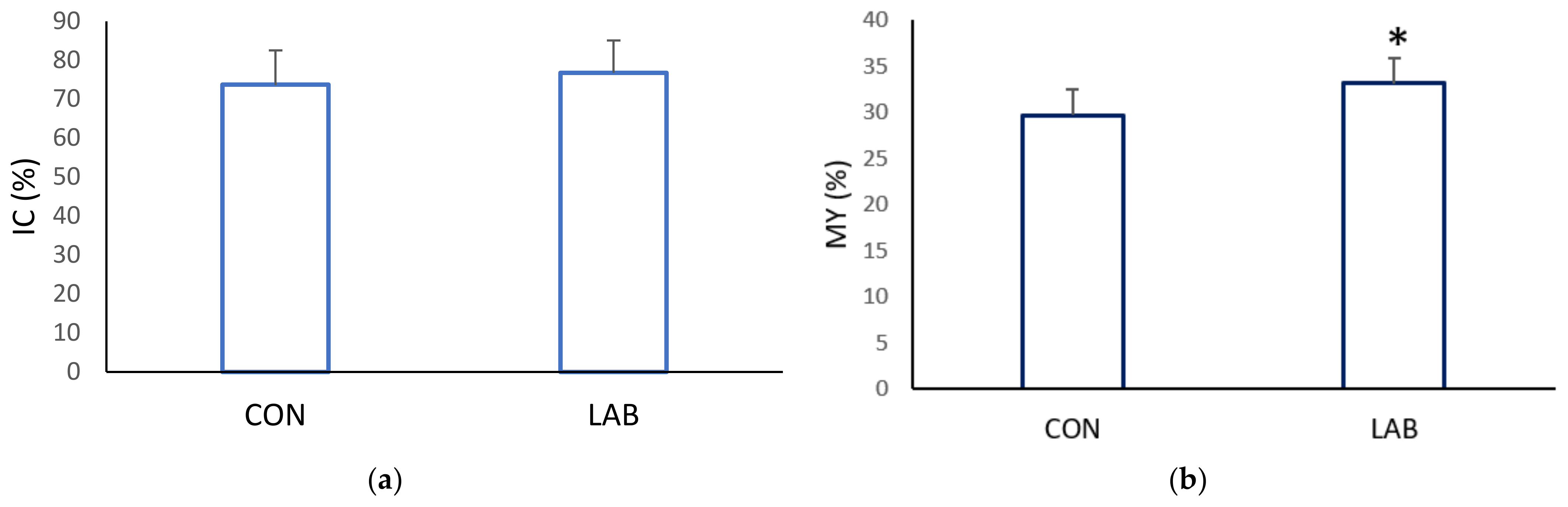
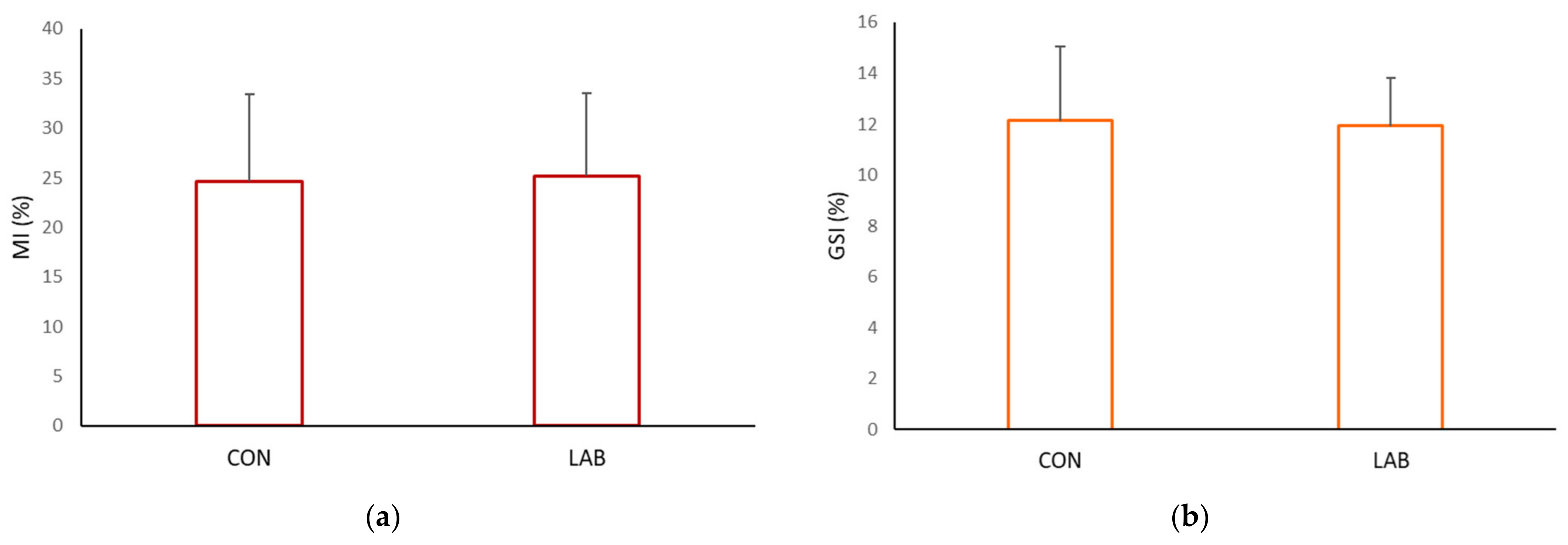
Data on the condition index from scallops in the control (73.71 ± 8.78%) and experimental tank (76.74 ± 8.34%) yielded similar results (Figure 4, p > 0.05). Scallops in a tank fed with Lpb. plantarum I gained, at the end of one month, a significantly higher meat yield (MY) (33.15 ± 2.63%) compared with the control tank (29.66 ± 2.87%). The muscle index and gonadosomatic index of the control and scallops fed additionally with Lpb. plantarum I showed no significant differences (Figure 5). The improved growth performance of shellfish fed with a diet containing Lpb. plantarum I can be attributed to the bacteria’s ability to stimulate digestive enzymes. This stimulation enhances feed digestibility, resulting in increased energy benefits that ultimately lead to a higher growth rate. Furthermore, the notable increase in indices observed in scallops treated with LAB can enhance digestion and improve the absorption of nutrients from the diet. As a result, this contributes to enhanced growth performance and commercial quality of the shellfish. According to studies, supplementing potential probiotic LAB has been found to increase the specific activities of the digestive enzymes in various marine species, such as lions-paw scallop [47], Catarina scallop (Argopecten ventricosus) [48], and white shrimp (Litopenaeus vannamei) [49]. It has been proposed that LAB supplementation improves gut maturation, which could explain the increased digestive enzyme activity in farmed shellfish fed with LAB [27]. Research by Van Doan et al. [50] has also confirmed the safety and effectiveness of Lactobacillus spp. as a probiotic in aquaculture, as it can improve nutrient assimilation in the host. In addition, treatment with Lactobacillus spp. isolated from lion’s paw scallop has been shown to help maintain the integrity of internal tissues and increase tissue weight [29], which may be reflected in the condition index and meat yield of the scallops in our study.
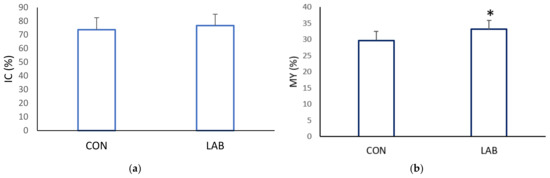
Figure 4.
Commercial quality: (a) condition index (CI) and (b) meat yield (MY), of the control (CON) A. opercularis and those exposed to LAB isolates (LAB), over four weeks. * p < 0.001.
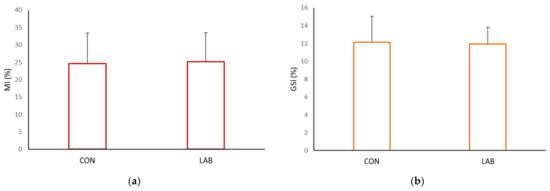
Figure 5.
Somatic indices: (a) muscle index (MI) and (b) gonadosomatic index (GSI), of the control (CON) A. opercularis and those exposed to LAB isolates (LAB), over four weeks.
4. Conclusions
To the best of our knowledge, this is the first study that isolated and identified LAB from the digestive tract of queen scallop A. opercularis, as well as further tested the best selected strain Lpb. plantarum I on the growth health status of this bivalve. Five different LAB strains were successfully isolated from the scallops’ digestive tract and identified with API and MALDI-TOF methods. Analysis of the strains indicated that Lpb. plantarum I exhibits the most potent antimicrobial activity. Additionally, this particular strain demonstrates remarkable resilience to adverse conditions, such as low pH and a broad range of temperatures, in addition to fulfilling the necessary safety requirements for its application.
Lpb. plantarum I also showed a good effect on the health status of A. opercularis during the one-month experiment in captivity. Our results suggest that the growth performance and commercial quality of the scallop A. opercularis can be stimulated by adding Lpb. plantarum I as food supplement in aquaculture during the one-month experiment in captivity. The findings presented in this study have significant value and can aid in gaining a better comprehension of the function of LAB in promoting shellfish health in both controlled and simulated conditions of climate change. As these changes are already occurring and inevitable, it is crucial to explore effective strategies for adapting to them. The use of protective LAB, particularly indigenous strains, could play a crucial role in controlling pathogens and promoting the growth of fish and shellfish culture and aquaculture in the future.
Author Contributions
Conceptualization, I.K., M.Š. and J.F.; data curation, D.K.; formal analysis, I.Č., I.K. and A.Ž.; investigation, K.M. and P.B.; methodology, I.Č., I.K. and A.Ž.; resources, A.Ž., Ž.J. and N.I.; validation, K.M. and N.I.; visualization, Ž.J. and D.K.; writing—original draft, I.Č., I.K. and A.Ž.; writing—review & editing, K.M., M.Š., P.B. and J.F. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the European Regional Development Fund [KK.05.1.1.02.0012] in the framework of the project “Integrated system of shellfish farming under climate change conditions”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this study are available from the corresponding author upon request (jfrece@pbf.hr).
Acknowledgments
The authors are grateful to Aquarium Pula for the use of their facilities.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Scientific, Technical and Economic Commitee for Fisheries (STECF). The EU Aquaculture Sector—Economic Report 2020 (STECF 21-02) EUR 28359 EN; Publications office of the European Union: Luxemburg, 2021; ISBN 978-92-76-36192-3.
- Ministry of Agriculture Directorate of Fisheries (MADF). Annual Report on Balance between Fishing Capacity and Fishing Opportunities for 2020, 2021 Zagreb Republic of Croatia. Available online: https://podaci.ribarstvo.hr/files/HR_Fleet-report-for-2020_FINAL_CLEAR.pdf (accessed on 16 March 2023).
- Hamer, D.P.; Kovačić, I.; Koščica, L.; Hamer, B. Physiological Indices of Maricultured Mussel Mytilus galloprovincialis Lamarck, 1819 in Istria, Croatia: Seasonal and Transplantation Effect. J. World Aquac. Soc. 2016, 47, 768–778. [Google Scholar] [CrossRef]
- Meulenberg, C.J.; Hawke, S.M.; Cavaion, I.; Kumer, P.; Lenarčić, B. Understanding interdisciplinarity through Adriatic maricultures and climate change adaptation. Vis. Sustain. 2022, 18, 1–26. [Google Scholar] [CrossRef]
- Kovačić, I.; Žunec, A.; Matešković, M.; Burić, P.; Iveša, N.; Štifanić, M.; Frece, J. Commercial Quality, Biological Indices and Biochemical Composition of Queen Scallop Aequipecten opercularis in Culture. Fishes 2023, 8, 48. [Google Scholar] [CrossRef]
- Nerlović, V. Exploitation of scallop Pecten jacobaeus (Linneaus, 1758) in the north western coastal region of Istria. In Proceedings of the 39th Croatian Symposium on Agriculture with International Participation, Zagreb, Croatia, 17–20 February 2004; Žimbrek, T., Ed.; Faculty of Agriculture, University of Zagreb: Zagreb, Croatia, 2004; pp. 606–608. [Google Scholar]
- Kovačić, I.; Pustijanac, E.; Ramšak, A.; Šebešćen, D.; Lipić, S. Variation of parasite and fungi infection between farmed and wild mussels (Mytilus galloprovincialis Lamarck, 1819) from the Adriatic Sea. J. Mar. Biol. Assoc. UK 2018, 98, 1871–1879. [Google Scholar] [CrossRef]
- Robinson, N.A.; Robledo, D.; Sveen, L.; Daniels, R.R.; Krasnov, A.; Coates, A.; Jin, Y.H.; Barrett, L.T.; Lillehammer, M.; Kettunen, A.H.; et al. Applying genetic technologies to combat infectious diseases in aquaculture. Rev. Aquac. 2022, 15, 491–535. [Google Scholar] [CrossRef]
- Mavraganis, T.; Constantina, C.; Kolygas, M.; Vidalis, K.; Nathanailides, C. Environmental issues of Aquaculture development. Egypt. J. Aquat. Biol. Fish. 2020, 24, 441–450. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- FAO; WHO (Eds.) Probiotics in Food: Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations: Rome, Italy; World Health Organization: Rome, Italy, 2006. [Google Scholar]
- Wang, G.; Chen, Y.; Xia, Y.; Song, X.; Ai, L. Characteristics of Probiotic Preparations and Their Applications. Foods 2022, 11, 2472. [Google Scholar] [CrossRef]
- Ranjha, M.M.A.N.; Shafique, B.; Batool, M.; Kowalczewski, P.; Shehzad, Q.; Usman, M.; Manzoor, M.F.; Zahra, S.M.; Yaqub, S.; Aadil, R.M. Nutritional and Health Potential of Probiotics: A Review. Appl. Sci. 2021, 11, 11204. [Google Scholar] [CrossRef]
- Alvanou, M.V.; Feidantsis, K.; Staikou, A.; Apostolidis, A.P.; Michaelidis, B.; Giantsis, I.A. Probiotics, Prebiotics, and Synbiotics Utilization in Crayfish Aquaculture and Factors Affecting Gut Microbiota. Microorganisms 2023, 11, 1232. [Google Scholar] [CrossRef]
- Jahangiri, L.; Esteban, M. Administration of Probiotics in the Water in Finfish Aquaculture Systems: A Review. Fishes 2018, 3, 33. [Google Scholar] [CrossRef]
- Pushparaj, K.; Bhotla, H.K.; Pappuswamy, M.; Issara, U.; Balasubramanian, B.; Al-Dhabi, N.A.; Arasu, M.V.; Meyyazhagan, A. Perspectives and implications of probiotics as beneficial mediators in aquaculture industry. In Aquaculture Science and Engineering; Balasubramanian, B., Liu, W.C., Sattanathan, G., Eds.; Springer: Singapore, 2022; pp. 79–97. [Google Scholar]
- Ringø, E. Probiotics in shellfish aquaculture. Aquac. Fish. 2020, 5, 1–27. [Google Scholar] [CrossRef]
- Mocanu, E.E.; Savin, V.; Popa, M.D.; Dima, F.M. The Effect of Probiotics on Growth Performance, Haematological and Biochemical Profiles in Siberian Sturgeon (Acipenser baerii Brandt, 1869). Fishes 2022, 7, 239. [Google Scholar] [CrossRef]
- Chizhayeva, A.; Amangeldi, A.; Oleinikova, Y.; Alybaeva, A.; Sadanov, A. Lactic acid bacteria as probiotics in sustainable development of aquaculture. Aquat. Living Resour. 2022, 35, 10. [Google Scholar] [CrossRef]
- Calo-Mata, P.; Arlindo, S.; Boehme, K.; de Miguel, T.; Pascoal, A.; Barros-Velazquez, J. Current Applications and Future Trends of Lactic Acid Bacteria and their Bacteriocins for the Biopreservation of Aquatic Food Products. Food Bioprocess Technol. 2007, 1, 43–63. [Google Scholar] [CrossRef]
- Petrova, P.; Arsov, A.; Tsvetanova, F.; Parvanova-Mancheva, T.; Vasileva, E.; Tsigoriyna, L.; Petrov, K. The Complex Role of Lactic Acid Bacteria in Food Detoxification. Nutrients 2022, 14, 2038. [Google Scholar] [CrossRef] [PubMed]
- Suzer, C.; Çoban, D.; Kamaci, H.O.; Saka, Ş.; Firat, K.; Otgucuoğlu, Ö.; Küçüksari, H. Lactobacillus spp. bacteria as probiotics in gilthead sea bream (Sparus aurata, L.) larvae: Effects on growth performance and digestive enzyme activities. Aquaculture 2008, 280, 140–145. [Google Scholar] [CrossRef]
- Dawood, M.A.; Koshio, S.; Ishikawa, M.; Yokoyama, S.; El Basuini, M.F.; Hossain, S.; Nhu, T.H.; Dossou, S.; Moss, A.S. Effects of dietary supplementation of Lactobacillus rhamnosus or/and Lactococcus lactis on the growth, gut microbiota and immune responses of red sea bream, Pagrus major. Fish Shellfish. Immunol. 2016, 49, 275–285. [Google Scholar] [CrossRef]
- Guimarães, M.C.; Cerezo, I.M.; Fernandez-Alarcon, M.F.; Natori, M.M.; Sato, L.Y.; Kato, C.A.T.; Moriñigo, M.A.; Tapia-Paniagua, S.; Dias, D.D.C.; Ishikawa, C.M.; et al. Oral Administration of Probiotics (Bacillus subtilis and Lactobacillus plantarum) in Nile Tilapia (Oreochromis niloticus) Vaccinated and Challenged with Streptococcus agalactiae. Fishes 2022, 7, 211. [Google Scholar] [CrossRef]
- Veisi, R.S.; Taghdir, M.; Abbaszadeh, S.; Hedayati, A. Dietary Effects of Probiotic Lactobacillus casei on Some Immunity Indices of Common Carp (Cyprinus carpio) Exposed to Cadmium. Biol. Trace Element Res. 2022, 201, 959–967. [Google Scholar] [CrossRef]
- Lim, H.J.; Kapareiko, D.; Schott, E.J.; Hanif, A.; Wikfors, G.H. Isolation and Evaluation of New Probiotic Bacteria for use in Shellfish Hatcheries: I. Isolation and Screening for Bioactivity. J. Shellfish. Res. 2011, 30, 609–615. [Google Scholar] [CrossRef]
- Kapareiko, D.; Lim, H.J.; Schott, E.J.; Hanif, A.; Wikfors, G.H. Isolation and Evaluation of New Probiotic Bacteria for use in Shellfish Hatcheries: II. Effects of a Vibrio sp. Probiotic Candidate Upon Survival of Oyster Larvae (Crassostrea virginica) in Pilot-Scale Trials. J. Shellfish. Res. 2011, 30, 617–625. [Google Scholar] [CrossRef]
- Zheng, X.; Duan, Y.; Dong, H.; Zhang, J. Effects of Dietary Lactobacillus plantarum on Growth Performance, Digestive Enzymes and Gut Morphology of Litopenaeus vannamei. Probiotics Antimicrob. Proteins 2017, 10, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Savin-Amador, M.; Rojas-Contreras, M.; Arce-Amézquita, P.M.; Rangel-Dávalos, C.; Vázquez-Juárez, R. Lactobacillus strains isolated from oysters improve the production of Crassostrea gigas larvae. Lat. Am. J. Aquat. Res. 2021, 49, 551–564. [Google Scholar] [CrossRef]
- Abasolo-Pacheco, F.; Saucedo, P.E.; Mazón-Suástegui, J.M.; Tovar-Ramírez, D.; Araya, R.; Ramírez-Orozco, J.M.; Campa-Córdova, Á.I. Isolation and use of beneficial microbiota from the digestive tract of lions-paw scallop Nodipecten subnodosus and winged pearl oyster Pteria sterna in oyster aquaculture. Aquac. Res. 2015, 47, 3042–3051. [Google Scholar] [CrossRef]
- Paillard, C.; Gueguen, Y.; Wegner, K.M.; Bass, D.; Pallavicini, A.; Vezzulli, L.; Arzul, I. Recent advances in bivalve-microbiota interactions for disease prevention in aquaculture. Curr. Opin. Biotechnol. 2021, 73, 225–232. [Google Scholar] [CrossRef]
- Ganesan, A.M.; Alfaro, A.C.; Brooks, J.D.; Higgins, C.M. The role of bacterial biofilms and exudates on the settlement of mussel (Perna canaliculus) larvae. Aquaculture 2010, 306, 388–392. [Google Scholar] [CrossRef]
- Kesarcodi-Watson, A.; Kaspar, H.; Lategan, M.J.; Gibson, L. Performance of single and multi-strain probiotics during hatchery production of Greenshell™ mussel larvae, Perna canaliculus. Aquaculture 2012, 354–355, 56–63. [Google Scholar] [CrossRef]
- Čanak, I.; Markov, K.; Melvan, E.; Starčević, A.; Živković, M.; Zadravec, M.; Pleadin, J.; Jakopović, Ž.; Kostelac, D.; Frece, J. Isolation and Characterisation of L. plantarum O1 Producer of Plantaricin as Potential Starter Culture for the Biopreservation of Aquatic Food Products. Food Technol. Biotechnol. 2018, 56, 581–589. [Google Scholar] [CrossRef]
- Frece, J.; Kos, B.; Svetec, I.K.; Zgaga, Z.; Beganović, J.; Leboš, A.; Šušković, J. Synbiotic effect of Lactobacillus helveticus M92 and prebiotics on the intestinal microflora and immune system of mice. J. Dairy Res. 2009, 76, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Joosten, H.M.L.J.; Northolt, M.D. Detection, Growth, and Amine-Producing Capacity of Lactobacilli in Cheese. Appl. Environ. Microbiol. 1989, 55, 2356–2359. [Google Scholar] [CrossRef] [PubMed]
- Halder, D.; Mandal, M.; Chatterjee, S.S.; Pal, N.K.; Mandal, S. Indigenous Probiotic Lactobacillus Isolates Presenting Antibiotic like Activity against Human Pathogenic Bacteria. Biomedicines 2017, 5, 31. [Google Scholar] [CrossRef]
- Okumus, I.; Stirling, H.P. Seasonal variations in the weight, condition index and biochemical composition of mussels (Mytilus edulis L.) in suspended culture in two Scottish sea lochs. Aquaculture 1998, 159, 249–261. [Google Scholar] [CrossRef]
- Orban, E.; Di Lena, G.; Nevigato, T.; Casini, I.; Caproni, R.; Santaroni, G.; Giulini, G. Nutritional and commercial quality of the striped venus clam, Chamelea gallina, from the Adriatic sea. Food Chem. 2007, 101, 1063–1070. [Google Scholar] [CrossRef]
- Jenkins, S.; Lart, W.; Vause, B.; Brand, A. Seasonal swimming behaviour in the queen scallop (Aequipecten opercularis) and its effect on dredge fisheries. J. Exp. Mar. Biol. Ecol. 2003, 289, 163–179. [Google Scholar] [CrossRef]
- Ringø, E.; Van Doan, H.; Lee, S.; Song, S.K. Lactic Acid Bacteria in Shellfish: Possibilities and Challenges. Rev. Fish. Sci. Aquac. 2019, 28, 139–169. [Google Scholar] [CrossRef]
- Reda, R.M.; Selim, K.M.; Mahmoud, R.; El-Araby, I.E. Effect of dietary yeast nucleotide on antioxidant activity, non-specific immunity, intestinal cytokines, and disease resistance in Nile Tilapia. Fish Shellfish. Immunol. 2018, 80, 281–290. [Google Scholar] [CrossRef]
- Cintas, L.M.; Casaus, M.P.; Herranz, C.; Nes, I.F.; Hernández, P.E. Review: Bacteriocins of Lactic Acid Bacteria. Food Sci. Technol. Int. 2001, 7, 281–305. [Google Scholar] [CrossRef]
- von Wright, A.; Axelsson, L. Lactic Acid Bacteria: An Introduction. In Lactic Acid Bacteria: Microbiological and Functional Aspects; Lahtinne, S., Salminen, S., von Wright, A., Ouwehand, A., Eds.; CRC Press Taylor and Francis: Boca Raton, FL, USA, 2011; pp. 1–17. [Google Scholar]
- Ishikawa, M.; Nakajima, K.; Yanagi, M.; Yamamoto, Y.; Yamasato, K. Marinilactibacillus psychrotolerans gen. nov., sp. nov., a halophilic and alkaliphilic marine lactic acid bacterium isolated from marine organisms in temperate and subtropical areas of Japan. Int. J. Syst. Evol. Microbiol. 2003, 53, 711–720. [Google Scholar] [CrossRef]
- Meidong, R.; Doolgindachbaporn, S.; Sakai, K.; Tongpim, S. Isolation and selection of lactic acid bacteria from Thai indigenous fermented foods for use as probiotics in tilapia fish Oreochromis niloticus. Aquacult. Aquarium Conserv. Legis. 2017, 10, 455–463. [Google Scholar]
- Muñoz-Atienza, E.; Landeta, G.; Rivas, B.d.L.; Gómez-Sala, B.; Muñoz, R.; Hernández, P.E.; Cintas, L.M.; Herranz, C. Phenotypic and genetic evaluations of biogenic amine production by lactic acid bacteria isolated from fish and fish products. Int. J. Food Microbiol. 2011, 146, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Granados-Amores, A.; Campa-Córdova, Á.I.; Araya, R.; Mazón-Suástegui, J.M.; Saucedo, P.E. Growth, survival and enzyme activity of lions-paw scallop (Nodipecten subnodosus) spat treated with probiotics at the hatchery. Aquac. Res. 2011, 43, 1335–1343. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chen, Y.-T. Lactobacillus spp. fermented soybean meal partially substitution to fish meal enhances innate immune responses and nutrient digestibility of white shrimp (Litopenaeus vannamei) fed diet with low fish meal. Aquaculture 2021, 548, 737634. [Google Scholar] [CrossRef]
- Van Doan, H.; Hoseinifar, S.H.; Ringø, E.; Esteban, M.Á.; Dadar, M.; Dawood, M.A.O.; Faggio, C. Host-Associated Probiotics: A Key Factor in Sustainable Aquaculture. Rev. Fish. Sci. Aquac. 2020, 28, 16–42. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).