Abstract
In this manuscript, we present the results of ecotoxicological tests conducted on the Baltic microphytobenthos community consisting largely of cosmopolitan species showing the responses at the community (taxonomic composition and structure), population (abundance), and cellular (chloroplast shape) levels. The tests were carried out for three chemicals with different modes of action on photosynthetic organisms, i.e., copper (II) chloride (at concentrations of 2·10−5 g·dm3 and 2·10−3 g·dm−3), glyphosate in the form of Roundup® (concentrations ranging from 4.2·10−2 to 8.5 g·dm−3), and the ionic liquid [BMIM]Cl (at concentrations of 1.13·10−3 g·dm−3 and 1.75·10−2 g·dm−3). The results of the study indicate that the responses of microphytobenthos at each level analysed are highly variable and difficult to predict a priori based on experiments performed on single strains. In addition, it was shown that microphytobenthic communities are relatively resistant to the substances tested, which is due to taxonomic richness and the resulting potential for substitution of sensitive taxa by more resistant taxa. The results obtained in the tests also indicate that the number of cells of individual taxa can remain at a similar level or increase over short periods of time despite the deformation of chloroplasts in a numerous group of cells.
1. Introduction
More than 300 million tons of industrial chemicals were used in the EU in 2018, of which more than two-thirds were classified by Eurostat as hazardous to health [1]. Many of these substances end up in surface waters and affect aquatic organisms through, for example, morphological changes, reduced reproductive capacity of aquatic mollusks, changes in animal behavior, or changes in the composition and structure of microphytobenthic communities [2]. Unfortunately, the effects of such changes are often difficult to predict.
Toxicological tests are currently carried out worldwide on a variety of organisms belonging to different levels of the food web, ranging from plants, herbivores, and carnivores to bacteria [3,4]. Photosynthetic organisms are particularly important for ecosystems due to the fact that they provide oxygen, participate in biogeochemical cycles, and provide food for other organisms. Hence, changes in plant organisms under the influence of various factors will not only limit their growth but may also directly affect the structure and functioning of the entire ecosystem [5]. Until now, ecotoxicological tests have mainly been conducted on monocultures of algae isolated from the environment. These are extremely valuable but provide information on the response of organisms only in terms of the fundamental niche, e.g., [6]. The use of whole communities in ecotoxicological testing is rare, despite the fact that they are easily obtained from the environment and do not require the labour-intensive and costly steps of isolating and maintaining individual strains in the laboratory [7,8,9]. Conducting toxicological tests that take into account whole communities makes it possible to understand the response of organisms in the context of actually existing interpopulation interactions, e.g., competition (antagonistic interaction) and symbiosis (non-antagonistic interaction). However, whole-community tests are still rare due to various challenges, including the lack of a recommended consistent methodology, the acquisition of a sizeable data set for a high number of taxa representing a variety of taxonomic groups with different life strategies, and the associated problems in interpreting the results. The microphytobenthos, which is the main element studied in this work, is ecologically very important for aquatic ecosystems, including those of coastal zones, estuaries, or shallow seas, as it is an important primary producer associated with the bottom. Organisms forming microphytobenthic communities, due to their short life cycles, are considered good indicators of environmental change [10], and their analysis provides important information on the state of the marine environment [11]. It is worth emphasizing that the tested Baltic microphytobenthic community includes many cosmopolitan species, which makes it possible to assume that similar reactions to selected chemical substances will be observed in communities occurring in brackish waters in other regions of the world.
The study aimed to test the effects of three compounds from different chemical groups with different levels of toxicity on natural microphytobenthic communities. The first substance used in the tests was copper in the form of copper (II) chloride. European refined copper consumption reached around 3.78 million metric tonnes in 2021, but consumption in the region has been gradually declining over the past decade [12]. Copper (II) chloride is produced on an industrial scale by the chlorination of copper. The use of copper chloride is very wide. CuCl2 is used as a wood preservative, fungicide, insecticide, and herbicide. Due to its oxidising properties, it is used as a purifying agent, e.g., in water treatment, and as a deodorising agent to prevent odour in the petroleum industry. Copper (II) chloride is an important element in the production of vinyl chloride and dichloroethane and has applications in the synthesis of organic and inorganic compounds as a catalyst. It is also used in pyrotechnics as a blue-green colouring agent [13]. Copper is a compound of very high functional importance to aquatic plant microorganisms and is a substance with a relatively well-recognised effect and mechanism of action, e.g., [14,15,16,17]. Copper has an essential role in the metabolism of photosynthetic organisms and is a component of many proteins and enzymes involved in a number of metabolic pathways [18]. However, high concentrations and prolonged exposure to copper ions inhibits photosynthesis and disrupts physiological processes [19,20]. Copper compounds are used as algicides, as they are toxic in large quantities to algae [21]. Copper (II) chloride may be a genotoxic compound, as it causes chromosomal aberrations and mitotic cycle disruption in cells of A. cepa model organisms [22]). Copper chloride also negatively affects aquatic and soil organisms [23]. In particular, denitrifying bacteria have been found to be very sensitive to its presence. Copper (II) chloride at a concentration of 0.95 mg·dm−3 causes 50% inhibition (IC50) of the metabolic activity of denitrifying microorganisms [24]. The US Environmental Protection Agency allows concentrations of no more than 1.3 ppm of aqueous copper ions in drinking water. Ingestion of copper ions in excessive quantities in humans can cause headache, diarrhoea, decreased blood pressure and fever, and, in extreme cases, haemolysis [25].
The second substance used in the tests was glyphosate in the form of Roundup®. Glyphosate in the form of Roundup is the most popular herbicide worldwide, with 825.8 million kilograms of glyphosate used worldwide in 2014 [26]. In Poland, 92 glyphosate-based herbicides are currently registered, with more still being registered. The amount of herbicides used worldwide is increasing despite the fact that the use of glyphosate has been shown not to lead to a positive effect on yields compared to alternative weed control methods such as mechanical and thermal weed control [27]. Glyphosate is the active substance used in non-selective herbicides. It is an extremely effective compound that has a broad spectrum of biological activity. The popularity of Roundup® herbicide has increased with the spread of genetically modified crops in recent years [28]. Therefore, it is very important to study the combined effect of the active substance glyphosate and the other components of the preparation, as this is the form to which the natural environment is most often exposed. Once in the plant, glyphosate inhibits, for example, the production of the enzyme EPSP (5-enolpyruvate-shikimo-3-phosphate) synthetase, which is responsible for the formation by plants of aromatic amino acids that are important for their growth and are included in the composition of many plant pigments [29] or the activity of microsomal ATPases [28]. Glyphosate at concentrations above 400 μg·dm−3 is potentially toxic to some aquatic species, including amphibians and fish [30,31,32]. Glyphosate and its breakdown product, so-called AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid), are observed in crops in non-target plant species even after the recommended withdrawal period. It is of concern that both glyphosate and AMPA have also been detected in crop plants and in the leaves of trees of native forests after glyphosate application to neighbouring crops [33]. The presence of glyphosate in marine ecosystems, as reported in some studies, e.g., [34], indicates its widespread use and high potential for spreading. However, glyphosate and AMPA pose a low risk to mammals, mainly due to low absorption through the skin and gastrointestinal tract [33,35]. Both substances are excreted in the urine and have a half-life of 3 to 15 h without any structural changes [36]. For these reasons, coupled with the acute toxicity data set, glyphosate and AMPA have been classified by the EPA in the category of least toxic substances (Category IV; virtually non-toxic and non-irritant) [33].
The third substance used was the ionic liquid 1-butyl-3-methylimidazolium chloride ([BMIM]Cl). Substances in this group are the new generation of polar organic solvents and catalysts referred to as ‘green chemistry’. Ionic liquids are ‘designer solvents’ in which the right choice of anions and cations allows the creation of chemical compounds with appropriate properties depending on the future application [37,38,39]. Due to this unique feature, it is difficult to determine the range of applications for ionic liquids worldwide. One very good example of the use of ionic liquids in cellulose processing is provided, for example, by [40]. However, after years of research, their ‘green’ nature has been questioned due to their negative environmental impact [41,42,43]. The properties of ionic liquids (e.g., good solubility, stability) result in the potential for these substances to accumulate in aquatic ecosystems, where they may enter, e.g., with wastewater [44,45]. Ionic liquids can pose a potential hazard by contaminating water and soil through accidental spills or ineffective wastewater treatment [46]. In studies conducted by different teams, imidazolium and pyridinium cations have been shown to adsorb on various types of soils and marine sediments, e.g., [47,48,49,50]. Furthermore, soil microorganisms also present in wastewater have been shown to have the capacity to degrade imidiazole ionic liquids, e.g., [37,51]. However, as shown by some authors [52], the way ionic liquids are degraded in the environment is determined by numerous biotic and abiotic factors. Certain classes of imidiazole cations of ionic liquids inhibit, in humans, the activity of acetylcholinesterase AchE, an enzyme that breaks down one of the primary neurotransmitters, acetylcholine, into choline and acetic acid residues. It is produced by the liver, among others, and is used in diagnostics as a marker of the liver’s efficiency in protein production [53]. In addition, 1-octyl-MIM bromide has been shown to cause liver damage in mice [54].
The research we conducted was aimed at updating the knowledge of the response of microphytobenthic communities to the presence in the environment of substances of anthropogenic origin with different toxic potential. The results of the study indicate that at the level of communities, populations, and cells, the responses of microphytobenthos are very diverse and difficult to predict a priori on the basis of experiments performed on single strains. An important aspect of the work is its versatility, because the taxa comprising the tested community are cosmopolitan (identified in various coastal waters in the world ocean). In addition, the laboratory tests performed made it possible to systematise knowledge of the methodological issues involved in this type of experimentation and indicate that the newly applied indicator based on the deformation state of chloroplasts does not correspond to the criteria of low labour and time consumption met by indicators commonly used in ecotoxicological tests.
2. Materials and Methods
In the first phase of the work, a number of preliminary tests were carried out during which the most appropriate exposure time of the culture panels in the environment [55], the duration of the ecotoxicological test, and the culture medium [9] were selected and the analytical methods used to assess the changes occurring in the communities were optimised [56].
2.1. Field and Laboratory Works
The microphytobenthos used in the laboratory study was collected from slides exposed in the coastal zone of the southern Baltic Sea (Gulf of Gdańsk) at a station located at 54°26′49″ N, 8°34′24″ E for a period of 14 days in summer (August) 2015. The methodology of the field work is presented in detail in Sylwestrzak et al. [57]. After transporting the panels to the laboratory, the material was prepared for further testing. After sonification and nitrogen desaturation to remove animal organisms, microphytobenthos cells of 44,273 (±2151) in 1 mL were placed in 250 mL flasks in 100 mL of seawater collected in situ. The measured concentrations of biogenic compounds in seawater were: N-NH4 9.4 mg·m−3, N-NO3 102 mg·m−3, P-PO4 36 mg·m−3, Si-SiO4 600 mg·m−3. Based on preliminary tests, it was shown that such concentrations of biogenic salts do not limit the growth of microalgae [57]. Light conditions with a PAR irradiance of 60 μmol photons m−2 s−1 and an L:D photoperiod of 16:8 were obtained using an artificial light source: Phillips halogen lamps (OSRAM L 36W/640-1).
In the tests, copper (II) chloride, glyphosate, and the ionic liquid BMIM[Cl] were used to compare effects of substances from different chemical groups with distinct mechanisms of action on living organisms and varying levels of toxicity on natural microphytobenthic communities. The concentration values of the test substances were selected on the basis of maximum acceptable concentrations of selected compounds in the environment introduced by legislation [58] and the literature [37,59] as well as on the basis of previous studies of our own [55]. The following concentrations were used in the ecotoxicological tests: for copper (II) chloride, 2·10−5 g·dm3 and 2·10−3 g·dm−3; for glyphosate, 4.2·10−2 g·dm−3, 8.5·10−1 g·dm−3, and 8.5 g·dm−3; and for ionic liquid [BMIMC]l, 1.13·10−3 g·dm−3 and 1.75·10−2 g·dm−3. All variants of the experiment were carried out in 3 replicates. The values used were high but were intended to reflect a sudden one-off influx of high concentrations of test substances into the environment. Undertaking the study, it was assumed that the microphytobenthic communities extracted from the environment had not adapted to the elevated values of the compounds tested. For example, the content of copper ions in the area of their cultivation is at the level of 1.3·10−7–1.1·10−6 g·dm−3 [60]. In turn, Skeff et al. [59] showed that in river mouths in the Baltic Sea, concentrations of glyphosate range from 2.8·10−8 g·dm−3 to 1.68·10−6 g·dm−3, while APMA is detected in all of them. In studies conducted in rivers in Germany, 1310 different ionic liquid pollutants were identified, but only ca. 20 compounds were detected in concentrations of up to μg·dm−3 [61].
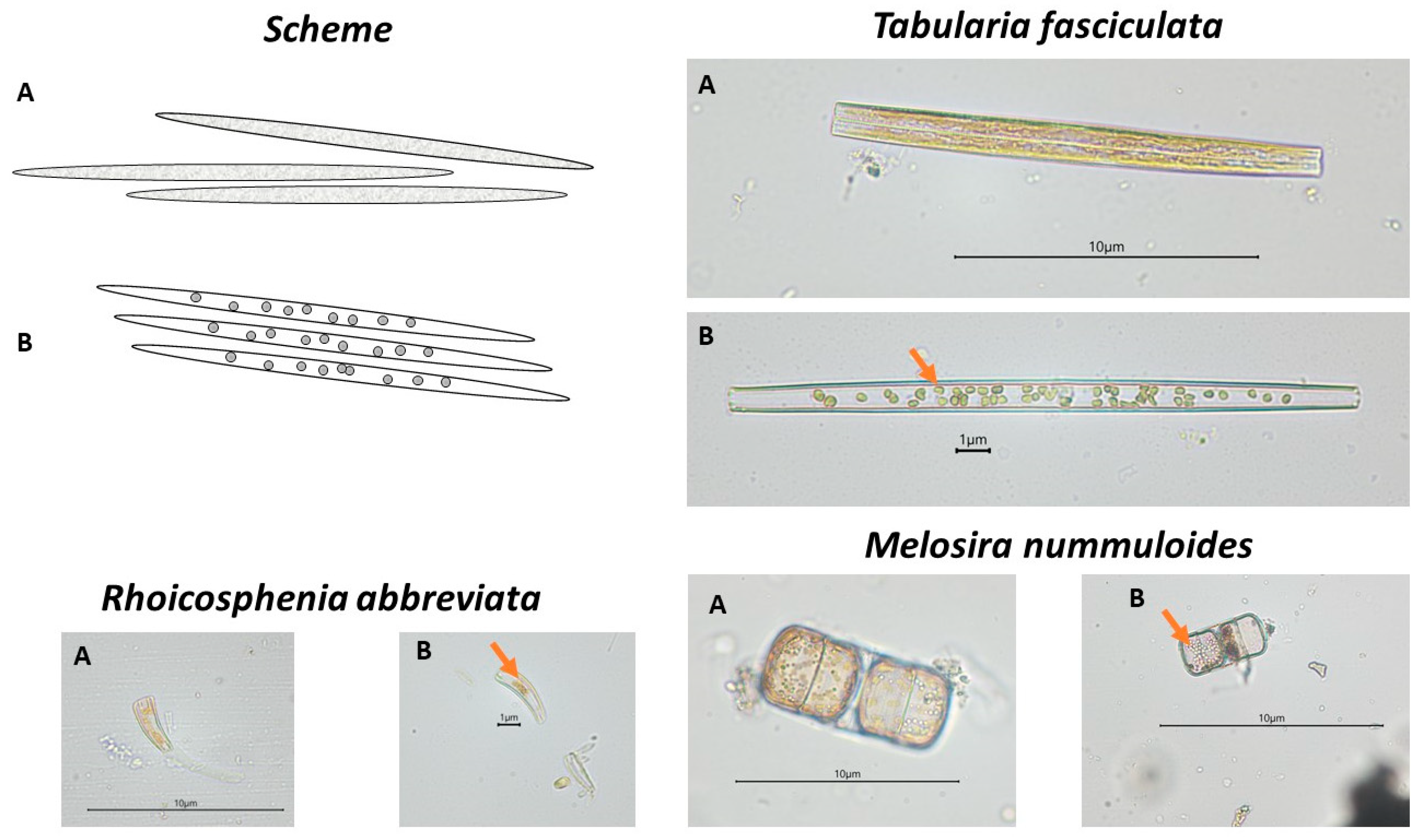
Observations of changes in the composition and structure of microphytobenthic communities were carried out using the principles adopted in the OECD Guidelines for the Assessment of the Effects of Chemical Toxicity on Plant Microorganisms [62]. The microalgae were identified using various taxonomic keys and floras [63,64,65,66,67,68,69,70]. Qualitative and quantitative analysis of communities was conducted using a Nikon (Japan) 80i microscope equipped with a DS-U2 camera at ×400 magnification. In addition, changes in the shape of chloroplasts of microphytobenthic cells were analysed according to a previously developed research methodology [9,55]. The state of the chloroplasts, determined by a change in shape and structure, was introduced as an additional indicator to supplement information on the cellular condition of the organisms composing the communities. Examples in the form of diagrams and microscopic photographs of cells with normally formed chloroplasts and chloroplasts with abnormal shape are shown in Figure 1.
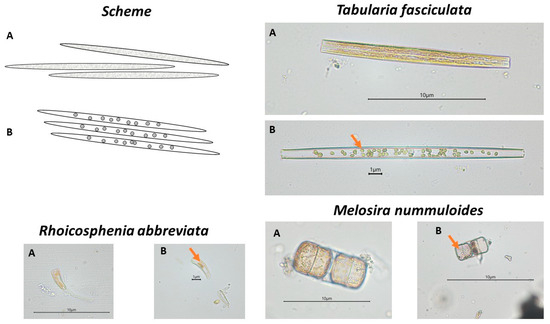
Figure 1.
Diagrams and photographs of plant microorganisms with normally formed chloroplasts (A) and with abnormal chloroplasts (marked with an arrow) (B). Tabularia fasciculata (Bacillariophyceae) in control solution at the start of the experiment (A) and after 7 days of testing at a concentration of 8.5 g·dm−3 glyphosate (B); Rhoicosphenia abbreviata (Bacillariophyceae) in control solution at the start of the experiment (A) and after 7 days at a concentration of 1.75·10−2 g·dm−3 ionic liquid [BMIM]Cl (B); Melosira nummuloides (Coscinodiscophyceae) in control solution at the start of the experiment (A) and after 7 days at a concentration of 2·10−3 g·dm−3 CuCl2 (B).
2.2. Statistical Analysis
The data obtained were processed with MS Excel. Student’s t-test was performed to compare the significance of differences in cell numbers between tested substances’ concentrations and the control solution and to designate differences among successive test days with STATISTICA version 10 (StatSoft, Kraków, Poland).
3. Results
One of the main elements analysed in the experiments was the qualitative and quantitative composition of the microphytobenthic communities. A total of 58 microalgal taxa were identified during the study (Appendix A). In the initial community, 87% of the taxa were represented by diatoms, 11% by cyanobacteria, and green algae accounted for less than 1%. Single Haptophyta cells were also identified, but their maximum percentage contribution to the community was 0.3%; hence, they were not included in the graph.
3.1. Response at Community Level
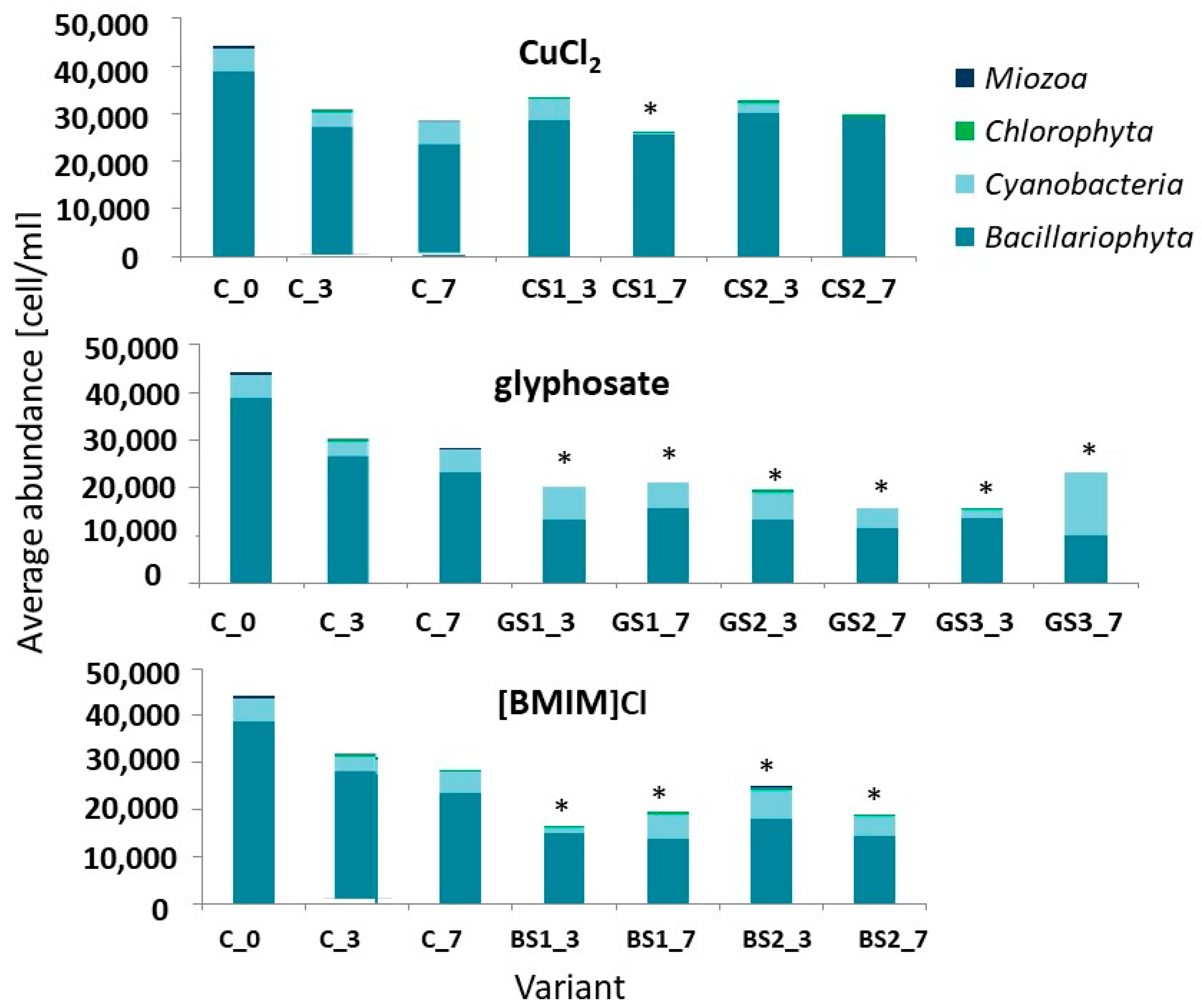
In the case of copper (II) chloride in the solutions tested, the total abundance of communities remained at a similar level throughout the study period, irrespective of the variant tested (Figure 2). Only the community structure, i.e., the abundance of representatives of individual microalgal taxa, changed. Glyphosate in the form of the Roundup® preparation influenced the reduction in the community abundance and its remodelling. At the highest applied concentration of 8.5 g∙dm3 of glyphosate, an increase in the proportion of blue-green algae to 51% of the total community was observed. In the case of communities treated with the ionic liquid [BMIM]Cl, most of the taxa composing the community under study responded with a reduction in cell abundance.
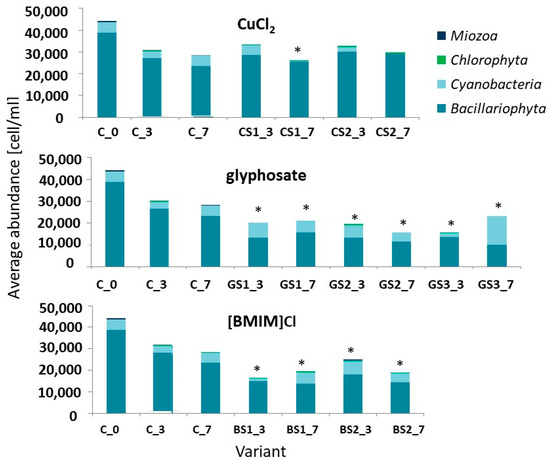
Figure 2.
Average abundance of taxonomic groups in communities during tests. Designations used: the first part of the caption encodes the concentration, e.g., C–control solution, 2∙10−3–CuCl2 concentration; the second part encodes the day of the test—e.g., _3 is the third day, _7 is the seventh day. Statistically significant differences were marked with * symbol.
3.2. Response at Population Level
Among the dominant species present in the initial communities and present in all variants of the experiments, mainly diatoms were observed. The most abundant species were Bacillaria paxillifera, Diatoma moniliformis, Diatoma vulgaris, Navicula perminuta, Tabularia fasciculata (Bacillariophyceae), and Melosira nummuloides (Coscinodiscophyceae), among others. Among Cyanobacteriota (formerly Cyanobacteria), the most abundant taxa were Spirulina sp. and Merismopedia sp. Regardless of the degree of dominance in the initial community, individual taxa showed different types of response to the presence of the tested substance. Three types of response were identified: an increase in abundance, which was interpreted as growth stimulation; no change in abundance, interpreted as no response (‘indifferent’ taxa); and a decrease in abundance, interpreted as growth inhibition.
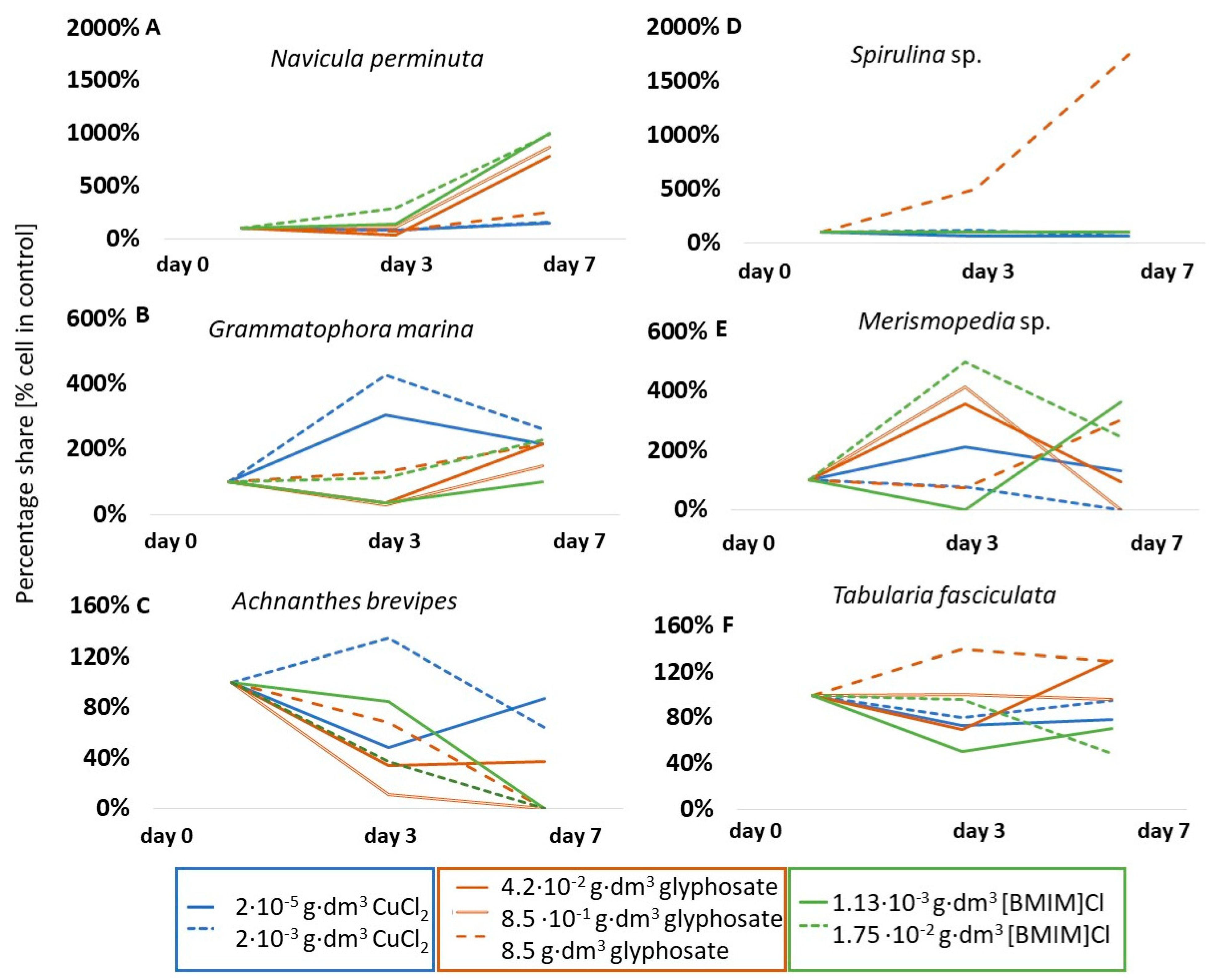
An example of a species that was stimulated by all the substances tested was Navicula perminuta (Figure 3A). This species showed an increase in cell number, by approximately 50% relative to the control, under the influence of copper (II) chloride, an eightfold increase in abundance in the presence of glyphosate, as well as a tenfold increase in abundance in the presence of ionic liquid [BMIM]Cl on the seventh day of testing. An unusually large, up to fourfold, increase in abundance under the influence of copper chloride was also observed in Grammatophora marina (Bacillariophyceae) (Figure 3B) on the third day, and, in the case of glyphosate and ionic liquid (1.75·10−2 g·dm−3), a twofold increase on the seventh day of testing. Some taxa were stimulated by the presence of chemicals only during the initial phase of testing. For example, the number of Achnanthes adnata (formerly Achnanthes brevipes) (Bacillariophyceae) cells in the presence of copper (II) chloride was 135% of the abundance in the control sample on the third day, but only 64% on the seventh day (Figure 3C). Other taxa responded differently depending on the substance and/or concentration. For example, glyphosate stimulated the cells of representatives of cyanobacteria to increase in abundance very intensively. Cell abundance of Spirulina sp. increased by 17.5 times that of the control on the last day (Figure 3D), and abundance of Merismopedia sp. increased by about four times for lower concentrations on the third day (Figure 3E). However, the other substances did not affect the change in abundance (neutral response for Spirulina sp. in [BMIM]Cl) or had an inhibitory effect (Spirulina sp. and Merismopedia sp. in CuCl2). Similarly different responses were observed for the diatom T. fasciculata (Figure 3F). In the case of glyphosate, a stimulation of up to 40% increase in cell numbers relative to the control solution was observed at the highest concentration, no response was observed at intermediate concentrations, and growth inhibition was observed at the lowest concentration. In contrast, copper chloride had no significant effect on changes in abundance for this taxon (p < 0.05).
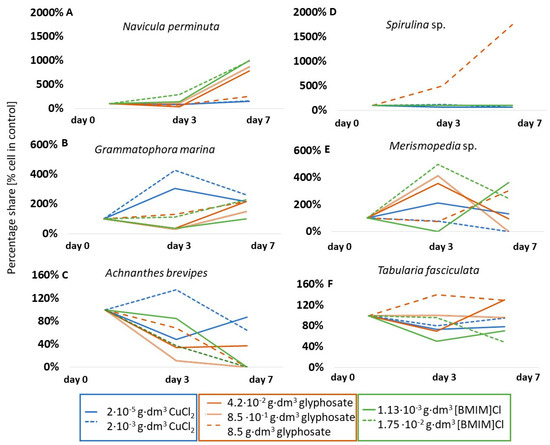
Figure 3.
Percentage share of cells of selected microalgal taxa compared to control solutions. Note that the changes in scale in each graph are due to different degrees of change in population size.
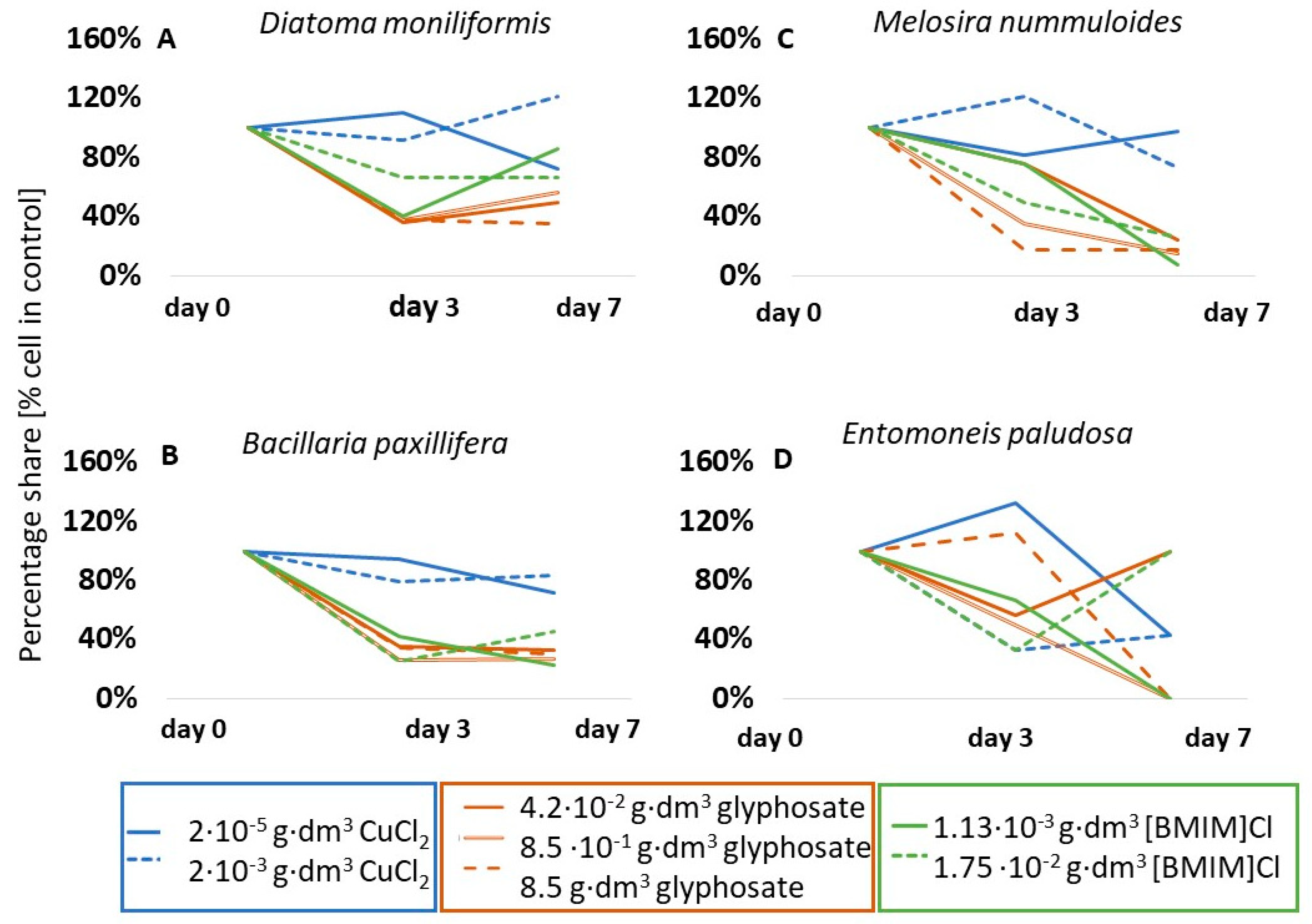
The group of taxa that showed a lack of response to copper (II) chloride also included, for example, Diatoma moniliformis (p < 0.05) (Figure 4A) and Bacillaria paxillifera (p < 0.05) (Figure 5B). However, in the presence of glyphosate and ionic liquid, few taxa showed a lack of response, such as the aforementioned Spirulina sp. which, at 0.85 g·dm−3 glyphosate and at both ionic liquid concentrations used, did not change its abundance over the course of the tests. A table showing the results of the significance tests for the species in question is included in Appendix B.
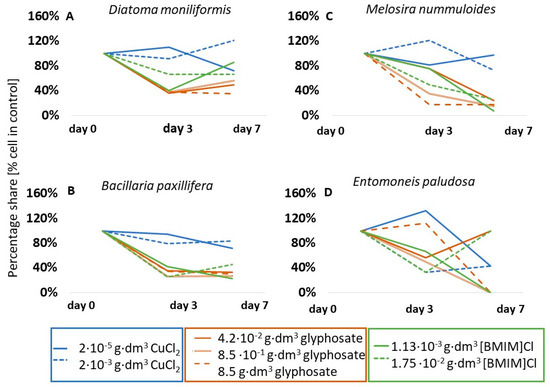
Figure 4.
Percentage share of cells of selected microalgal taxa compared to control solutions.
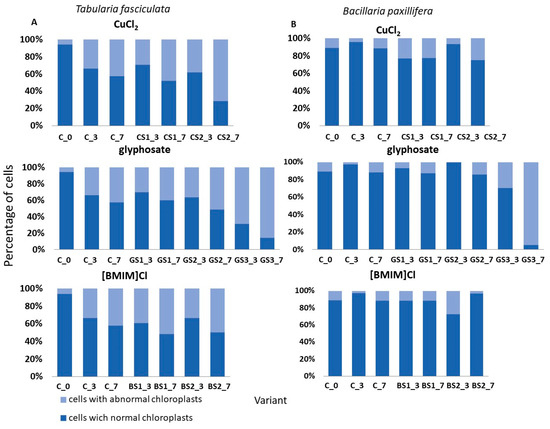
Figure 5.
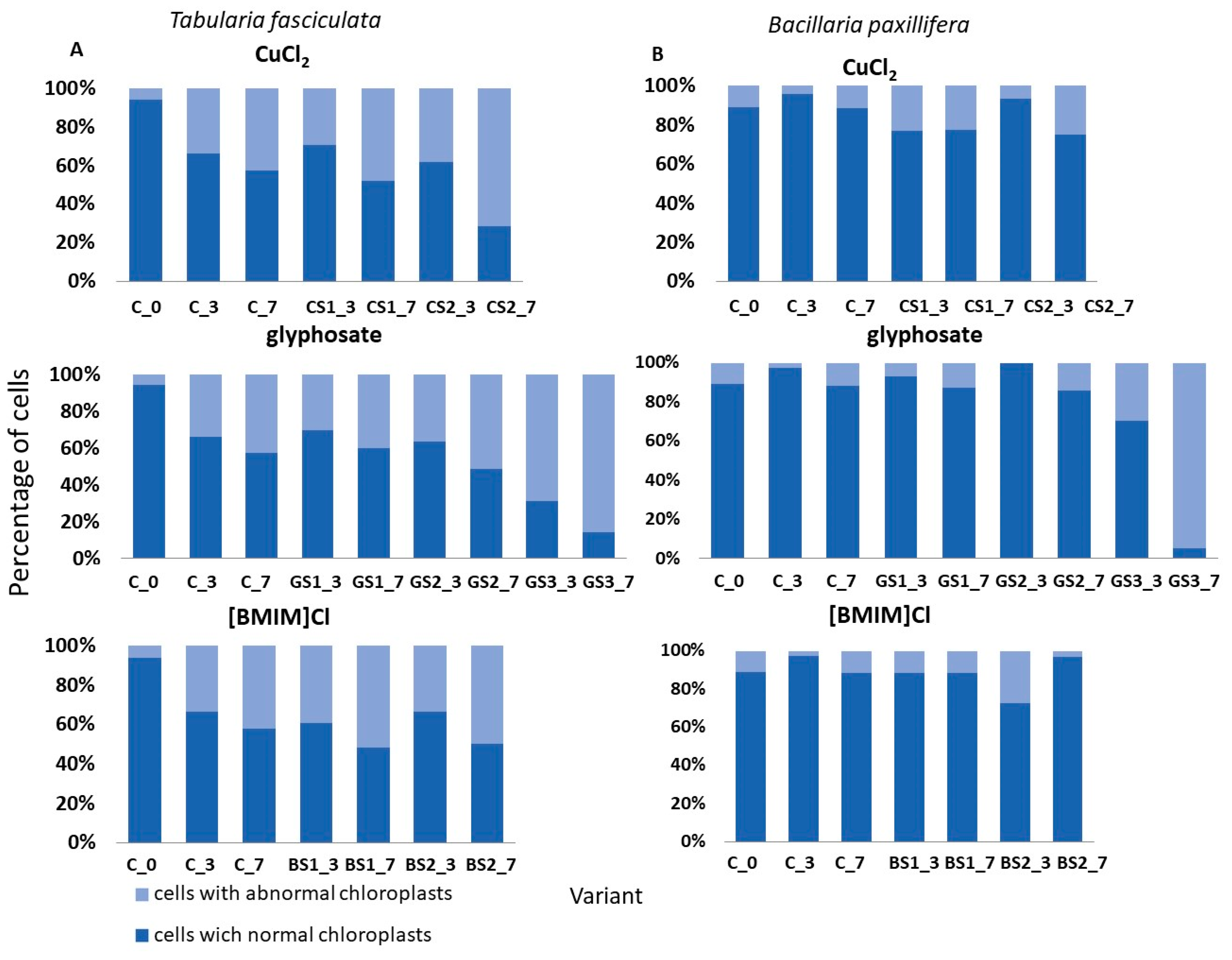
Changes in the condition of selected species expressed as the percentage share of cells with normal chloroplasts and abnormal chloroplasts in the control solution (C) and in the tested substances. Designations used: C—control solution, 2∙10−3–CuCl2 concentration; the second part encodes the day of test—e.g., _3 is the third day of test, _7 is the seventh day of test. (A) Tabularia fasciculata. (B) Bacillaria paxilifera.
A significant inhibition of growth was presented by one of the dominant diatom species, B. paxillifera, in both copper chloride and ionic liquid tests (Figure 4B). In contrast, growth inhibition of 15% in the presence of glyphosate and as much as 92% in the presence of ionic liquid was observed for the diatom M. nummuloides (Figure 4C). For A. brevipes, no cells of this taxon were observed in glyphosate and in ionic liquid on the seventh day of tests (Figure 3C). The species Entomoneis paludosa (Bacillariophyceae) also reacted negatively to the presence of ionic liquid and showed a 57% reduction in abundance on the seventh day of tests, while glyphosate induced a complete growth inhibition of this taxon (Figure 4D). It is noteworthy that, in the case of ionic liquid, more than 75% of the identified taxa were sensitive to its effects, as manifested by growth inhibition.
3.3. Response at the Cellular Level
The effects of the tested chemicals on changes in the shape of chloroplasts in cells of the selected two dominant diatom taxa are presented in Figure 5. Although the abundances of T. fasciculata and B. paxillifera were similar and constituted about 17% of the total community at the start of the tests, their responses at the cellular level differed depending on the substance used and between concentrations. Chloroplast deformations were observed in the presence of all tested substances in both species (Figure 5A). However, on the seventh day at a copper chloride concentration of 2·10−3 g·dm3, 71% of the cells had abnormally formed chloroplasts, while at the highest concentration of glyphosate, up to 86% of the cells had abnormally formed chloroplasts. In contrast, in B. paxillifera, only 6% to 24% of cells with abnormally shaped chloroplasts were observed at the copper chloride and ionic liquid concentrations tested, while glyphosate at 8.5 g·dm3 caused changes in chloroplasts in 95% of cells (Figure 5B).
4. Discussion
As shown in previous studies, the presence of substances of anthropogenic origin in river basins, estuaries, or coastal zones can alter community structure and primary microphytobenthic production, even if the pollution does not exceed acute toxicity levels [71]. To test the response of microphytobenthic communities developing in brackish waters of the Baltic Sea, compounds were selected whose observed concentrations are not currently very high, but whose popularity continues to grow. Previously, copper has been used in antifouling products used for ship maintenance, which have been a source of copper compounds released into marine waters [72]. Despite its use being banned [73], the use of copper ions in many other industries means that the risk of introducing them in quantities exceeding the detoxification capacity of organisms into surface waters is increasing. For example, in recent years, there has been an increased interest in copper-based nanoparticles for applications in cosmetics (cream additives), industry (e.g., metal coatings, inks, and food packaging plastics), and medicine (e.g., disinfection and antimicrobial coatings) [74,75,76]. Coastal ecosystems and estuaries are also increasingly exposed to herbicide contamination [71]. A multiyear study of pesticide content in surface waters has shown that the most common pesticides are bentazone and glyphosate [77]. Glyphosate as a pesticide is used on an increasing scale due to the massive expansion of agricultural production, the high yield of herbicide formulations with this substance, their low cost of production, and the still liberal laws in many countries with highly developed agricultural economies [78]. In the meantime, ionic liquids are gaining popularity due to their reputation as ‘green chemistry’ and are finding use in a variety of chemical processes, where they represent a new alternative to traditional organic solvents [79]. However, inadequate treatment of wastewater or accidental spillage of ionic liquids can cause negative effects on the ecosystem [43].
4.1. Response at Community Level
Microphytobenthic communities are specific microecosystems with a complex taxonomic structure. Each taxon has a unique tolerance to a specific environmental stressor, so it can be expected that the response of communities will vary according to the stressor and its intensity. Tests showed that copper (II) chloride mainly altered the taxonomic composition of the community, with no significant change in total cell abundance. Similar observations on compositional and structural changes in freshwater microalgal communities treated with copper ions were carried out by Sabater et al. [80]. In our tests, we have shown that in the presence of Roundup® the proportion of cyanobacteria increased significantly. On the one hand, glyphosate may have eliminated diatoms from the community as cyanobacteria took advantage of the niches that formed; on the other hand, glyphosate decomposes relatively quickly, and cyanobacteria show a competitive advantage to the introduction of an additional carbon source, which is in line with the results of studies conducted on the toxicity of glyphosate on six species of Cyanobacteriota [81]. In our tests, we also showed that the ionic liquid [BMIM]Cl causes a reduction in the abundance of the entire community, which is dominated by diatoms. Latała et al. [82] also suggested that diatoms, in particular, are extremely sensitive to ionic liquids.
4.2. Response at Population Level
Among the dominant species present in the initial community and present during the experiments, mainly diatoms and single cyanobacterial species were distinguished, including numerous cosmopolitan taxa, e.g., B. paxilifera, N. perminuta, N. ramossisima, T. fasciculata, M. nummuloides, and S. subsalsa [83]. Irrespective of the degree of dominance in the initial community, cell size, or taxonomic group, individual taxa showed a variety of responses to the presence of the chemicals tested. The rate of uptake of selected ions across cell membranes and the internal detoxification mechanism were likely to be factors that significantly influenced the toxicity of the tested substances [84]. We distinguished three main types of response to the presence of potentially toxic chemicals. Some organisms responded with an increase in cell numbers relative to control samples. This was found to be a response characteristic of resistant species in which the chemical stimulates growth. Some taxa remained indifferent to the presence of the toxicant and showed no significant change in abundance. The last group was made up of sensitive taxa, which reacted by decreasing cell numbers under the influence of the test substances. Unfortunately, in the case of our tests, we are unable to determine whether the selected substances stimulated growth or reduced competition, which ultimately manifested itself in an increase in population size.
An example of a particularly resistant species, which was stimulated by all the factors tested, was N. perminuta. Other resistant taxa that increased their abundance, contributing to the remodelling of the community structure were, e.g., G. marina (copper chloride II), Cylindrotheca closterium, Spirulina sp. (glyphosate), and Merismopedia sp. ([BMIM]Cl). Plants, including aquatic plants, have many defence mechanisms that enable them to survive under unfavourable conditions. As a result of oxidative stress caused by pollution, the activation of detoxification mechanisms and defensive antioxidant processes are observed, which enable microalgae cells to defend themselves, but these are taxon-specific responses [85]. Important regulatory factors in the adaptation strategies of algae to chemical stress are auxins and cytokinins, whose production rates vary between species [86]. Taxa identified as indifferent to the tested chemicals in which these mechanisms were likely to have acted included: C. pediculus, M. nummuloides, and N. gregaria in the case of copper (II) chloride. In contrast, glyphosate did not affect changes in abundance in, e.g., Licmophora sp. and T. fasciculata. In the case of ionic liquid, no species described as indifferent were observed.
A study by Yu et al. [87] showed that multispecies algal communities with interspecies interactions respond differently to stress caused, for example, by the presence of chemicals. The authors determined the sensitivity of algae to the presence of copper ions by measuring the inhibition of the production of certain cellular enzymes and showed, among other things, that the EC50 for Microcystis aeruginosa (Cyanobacteriota) in the community was significantly higher compared to monocultures of this species and that cell metabolism is also affected by interspecies interactions. Also, our previous studies indicate that taxa in communities have a higher resistance to the stressors tested than individual microalgal strains [45]. Microphytobenthic communities usually consist of a large number of species, even at early stages of succession [88]; hence, it is to be expected that the response of taxa to the presence of toxic substances in communities will be different than in monocultures and further enhanced or weakened by competitive relationships. In our tests, we showed that high concentrations of Roundup® contributed to complete community conversion. Cyanobacteriota were particularly resistant to this preparation. The presence of glyphosate probably resulted in enrichment of the culture medium with phosphorus biogenic compounds [89], leading to a dominance of the microphytobenthic community by them (e.g., Spirulina sp.). Similar results were obtained in community tests conducted in mesocosms, where a receding of diatom species in favour of cyanobacteria was observed [90], and in tests on pesticide toxicity in South American lakes [91]. It is probable that the receding of diatoms contributes to the release of ecological niches that cyanobacteria exploit, as in the case of our study; e.g., Spirulina sp. and Mersimopedia sp. showed an increase in abundance.
Copper at high doses reduces cell growth of individual taxa, as it inhibits photosynthesis [19]. In the experiment presented here, at concentrations of 2·10−5 g·dm3 and 2·10−3 g·dm3, the presence of copper resulted in a 57% reduction in the abundance of E. paludosa compared to the control solution after seven days, while no representatives of C. closterium were observed. In tests conducted on microalgae isolated from the Cochin estuary in India, it was shown that the EC50 for the diatom Nitzschia closterium (synonym of Cylindrotheca closterium) was 2.045·10−6 g·dm−3 [92]. In tests on freshwater communities [80] at a concentration of 1.5·10−5 g·dm−3 copper ions, a significant change in community structure was observed with a marked decrease in the abundance of the diatoms Synedra ulna and Achnanthes minutissima after seven days of testing. In our tests on brackish-water microphytobenthic communities, the presence of copper ions at a concentration of 2·10−5 g·dm3 induced a reduction in the abundance of diatom species, e.g., B. paxillifera and T. fasciculata, and at concentrations of 2·10−5 g·dm3 and 2·10−3 g·dm3 of the cyanobacterium Spirulina sp. In the course of the experiments described here, it was shown that many diatom species, including A. brevipes, B. paxillifera, M. nummuloides, and Peridinium sp., are sensitive to glyphosate in the formulation Roundup® at concentrations ranging from 4.2·10−2 to 8.5 g·dm−3. Glyphosate-based herbicides are chemical stressors for microalgae that can affect the structure of, among others, freshwater plankton [93]. Furthermore, a study by Fan et al.in [94] showed that the ionic liquids [C10IM]Cl and [C10MIM]Cl, at concentrations ranging from 0.2·10−4 g·dm3 to 0.1·10−3 g·dm3, inhibited microalgal growth by 50%. In our tests, a reduction in abundance at concentrations of 1.13·10−3 g·dm−3 and 1.75·10−2 g·dm−3 [BMIM]Cl was observed on the third day in dominant taxa such as B. paxillifera and T. fasciculata, while some taxa, such as A. brevipes and C. closterium, were not observed at all on the seventh day of testing.
4.3. Response at the Cellular Level
An innovative aspect of the study was the analysis of the state of chloroplasts, which provides insight into the response of the taxa comprising the communities to the applied chemical agents at the cellular level. Based on the tests carried out, it was observed that species, despite a similar response at the population level, i.e., similar changes in abundance, showed different proportions of cells with deformed chloroplasts compared to cells with normally formed chloroplasts. For example, in T. fasciculata, the proportion of cells with abnormally shaped chloroplasts ranged from 30% to 70% for copper (II) chloride, from 30% to 85% for glyphosate, and from 10% to about 50% for ionic liquid. In contrast, in B. paxillifera, the number of cells with abnormally shaped chloroplasts was 20% to 30% higher than in T. fasciculata at the same concentrations. Information on chloroplast structure damage induced by the presence of chemicals can be found in the literature, e.g., [55,95], but the value of such indicators seems small in relation to the effort and time required for the analysis.
5. Conclusions
Based on tests conducted on microphytobenthic communities with chemicals with documented toxic effects on living organisms, changes were observed both at the community level (taxonomic composition and structure) and at the cellular level (chloroplast shape). The relatively high resistance of microphytobenthic communities to the tested substances was due to the taxonomic richness of the studied formation. Sensitive taxa (e.g., E. paludosa, M. nummuloides) were replaced by species with higher resistance (e.g., N. perminuta, Spirulina sp.) so that the community abundance did not undergo significant changes. Changes in the taxonomic composition and community structure of Baltic microphytobenthos depended on the substance tested and its concentration. The results obtained in the tests indicate that the number of cells of individual taxa can remain at a similar level or increase over short periods of time despite significant deformation of chloroplasts. The novel method of analysing the degree of chloroplast deformation makes it possible to observe early responses of cells to a stress factor before the population size noticeably changes, but the analysis of chloroplast status is difficult and time-consuming; hence, its potential is limited.
Author Contributions
Conceptualization, Z.S. and A.Z.; methodology, Z.S. and A.Z.; software, Z.S. and A.Z.; validation, Z.S. and A.Z.; investigation, Z.S.; resources, Z.S. and A.Z.; data curation, Z.S.; writing—original draft preparation, Z.S. and A.Z.; writing—review and editing, Z.S. and A.Z.; visualization, Z.S.; supervision, A.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Research Projects for Young Scientists from the Faculty of Oceanography and Geography, University of Gdańsk (No. 538-G245-B888-15, 538-G245-B209-16, 538-G245-B567-17).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We would like to express our gratitude to Filip Pniewski for his valuable suggestions during the development of this research work.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
List of taxa identified during experiments with various variants of experiments (+ sign refers to presence).
Table A1.
List of taxa identified during experiments with various variants of experiments (+ sign refers to presence).
| Taxa | Author | CuCl2 | Roundup® | [BMIM]Cl |
|---|---|---|---|---|
| Bacillariophyta | ||||
| Achnanthes brevipes | Bory | + | + | + |
| Achnanthes lemmermannii | Hustedt | + | + | + |
| Amphora ovalis | (Kützing) Kützing | + | + | |
| Amphora pediculus | (Kützing) Grunow | + | + | + |
| Amphora sp. | Kützing | + | + | |
| Bacillaria paxillifera | (O.F.Müller) T.Marsson | + | + | + |
| Berkeleya rutilans | (Trentepohl ex Roth) Grunow | + | + | |
| Brebissonia lanceolata | (C.Agardh) R.K.Mahoney & Reimer | + | + | + |
| Chaetoceros wighamii | Brightwell | + | + | |
| Cocconeis pediculus | Ehrenberg | + | + | |
| Cocconeis placentula | Ehrenberg | + | ||
| Cocconeis sp. | Ehrenberg | + | + | |
| Cyclotella sp. | (Kützing) Brėbisson | + | + | |
| Cylindrotheca closterium | (Ehrenberg) Reimann & J.C.Lewin | + | + | + |
| Diatoma moniliformis | Kützing | + | + | |
| Diatoma tenuis | C.Agardh | + | + | |
| Diatoma vulgaris | Bory | + | + | |
| Diploneis didyma | (Ehrenberg) Ehrenberg | + | + | |
| Diploneis interrupta | (Kützing) Cleve | + | + | |
| Encyonema prostratum | (Berkeley) Kützing | + | + | |
| Entomoneis paludosa | (W.Smith) Reimer | + | + | + |
| Epithemia gibba | (Ehrenberg) Kützing | + | ||
| Epithemia sp. | Kützing | + | + | |
| Fallacia sp. | Kütz | + | + | + |
| Gomphonella olivacea | (Hornemann) Rabenhorst | + | + | + |
| Grammatophora marina | (Lyngbye) Kützing | + | + | + |
| Gyrosigma sp. | Kützing | + | + | |
| Halamphora coffeiformis | (C.Agardh) Mereschkowsky | + | + | + |
| Licmophora gracilis | (Ehrenberg) Grunow | + | + | |
| Licmophora sp. | C.Agardh | + | ||
| Melosira moniliformis | C.Agardh | + | + | + |
| Melosira nummuloides | C.Agardh | + | + | + |
| Navicula gregaria | Donkin | + | + | + |
| Navicula meniscus | Schumann | + | ||
| Navicula palpebralis | Brébisson ex W.Smith | + | + | + |
| Navicula perminuta | Grunow | + | + | + |
| Navicula ramosissima | (C.Agardh) Cleve | + | + | + |
| Navicula sp. | Bory de Saint-Vincent | + | + | |
| Nitzschia dissipata | (Kützing) Rabenhorst | + | ||
| Nitzschia sigma | (Kützing) W.Smith | + | + | |
| Opephora sp. | Petit | + | ||
| Planothidium delicatulum | (Kützing) Round & Bukhtiyarova | + | + | |
| Pleurosigma aestuarii | (Brébisson ex Kützing) W.Smith | + | ||
| Pleurosigma sp. | W. Smith | + | + | + |
| Proschkinia poretzkajae | (Koretkevich) D.G.Mann | + | + | + |
| Rhoicosphenia abbreviata | (C.Agardh) Lange-Bertalot | + | + | + |
| Rhopalodia gibba | (Ehrenberg) Otto Müller | + | + | |
| Surirella brebissonii | Krammer & Lange-Bertalot | + | ||
| Tabularia fasciculata | (C.Agardh) D.M.Williams & Round | + | + | + |
| Tryblionella sp. | (Grunow) | + | + | |
| Cyanobacteria | ||||
| Dolichospermum flos-aquae | (Bornet & Flahault) P.Wacklin | + | + | + |
| Cyanobacteria sp. | + | |||
| Merismopedia sp. | Meyen | + | + | + |
| Microcystis sp. | Lemmermann | + | + | |
| Nodularia sp. | Mertens ex Bornet & Flahault | + | + | |
| Oscillatoria sp. | Vaucher ex Gomont | + | ||
| Spirulina major | Kützing ex Gomont | + | + | |
| Spirulina subsalsa | Oersted ex Gomont | + | + | + |
| Woronichinia sp. | A.A.Elenkin | + | + | |
| Dinophyceae | ||||
| Peridinium sp. | Ehrenberg | + | + | |
| Chlorophyta | ||||
| Pseudopediastrum boryanum | (Turpin) E.Hegewald | + | ||
| Scenedesmus sp. | Meyen | + | + | |
| Haptophyta | ||||
| Prymnesium sp. | N.Carter | + | + | |
Appendix B

Table A2.
Tables of the statistical analysis results for chosen taxa.
Table A2.
Tables of the statistical analysis results for chosen taxa.
| Taxon | Day of Experiment | CuCl_0.0002 g/dm3 | CuCl_0.002 g/dm3 | Glyphosate_0.042 g/dm3 | Glyphosate _0.85 g/dm3 | Glyphosate _8.5 g/dm3 | [BMIM]Cl_0.00113 g/dm3 | [BMIM]Cl_0.0175 g/dm3 |
|---|---|---|---|---|---|---|---|---|
| Achnanthes brevipes | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0.033 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Bacillaria paxillifera | 3 | 0.011 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Diatoma moniliformis | 3 | 0.0001 | 0.012 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Entomoneis paludosa | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 1 | 0 | 0 | 0 | 1 | |
| Grammatophora marina | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | |
| Melosira nummuloides | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0.322 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Merismpoedia sp. | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0.0589 | 0 | 0 | 0 | 0 | |
| Navicula perminuta | 3 | 0 | 0 | 0 | 0.0002 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Spirulina sp. | 3 | 0 | 0 | 1 | 1 | 0 | 1 | 1 |
| 7 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | |
| Tabularia fasciculata | 3 | 0 | 0 | 0 | 0.682 | 0 | 0 | 0.093 |
| 7 | 0 | 0 | 0 | 0.238 | 0 | 0 | 0 |
References
- European Environment Agency. The European Environment—State and Outlook 2020: Knowledge for Transition to a Sustainable Europe; Publications Office of the European Union: Luxembourg, 2020. [Google Scholar]
- Rocha, A.C.S.; Reis-Henriques, M.A.; Galhano, V.; Ferreira, M.; Guimarães, L. Toxicity of seven priority hazardous and noxious substances (HNSs) to marine organisms: Current status, knowledge gaps and recommendations for future research. Sci. Total Environ. 2016, 542, 728–749. [Google Scholar] [CrossRef] [PubMed]
- Radix, P.; Léonard, M.; Papantoniou, C.; Roman, G.; Saouter, E.; Gallotti-Schmitt, S.; Vasseur, P. Comparison of four chronic toxicity tests using algae, bacteria, and invertebrates assessed with sixteen chemicals. Ecotoxicol. Environ. Saf. 2000, 47, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Forouhar Vajargah, M.; Mohamadi Yalsuyi, A.; Sattari, M.; Prokić, M.D.; Faggio, C. Effects of copper oxide nanoparticles (CuO-NPs) on parturition time, survival rate and reproductive success of guppy fish, Poecilia reticulata. J. Clust. Sci. 2020, 31, 499–506. [Google Scholar] [CrossRef]
- Lewis, M.A. Use of freshwater plants for phytotoxicity testing: A review. Environ. Pollut. 1995, 87, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhong, X.; Wang, Y.; Zhao, Q.; Huang, H. Growth Performance and Antioxidative Response of Chlorella pyrenoidesa, Dunaliella salina, and Anabaena cylindrica to Four Kinds of Ionic Liquids. Appl. Biochem. Biotechnol. 2021, 193, 1945–1966. [Google Scholar] [CrossRef]
- Dahl, B.; Blanck, H. Pollution-induced community tolerance (PICT) in periphyton communities established under tri-n-butyltin (TBT) stress in marine microcosms. Aquat. Toxicol. 1995, 62, 35–44. [Google Scholar] [CrossRef]
- Blanck, H.; Eriksson, K.M.; Grönvall, F.; Dahl, B.; Guijarro, K.M.; Birgersson, G.; Kylin, H. A retrospective analysis of contamination and periphyton PICT patterns for the antifoulantirgarol 1051, around a small marina on the Swedish west coast. Mar. Poll. Bull. 2009, 58, 230–237. [Google Scholar] [CrossRef]
- Sylwestrzak, Z.; Pniewski, F. The influence of the culture medium on the results of experiments testing the impact of a toxic substance on microphytobenthos communities. In Achievements of Young Scientists; Kuczera, M., Piech, K., Eds.; Creativetime: Kraków, Poland, 2014. (In Polish) [Google Scholar]
- Potapova, M.; Charles, D.F. Diatom metrics for monitoring eutrophication in rivers of the United States. Ecol. Indic. 2007, 7, 48–70. [Google Scholar] [CrossRef]
- Pennesi, C.; Danovaro, R. Assessing marine environmental status through microphytobenthos assemblages colonizing the Autonomous Reef Monitoring Structures (ARMS) and their potential in coastal marine restoration. Mar. Pollut. Bull. 2017, 125, 56–65. [Google Scholar] [CrossRef]
- Garside, M. Statista. 2023. Available online: https://www.statista.com/statistics/1236733/europe-annual-refined-copper-consumption/ (accessed on 17 October 2023).
- Coates, R.M.; Denmark, S.E. (Eds.) Reagents, Auxiliaries, and Catalysts for CC Bond Formation; John Wiley & Sons: Hoboken, NJ, USA, 1999. [Google Scholar]
- Stauber, J.L.; Florence, T.M. Mechanism of toxicity of ionic copper and copper complexes to algae. Mar. Biol. 1987, 94, 511–519. [Google Scholar] [CrossRef]
- Manimaran, K.; Karthikeyan, P.; Ashokkumar, S.; Prabu, V.A.; Sampathkumar, P. Effect of copper on growth and enzyme activities of marine diatom, Odontella mobiliensis. Bull. Environ. Contam. Toxicol. 2012, 88, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Serwatka, M.; Zgrundo, A.; Sylwestrzak, Z.; Śliwińska, S. Effect of CuCl2 on growth and motility of the marine diatom Cylinrotheca closterium (Ehremberg) Lewin and Reimann. Eur. J. Phycol. 2015, 50 (Suppl. 1), 121–217. [Google Scholar]
- Li, B.; Zhang, X.; Deng, J.; Cheng, Y.; Chen, Z.; Qin, B.; Tefsen, B.; Wells, M. A new perspective of copper-iron effects on bloom-forming algae in a highly impacted environment. Water Res. 2021, 195, 116889. [Google Scholar] [CrossRef] [PubMed]
- Morelli, E.; Scarano, G. Copper-induced changes of non-protein thiols and antioxidant enzymes in the marine microalga Phaeodactylum tricornutum. Plant Sci. 2004, 167, 289–296. [Google Scholar] [CrossRef]
- Fernandes, J.C.; Henriques, F.S. Biochemical, physiological, and structural effects of excess copper in plants. Bot. Rev. 1991, 57, 246–273. [Google Scholar] [CrossRef]
- Guasch, H.; Navarro, E.; Serra, A.; Sabater, S. Phosphate limitation influences the sensitivity to copper in periphytic algae. Freshw. Biol. 2004, 49, 463–473. [Google Scholar] [CrossRef]
- Kierzkowski, D.J.; Puetz, J.D.; Wei, G. Formulated Copper Algaecides. U.S. Patent No. 6,069,113, 30 May 2000. [Google Scholar]
- Macar, T.K.; Macar, O.; Yalçın, E. Resveratrol ameliorates the physiological, biochemical, cytogenetic, and anatomical toxicities induced by copper(II) chloride exposure in Allium cepa L. Environ. Sci. Pollut. Res. 2020, 27, 657–667. [Google Scholar] [CrossRef] [PubMed]
- Shiyab, S. Phytoaccumulation of copper from irrigation water and its effect on the internal structure of lettuce. Agriculture 2018, 8, 29. [Google Scholar] [CrossRef]
- Ochoa-Herrera, V.; León, G.; Banihani, Q.; Field, J.A.; Sierra-Alvarez, R. Toxicity of copper (II) ions to microorganisms in biological wastewater treatment systems. Sci. Total Environ. 2011, 412, 380–385. [Google Scholar] [CrossRef]
- National Primary Drinking Water Regulations; EPA: Washington, DC, USA, 2015.
- Statista. Available online: https://www.statista.com/statistics/567250/glyphosate-use-worldwide (accessed on 17 October 2023).
- Wynn, S.; Elise, W. Impact assessment of the loss of glyphosate within the EU: A literature review. Environ. Sci. Eur. 2022, 34, 91. [Google Scholar] [CrossRef]
- Steinmann, H.H.; Dickeduisberg, M.; Theuvsen, L. Uses and benefits of glyphosate in German arable farming. Crop Prot. 2012, 42, 164–169. [Google Scholar] [CrossRef]
- Franz, J.E.; Mao, M.K.; Sikorski, J.A. Glyphosate: A Unique Global Herbicyde; ACS Monograph 189; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- King, J.J.; Wagner, R.S. Toxic effects of the herbicide Roundup® Regular on Pacific Northwestern amphibians. Northwestern Nat. 2010, 91, 318–324. [Google Scholar] [CrossRef]
- Braz-Mota, S.; Sadauskas-Henrique, H.; Duarte, R.M.; Val, A.L.; Almeida-Val, V.M. Roundup® exposure promotes gills and liver impairments, DNA damage and inhibition of brain cholinergic activity in the Amazon teleost fish Colossoma macropomum. Chemosphere 2015, 135, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Annett, R.; Habibi, H.R.; Hontela, A. Impact of glyphosate and glyphosate-based herbicides on the freshwater environment. J. Appl. Toxicol. 2014, 34, 458–479. [Google Scholar] [CrossRef] [PubMed]
- Mercurio, P.; Flores, F.; Mueller, J.F.; Carter, S.; Negri, A.P. Glyphosate persistence in seawater. Mar. Pollut. Bull. 2015, 85, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.M.; Kroes, R.; Munro, I.C. Safety evaluation and risk assessment of the herbicide roundup and its active ingredient, glyphosate, for humans. Regul. Toxicol. Pharmacol. 2000, 31, 117–165. [Google Scholar] [CrossRef] [PubMed]
- Greim, H.; Saltmiras, D.; Mostert, V.; Strupp, C. Evaluation of carcinogenic potential of the herbicide glyphosate, drawing on tumor incidence data from fourteen chronic/carcinogenicity rodent studies. Crit. Rev. Toxicol. 2015, 45, 185–208. [Google Scholar] [CrossRef]
- Anadon, A.; Martinez-Larranaga, M.; Martínez, M.; Castellano, V.; Martínez, M.; Martin, M.; Nozal, M.; Bernal, J. Toxicokinetics of glyphosate and its metabolite aminomethyl phosphonic acid in rats. Toxicol. Lett. 2009, 190, 91–99. [Google Scholar] [CrossRef]
- Kulacki, K.J.; Lamberti, G.A. Toxicity of imidazolium ionic liquids to freshwater algae. Green Chem. 2008, 10, 104–110. [Google Scholar] [CrossRef]
- Isosaari, P.; Srivastava, V.; Sillanpää, M. Ionic liquid-based water treatment technologies for organic pollutants: Current status and future prospects of ionic liquid mediated technologies. Sci. Total Environ. 2019, 690, 604–619. [Google Scholar] [CrossRef] [PubMed]
- Szepiński, E. Synthesis, Properties and Application of Ionic Liquids, Derivatives of Natural Organic Compounds. Ph.D. Thesis, Politechnika Gdańska, Gdańsk, Poland, 2019. (In Polish). [Google Scholar]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of cellose with ionic liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Nikitenko, S.I.; Berthon, C.; Moisy, P. Instability of actinide (IV) hexachloro complexes in room-temperature ionic liquid [BuMeIm] PF6 due to hydrolysis of the hexafluorophosphate anion. Comptes Rendus Chim. 2007, 10, 1122–1127. [Google Scholar] [CrossRef]
- Freire, M.G.; Neves, C.M.; Marrucho, I.M.; Coutinho, J.A.; Fernandes, A.M. Hydrolysis of tetrafluoroborate and hexafluorophosphate counter ions in imidazolium-based ionic liquids. J. Phys. Chem. A 2010, 114, 3744–3749. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dong, J.; Li, B.; Xue, C.; Tetteh, P.A.; Li, D.; Gao, K.; Deng, X. Using a freshwater green alga Chlorella pyrenoidosa to evaluate the biotoxicity of ionic liquids with different cations and anions. Ecotoxicol. Environ. Saf. 2020, 198, 110604. [Google Scholar] [CrossRef]
- Ma, J.M.; Cai, L.L.; Zhang, B.J.; Hu, L.W.; Li, X.Y.; Wang, J.J. Acute toxicity and effects of 1-alkyl-3-methylimidazolium bromide ionic liquids on green algae. Ecotoxicol. Environ. Saf. 2010, 73, 1465–1469. [Google Scholar] [CrossRef]
- Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F. Chapter 9. The use of microphytobenthos in ecotoxicological tests–methodology. In Research Methodology Used by Young Scientists; Bochentyn, B., Walkowicz, M., Eds.; Creativetime: Kraków, Poland, 2014. (In Polish) [Google Scholar]
- Bubalo, M.C.; Radošević, K.; Redovniković, I.R.; Halambek, J.; Srček, V.G. A brief overview of the potential environmental hazards of ionic liquids. Ecotoxicol. Environ. Saf. 2014, 99, 1–12. [Google Scholar] [CrossRef]
- Matzke, M.; Thiele, K.; Müller, A.; Filser, J. Sorption and desorption of imidazolium based ionic liquids in different soil types. Chemosphere 2009, 74, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Mrozik, W.; Kotłowska, A.; Kamysz, W.; Stepnowski, P. Sorption of ionic liquids onto soils: Experimental and chemometric studies. Chemosphere 2012, 88, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Stepnowski, P.; Mrozik, W.; Nichthauser, J. Adsorption of alkylimidazolium and alkylpyridinium ionic liquids onto natural soils. Environ. Sci. Technol. 2007, 41, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Studzińska, S.; Kowalkowski, T.; Buszewski, B. Study of ionic liquid cations transport in soil. J. Hazard. Mater. 2009, 168, 1542–1547. [Google Scholar] [CrossRef]
- Kumar, M.; Trivedi, N.; Reddy, C.R.K.; Jha, B. Toxic effects of imidazolium ionic liquids on the green seaweed Ulva lactuca: Oxidative stress and DNA damage. Chem. Res. Toxicol. 2011, 24, 1882–1890. [Google Scholar] [CrossRef] [PubMed]
- Steudte, S.; Stepnowski, P.; Cho, C.W.; Thöming, J.; Stolte, S. (Eco) toxicity of fluoro-organic and cyano-based ionic liquid anions. Chem. Commun. 2012, 48, 9382–9384. [Google Scholar] [CrossRef] [PubMed]
- Frade, R.F.; Afonso, C.A. Impact of ionic liquids in environment and humans: An overview. Hum. Exp. Toxicol. 2010, 29, 1038–1054. [Google Scholar] [CrossRef] [PubMed]
- Ranke, J.; Müller, A.; Bottin-Weber, U.; Stock, F.; Stolte, S.; Arning, J.; Jastorff, B. Lipophilicity parameters for ionic liquid cations and their correlation to in vitro cytotoxicity. Ecotoxicol. Environ. Saf. 2007, 67, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Sylwestrzak, Z. Effect of Selected Abiotic Factors on Baltic Microphytobenthic Communities. Ph.D. Thesis, Faculty of Oceanography and Geography, University of Gdańsk, Gdańsk, Poland, 2023; 112p. (In Polish). [Google Scholar]
- Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F. Ecotoxicological Studies on the Effect of Roundup®(Glyphosate Formulation) on Marine Benthic Microalgae. Int. J. Environ. Res. Public Health 2021, 18, 884. [Google Scholar] [CrossRef]
- Sylwestrzak, Z.; Zgrundo, A.; Pniewski, F. Copper chloride (II) effect on the composition and structure of marine microphytobenthic communities. Environ. Monit. Assess. 2022, 194, 443. [Google Scholar] [CrossRef] [PubMed]
- Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on Environmental Quality Standards in the Field of Water Policy, Amending and Subsequently Repealing Council Directives 82/176/EEC, 83/513/EEC, 84/156/EEC, 84/491/EEC, 86/280/EEC and Amending Directive 2000/60/EC of the European Parliament and of the Council. Available online: https://eur-lex.europa.eu/eli/dir/2008/105/oj (accessed on 22 April 2023).
- Skeff, W.; Neumann, C.; Schulz-Bull, D.E. Glyphosate and AMPA in the estuaries of the Baltic Sea method optimization and field study. Mar. Pollut. Bull. 2015, 100, 577–585. [Google Scholar] [CrossRef]
- Bakierowska, A.; Wojtaszek, A.; Kopiec, J. Assessment of the State of the Environment of the Polish Maritime Areas of the Baltic Sea Based on 2019 Monitoring Data against the Background of the Decade 2009–2018; Zalewska, T., Kraśniewski, W., Eds.; Inspekcja Ochrony Środowiska Warszawa: Warszawa, Poland, 2020. (In Polish) [Google Scholar]
- Ahrens, L. Polyfluoroalkyl Compounds in the Aquatic Environment: A Review of Their Occurrence and Fate. J. Environ. Monit. 2011, 13, 20–31. [Google Scholar] [CrossRef]
- OECD. Test No. 201: Freshwater Alga and Cyanobacteria, Growth Inhibition Test; OECD Publishing: Paris, France, 2011. [Google Scholar]
- Snoeijs, P.; Potapova, M. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1993; Volume 1, 129p. [Google Scholar]
- Snoeijs, P.; Vilbaste, S. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1994; Volume 2, 126p. [Google Scholar]
- Snoeijs, P.; Potapova, M. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1995; Volume 3, 126p. [Google Scholar]
- Snoeijs, P.; Kasperovičiene, J. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1996; Volume 4, 126p. [Google Scholar]
- Snoeijs, P.; Balashova, N. Intercalibration and Distribution of Diatom Species in the Baltic Sea; Opulus Press: Uppsala, Sweden, 1998; Volume 5, 127p. [Google Scholar]
- Cox, E. Identification of Freshwater Diatoms from Live Material; Chapman and Hall: Boca Raton, FL, USA, 1996; 168p. [Google Scholar]
- Pliński, M.; Komárek, J. Cyanoprokaryota with the English key for the identification to the genus. In Flora of the Gulf of Gdańsk and Adjacent Waters (the Southern Baltic Sea); Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2007; 172p. (In Polish) [Google Scholar]
- Pliński, M.; Hindák, F. Green Algae with the English key for the identification to the genus. In Flora of the Gulf of Gdańsk and Adjacent Waters (the Southern Baltic Sea); Wydawnictwo Uniwersytetu Gdańskiego: Gdańsk, Poland, 2010; 140p. (In Polish) [Google Scholar]
- Schmitt-Jansen, M.; Altenburger, R. Toxic effects of isoproturon on periphyton communities–a microcosm study. Estuar. Coast. Shelf Sci. 2005, 62, 539–545. [Google Scholar] [CrossRef]
- Andersson, S.; Kautsky, L. Copper effects on reproductive stages of Baltic Sea Fucus vesiculosus. Mar. Biol. 1996, 125, 171–176. [Google Scholar] [CrossRef]
- AFS. International Convention on the Control of Harmful Anti-Fouling Systems on Ships; AFS: New York, NY, USA, 2001. [Google Scholar]
- Poggio, C.; Colombo, M.; Arciola, C.R.; Greggi, T.; Scribante, A.; Dagna, A. Copper-alloy surfaces and cleaning regimens against the spread of SARS-CoV-2 in dentistry and orthopedics. From fomites to anti-infective nanocoatings. Materials 2020, 13, 3244. [Google Scholar] [CrossRef]
- Ramteke, L.; Gawali, P.; Jadhav, B.L.; Chopade, B.A. Comparative study on antibacterial activity of metal ions, monometallic and alloy noble metal nanoparticles against nosocomial pathogens. BioNanoScience 2020, 10, 1018–1036. [Google Scholar] [CrossRef]
- Ermini, M.L.; Voliani, V. Antimicrobial nano-agents: The copper age. ACS Nano 2021, 15, 6008–6029. [Google Scholar] [CrossRef]
- Stenström, J.R.; Kreuger, J.; Goedkoop, W. Pesticide mixture toxicity to algae in agricultural streams–Field observations and laboratory studies with in situ samples and reconstituted water. Ecotoxicol. Environ. Saf. 2021, 215, 112153. [Google Scholar] [CrossRef]
- Brovini, E.M.; de Deus, B.C.T.; Vilas-Boas, J.A.; Quadra, G.R.; Carvalho, L.; Mendonca, R.F.; de Oliveira Pereira, R.; Cardoso, S.J. Three-bestseller pesticides in Brazil: Freshwater concentrations and potential environmental risks. Sci. Total Environ. 2021, 771, 144754. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, X.; Chen, C.; Du, S.; Dong, Y. Effects of imidazolium chloride ionic liquids and their toxicity to Scenedesmus obliquus. Ecotoxicol. Environ. Saf. 2015, 122, 83–90. [Google Scholar] [CrossRef]
- Sabater, S.; Guasch, H.; Romani, A.; Munoz, I. The effect of biological factors on the efficiency of river biofilms in improving water quality. Hydrobiologia 2002, 469, 149–156. [Google Scholar] [CrossRef]
- Lin, W.; Zhang, Z.; Chen, Y.; Zhang, Q.; Ke, M.; Lu, T.; Qian, H. The mechanism of different cyanobacterial responses to glyphosate. J. Environ. Sci. 2023, 125, 258–265. [Google Scholar] [CrossRef]
- Latała, A.; Nędzi, M.; Stepnowski, P. Toxicity of imidazolium and pyridinium based ionic liquids towards algae. Chlorella vulgaris, Oocystis submarina (green algae) and Cyclotella meneghiniana, Skeletonema marinoi (diatoms). Green Chem. 2009, 11, 580–588. [Google Scholar] [CrossRef]
- Guiry, M.D.; Guiry, G.M.; AlgaeBase. World-Wide Electronic Publication, National University of Ireland, Galway. 7 July 2021. Available online: https://www.algaebase.org (accessed on 20 September 2023).
- Levy, J.L.; Stauber, J.L.; Jolley, D.F. Sensitivity of marine microalgae to copper: The efect of biotic factors on copper adsorption and toxicity. Sci. Total Environ. 2007, 387, 141–154. [Google Scholar] [CrossRef]
- Iummato, M.M.; Fassiano, A.; Graziano, M.; dos Santos Afonso, M.; de Molina, M.D.C.R.; Juárez, Á.B. Effect of glyphosate on the growth, morphology, ultrastructure and metabolism of Scenedesmus vacuolatus. Ecotoxicol. Environ. Saf. 2019, 172, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska-Niczyporuk, A.; Bajguz, A.; Kotowska, U.; Zambrzycka-Szelewa, E.; Sienkiewicz, A. Auxins and cytokinins regulate phytohormone homeostasis and thiol-mediated detoxification in the green alga Acutodesmus obliquus exposed to lead stress. Sci. Rep. 2020, 10, 10193. [Google Scholar] [CrossRef]
- Yu, Y.; Kong, F.; Wang, M.; Qian, L.; Shi, X. Determination of short-term copper toxicity in a multispecies microalgal population using flow cytometry. Ecotoxicol. Environ. Saf. 2007, 66, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Arrhenius, Å.; Backhaus, T.; Hilvarsson, A.; Wendt, I.; Zgrundo, A.; Blanck, H. A novel bioassay for evaluating the efficacy of biocides to inhibit settling and early establishment of marine biofilms. Mar. Pollut. Bull. 2014, 87, 292–299. [Google Scholar] [CrossRef] [PubMed]
- Delpy, F.; Lucas, Y.; Merdy, P. Evaluation of Roundup® effects on Chlorella vulgaris through spectral changes in photosynthetic pigments in fresh and marine water. Environ. Adv. 2022, 8, 100240. [Google Scholar] [CrossRef]
- Vera, M.S.; Lagomarsino, L.; Sylvester, M.; Pérez, G.L.; Rodríguez, P.; Mugni, H.; Pizarro, H. New evidences of Roundup®(glyphosate formulation) impact on the periphyton community and the water quality of freshwater ecosystems. Ecotoxicology 2010, 19, 710–721. [Google Scholar] [CrossRef] [PubMed]
- Benegas, G.S.; Correa-Perez, C.; Mendez-Gaona, S. Impact of Pesticides on Cyanobacteria in Aquatic Ecosystems. In Cyanobacterial Biotechnology in the 21st Century; Springer Nature: Singapore, 2023; pp. 197–220. [Google Scholar]
- Neethu, K.V.; Saranya, K.S.; Krishna, N.G.A.; Praved, P.H.; Aneesh, B.P.; Nandan, S.B.; Marigoudar, S.R. Toxicity of copper on marine diatoms, Chaetoceros calcitrans and Nitzchia closterium from Cochin estuary, India. Ecotoxicology 2021, 30, 783–793. [Google Scholar] [CrossRef]
- Hernández-García, C.I.; Martínez-Jerónimo, F. Multistressor negative effects on an experimental phytoplankton community. The case of glyphosate and one toxigenic cyanobacterium on Chlorophycean microalgae. Sci. Total Environ. 2020, 717, 137186. [Google Scholar] [CrossRef]
- Fan, H.; Jin, M.; Wang, H.; Xu, Q.; Xu, L.; Wang, C.; Liu, H. Effect of differently methyl-substituted ionic liquids on Scenedesmus obliquus growth, photosynthesis, respiration, and ultrastructure. Environ. Pollut. 2019, 250, 155–165. [Google Scholar] [CrossRef]
- Kim, Y.; Ponomarev, A.V. Low-dose electron beam treatment of red tide blooms microalgae. Radiat. Phys. Chem. 2021, 179, 109201. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).