1. Background
The concept of a digital twin refers to a virtual model of a tangible entity or physical system, which can be used to study, monitor, and optimize the composition and functions of its physical counterpart [
1,
2]. DTs can be used to simulate a variety of entities with increasing levels of complexity, ranging from everyday consumer objects to transportation systems, cities, ecosystems, and the human body [
3].
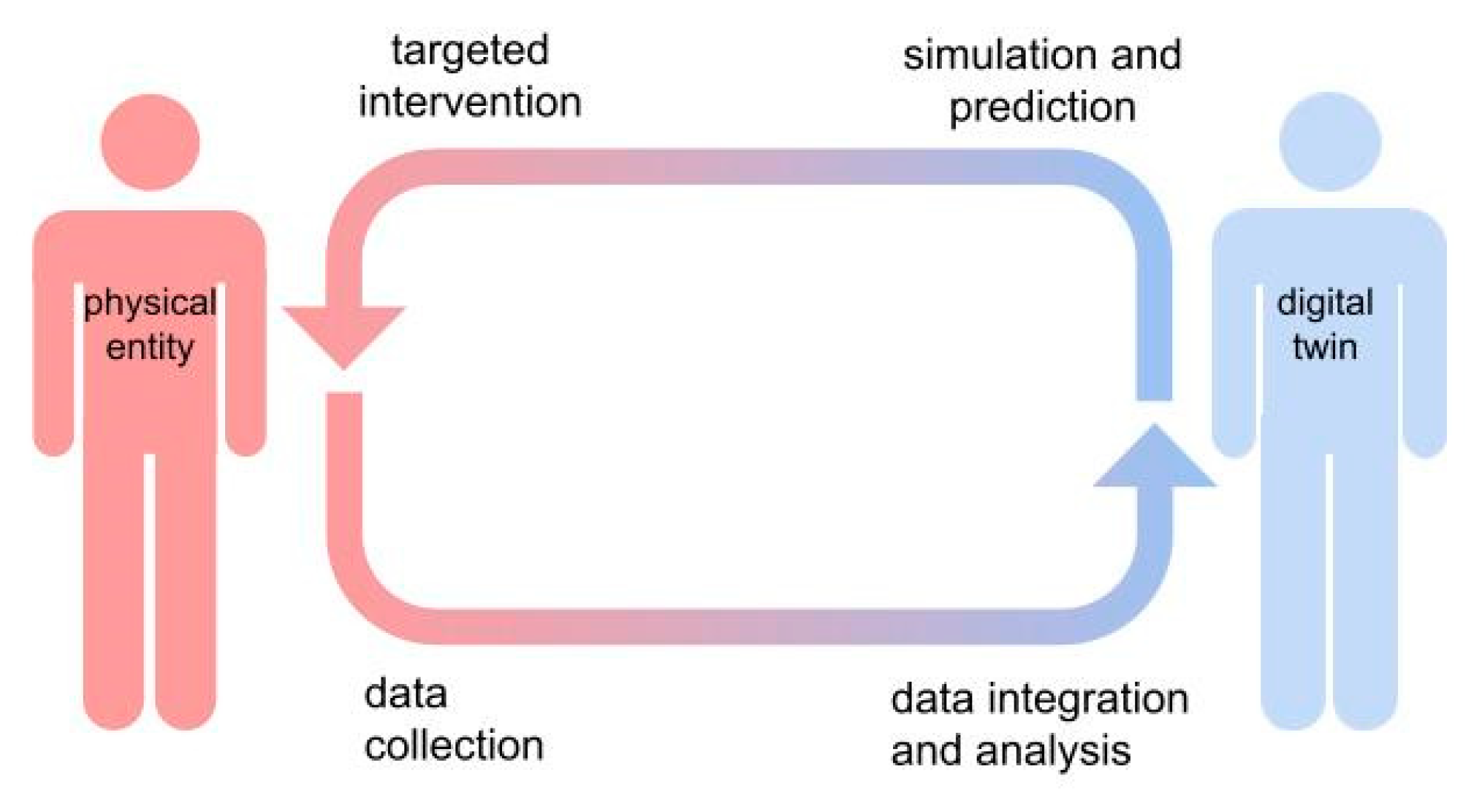
This concept was first introduced in 2003 by Dr. Michael Grieves, a professor at the University of Michigan, who described digital twins as a way to create a “real-time digital representation of a physical object or process, which allows for simulation, prediction, and optimization” [
4] (
Figure 1). Digital twins provide powerful insight into the inner workings of a physical system, allowing for in silico simulations of several real-world scenarios through the arbitrary manipulation of specific variables and parameters of interest, obtaining accurate predictions of the behavior of the system under different conditions.
First conceptualized in the aerospace industry, the digital twin idea has become increasingly popular in the context of Industry 4.0 along with the growing trend of automation and digitization, attracting considerable interest in a variety of sectors, including manufacturing [
5], transportation [
6,
7], and healthcare [
3]. In manufacturing, they have demonstrated significant results in predictive maintenance and performance optimization, without tampering with or exhausting the original physical product [
5]. In the automotive industry, sensors allow for continuous real-time data detection and interchange with remote control centers, increasing the flexibility and customization of services and user safety [
6,
7].
In medicine, the most ambitious application of digital twins aims to achieve a complete digital representation of the physical patient (hereinafter referred to Digital Human Twin, DHT) in order to improve the prevention, diagnosis, and treatment of diseases. Despite this long-term perspective, currently available applications mostly concern the simulation of single anatomical structures (tissues or organs), physiological processes, or metabolic interactions [
8]. At another level, the evolution of digital twins will concern the simulation of healthcare institutions or processes, aimed at improving organizations’ workflow and resource management [
8]. These models would obviously benefit, in perspective, from the development of DHTs that represent virtual copies of individual patients and that could be integrated into higher-level representations. In all these settings, digital twin applications could improve the study and monitoring of highly complex systems characterized by many interacting components and thousands of variables that can be difficult to characterize with traditional approaches.
With the rise in the Internet of Things paradigm, advancements in medical technology, and the growing popularity of wearable devices for biosignal monitoring and health promotion, there exists a vast amount of data available for the development of digital twins.
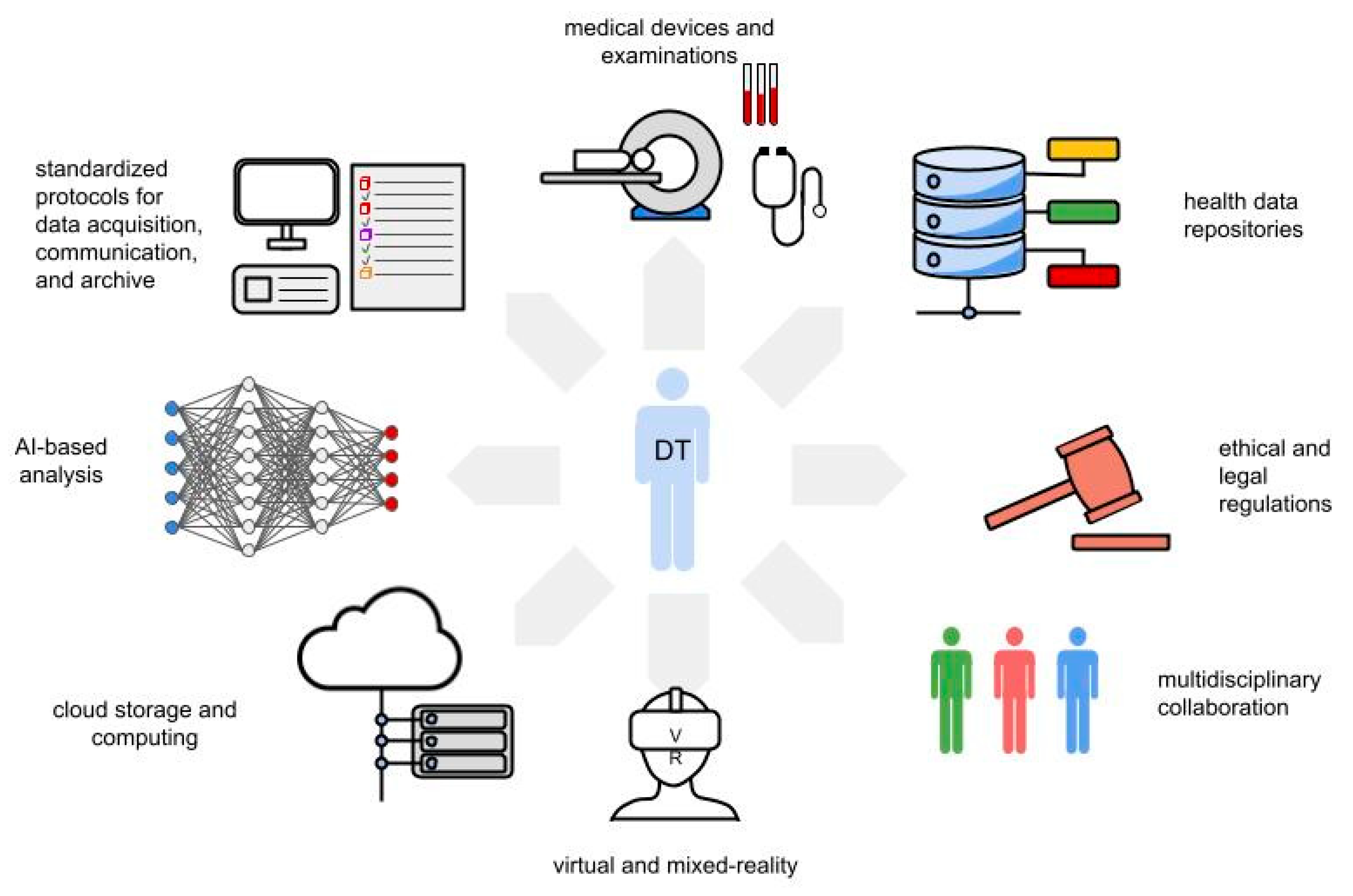
The construction of a digital twin is based on an intricate constellation of skills, professional figures, and technological devices that make up the digital twin framework (
Figure 2). This includes large-scale biological data repositories, cloud storage, and computing; standardized protocols for data acquisition and communication; multidisciplinary collaboration; artificial-intelligence-based algorithms; virtual reality technologies; and robust legal and ethical frameworks.
While the digital twin idea has been extensively studied in manufacturing and engineering, its potential application to the human body to create a DHT is still unfolding with vast open prospects [
3]. Thanks to the evolution of underlying technologies, the applications of digital twins in medicine hold immense potential for revolutionizing personalized medicine and transforming healthcare practices.
In this article, two physicians, experienced in imaging research, artificial intelligence and digital twin, conducted a comprehensive literature research on Pubmed and Google Scholar using the keywords “digital twin”, “human digital twin”, “digital twin & healthcare”, “digital twin & medicine”, “digital twin & drug development”, “digital twin & transplant”, “digital twin & surgery”. The abstracts were evaluated, and the appropriate article collected on an Excel database to create this narrative review.
Through this research we aim to examine the definition and applications of digital twin in healthcare with a special focus on DHT (virtual replicas of patients), and discuss its potential applications, current status, challenges, and future implications in medicine.
2. The Digital Human Twin (DHT) Framework
DHT consists of virtual replicas of an individual which encompasses all the morphological (anatomical), physiological, and-ideally-cognitive properties, to establish an all-round digital representation of the human body. A key feature of the DHT is the bi-directional data flow between the physical entity and its virtual replica, allowing for real-time continuous updating of the virtual model and, conversely, targeted interventions on the physical systems based on predictive simulations performed on DHTs.
Due to the complexity of biological organisms, the construction of DHTs requires advanced data analytics and visualization tools for multi-scale biological data including three main domains: (1) so-called multi-omics sciences, such as genomics, transcriptomics, proteomics and metabolomics, (2) morphological (anatomical) data, and (3) biofunctional data (for example, cardiac electrophysiology) (
Figure 3). A DHT aims to represent the virtual replica of an individual or patient with its distinctive genotypic and phenotypic signature, ideally accounting also for the complex interplay between organisms and the environment.
The idea of building a well-rounded DHT is still a future prospect, however, many companies and research institutes have been exploring the possibility of building digital copies of body parts or physiological systems aimed at specific applications [
8]. These accelerate the paradigm shift towards personalized medicine, helping to optimize the therapeutic approach in a tailored way, to predict the future health needs of individuals and populations, and to plan public health policies and interventions [
8].
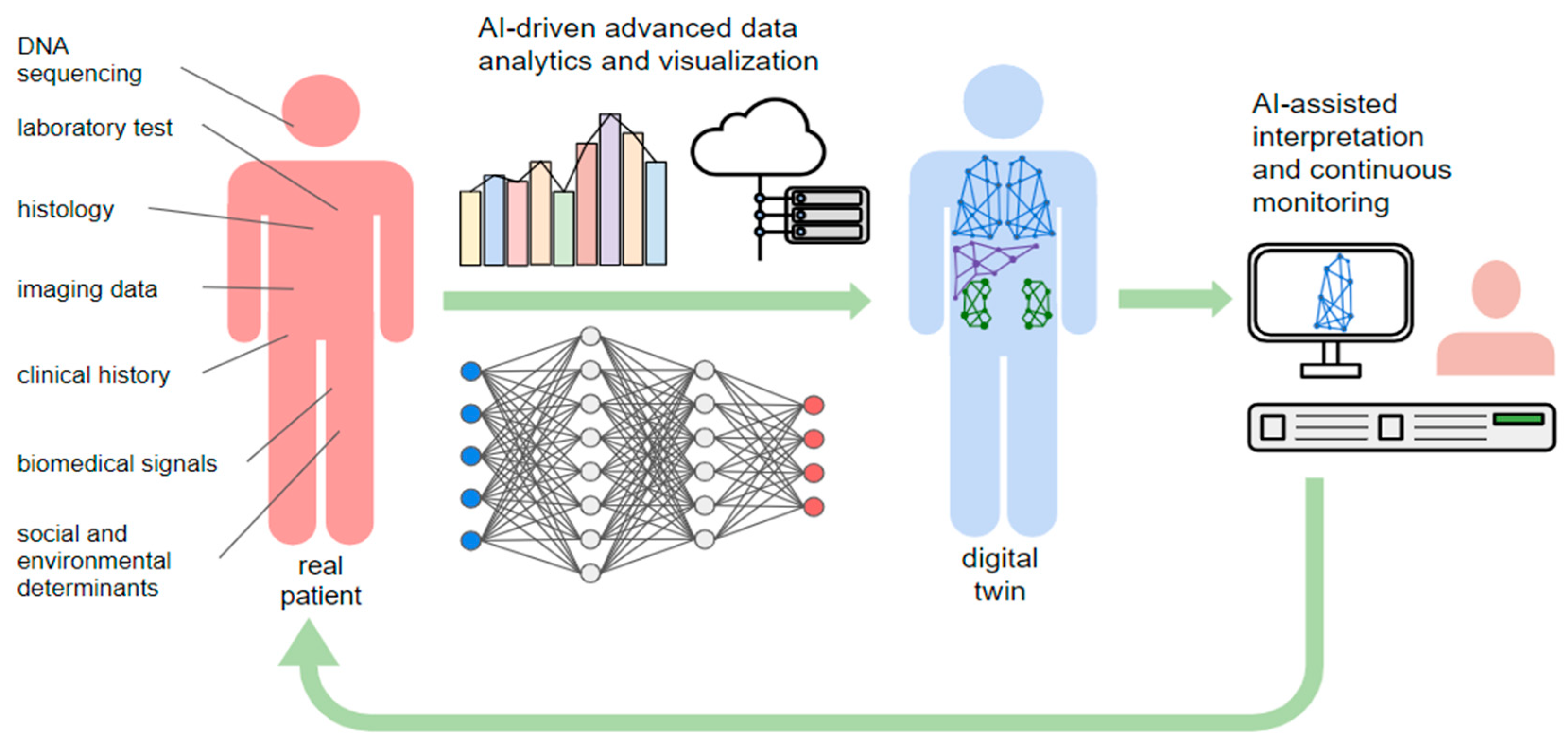
The development of a DHT requires access to large-scale healthcare data repositories, including genetic data and data from medical records, imaging, histopathology, and other relevant sources, that must be high quality, accurate, and complete [
9] (
Figure 4).
Data are processed through advanced analytics and AI-based techniques, including Deep Learning models, which can enhance the sophistication of the DHT simulations, enabling predictive simulations and decision support tools with a high throughput processing of thousands of disease-relevant variables. Deep learning methods address this challenge by using a computationally convenient representation of non-linear maps via artificial neural networks [
8,
10].
Virtual modeling requires high-performance computing capabilities for processing and analyzing extensive biological data, while maintaining effective predictions of real-time interactions [
8,
10]. For this reason, the construction of a digital twin has especially led to growing links between big data companies, which own the computational and storage resources, healthcare companies which provide the data, and biomedical research centers which guide the analysis and interpretation of models [
8,
10].
A critical aspect of the implementation of digital twins is the lack of interconnectivity among different systems and the medical devices involved in the DHT framework. The adoption of standardized data representation and exchange protocols facilitates seamless communication and the integration of electronic health records, medical devices, and other healthcare systems [
11]. This interoperability enables the exchange of patient-specific information, enhancing the accuracy and completeness of the DHT model [
12].
Digital twin implementation requires multidisciplinary collaboration and cross-fertilization between different figures, including physicians, radiologists and image processing experts, molecular biologists, geneticists and bioinformaticians, computer scientists, data scientists, and engineers, who need to work collectively to address the complex challenges associated with building an accurate and reliable digital replica [
13].
Finally, alongside technical advances, ethical and legal issues are of paramount importance in establishing this new paradigm. Ensuring data privacy, security, and consent management is critical in building trust and safeguarding the sensitive nature of healthcare data [
14,
15].
The potential benefits of DHTs in healthcare are enormous, and as the technology continues to evolve and improve, we can expect to see even more innovative applications in the future [
16]. Potential applications in healthcare include (
Figure 5):
- -
Person-centric prevention and risk stratification: risk stratification and personalized screening strategies aimed at early diagnosis of diseases [
12];
- -
Disease modeling and clinical decision support: molecular, morphological, and physiological disease modeling that enables accurate prediction of symptom onset, disease progression, and complications, to support clinical decision-making and worklist prioritizations [
8];
- -
Targeted therapy: DHTs allow the development of highly effective personalized therapies to target the specific molecular mechanism underlying the disease to personalize treatment planning for patients: the right molecule for the right disease to the right patient [
8];
- -
Surgical planning: virtual replicas of organs (organs DHT) and systems can be created using imaging data and other pertinent data sources, allowing doctors to simulate the procedure and optimize the surgical plan before the actual surgery [
17];
- -
Organizations: high-throughput analyses of large amounts of clinical data based on DHTs that are highly reproducible replicas of real patients could ultimately enable the healthcare system to better plan public health interventions, resulting in significant savings in time and resources and reduced physician and cognitive workload for operators [
18];
- -
Medical training: thanks to the use of virtual and augmented reality tools, it will be possible to create increasingly realistic scenarios in which DHTs can be used as educational tools for training medical students and residents, allowing the simulation of challenging procedures in a safe environment reproducing the features and behaviors of real-world patients [
19].
3. Person-Centered Risk Stratification and Prevention
As digital replicas of individual patients, ideally incorporating the complex and unique interplay between physiology, anatomy, and the environment, DHTs could play a key role in risk stratification of disease development by integrating multiclass data from healthcare archives, live medical interviews, and wearable devices. DHTs ideally represent a valid framework for structuring and representing this large amount of data in a unified way, enabling the development of tailored screening strategies for earlier diagnosis and better prognosis [
12].
Leveraging advanced AI-driven algorithms and data mining techniques, DHT can integrate multi-level patient information such as social determinants, medical images, laboratory data, histology, and biological signals into complex virtual representations of the individual. Moreover, DHT can also provide useful insights into the role of different genetic, behavioral, and environmental factors in pathogenesis, clarifying their contribution to the development of lifestyle and work-related diseases [
8].
DHTs can help care providers and institutions optimize screening strategies at the population level, improve patient outcomes, and support more personalized and proactive approaches to healthcare [
20]. Additionally, these virtual replicas enable predictive assessment of the impact of different screening strategies and interventions on health and patient populations, allowing healthcare providers to simulate and compare the effectiveness of different screening strategies and algorithms, improving their accuracy, and reducing false positive/negative results [
8]. This information can aid in the development of targeted screening strategies for high-risk individuals or specific populations [
21,
22]. Moreover, by combining data from multiple sources, including electronic health records, wearable devices, and environmental sensors, DHTs can analyze trends and patterns to predict the likelihood of disease occurrence or progression, anticipating the need for treatment [
21]. For example, Suzuki et al. used computational fluid dynamics to derive hemodynamic parameters from CT images and develop a personalized risk model of small intracranial aneurysm ruptures guided by anatomical images and using a multivariate logistic regression model [
23].
4. Disease Modeling and Clinical Decision Support
Organs, tissues, and metabolic interactions can be virtually recreated and modeled to study the progression of the disease and predict how it will affect the patient over time and their response to therapy. This computer-generated simulation of the disease state can be run within the DHT and used to monitor and predict clinical outcomes and optimize treatment plans in a truly ‘holistic’ approach [
8].
Data from historical medical records, genetic, histological, imaging, behavioral, and social data which are integrated to create the virtual replica of the patient or the affected organs, can be analyzed by AI-driven algorithms, and associated with similar cases to predict the progression and efficacy of therapies. Real-time supervision tools can actually help healthcare workers and organizations improve patient management. DHTs can be used to implement an alerting system in case of critical abnormalities, unexpected changes, or lack of treatment-beneficial effects, allowing a timely intervention or a proper treatment modification.
These concepts seem particularly suitable for highly complex chronic diseases, such as multiple sclerosis, characterized by a heterogeneous course and multiple treatment strategies [
21]. So far, DT systems are mostly confined to the research field and the daily clinical use is still limited, even if some organ replicas have already been developed and can be potentially used for remote monitoring of the organ functionality and to test new treatments before the actual use on patients [
8].
Some heart models have been developed combining electrophysiological ECG signals with morphological CT scan data (ECVUE™, CardioInsight Inc., Cleveland), yielding a round virtual representation of the patient’s heart functionality, including blood flow dynamics, contracting mechanics, and electrical impulses, generating a simultaneous 3D cardiac map [
24].
These systems could be used to better characterize the disease, plan interventional procedures, and simulate the effect of a drug on cardiac dynamics and the effects of a pacemaker before actual implantation, predicting the efficacy of the treatment on the patient [
8]. Similar maps are already being used to continuously remotely monitor a patient’s heart activity and alert doctors when a dangerous arrhythmia occurs [
25].
Another emblematic example is provided by the elderly with type 2 diabetes, who need continuous glucose monitoring and a precise personalized drug balance in order to avoid hypo and hyperglycemia in the short term and to prevent complications in the long term. In a recent paper, Thamotharan et al. presented a DHT framework tested on 15 elderly patients, based on complex mathematical and deep-learning models able to mimic each diabetic patient, updating the data at the same time from glycemia measurements. The results show an improvement of the time-in-range from 3–75% to 86–97%, a reduction in hyper and hypo-glycemia events, and a decrease in insulin infusions by 14–29% [
26].
Furthermore, older people would particularly benefit from these technologies, as they often live alone, are not fully aware of their debilitating condition, and are unable to adequately communicate their symptoms [
26].
5. Surgical Planning
DHT enables the creation of accurate, three-dimensional models of a patient’s anatomy based on medical imaging data, such as CT scans or MRI sequences. These virtual replicas provide surgeons with a comprehensive and detailed visualization of the patient’s anatomy, allowing for better lesion localization and a deeper understanding of complex structures, spatial relationships, and potential anatomical variations [
2,
27].
Creating virtual replicas of DHT-embedded organs and systems using imaging data and other pertinent data sources allows physicians to plan and simulate surgical procedures before they are performed on real patients.
DHTs enable in-depth assessment of the patient’s anatomy and the morphological characteristics of the lesion, the lesion’s relationships with blood vessels, and other critical structures, helping to determine optimal incision sites and guiding surgical resection in real time, improving patient safety and surgical outcomes [
28]. Furthermore, surgeons can virtually experiment with different approaches and evaluate and compare the feasibility and results of different techniques, anticipating potential challenges or complications. For example, Gols et al. demonstrated that by using estimated hepatic flow velocity as input data, a DHT model could correctly predict postoperative portal hypertension [
29].
DHTs can also be integrated with virtual or augmented reality technologies to provide surgeons with an immersive and interactive planning environment, allowing for a more intuitive understanding of the patient’s anatomy and facilitating better surgical planning and decision making [
17].
In brain tumor surgery, the development of DHTs that faithfully replicate the morpho-functional organization of cortico-subcortical circuits will assist neurosurgeons in planning the operation, delimiting the extent of the lesion and its relationships with the cerebral connectome, thus allowing precision brain surgery as radical as reasonably acceptable to preserve the quality of life [
30]. The imaging and functional data acquired in a preliminary phase will be used for the development of models of neuronavigation in the operating room to guide access and the first phases of the intervention. During the procedure, DHTs can serve as a reference tool for surgeons: by overlaying the virtual model on real-time imaging data from the operating room, surgeons can navigate and visualize patient anatomy with greater accuracy, in order to identify critical structures, avoid complications, and achieve more radical excision. Furthermore, DHT can be enriched in real-time with information collected in the operating room, for example through direct electrostimulation techniques, which allow real-time mapping of the actual connectivity in the patient’s brain.
The development of DHTs may also play a significant role in organ transplant procedures by improving pre-transplant planning, surgical outcomes, and post-transplant monitoring [
18]. Leveraging machine learning and deep learning algorithms, DHTs can provide a prediction of transplant progress from historical data of thousands of similar cases and combine typical tendencies with the specific characteristics of the candidate patient to predict the likelihood of post-transplant complications, organ rejection, or organ failure.
DHTs can be created based on anatomical data from the recipient, including medical images such as CT or MRI scans. These virtual replicas allow surgeons to visualize the patient’s organs, blood vessels, and other relevant structures in a three-dimensional model: this helps to evaluate the feasibility of the transplant, identify potential challenges, and plan the surgical procedure.
DHTs can help match donors with potential recipients by analyzing various factors such as blood type, tissue compatibility, and other specific patient characteristics.
In the post-transplant phase, DHTs can be continuously updated with data from the transplanted organ to monitor its function and detect any anomalies. This includes integrating real-time data from monitoring devices, such as organ-specific sensors or implantable devices. By comparing the virtual twin’s predicted behavior with real-time data, healthcare professionals can identify potential complications or rejections at an early stage, enabling timely interventions.
Finally, it is also possible to better customize implants or prostheses based on the patient’s unique anatomy, as DHTs can be applied in the accurate measurement and analysis of anatomical structures, enabling the design and fabrication of patient-specific implants to improve the fit and functionality of the implant, leading to better patient outcomes and reducing the risk of complications.
6. Targeted Therapies
Many diseases with high morbidity and mortality rates have complex and multifactorial pathogenesis, with several intricate molecular pathways and environmental factors involved that can affect the efficacy of a treatment. The physician can hardly predict the effectiveness of therapy with inferences based on the patient’s clinical data. For this reason, despite the extensive literature on the importance of targeted therapies, it is difficult to apply tailor-made management strategies. However, there is evidence especially in the oncology setting that targeted therapy is the key to improving patient survival in a variety of diseases. In cancer therapy, there is evidence that patients receiving targeted therapies have significantly improved overall survival and progression-free survival compared to those receiving generic treatments [
31]. The development of DHTs could in the near future bridge the gap in treatment response between clinical trials and real-world clinical practice highlighting an unmet need to develop therapies tailored to individual patients.
Indeed, despite the remarkable technical and functional advances in next-generation sequencing technologies that have led to a deeper understanding of the molecular basis of many diseases, including cancers and autoimmune diseases, each patient responds to targeted therapy differently [
32].
DHTs can be used to create virtual models of individual patients with the ideal aim of digitally representing the unique and complex interaction between genetic, metabolic, and environmental factors. In this way, it would be possible to recognize and select highly effective personalized therapies to address the specific molecular mechanism of disease within the patient’s unique metabolic, immune, and behavioral ecosystem. Each virtual twin could be treated with various drugs as a potential treatment for the disease, enabling the prediction of specific patient responses on the base of a vast number of variables.
Powerful support for the construction of these models comes from quantitative imaging, driven by the extensive application of artificial intelligence to the analysis of imaging data. In oncology, this approach allows for extrapolating the unique tumor imaging signature and powerful non-invasive assessment of the intra- and peritumoral molecular landscape. Overall, the development of morphologically and functionally virtual replicas of the organism could bridge the gap between clinical practice and the ideal of personalized medicine, leading to a true patient-centered strategy: the right molecule for the right disease at the right dosage for the right patient [
8].
DHT for the selection of target therapies are built on the integration of multilevel data omics and applications, which includes genomics and proteomics, and, from a radiological point of view, imaging features extraction with texture analysis, radiomics, and radiogenomics: these models can be used to simulate the effects of different treatments and predict outcomes, allowing doctors to develop new therapies and improve existing ones and tailor treatments to the individual patient (
Figure 6) [
33]. Since it is derived from computationally predicted effects of a patient’s high-resolution model instead of previously observed effects on a small number of phenotypic and molecular variables in groups of patients, this prioritization would be far more precise than current treatment methods [
19].
Using the DHT, it is possible to test the effect of different drugs, at different dosages, simulating pharmacokinetic and pharmacodynamic interactions, until the correct therapy is correctly selected for that patient.The circles with different colors represent different possible therapies that are tested on a virtual model to find the right one for the patient
In the clinical setting, a DHT of a type 1 diabetes pancreas model was created to adjust insulin dosage depending on continuous blood glucose monitoring [
34].
Voigt et al. explored the world of multiple sclerosis and the impact of DT on the management of this common cause of neurological disability that affects especially young adults; a DHT paired to the patient’s characteristics can be created through AI-based analysis of several disease parameters including genomic, clinical, patient-related data, environmental factors, and previous medical procedures to allow the creation of personalized therapeutic pathways [
21].
7. DHT and Pharma Industry: Drug Development
DHTs promise to revolutionize the pharmaceutical field not only in clinical applications but especially in drug development. As in the Phase I study clinical dose-finding of new drugs can be difficult primarily due to the small sample size, the simulation of the molecular landscape of an organism derived from the integration of genetic, transcriptomic, and metabolomic information will allow forecasting the effects of new drugs or interactions between drugs on a virtual body, allowing researchers to test the safety and efficacy of new medicines before conducting clinical trials, protecting humans from potential adverse reactions by discarding potentially harmful molecules in advance and potentially reducing costs for pharmaceutical companies [
19].
In a recent study, Susilo et al. have challenged this paradigm by developing a virtual model of non-Hodgkin’s lymphoma to facilitate the phase I study of a specific antibody drug (monsunetuzumab). The authors used a DHT to study clinical dose-response relationships and to explore potential underlying biomarkers of treatment response. Virtual patients reproduced the clinical measurement for the corresponding clinical patient and were derived by combining specific clinical data of real patients enrolled in the Phase I study (e.g., pharmacokinetics, tumor size, and biomarker data) with other in vitro/in vivo data obtained with a quantitative systems pharmacology model. To account for biological variability and model uncertainty, multiple DHTs with different model parameterizations were identified for each clinical patient. In this way, they were able to predict the effective dose and to compare responder vs. non-responder patterns, highlighting the key role of tumor size, proliferation rate, and baseline T-cell infiltration in determining response to the drug [
35]. Despite promising results, open issues remain the characterization of disease-associated environmental variables (“exposome”) is still difficult, especially for the large amount of data required to build a built reality and reproducible DHT.
In neurosciences, Spitze et al. described the main application of DHT in mental health; they affirm that the physical asset (the alliance between patient and therapist) and its DHT are two connected dynamical systems that evolve across their separate state spaces over time [
36]. They employed a probabilistic graphical model (dynamic Bayesian network with decision nodes) to establish the constituents of this coupled dynamical system over therapy sessions (treatment efficacy, strength of alliance at the end of each session, and rupture during the session). The digital states are the results of validated scales that measure the efficacy and effectiveness of treatment as well as the strength of the alliance in each session. These values are determined by a previously trained statistical/machine learning model that calculates these quantities based on current and predicted nonverbal synchrony values calculated over sessions [
36]. Ruptures in the alliance are described as deterioration or tension in the alliance, evidenced by a disagreement between the patient and therapist on treatment objectives, a lack of participation in therapeutic activities, or pressure on their emotional connection [
37]. Ruptures are correlated with improved therapy outcomes when they are solved, to resolve a rupture, therapists must first recognize it, then they must use specific resolution procedures to heal it; as a result, anticipating the progression of therapy using dt enables the implementation of strategies aiming at a certain outcome.
8. DT and Organizations
The use of patients’ DHT can support clinical decisions not only at the patient’s level but also at the organizational level through the creation of hospital and organizational digital twin that can be used to simulate operational strategies or medical interventions to help decide what actions to take based on exam priority and available resources [
38], to respond to challenges such as growing patient demand, increasing clinical complexity, aging infrastructure, lack of space, increasing waiting times, and rapid advances in medical technology requiring additional equipment implementation [
8]. Algorithms based on deep learning have already proved their usefulness in image acquisition, aided detection, prioritization, reporting [
39,
40] and these applications could be particularly useful in trauma management which represents the most critical time-dependent process, including the pre-hospital phase and the operative phase in the emergency department [
40]. In this setting DHTs can improve the effects of decisions in high-risk contexts, anticipating the appearance of complications and improving decision-making processes [
8]. In the last years, Croatti et al. have presented a project of agent-based DT, designed to support the severe traumas management process [
41].
For example, in a car accident case, the digital twin collects real-time information provided by the central unit that received the call, the GPS System of the vehicle, and the smart devices held by rescuers; in the meantime, a so-called Trauma Tracker automatically reports the trauma events during the resuscitation, creating official documentation in a faster and more precise way compared to the traditional handwritten papers Meanwhile, the in-hospital trauma team starts to receive real-time information directly from the accident site about the incoming patient [
41].
The system would act as a personal assistant of the trauma leader because it receives real-time clinical data, generates alerts, and suggests actions to perform that are visualized on room displays or on wearable hands-free technologies, like smart glasses [
42].
Moreover, digital twin can be exploited in the hospital management field, creating simulations and hypothetical scenarios with planning and scheduling purposes, to improve the efficiency of healthcare services and optimize the resources.
In a case study, Liu et al. tested a smart healthcare system applied to the elderly care service. In their experiment, a virtual platform was used to study the bed availability conditions of a hospital, considering the dynamic interaction between emergency patients and patients scheduled to be hospitalized. The system creates a simulation that considers external factors such as weather and season because when temperatures are high, the number of elderly patients’ increases, and the number of free beds decreases as well. In this way, the reception capacity is automatically calculated in each season and the availability of beds can be adjusted, scheduling a proper number of elective admissions [
25].
9. Ethical and Legal Concerns
DHT presents various ethical issues that must be thoughtfully addressed for a responsible and ethical application of this technology. Primary ethical concerns [
43,
44,
45,
46,
47] associated with DHT in healthcare are:
Privacy and Data Security: Creating and using a DHT demands extensive data gathering, including personal health information, genetic data, and lifestyle habits. Robust data privacy and security measures are crucial to avoid unauthorized access, breaches, and misuse of sensitive information. Compliance with data protection regulations, such as the General Data Protection Regulation or Health Insurance Portability and Accountability Act, is crucial to ensure the privacy and security of individuals’ sensitive health data [
48,
49,
50,
51]. Furthermore, it is essential to prevent unauthorized access, data violations, or possible misuse of sensitive data. Robust security measures, including encryption and access restrictions, must be implemented to safeguard patients’ data from cyber threats [
52,
53]. Conformity with established security standards such as ISO/IEC 27001 is indispensable for mitigating risks related to unauthorized data acquisition [
54].
Informed Consent: Producing a DHT may entail exhaustive monitoring of an individual’s health through real-time and historical data analysis. Obtaining informed consent from patients is vital; they must fully comprehend the implications of sharing their personal data and the possible uses and risks associated with creating and using a DHT. Transparent consent processes and strict adherence to data protection regulations are essential for maintaining patient trust [
55].
Equity and Accessibility: The development and adoption of the DHT should follow principles of equity and accessibility. It is vital to ensure equal access to this technology for all individuals, regardless of socioeconomic status, race, or location, to avoid worsening healthcare disparities. Affordability, availability, algorithmic biases, and other ethical factors must be evaluated [
56].
Trust and Autonomy: As DHTs access more personal health data and integrate further into healthcare decision-making processes, trust, and autonomy become pressing concerns. Patients should have confidence that their DHT is being used responsibly in their best interest while maintaining control over its use, including opting out or altering the extent of data sharing. [
57]
Psychological Impact: Interaction with an ever-changing DHT can have a psychological impact on the individual as it reflects aging and health changes over time. This awareness can affect self-esteem, self-perception, and mental health. Addressing the psychological aspect of aging in the context of the DHT is an ethical challenge. It is essential to provide supportive resources, such as counseling and psychological support, to help people cope with the psychological challenges related to aging. [
57]
Algorithmic Bias and Fairness: Developing algorithms for human DHTs relies on extensive data training; biased or non-diverse training sets can perpetuate unequal treatment resulting in healthcare disparities. Ensuring fairness in algorithms is imperative to prevent the exacerbation of existing healthcare inequities. [
58]
Liability: The integration of DHTs raises several questions concerning liability. Indeed, if errors occur, for instance, inaccurate prediction or wrong diagnoses, determining liability becomes challenging because of the legal gap. Clear legal frameworks must be established to define the responsibilities of healthcare providers, technology developers, and other relevant stakeholders [
59,
60].
Regulatory Compliance: The development of DHTs involves different regulatory frameworks governing healthcare, data protection, medical devices, and artificial intelligence. Compliance with existing regulations stipulated by entities such as the Food and Drug Administration and European Medicines Agency may be mandatory [
60].
Addressing these concerns requires collaboration among healthcare providers, researchers, policymakers, and technology developers. Open and transparent discussions, robust regulatory frameworks, and ongoing monitoring of the ethical implications of the DHT are necessary to harness its potential while safeguarding patient rights, privacy, and well-being.
10. Limitations and Challenges
Different limitations and challenges need to be considered when implementing DHTs in healthcare.
First, DHTs rely on accurate and comprehensive data from various sources, including electronic health records, medical devices, and wearables. Ensuring the quality, integrity, and interoperability of these diverse data sets can be a significant challenge, as Inconsistent or incomplete data can lead to inaccurate or unreliable digital twin models [
61,
62].
The lack of standardized models and protocols can represent scalability and interoperability challenges. Each healthcare system may have unique requirements and data formats, making it difficult to exchange or combine digital twin models between different organizations.
Moreover, the development of accurate and reliable models for complex physiological systems or diseases can be challenging, as related to a deep understanding of the underlying biological processes and the ability to represent them effectively in the virtual model.
The validation and calibration of these models with real-world data are crucial but can be time-consuming and resource-intensive [
63].
Building and maintaining digital twin infrastructure can be costly, requiring significant investments in computational resources, storage, and expertise. Smaller healthcare providers or resource-constrained settings may face challenges in implementing and sustaining digital twin s due to financial and resource limitations.
Addressing these limitations and challenges requires collaboration between healthcare professionals, data scientists, technologists, policymakers, and regulatory bodies. It is crucial to invest in research, standardization efforts, and robust governance frameworks to unlock the full potential of digital twins in healthcare while addressing these concerns.
11. Conclusions
DHT can capture an individual’s health data, including genetic information, medical history, and lifestyle factors. This comprehensive data can be used to create personalized treatment plans, tailored to each individual’s unique needs. By analyzing vast amounts of health data from multiple sources, they can allow the development of personalized screening programs for early diagnostics. They can help identify patterns and markers that may indicate the presence of diseases or predict the risk of developing certain conditions, as well as the progression of a disease.
The possibility of remote monitoring of patients, allows healthcare professionals to track vital signs, symptoms, and treatment adherence from a distance and can enhance telemedicine capabilities and provide timely interventions for patients in remote locations.
DHTs have also a crucial role in the simulation of patients’ management, both from a therapeutic and a surgical point of view.
However, the use of DHT raises ethical questions regarding informed consent, data ownership, and potential discrimination based on health profiles. Guidelines and regulations should be established to ensure ethical use and protection of patient data.
Moreover, integrating digital human twins into existing healthcare systems and workflows can be complex, as it requires interoperability with electronic health records, medical devices, and other healthcare technologies. Standardization efforts and collaboration among stakeholders are needed to ensure seamless integration.
We provided an overview of the current applications of digital twin s in healthcare, with the potential of their use and benefits for their applications in the clinical practice, but also the challenges currently hampering their diffusion.
The further development of DHTs and their possible diffusion will help the creation of truly personalized medicine.