On the Development of an Intelligent Poly(aniline-co-o-toluidine)/Fe3O4/Alkyd Coating for Corrosion Protection in Carbon Steel
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Synthesis of Copolymer Poly(aniline-co-o-toluidine)
2.3. Synthesis of Magnetite Nanoparticles (Fe3O4)
2.4. Synthesis of Copolymer Poly(aniline-co-o-toluidine)/Iron Oxide Magnetic Nanoparticle Composites
2.5. Preparation of the Carbon Steel
2.6. Coating of the Carbon Steel
2.7. Electrochemical Impedance Spectroscopy
2.8. Characterization Techniques
3. Results and Discussion
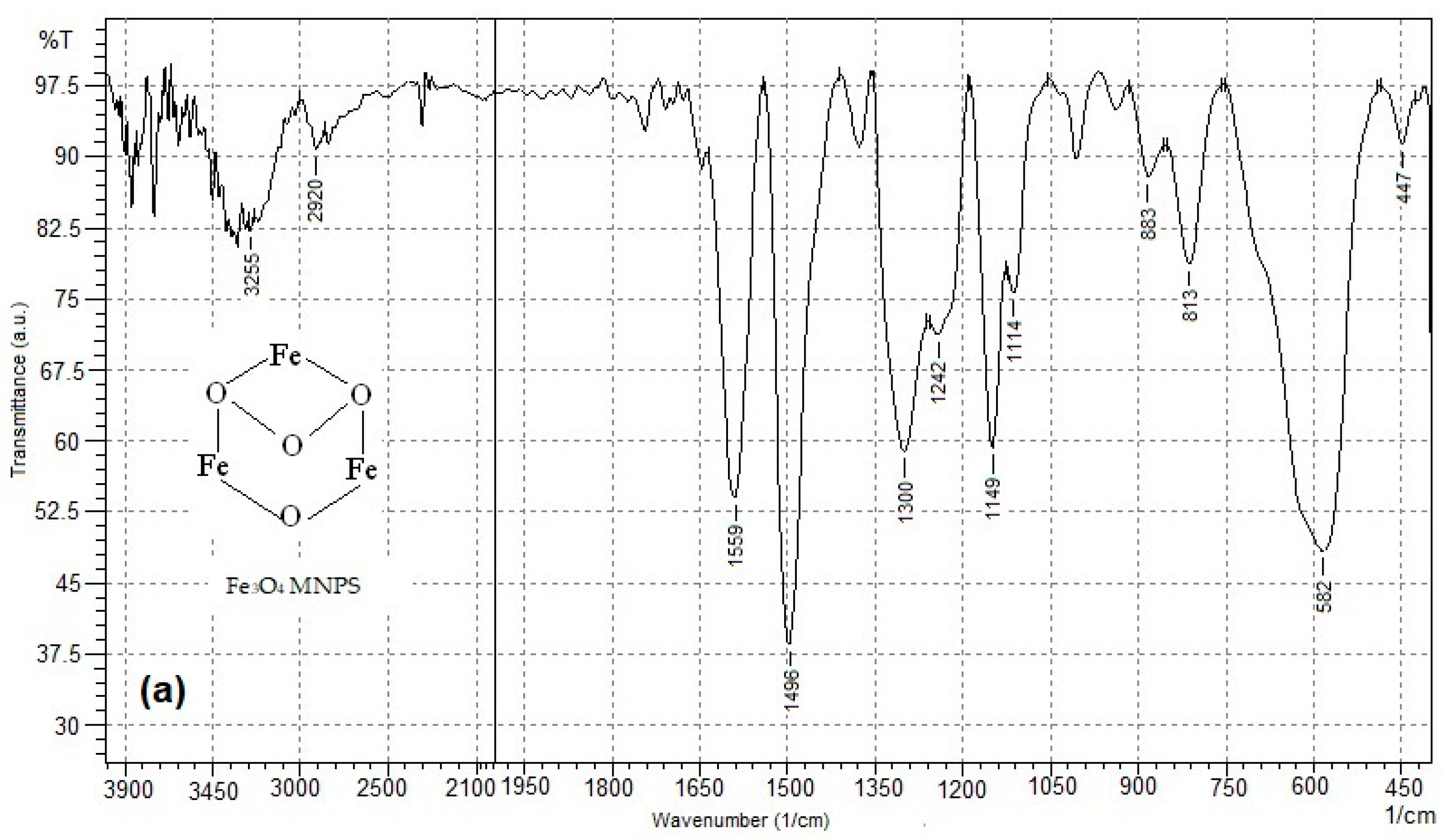
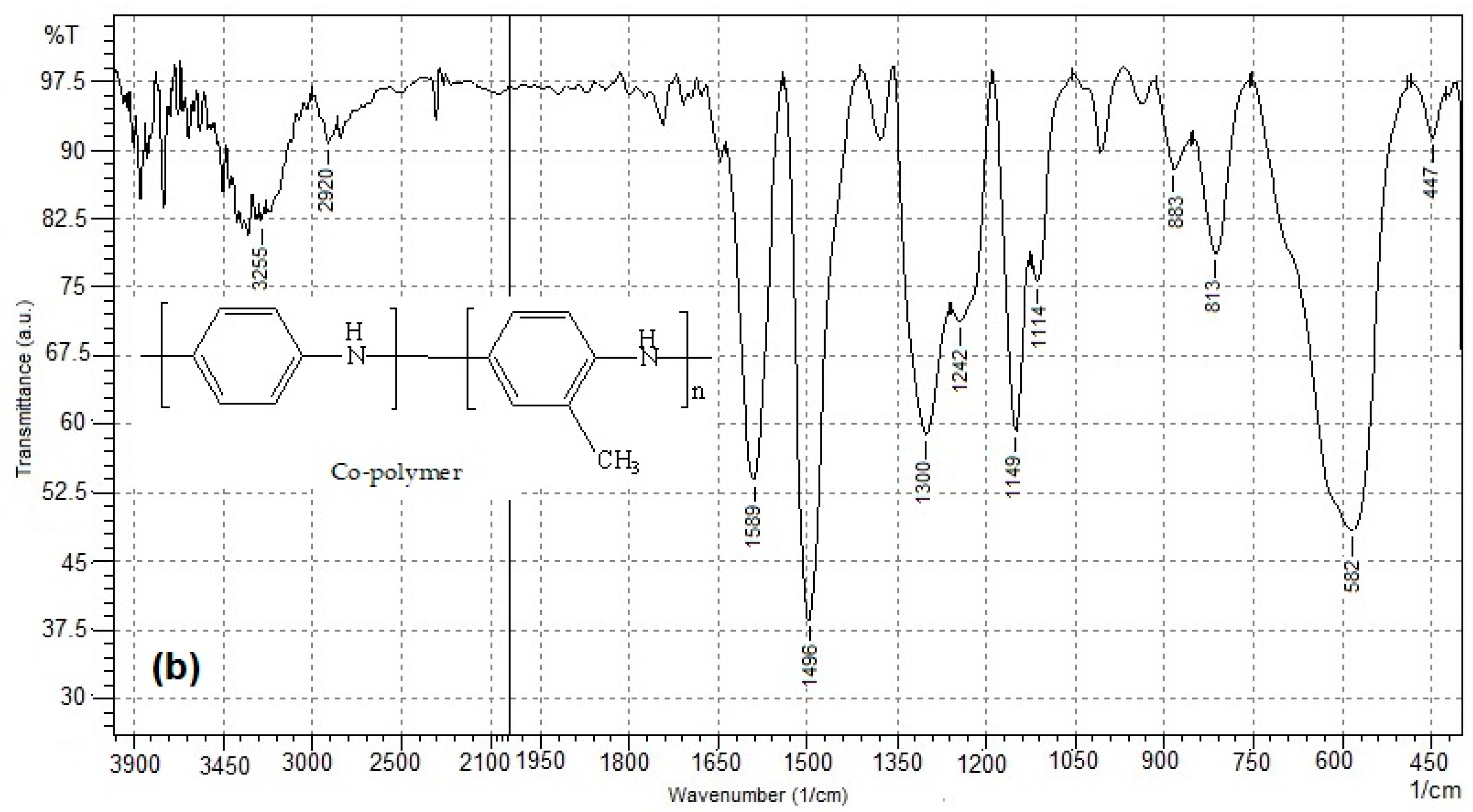
3.1. FT-IR Analysis
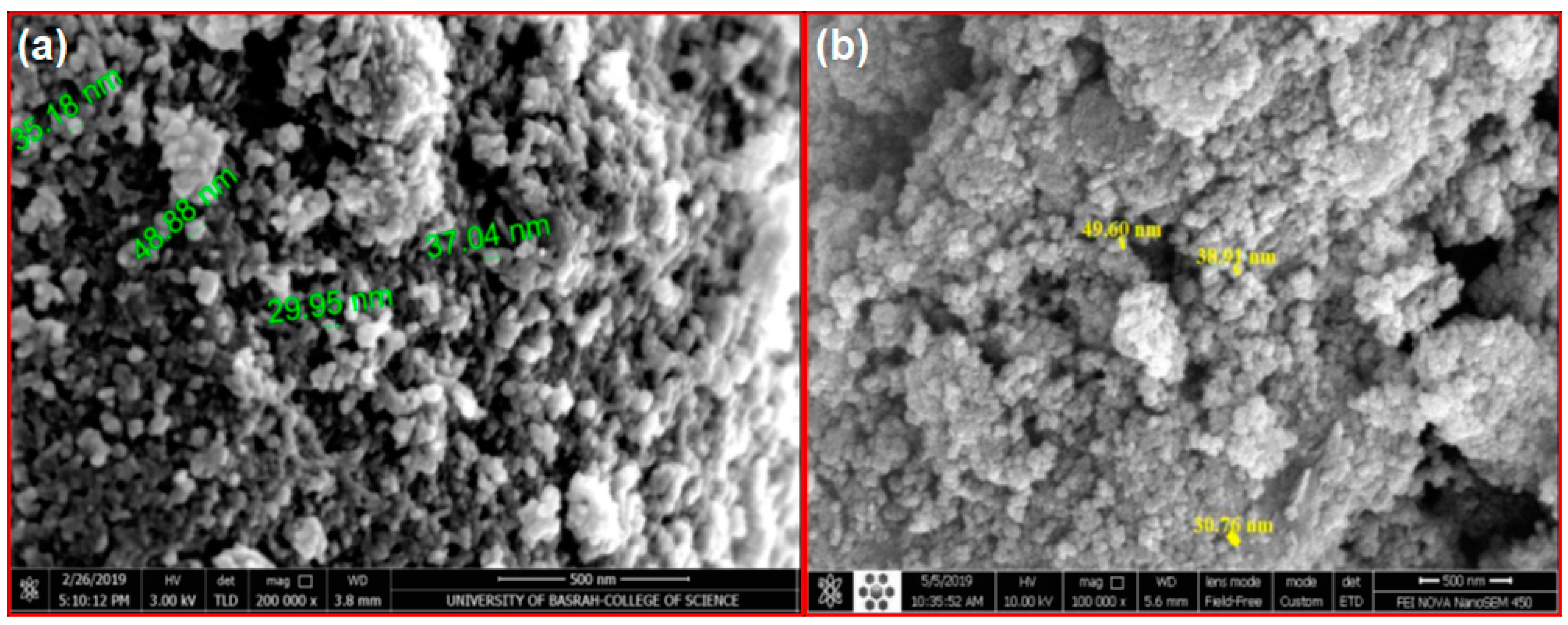
3.2. SEM Analysis
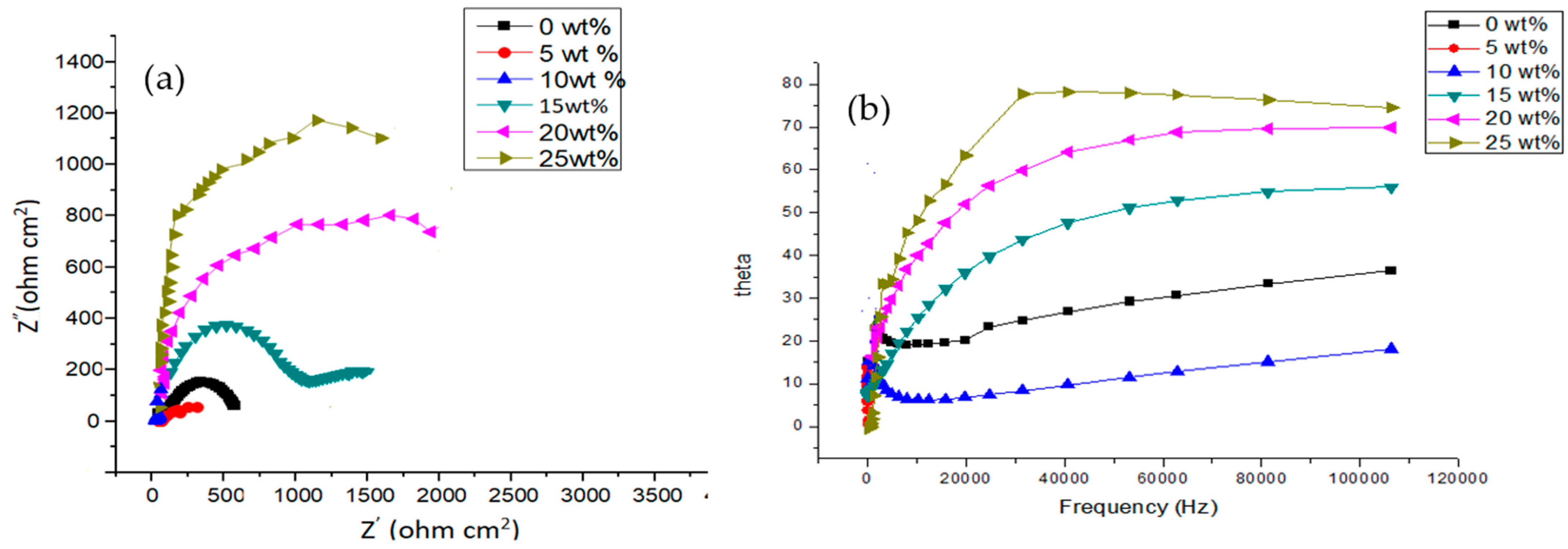
3.3. Corrosion Resistance Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deyab, M.A.; Zaky, M.T.; Nessim, M.I. Inhibition of Acid Corrosion of Carbon Steel Using Four Imidazolium Tetrafluoroborates Ionic Liquids. J. Mol. Liq. 2017, 229, 396–404. [Google Scholar] [CrossRef]
- Swetha, G.A.; Sachin, H.P.; Guruprasad, A.M.; Prasanna, B.M.; Sudheer, K. Use of Seroquel as an Effective Corrosion Inhibitor for Low Carbon Steel in 1 M HCl. J. Bio-Tribo-Corros. 2018, 4, 57. [Google Scholar] [CrossRef]
- Lgaz, H.; Salghi, R.; Ali, I.H. Corrosion Inhibition Behavior of 9-Hydroxyrisperidone as a Green Corrosion Inhibitor for Mild Steel in Hydrochloric Acid: Electrochemical, DFT and MD Simulations Studies. Int. J. Electrochem. Sci. 2018, 13, 250–264. [Google Scholar] [CrossRef]
- Shi, H.W.; Liu, F.C.; Han, E.H.; Wei, Y.H. Effects of Nano Pigments on the Corrosion Resistance of Alkyd Coating. J. Mater. Sci. Technol. 2007, 23, 551–558. [Google Scholar]
- Li, J.; Ecco, L.; Fedel, M.; Ermini, V.; Delmas, G.; Pan, J. In-Situ AFM and EIS Study of a Solventborne Alkyd Coating with Nanoclay for Corrosion Protection of Carbon Steel. Prog. Org. Coat. 2015, 87, 179–188. [Google Scholar] [CrossRef]
- Ecco, L.G.; Fedel, M.; Ahniyaz, A.; Deflorian, F. Influence of Polyaniline and Cerium Oxide Nanoparticles on the Corrosion Protection Properties of Alkyd Coating. Prog. Org. Coat. 2014, 77, 2031–2038. [Google Scholar] [CrossRef]
- Deyab, M.A.; Keera, S.T. Effect of Nano-TiO2 Particles Size on the Corrosion Resistance of Alkyd Coating. Mater. Chem. Phys. 2014, 146, 406–411. [Google Scholar] [CrossRef]
- Wang, H.; Hu, G.; Liu, X.; Guo, L.; Li, X.; Guo, R.; Li, Y. Concurrent Alkylation and Crosslinking of Polyaniline for Enhanced Anticorrosive Performance of Waterborne Alkyd Coating. Prog. Org. Coat. 2022, 168, 106865. [Google Scholar] [CrossRef]
- Piao, J.; Wang, W.; Cao, L.; Qin, X.; Wang, T.; Chen, S. Self-Healing Performance and Long-Term Corrosive Resistance of Polyvinylidene Fluoride Nanofiber Alkyd Coating. Compos. Commun. 2022, 36, 101404. [Google Scholar] [CrossRef]
- Guo, L.; Wang, H.; Li, X.; Fei, G.; Yuan, Y.; Li, Y. A Synergistic System of Polyaniline@ Graphene-Alkyd Resin via a Gemini Surfactant for Enhanced Anti-Corrosion Properties. Prog. Org. Coat. 2022, 170, 106944. [Google Scholar] [CrossRef]
- Deyab, M.A.; Eddahaoui, K.; Essehli, R.; Benmokhtar, S.; Rhadfi, T.; De Riccardis, A.; Mele, G. Influence of Newly Synthesized Titanium Phosphates on the Corrosion Protection Properties of Alkyd Coating. J. Mol. Liq. 2016, 216, 699–703. [Google Scholar] [CrossRef]
- Benitha, V.S.; Jeyasubramanian, K.; Hikku, G.S. Investigation of Anti-Corrosion Ability of Nano Mixed Metal Oxide Pigment Dispersed Alkyd Coating and Its Optimization for A36 Steel. J. Alloys Compd. 2017, 721, 563–576. [Google Scholar] [CrossRef]
- Alam, J.; Riaz, U.; Ahmad, S. High Performance Corrosion Resistant Polyaniline/Alkyd Ecofriendly Coatings. Curr. Appl. Phys. 2009, 9, 80–86. [Google Scholar] [CrossRef]
- Ifijen, I.H.; Maliki, M.; Omorogbe, S.O.; Ibrahim, S.D. Incorporation of Metallic Nanoparticles Into Alkyd Resin: A Review of Their Coating Performance. In Proceedings of the TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings, Anaheim, CA, USA, 27 February–3 March 2022; Springer International Publishing: Cham, Switzerland, 2022; pp. 338–349. [Google Scholar]
- Jeyasubramanian, K.; Benitha, V.S.; Parkavi, V. Nano Iron Oxide Dispersed Alkyd Coating as an Efficient Anticorrosive Coating for Industrial Structures. Prog. Org. Coat. 2019, 132, 76–85. [Google Scholar] [CrossRef]
- Wang, W.; Xu, L.; Li, X.; Lin, Z.; Yang, Y.; An, E. Self-Healing Mechanisms of Water Triggered Smart Coating in Seawater. J. Mater. Chem. A 2014, 2, 1914–1921. [Google Scholar] [CrossRef]
- Neto, A.G.C.; Pellanda, A.C.; de Carvalho Jorge, A.R.; Floriano, J.B.; Berton, M.A.C. Preparation and Evaluation of Corrosion Resistance of a Self-Healing Alkyd Coating Based on Microcapsules Containing Tung Oil. Prog. Org. Coat. 2020, 147, 105874. [Google Scholar] [CrossRef]
- Karekar, S.E.; Bagale, U.D.; Sonawane, S.H.; Bhanvase, B.A.; Pinjari, D.V. A Smart Coating Established with Encapsulation of Zinc Molybdate Centred Nanocontainer for Active Corrosion Protection of Mild Steel: Release Kinetics of Corrosion Inhibitor. Compos. Interfaces 2018, 25, 785–808. [Google Scholar] [CrossRef]
- Li, W.; Hintze, P.; Calle, L.M.; Buhrow, J.; Curran, J.; Muehlberg, A.J.; Gelling, V.J.; Webster, D.C.; Croll, S.G.; Contu, F.; et al. Smart Coating for Corrosion Indication and Prevention: Recent Progress; OnePetro: Richardson, TX, USA, 2009. [Google Scholar]
- Vijayan, P.P.; Al-Maadeed, M. Self-Repairing Composites for Corrosion Protection: A Review on Recent Strategies and Evaluation Methods. Materials 2019, 12, 2754. [Google Scholar]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Yu, C.H.; Caiulo, N.; Lo, C.C.; Tam, K.; Tsang, S.C. Synthesis and Fabrication of a Thin Film Containing Silica-Encapsulated Face-Centered Tetragonal FePt Nanoparticles. Adv. Mater. 2006, 18, 2312–2314. [Google Scholar] [CrossRef]
- Naser, A.A.; Al-Mubarak, A.S.; Al-Sawaad, H.Z. Synthesis, Characterization and Evaluation of Some Graphene Oxide Derivatives and Their Application as Corrosion Inhibitors for Carbon Steel Alloy Type C1025 in Hydrochloric Acid. Int. J. Corros. Scale Inhib. 2019, 8, 974. [Google Scholar]
- Mohamed, M.G.; Mahdy, A.; Obaid, R.J.; Hegazy, M.A.; Kuo, S.-W.; Aly, K.I. Synthesis and Characterization of Polybenzoxazine/Clay Hybrid Nanocomposites for UV Light Shielding and Anti-Corrosion Coatings on Mild Steel. J. Polym. Res. 2021, 28, 297. [Google Scholar] [CrossRef]
- Aly, K.I.; Abd El-Lateef, H.M.; Yehia, N.; Khodairy, A.; Sayed, M.M.; El-Remaily, M.A.E.A.A.A. Novel Polyesters Based on Indazole Moiety: Synthesis, Characterization and Applicability as Efficient Inhibitors for Acidic X-65-Steel Corrosion. React. Funct. Polym. 2021, 166, 105001. [Google Scholar] [CrossRef]
- Aly, K.I.; Mahdy, A.; Hegazy, M.A.; Al-Muaikel, N.S.; Kuo, S.-W.; Gamal Mohamed, M. Corrosion Resistance of Mild Steel Coated with Phthalimide-Functionalized Polybenzoxazines. Coatings 2020, 10, 1114. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Kuo, S.W.; Mahdy, A.; Ghayd, I.M.; Aly Kamal, I. Bisbenzylidene Cyclopentanone and Cyclohexanone-Functionalized Polybenzoxazine Nanocomposites: Synthesis, Characterization, and Use for Corrosion Protection on Mild Steel. Mater. Today Commun. 2020, 25, 101418. [Google Scholar] [CrossRef]
- Cayton, R.H.; Sawitowski, T. The Impact of Nano-Materials on Coating Technologies. In Proceedings of the Technical Proceedings of the 2005 NSTI Nanotechnology Conference and Trade Show; NSTI: Mumbai, India, 2005; Volume 2, pp. 8–12. [Google Scholar]
- Fernando, R. Nanomaterial Technology Applications in Coatings. JCT Coat. 2004, 1, 32–38. [Google Scholar]
- Riaz, U.; Ahmad, S.; Ashraf, S.M. Comparison of Corrosion Protective Performance of Nanostructured Polyaniline and Poly(1-Naphthylamine)-Based Alkyd Coatings on Mild Steel. Mater. Corros. 2009, 60, 280–286. [Google Scholar] [CrossRef]
- Jadhav, R.S.; Hundiwale, D.G.; Mahulikar, P.P. Synthesis of Nano Polyaniline and Poly-o-Anisidine and Applications in Alkyd Paint Formulation to Enhance the Corrosion Resistivity of Mild Steel. J. Coat. Technol. Res. 2010, 7, 449–454. [Google Scholar] [CrossRef]
- Sweeton, F.H.; Baes, C.F., Jr. The Solubility of Magnetite and Hydrolysis of Ferrous Ion in Aqueous Solutions at Elevated Temperatures. J. Chem. Thermodyn. 1970, 2, 479–500. [Google Scholar] [CrossRef]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Physical Analysis and Statistical Investigation of Tensile and Fatigue Behaviors of Glass Fiber-Reinforced Polyester via Novel Fibers Arrangement. J. Compos. Mater. 2023, 57, 147–166. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.M. Mechanical Properties of Linear Low-Density Polyethylene Fire-Retarded with Melamine Polyphosphate. J. Appl. Polym. Sci. 2018, 135, 46770. [Google Scholar] [CrossRef]
- Mahmoud Zaghloul, M.Y.; Yousry Zaghloul, M.M.; Yousry Zaghloul, M.M. Developments in Polyester Composite Materials–An in-Depth Review on Natural Fibres and Nano Fillers. Compos. Struct. 2021, 278, 114698. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Zaghloul, M.Y.M.; Zaghloul, M.M.Y. Experimental and Modeling Analysis of Mechanical-Electrical Behaviors of Polypropylene Composites Filled with Graphite and MWCNT Fillers. Polym. Test. 2017, 63, 467–474. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Steel, K.; Veidt, M.; Heitzmann, M.T. Wear Behaviour of Polymeric Materials Reinforced with Man-Made Fibres: A Comprehensive Review about Fibre Volume Fraction Influence on Wear Performance. J. Reinf. Plast. Compos. 2022, 41, 215–241. [Google Scholar] [CrossRef]
- Zaghloul, M.M.Y.; Steel, K.; Veidt, M.; Heitzmann, M.T. Mechanical and Tribological Performances of Thermoplastic Polymers Reinforced with Glass Fibres at Variable Fibre Volume Fractions. Polymers 2023, 15, 694. [Google Scholar] [CrossRef]
- Fuseini, M.; Zaghloul, M.M.Y. Investigation of Electrophoretic Deposition of PANI Nano Fibers as a Manufacturing Technology for Corrosion Protection. Prog. Org. Coat. 2022, 171, 107015. [Google Scholar] [CrossRef]
- Kulandaivalu, S.; Zainal, Z.; Sulaiman, Y. A New Approach for Electrodeposition of Poly (3, 4-Ethylenedioxythiophene)/Polyaniline (PEDOT/PANI) Copolymer. Int. J. Electrochem. Sci. 2015, 10, 8926–8940. [Google Scholar] [CrossRef]
- Jeyakumari, J.J.L.; Yelilarasi, A.; Sundaresan, B.; Dhanalakshmi, V.; Anbarasan, R. Chemical Synthesis of Poly (Aniline-Co-o/m-Toluidine)/V2O5 Nano Composites and Their Characterizations. Synth. Met. 2010, 160, 2605–2612. [Google Scholar] [CrossRef]
- Zhang, Y.; Shao, Y.; Meng, G.; Zhang, T.; Li, P.; Wang, F. Evaluation of the Corrosion Protection of Defective Polyaniline/Epoxy Coating by Localized Electrochemical Impedance Spectroscopy. J. Coat. Technol. Res. 2015, 12, 777–785. [Google Scholar] [CrossRef]
- Mahudeswaran, A.; Manoharan, D.; Chandrasekaran, J.; Vivekanandan, J.; Vijayanand, P.S. CSA Doped Poly (Aniline-Co-o-Toluidine) and Dispersed Zinc Oxide Nanoparticles: A Promising Material for Photovoltaics. Mater. Res. 2015, 18, 482–488. [Google Scholar] [CrossRef]
- Shirinova, H.A.; Palma, L.D.; Sarasini, F. Synthesis and Characterization of Magnetic Nanocomposites for Environmental Remediation. Chem. Eng. 2016, 47. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Magneto-Electro-Responsive Material Based on Magnetite Nanoparticles/Polyurethane Composites. Mater. Sci. Eng. C 2016, 61, 312–323. [Google Scholar] [CrossRef] [PubMed]
- Bian, L.; Bao, L.; Wang, J.; Lei, J. In Situ Preparation of Monodispersed Ag/Polyaniline/Fe3O4 Nanoparticles via Heterogeneous Nucleation. Nanoscale Res. Lett. 2013, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Bentiss, F. Evaluation of Alkaloids Extract of Retama monosperma (L.) Boiss. Stems as a Green Corrosion Inhibitor for Carbon Steel in Pickling Acidic Medium by Means of Gravimetric, AC Impedance and Surface Studies. J. Mater. Environ. Sci. 2017, 9, 358–369. [Google Scholar]
- Simonović, A.T.; Tasić, Ž.Z.; Radovanović, M.B.; Petrović Mihajlović, M.B.; Antonijević, M.M. Influence of 5-Chlorobenzotriazole on Inhibition of Copper Corrosion in Acid Rain Solution. ACS Omega 2020, 5, 12832–12841. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Pyridine Derivatives as Corrosion Inhibitors for N80 Steel in 15% HCl: Electrochemical, Surface and Quantum Chemical Studies. Measurement 2015, 76, 136–147. [Google Scholar] [CrossRef]
- Lgaz, H.; Lee, H. Facile Preparation of New Hydrazone Compounds and Their Application for Long-Term Corrosion Inhibition of N80 Steel in 15% HCl: An Experimental Study Combined with DFTB Calculations. J. Mol. Liq. 2022, 347, 117952. [Google Scholar] [CrossRef]
- Zaher, A.; Aslam, R.; Lee, H.-S.; Khafouri, A.; Boufellous, M.; Alrashdi, A.A.; El Aoufir, Y.; Lgaz, H.; Ouhssine, M. A Combined Computational & Electrochemical Exploration of the Ammi visnaga L. Extract as a Green Corrosion Inhibitor for Carbon Steel in HCl Solution. Arab. J. Chem. 2022, 15, 103573. [Google Scholar] [CrossRef]
- Dehghani, A.; Bahlakeh, G.; Ramezanzadeh, B.; Ramezanzadeh, M. Electronic/Atomic Level Fundamental Theoretical Evaluations Combined with Electrochemical/Surface Examinations of Tamarindus Indiaca Aqueous Extract as a New Green Inhibitor for Mild Steel in Acidic Solution (HCl 1 M). J. Taiwan Inst. Chem. Eng. 2019, 102, 349–377. [Google Scholar] [CrossRef]
- Saha, S.K.; Murmu, M.; Murmu, N.C.; Banerjee, P. Benzothiazolylhydrazine Azomethine Derivatives for Efficient Corrosion Inhibition of Mild Steel in Acidic Environment: Integrated Experimental and Density Functional Theory Cum Molecular Dynamics Simulation Approach. J. Mol. Liq. 2022, 364, 120033. [Google Scholar] [CrossRef]
- Saha, S.K.; Dutta, A.; Ghosh, P.; Sukul, D.; Banerjee, P. Adsorption and Corrosion Inhibition Effect of Schiff Base Molecules on the Mild Steel Surface in 1 M HCl Medium: A Combined Experimental and Theoretical Approach. Phys. Chem. Chem. Phys. 2015, 17, 5679–5690. [Google Scholar] [CrossRef]
- Umoren, S.A.; Solomon, M.M.; Obot, I.B.; Suleiman, R.K. Date Palm Leaves Extract as a Green and Sustainable Corrosion Inhibitor for Low Carbon Steel in 15 Wt.% HCl Solution: The Role of Extraction Solvent on Inhibition Effect. Environ. Sci. Pollut. Res. 2021, 28, 40879–40894. [Google Scholar] [CrossRef]
- Solomon, M.M.; Umoren, S.A.; Quraishi, M.A.; Jafar Mazumder, M.A. Corrosion Inhibition of N80 Steel in Simulated Acidizing Environment by N-(2-(2-Pentadecyl-4,5-Dihydro-1H-Imidazol-1-YL) Ethyl) Palmitamide. J. Mol. Liq. 2019, 273, 476–487. [Google Scholar] [CrossRef]
- Naderi, R.; Attar, M.M. Electrochemical Study of Protective Behavior of Organic Coating Pigmented with Zinc Aluminum Polyphosphate as a Modified Zinc Phosphate at Different Pigment Volume Concentrations. Prog. Org. Coat. 2009, 66, 314–320. [Google Scholar] [CrossRef]
- Abdel-Gaber, A.M.; Abd-El Nabey, B.A.; Khamis, E.; Abdelattef, O.A.; Aglan, H.; Ludwick, A. Influence of Natural Inhibitor, Pigment and Extender on Corrosion of Polymer Coated Steel. Prog. Org. Coat. 2010, 69, 402–409. [Google Scholar] [CrossRef]
- Yeh, J.-M.; Hsieh, C.-F.; Yeh, C.-W.; Wu, M.-J.; Yang, H.-C. Organic Base-Catalyzed Sol–Gel Route to Prepare PMMA–Silica Hybrid Materials. Polym. Int. 2007, 56, 343–349. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, F.; Du, Y. Effect of Nano-Sized Titanium Powder Addition on Corrosion Performance of Epoxy Coatings. Surf. Coat. Technol. 2007, 201, 7241–7245. [Google Scholar] [CrossRef]
- Pavithra, M.K.; Venkatesha, T.V.; Kumar, M.K.P.; Anantha, N.S.R. Outstanding Inhibitive Effect of Colchicine on Aluminium Alloy 6061 Corrosion. J. Electrochem. Sci. Eng. 2015, 5, 197–208. [Google Scholar] [CrossRef]
- Zhu, C.; Xie, R.; Xue, J.; Song, L. Studies of the Impedance Models and Water Transport Behaviors of Cathodically Polarized Coating. Electrochim. Acta 2011, 56, 5828–5835. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and Characterization of Magnetite Nanoparticles via the Chemical Co-Precipitation Method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Baig, N.; Chauhan, D.S.; Saleh, T.A.; Quraishi, M.A. Diethylenetriamine Functionalized Graphene Oxide as a Novel Corrosion Inhibitor for Mild Steel in Hydrochloric Acid Solutions. New J. Chem. 2019, 43, 2328–2337. [Google Scholar] [CrossRef]
- Gupta, R.K.; Malviya, M.; Verma, C.; Quraishi, M.A. Aminoazobenzene and Diaminoazobenzene Functionalized Graphene Oxides as Novel Class of Corrosion Inhibitors for Mild Steel: Experimental and DFT Studies. Mater. Chem. Phys. 2017, 198, 360–373. [Google Scholar] [CrossRef]
| Shade No./Color | Finish | Solids Volume, % | Theoretical Spreading Rate | Flashpoint |
|---|---|---|---|---|
| Grey 12170 | Matt | Grey 12170: 48% | 10.8 m2/L - 0.035 mm | >35 °C |
| Surface dry | Through-dry | Volatile organic compounds content | Specific gravity | |
| 4 h (10 °C) | 12 h (10 °C) | 405 g/L | 1.26 kg/L |
| C% | S% | Mn% | P% | Si% | Cr% | Ni% | Cu% | As% | Fe% |
|---|---|---|---|---|---|---|---|---|---|
| 0.083 | 0.002 | 0.50 | 0.003 | 0.011 | 0.019 | 0.0111 | 0.0183 | 0.097 | 99.1 |
| Formulation | Wt.% of Inhibitor | Rp (Ω cm2) | n |
Y° × 10−5 (Ω−1 Sn cm−2) | Cdl × 10−7 (µF cm−2) | τ (s) | fmax (Hz) | % IE |
|---|---|---|---|---|---|---|---|---|
| Alkyd | - | 577.2 | 0.5707 | 7.598 | 1.04 × 103 | 0.67 | 236.69 | - |
| Fe3O4 MNPs | 5 | 986.1 | 0.4287 | 10.437 | 5.23 | 0.12 | 1263.7 | 41 |
| 10 | 1073 | 0.5286 | 0.024 | 2.79 × 10−3 | 0.02 | 5441.2 | 46 | |
| 15 | 1143 | 0.7363 | 0.022 | 7.94 | 0.18 | 856.72 | 50 | |
| 20 | 1235 | 1.201 | 0.001 | 1.76 × 103 | 0.21 | 734.38 | 53 | |
| 25 | 1304.7 | 1.079 | 0.004 | 1.06 × 103 | 0.10 | 1508 | 56 | |
| [Poly(aniline-co-o-toluidine)/Fe3O4] | 5 | 1167 | 1.294 | 0.026 | 6.98 × 104 | 0.61 | 258.61 | 50 |
| 10 | 1195 | 0.1138 | 5.117 | 1.64 × 10−28 | 7.96 | 20.934 | 52 | |
| 15 | 2651 | 0.2900 | 1.158 | 3.58 × 10−8 | 3.90 | 40.792 | 78 | |
| 20 | 2810 | 0.6795 | 0.039 | 1.31 | 2.77 | 23.365 | 80 | |
| MNPs | 25 | 2749 | 0.8284 | 0.040 | 68.7 | 0.39 | 404.57 | 79 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mousa, O.I.; Al-Luaibi, S.S.; Al-Mubarak, A.S.; Lgaz, H.; Hammouti, B.; Chaouiki, A.; Ko, Y.G. On the Development of an Intelligent Poly(aniline-co-o-toluidine)/Fe3O4/Alkyd Coating for Corrosion Protection in Carbon Steel. Appl. Sci. 2023, 13, 8189. https://doi.org/10.3390/app13148189
Mousa OI, Al-Luaibi SS, Al-Mubarak AS, Lgaz H, Hammouti B, Chaouiki A, Ko YG. On the Development of an Intelligent Poly(aniline-co-o-toluidine)/Fe3O4/Alkyd Coating for Corrosion Protection in Carbon Steel. Applied Sciences. 2023; 13(14):8189. https://doi.org/10.3390/app13148189
Chicago/Turabian StyleMousa, Oday I., Salah S. Al-Luaibi, Alaa S. Al-Mubarak, Hassane Lgaz, Belkheir Hammouti, Abdelkarim Chaouiki, and Young Gun Ko. 2023. "On the Development of an Intelligent Poly(aniline-co-o-toluidine)/Fe3O4/Alkyd Coating for Corrosion Protection in Carbon Steel" Applied Sciences 13, no. 14: 8189. https://doi.org/10.3390/app13148189
APA StyleMousa, O. I., Al-Luaibi, S. S., Al-Mubarak, A. S., Lgaz, H., Hammouti, B., Chaouiki, A., & Ko, Y. G. (2023). On the Development of an Intelligent Poly(aniline-co-o-toluidine)/Fe3O4/Alkyd Coating for Corrosion Protection in Carbon Steel. Applied Sciences, 13(14), 8189. https://doi.org/10.3390/app13148189










