Chemical Signatures of Ge in Magnetite of Wugang BIF, China
Abstract
1. Introduction
2. Geological Setting and Methods
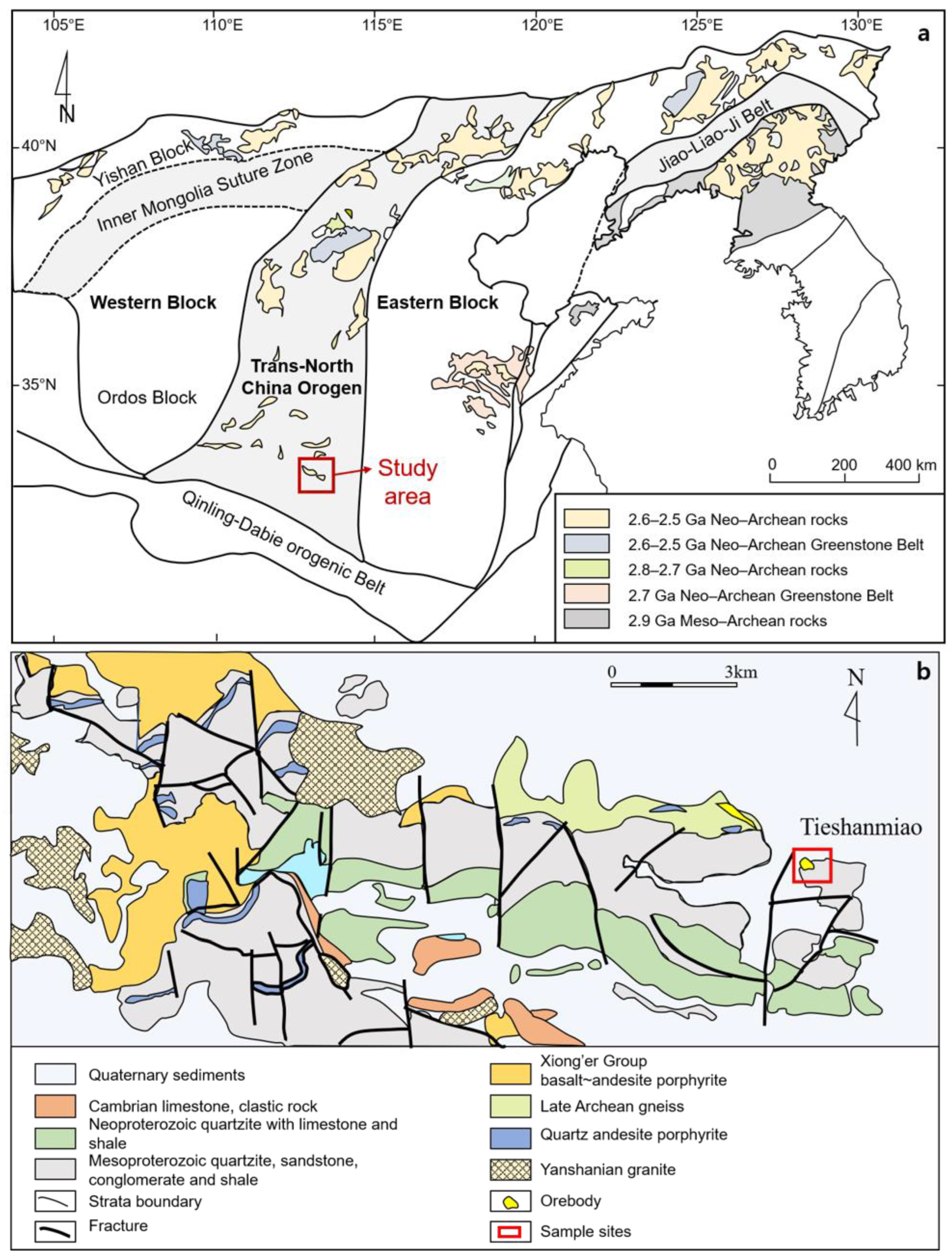
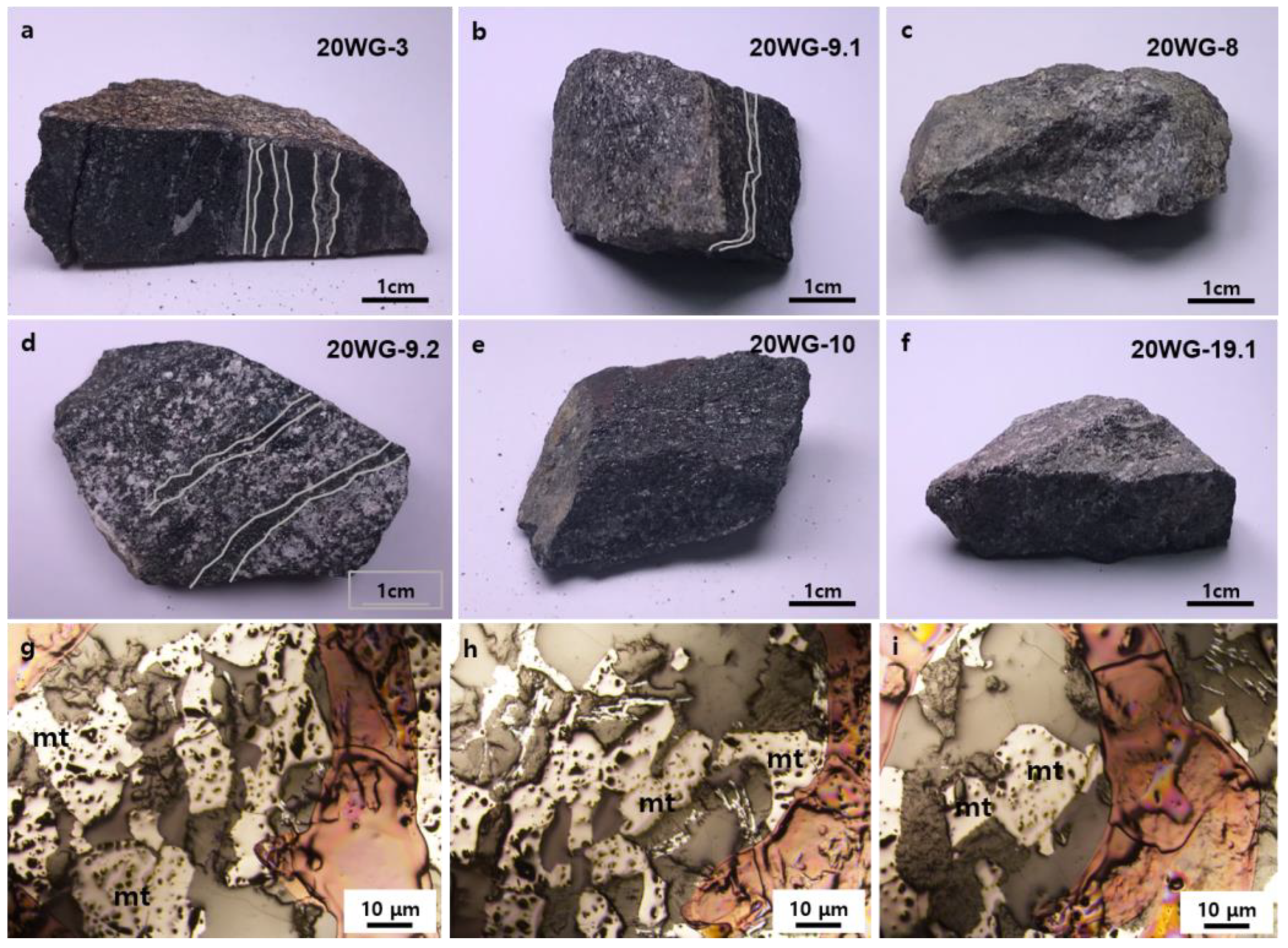
2.1. Geological Setting and Samples
2.2. Analytical Methods
3. Results and Discussion
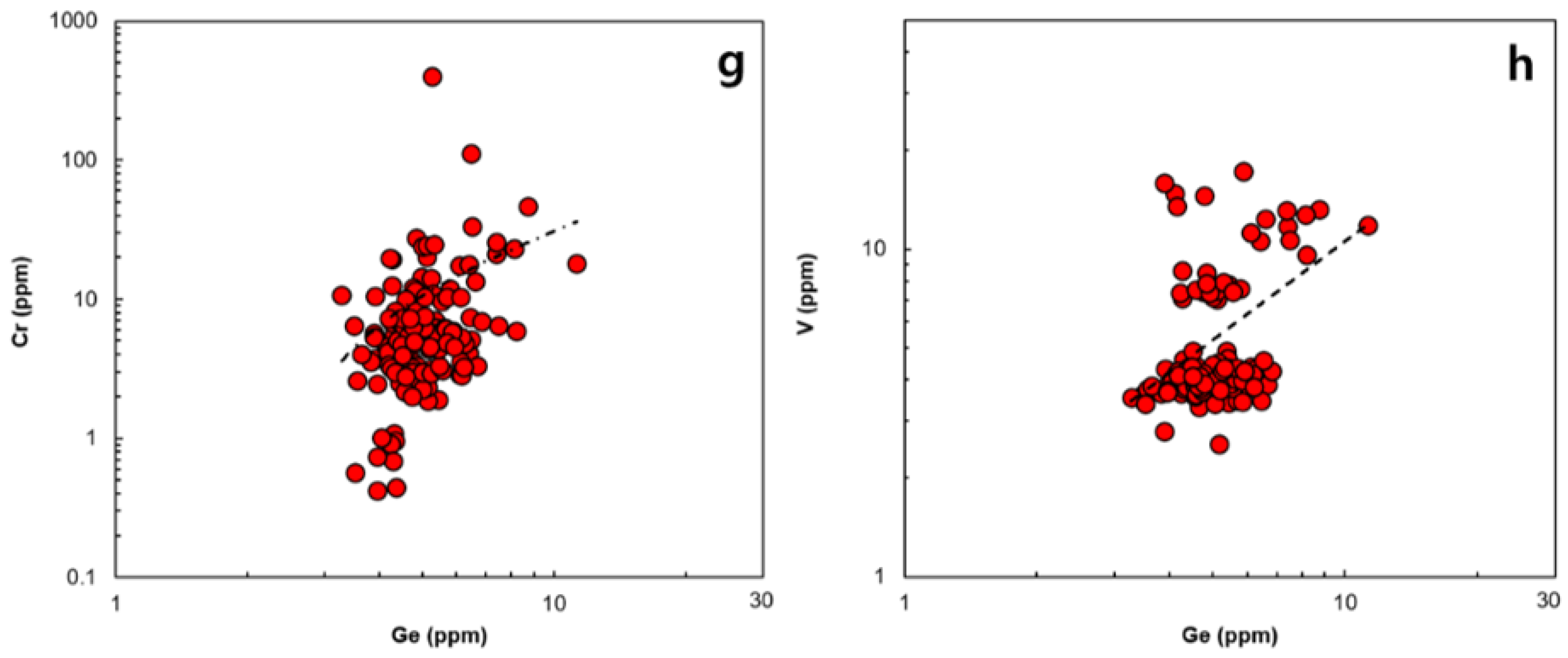
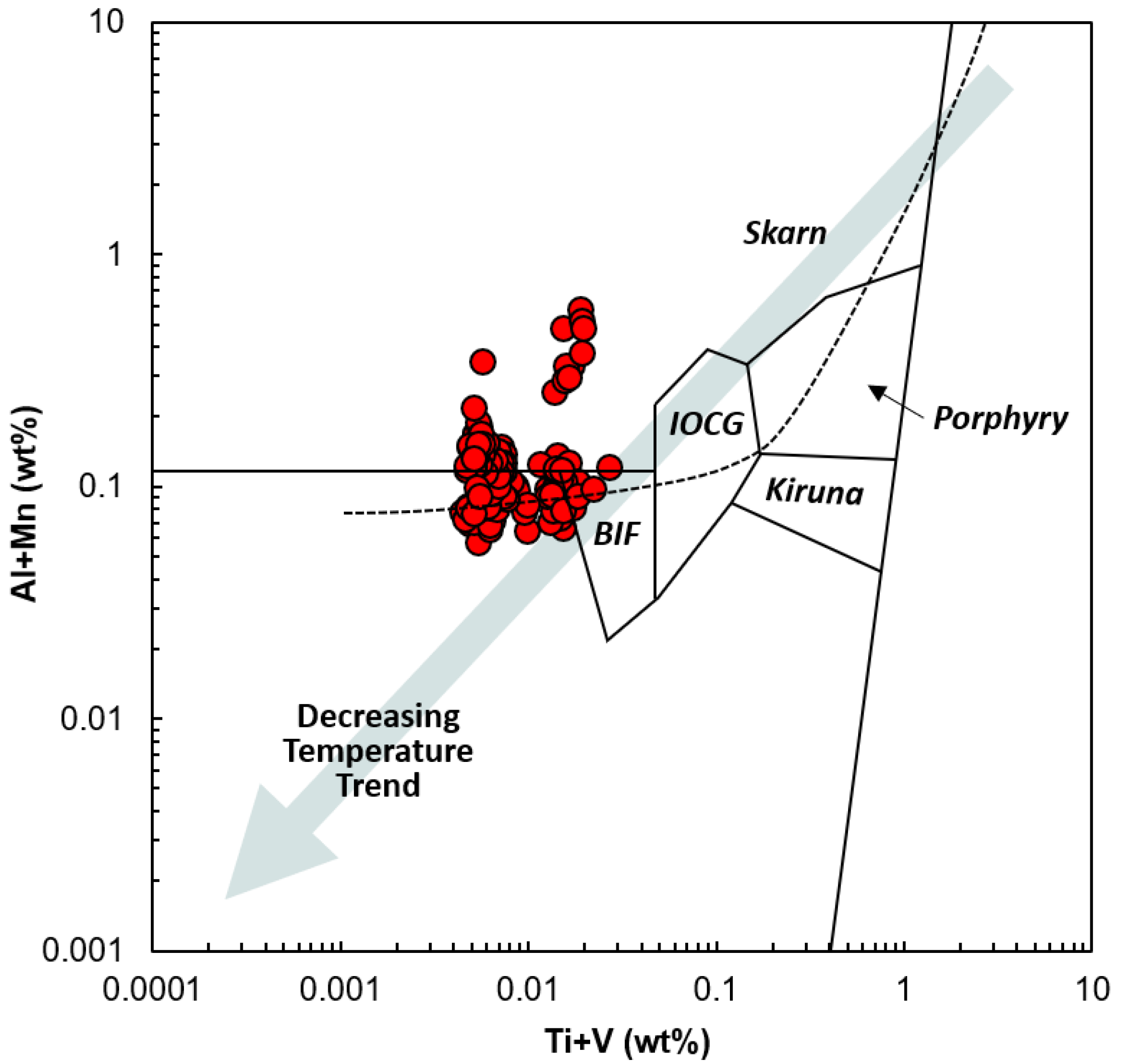
3.1. Geochemical Properties of Ge in Magnetite in Various Ore Deposits
3.2. Controlling Factor of Ge in Magnetite in the Hydrothermal System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Poulton, S.W.; Canfield, D.E. Ferruginous conditions: A dominant feature of the ocean through Earth’s history. Elements 2011, 7, 107–112. [Google Scholar] [CrossRef]
- James, H.L. Sedimentary facies of iron-formation. Econ. Geol. 1954, 49, 235–293. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Zhang, L.-C.; Xue, C.-J.; Zheng, M.-T.; Zhu, M.-T.; Robbins, L.J.; Slack, J.F.; Planavsky, N.J.; Konhauser, K.O. Earth’s youngest banded iron formation implies ferruginous conditions in the Early Cambrian ocean. Sci. Rep. 2018, 8, 9970. [Google Scholar] [CrossRef] [PubMed]
- Heimann, A.; Johnson, C.M.; Beard, B.L.; Valley, J.W.; Roden, E.E.; Spicuzza, M.J.; Beukes, N.J. Fe, C, and O isotope compositions of banded iron formation carbonates demonstrate a major role for dissimilatory iron reduction in~2.5 Ga marine environments. Earth Planet. Sci. Lett. 2010, 294, 8–18. [Google Scholar] [CrossRef]
- Bekker, A.; Slack, J.F.; Planavsky, N.; Krapež, B.; Hofmann, A.; Konhauser, K.O.; Rouxel, O.J. Iron formation: The sedimentary product of a complex interplay among mantle, tectonic, oceanic, and biospheric processes. Econ. Geol. 2010, 105, 467–508. [Google Scholar] [CrossRef]
- Klein, C. Some Precambrian banded iron-formations (BIFs) from around the world: Their age, geologic setting, mineralogy, metamorphism, geochemistry, and origins. Am. Mineral. 2005, 90, 1473–1499. [Google Scholar] [CrossRef]
- Shibuya, T.; Komiya, T.; Nakamura, K.; Takai, K.; Maruyama, S. Highly alkaline, high-temperature hydrothermal fluids in the early Archean ocean. Precambrian Res. 2010, 182, 230–238. [Google Scholar] [CrossRef]
- Derry, L.A.; Jacobsen, S.B. The chemical evolution of Precambrian seawater: Evidence from REEs in banded iron formations. Geochim. Cosmochim. Acta 1990, 54, 2965–2977. [Google Scholar] [CrossRef]
- Schad, M.; Byrne, J.M.; ThomasArrigo, L.K.; Kretzschmar, R.; Konhauser, K.O.; Kappler, A. Microbial Fe cycling in a simulated Precambrian ocean environment: Implications for secondary mineral (trans) formation and deposition during BIF genesis. Geochim. Cosmochim. Acta 2022, 331, 165–191. [Google Scholar] [CrossRef]
- Araújo, J.C.S.; Lobato, L.M. Depositional model for banded iron formation host to gold in the Archean Rio das Velhas greenstone belt, Brazil, based on geochemistry and LA-ICP-MS magnetite analyses. J. S. Am. Earth Sci. 2019, 94, 102205. [Google Scholar] [CrossRef]
- Chung, D.; Zhou, M.-F.; Gao, J.-F.; Chen, W.T. In-situ LA–ICP-MS trace elemental analyses of magnetite: The late Palaeoproterozoic Sokoman Iron Formation in the Labrador Trough, Canada. Ore Geol. Rev. 2015, 65, 917–928. [Google Scholar] [CrossRef]
- Dupuis, C.; Beaudoin, G. Discriminant diagrams for iron oxide trace element fingerprinting of mineral deposit types. Miner. Depos. 2011, 46, 319–335. [Google Scholar] [CrossRef]
- Gourcerol, B.; Kontak, D.J.; Thurston, P.C.; Duparc, Q. Do Magnetite Layers in Algoma-Type Banded Iron Formations (BIF) Preserve Their Primary Geochemical Signature? A Case Study of Samples from Three Archean BIF-Hosted Gold Deposits. Can. Mineral. 2016, 54, 605–624. [Google Scholar] [CrossRef]
- Nadoll, P.; Angerer, T.; Mauk, J.L.; French, D.; Walshe, J. The chemistry of hydrothermal magnetite: A review. Ore Geol. Rev. 2014, 61, 1–32. [Google Scholar] [CrossRef]
- Sun, X.; Zhu, X.; Tang, H.; Luan, Y. In situ LA-ICP-MS trace element analysis of magnetite from the late Neoarchean Gongchangling BIFs, NE China: Constraints on the genesis of high-grade iron ore. Geol. J. 2018, 53, 8–20. [Google Scholar] [CrossRef]
- Fleet, M. The structure of magnetite. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1981, 37, 917–920. [Google Scholar] [CrossRef]
- Lindsely, D.H. The crystal chemistry and structure of oxide minerals as exemplified by the Fe-Ti oxides. Oxide Miner. 1976, L1–L60. [Google Scholar]
- Wechsler, B.A.; Lindsley, D.H.; Prewitt, C.T. Crystal structure and cation distribution in titanomagnetites (Fe3-xTixO4). Am. Mineral. 1984, 69, 754–770. [Google Scholar]
- Deditius, A.P.; Reich, M.; Simon, A.C.; Suvorova, A.; Knipping, J.; Roberts, M.P.; Rubanov, S.; Dodd, A.; Saunders, M. Nanogeochemistry of hydrothermal magnetite. Contrib. Mineral. Petrol. 2018, 173, 46. [Google Scholar] [CrossRef]
- Bernstein, L.R. Germanium geochemistry and mineralogy. Geochim. Cosmochim. Acta 1985, 49, 2409–2422. [Google Scholar] [CrossRef]
- Höll, R.; Kling, M.; Schroll, E. Metallogenesis of germanium—A review. Ore Geol. Rev. 2007, 30, 145–180. [Google Scholar] [CrossRef]
- Meng, Y.; Hu, R.; Huang, X.; Gao, J. Germanium in magnetite: A preliminary review. Acta Geol. Sin. Engl. Ed. 2017, 91, 711–726. [Google Scholar] [CrossRef]
- Liu, L.; Zhang, H.; Yang, X.; Li, Y. Age, origin and significance of the Wugang BIF in the Taihua complex, Southern North China Craton. Ore Geol. Rev. 2018, 95, 880–898. [Google Scholar] [CrossRef]
- Zhai, M.; Santosh, M. Metallogeny of the North China Craton: Link with secular changes in the evolving Earth. Gondwana Res. 2013, 24, 275–297. [Google Scholar] [CrossRef]
- Zhao, G.; Zhai, M. Lithotectonic elements of Precambrian basement in the North China Craton: Review and tectonic implications. Gondwana Res. 2013, 23, 1207–1240. [Google Scholar] [CrossRef]
- Huang, X.-L.; Niu, Y.; Xu, Y.-G.; Yang, Q.-J.; Zhong, J.-W. Geochemistry of TTG and TTG-like gneisses from Lushan-Taihua complex in the southern North China Craton: Implications for late Archean crustal accretion. Precambrian Res. 2010, 182, 43–56. [Google Scholar] [CrossRef]
- Zhao, G.; Sun, M.; Wilde, S.A.; Sanzhong, L. Late Archean to Paleoproterozoic evolution of the North China Craton: Key issues revisited. Precambrian Res. 2005, 136, 177–202. [Google Scholar] [CrossRef]
- Liu, D.; Wilde, S.A.; Wan, Y.; Wang, S.; Valley, J.W.; Kita, N.; Dong, C.; Xie, H.; Yang, C.; Zhang, Y. Combined U–Pb, hafnium and oxygen isotope analysis of zircons from meta-igneous rocks in the southern North China Craton reveal multiple events in the Late Mesoarchean–Early Neoarchean. Chem. Geol. 2009, 261, 140–154. [Google Scholar] [CrossRef]
- Lu, J.-S.; Wang, G.-D.; Wang, H.; Chen, H.-X.; Wu, C.-M. Palaeoproterozoic metamorphic evolution and geochronology of the Wugang block, southeastern terminal of the Trans-North China Orogen. Precambrian Res. 2014, 251, 197–211. [Google Scholar] [CrossRef]
- Lan, C.Y.; Zhao, T.P.; Luo, Z.Z.; Wang, C.L.; Wen, Q.F.; Liu, L.X. The genesis of the Zhaoanzhuang Fe oxide deposit in Wuyang region of Henan Province: Insights from magnetite and apatite. Acta Petrol. Sin. 2015, 31, 1653–1670. [Google Scholar]
- Moon, I.; Lee, I.; Yang, X. Geochemical Studies of BIF in Wugang, North China Craton: Implication for the Genesis. Econ. Environ. Geol. 2019, 52, 213–221. [Google Scholar]
- Lan, C.; Zhang, L.; Zhao, T.; Wang, C.; Li, H.; Zhou, Y. Mineral and geochemical characteristics of the Tieshanmiao-type BIF-iron deposit in Wuyang region of Henan Province and its implications for ore-forming processes. Acta Petrol. Sin. 2013, 29, 2567–2582. [Google Scholar]
- Dare, S.A.; Barnes, S.-J.; Beaudoin, G. Variation in trace element content of magnetite crystallized from a fractionating sulfide liquid, Sudbury, Canada: Implications for provenance discrimination. Geochim. Cosmochim. Acta 2012, 88, 27–50. [Google Scholar] [CrossRef]
- Audetat, A.; Gunther, D.; Heinrich, C.A. Formation of a magmatic-hydrothermal ore deposit: Insights with LA-ICP-MS analysis of fluid inclusions. Science 1998, 279, 2091–2094. [Google Scholar] [CrossRef]
- Belissont, R.; Boiron, M.-C.; Luais, B.; Cathelineau, M. LA-ICP-MS analyses of minor and trace elements and bulk Ge isotopes in zoned Ge-rich sphalerites from the Noailhac–Saint-Salvy deposit (France): Insights into incorporation mechanisms and ore deposition processes. Geochim. Cosmochim. Acta 2014, 126, 518–540. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, X.; Zhou, H.; Lin, H.; Jiang, L.; Yang, T. In-situ LA-ICP-MS trace elements analysis of scheelites from the giant Beiya gold–polymetallic deposit in Yunnan Province, Southwest China and its metallogenic implications. Ore Geol. Rev. 2017, 80, 828–837. [Google Scholar] [CrossRef]
- Zhao, H.-X.; Frimmel, H.E.; Jiang, S.-Y.; Dai, B.-Z. LA-ICP-MS trace element analysis of pyrite from the Xiaoqinling gold district, China: Implications for ore genesis. Ore Geol. Rev. 2011, 43, 142–153. [Google Scholar] [CrossRef]
- Zhao, W.W.; Zhou, M.-F. In-situ LA–ICP-MS trace elemental analyses of magnetite: The Mesozoic Tengtie skarn Fe deposit in the Nanling Range, South China. Ore Geol. Rev. 2015, 65, 872–883. [Google Scholar] [CrossRef]
- Dare, S.A.; Barnes, S.-J.; Beaudoin, G.; Méric, J.; Boutroy, E.; Potvin-Doucet, C. Trace elements in magnetite as petrogenetic indicators. Miner. Depos. 2014, 49, 785–796. [Google Scholar] [CrossRef]
- Hawley, J.; Stanton, R. The Sudbury ores, their mineralogy and origin; Part 2, The facts; The ores, their minerals, metals and distribution. Can. Mineral. 1962, 7, 30–145. [Google Scholar]
- Naldrett, A.; Singh, J.; Krstic, S.; Li, C. The mineralogy of the Voisey’s Bay Ni-Cu-Co deposit, northern Labrador, Canada: Influence of oxidation state on textures and mineral compositions. Econ. Geol. 2000, 95, 889–900. [Google Scholar]
- Czamanske, G.K.; Kunilov, V.E.; Zientek, M.L.; Cabri, L.J.; Likhachev, A.P.; Calk, L.C.; Oscarson, R.L. A proton microprobe study of magmatic sulfide ores from the Noril’sk-Talnakh District, Siberia. Can. Mineral. 1992, 30, 249–287. [Google Scholar]
- Gao, J.-F.; Zhou, M.-F.; Lightfoot, P.C.; Wang, C.Y.; Qi, L.; Sun, M. Sulfide saturation and magma emplacement in the formation of the Permian Huangshandong Ni-Cu sulfide deposit, Xinjiang, northwestern China. Econ. Geol. 2013, 108, 1833–1848. [Google Scholar] [CrossRef]
- Zhou, M.-F.; Lesher, C.M.; Yang, Z.; Li, J.; Sun, M. Geochemistry and petrogenesis of 270 Ma Ni–Cu–(PGE) sulfide-bearing mafic intrusions in the Huangshan district, Eastern Xinjiang, Northwest China: Implications for the tectonic evolution of the Central Asian orogenic belt. Chem. Geol. 2004, 209, 233–257. [Google Scholar] [CrossRef]
- Yin, J.; Xing, S.; Xiao, K. Metallogenic characteristics and resource potential analysis of the Middle-Lower Yangtze River Fe-Cu-Au-Pb-Zn Metallogenic Belt. Acta Geol. Sin. 2016, 90, 1525–1536. [Google Scholar]
- Zhao, X.-F.; Zhou, M.-F. Fe–Cu deposits in the Kangdian region, SW China: A Proterozoic IOCG (iron-oxide–copper–gold) metallogenic province. Miner. Depos. 2011, 46, 731–747. [Google Scholar] [CrossRef]
- Zhao, X.-F.; Zhou, M.-F.; Li, J.-W.; Selby, D.; Li, X.-H.; Qi, L. Sulfide Re-Os and Rb-Sr isotope dating of the Kangdian IOCG metallogenic province, southwest China: Implications for regional metallogenesis. Econ. Geol. 2013, 108, 1489–1498. [Google Scholar] [CrossRef]
- Huang, X.-W.; Zhou, M.-F.; Qi, L.; Gao, J.-F.; Wang, Y.-W. Re–Os isotopic ages of pyrite and chemical composition of magnetite from the Cihai magmatic–hydrothermal Fe deposit, NW China. Miner. Depos. 2013, 48, 925–946. [Google Scholar] [CrossRef]
- Tianfeng, W.; Qingle, Z.; Qianqian, W. Paleozoic Tectono-Metallogeny in the Tianshan-Altay Region, Central Asia. Acta Geol. Sin. Engl. Ed. 2015, 89, 1120–1132. [Google Scholar] [CrossRef]
- Liu, P.-P.; Zhou, M.-F.; Chen, W.T.; Gao, J.-F.; Huang, X.-W. In-situ LA-ICP-MS trace elemental analyses of magnetite: Fe–Ti–(V) oxide-bearing mafic–ultramafic layered intrusions of the Emeishan Large Igneous Province, SW China. Ore Geol. Rev. 2015, 65, 853–871. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. 3.01—Composition of the Continental Crust. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Pergamon: Oxford, UK, 2003; pp. 1–64. [Google Scholar]
- Hu, H.; Li, J.-W.; Lentz, D.; Ren, Z.; Zhao, X.-F.; Deng, X.-D.; Hall, D. Dissolution—Reprecipitation process of magnetite from the Chengchao iron deposit: Insights into ore genesis and implication for in-situ chemical analysis of magnetite. Ore Geol. Rev. 2014, 57, 393–405. [Google Scholar] [CrossRef]
- McIntire, W. Trace element partition coefficients—A review of theory and applications to geology. Geochim. Cosmochim. Acta 1963, 27, 1209–1264. [Google Scholar] [CrossRef]
- Arnórsson, S. Germanium in Icelandic geothermal systems. Geochim. Cosmochim. Acta 1984, 48, 2489–2502. [Google Scholar] [CrossRef]
- Davy, R. Part A: A contribution on the chemical composition of Precambrian iron-formations. In Developments in Precambrian Geology; Elsevier: Amsterdam, The Netherlands, 1983; Volume 6, pp. 325–343. [Google Scholar]
- Burton, J.; Culkin, F.; Riley, J. The abundances of gallium and germanium in terrestrial materials. Geochim. Cosmochim. Acta 1959, 16, 151–180. [Google Scholar] [CrossRef]
- Escoube, R.; Rouxel, O.J.; Edwards, K.; Glazer, B.; Donard, O.F. Coupled Ge/Si and Ge isotope ratios as geochemical tracers of seafloor hydrothermal systems: Case studies at Loihi Seamount and East Pacific Rise 9 50′ N. Geochim. Cosmochim. Acta 2015, 167, 93–112. [Google Scholar] [CrossRef]

| FeOT | MnO | Cr2O3 | MgO | SiO2 | K2O | CaO | TiO2 | Na2O | Al2O3 | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 89.2 | - | - | - | - | - | - | - | - | - | 89.6 |
| Max | 94.2 | 0.2 | 0.1 | 0.1 | 0.3 | 0.2 | - | 0.1 | 0.4 | 0.1 | 94.5 |
| Median | 92.2 | 0.1 | - | - | - | - | - | - | - | - | - |
| Standard deviation | 1.0 | - | - | - | - | - | - | - | - | - | - |
| Ge | Ti | V | Mg | Al | Cr | Mn | Co | Zn | Ga | Ni | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Min | 3.3 | 40 | 2.5 | 17 | 78 | 0.4 | 244 | 0.4 | 6.8 | 0.5 | <0.01 |
| Max | 11 | 262 | 17 | 953 | 1764 | 405 | 4205 | 22 | 119 | 6.5 | 22 |
| Median | 4.7 | 54 | 3.9 | 135 | 341 | 4.5 | 622 | 1.2 | 43 | 0.8 | 4.0 |
| Standard deviation | 1.0 | 46 | 3.1 | 240 | 356 | 32 | 594 | 5.1 | 25 | 1.2 | 3.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moon, I.; Liu, L.; Yang, X.; Suh, Y.J.; Jung, J.; Ha, Y. Chemical Signatures of Ge in Magnetite of Wugang BIF, China. Appl. Sci. 2023, 13, 8246. https://doi.org/10.3390/app13148246
Moon I, Liu L, Yang X, Suh YJ, Jung J, Ha Y. Chemical Signatures of Ge in Magnetite of Wugang BIF, China. Applied Sciences. 2023; 13(14):8246. https://doi.org/10.3390/app13148246
Chicago/Turabian StyleMoon, Inkyeong, Lei Liu, Xiaoyong Yang, Yeon Jee Suh, Jaewoo Jung, and Youngji Ha. 2023. "Chemical Signatures of Ge in Magnetite of Wugang BIF, China" Applied Sciences 13, no. 14: 8246. https://doi.org/10.3390/app13148246
APA StyleMoon, I., Liu, L., Yang, X., Suh, Y. J., Jung, J., & Ha, Y. (2023). Chemical Signatures of Ge in Magnetite of Wugang BIF, China. Applied Sciences, 13(14), 8246. https://doi.org/10.3390/app13148246









