Liquid to Fused Deposition Modeling (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology—Mechanical and Functional Properties
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Polymers
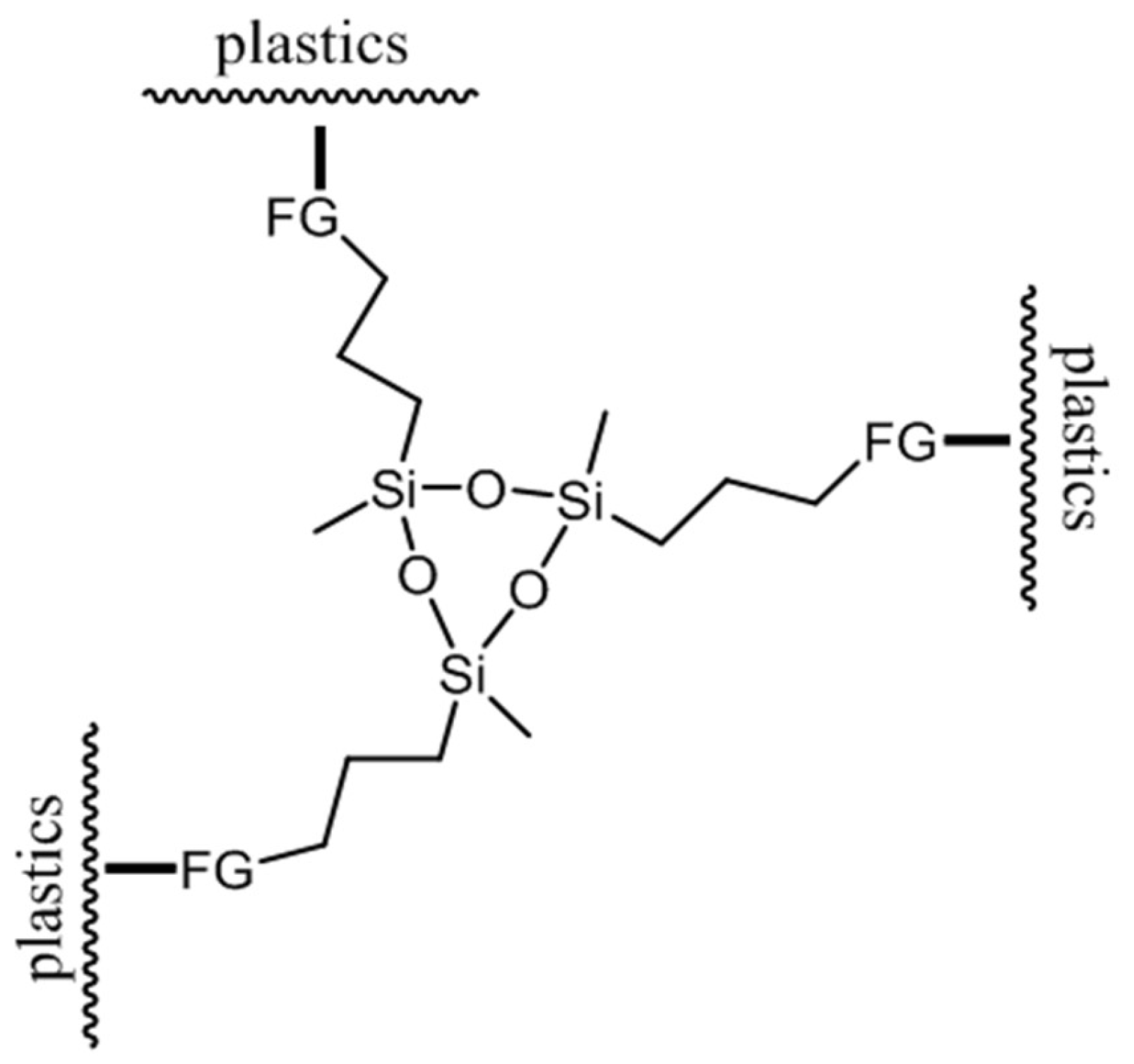
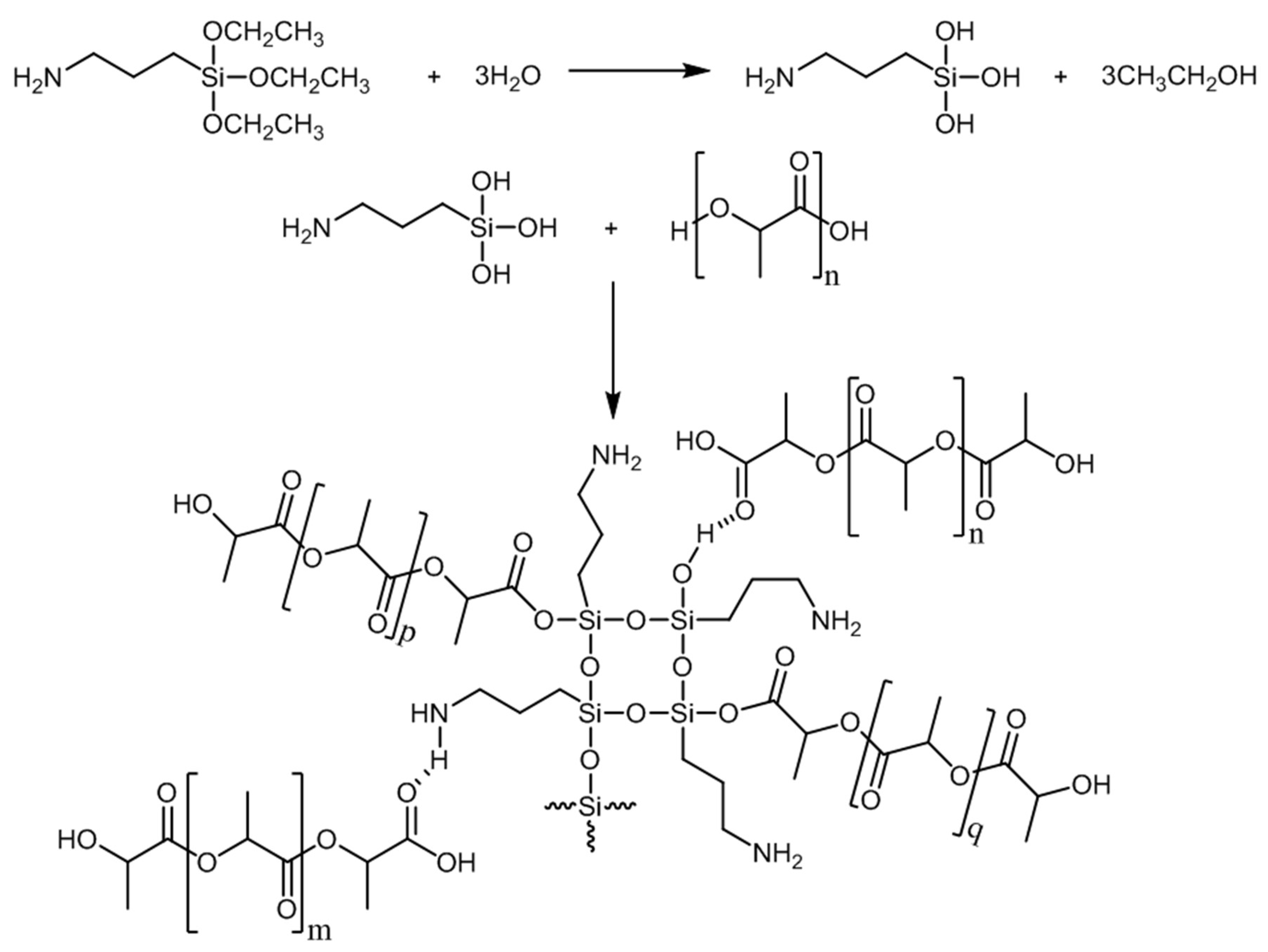
2.1.2. Solvents and Liquid Chemicals
2.2. Devices and Methods
2.3. Analyses—Instrumental Methods and Measurements
3. Results and Discussion
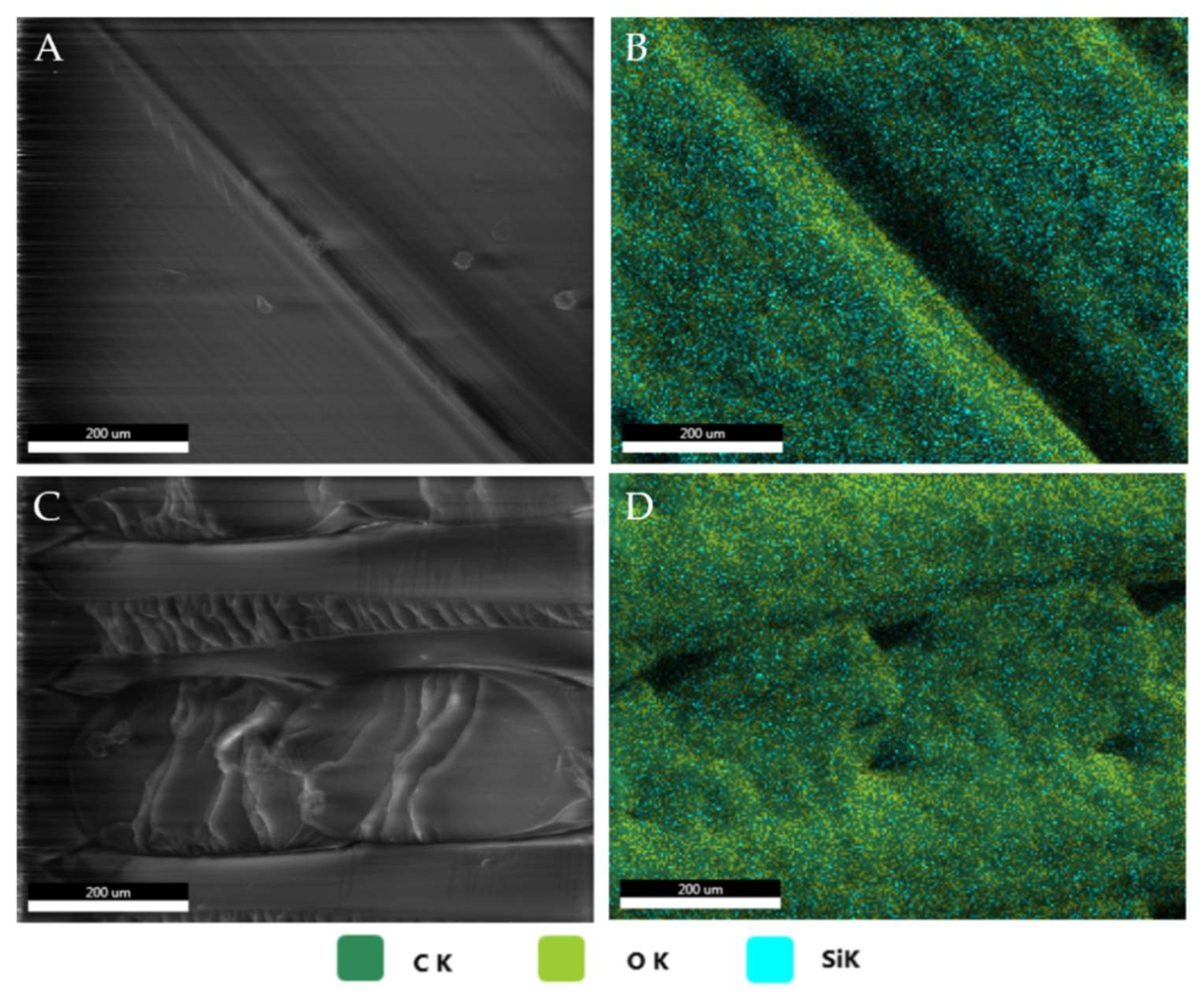
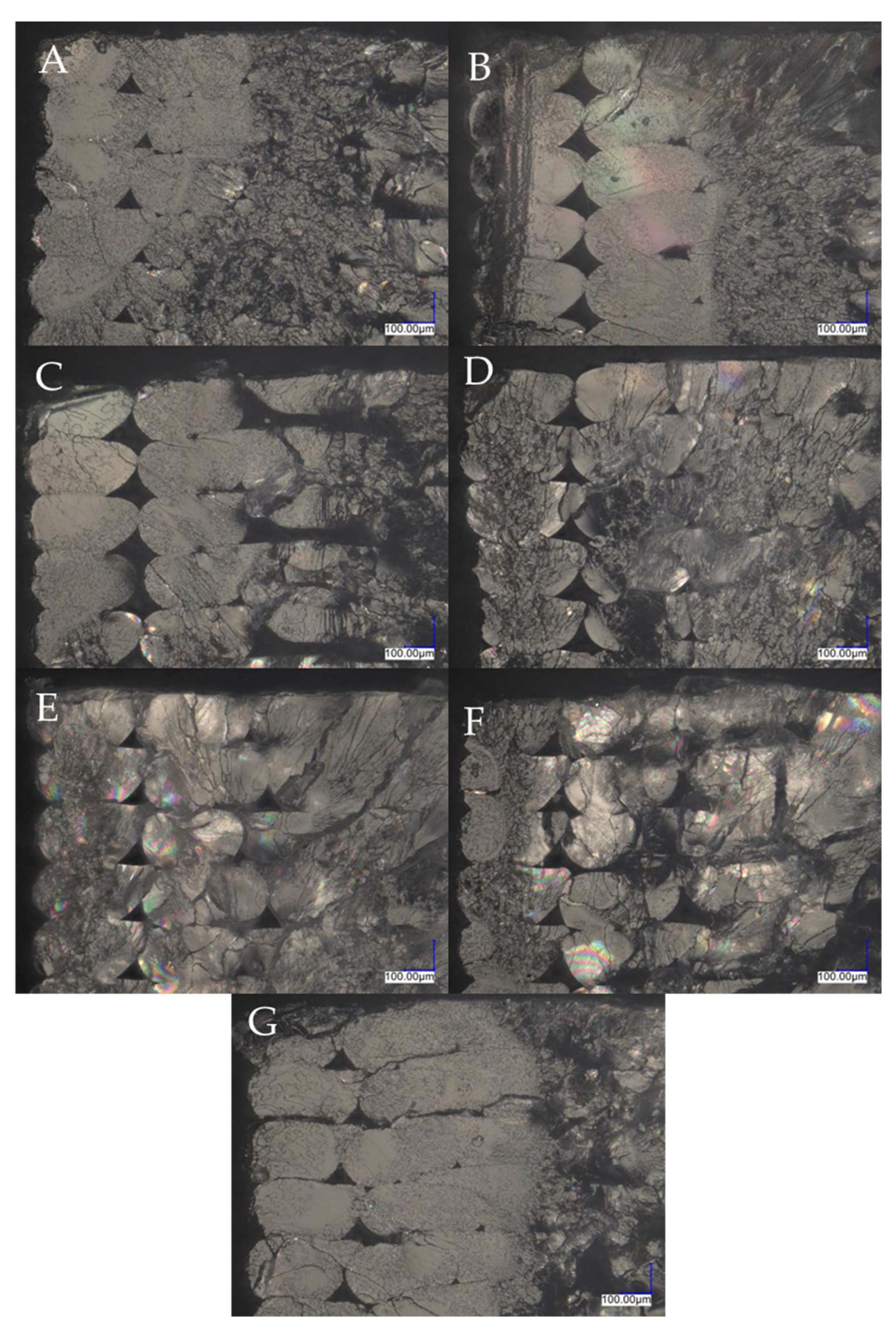
3.1. SEM/EDS
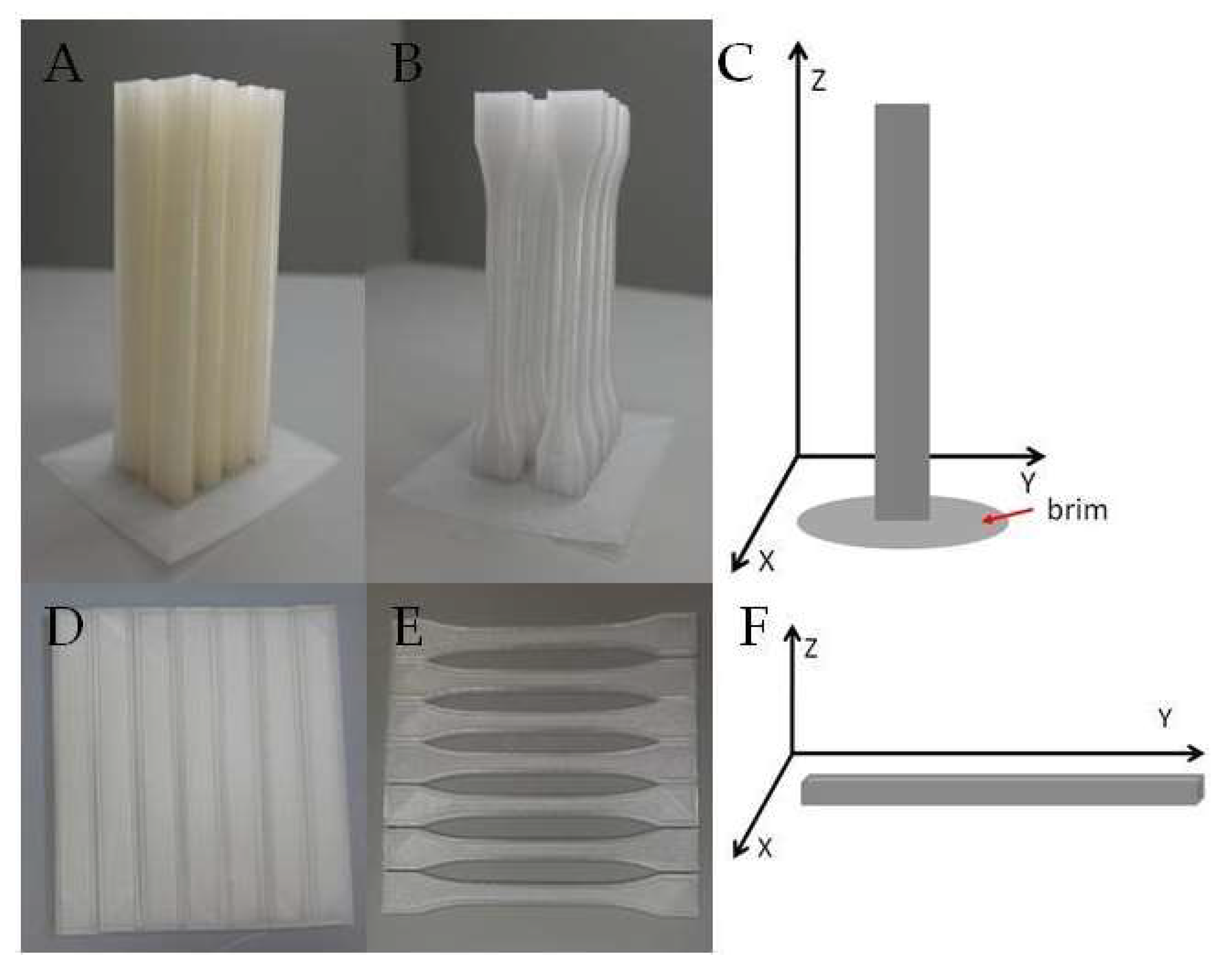
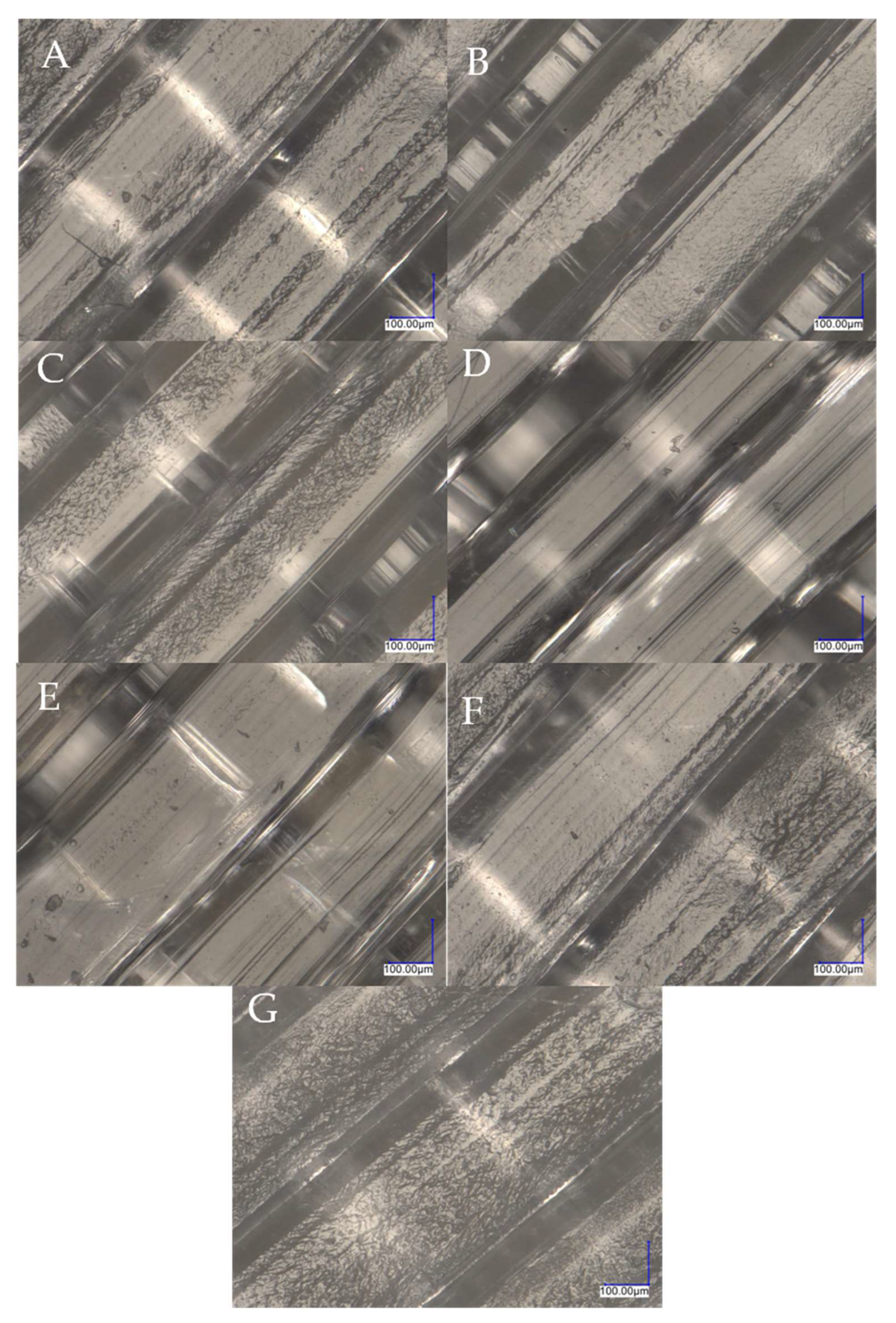
3.2. Digital Optical Microscope
3.3. Mechanical Properties
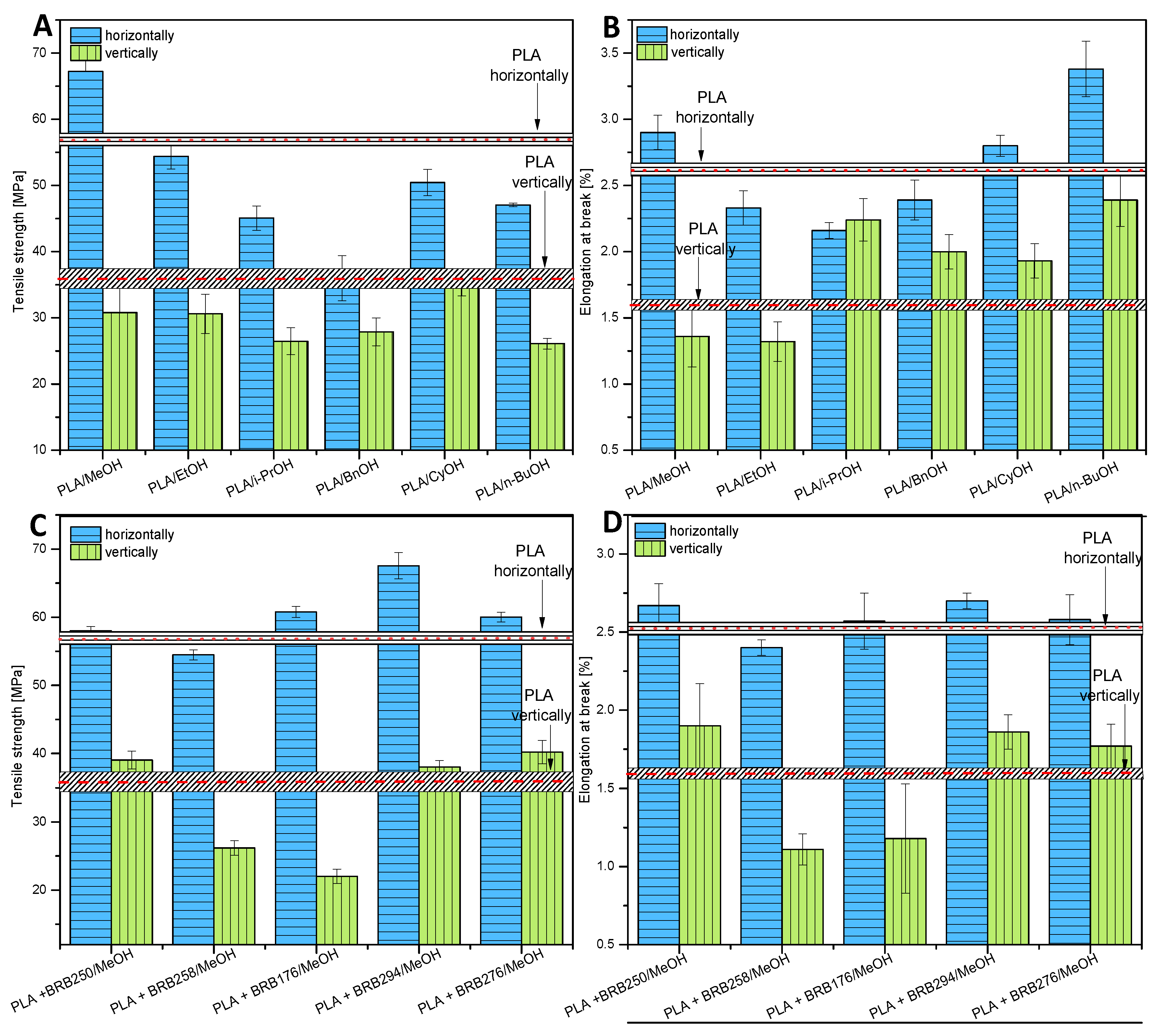
3.3.1. Tensile Strength
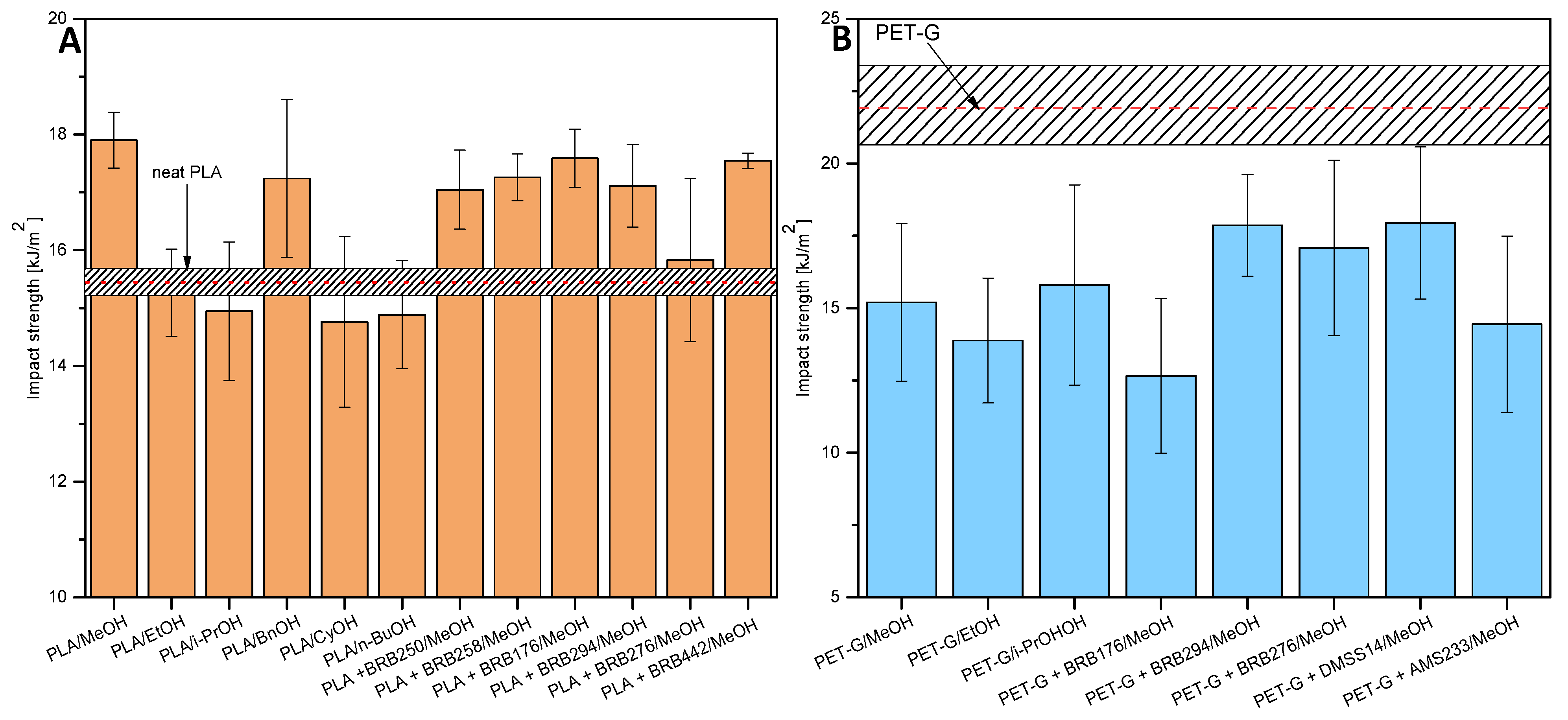
3.3.2. Impact Strength
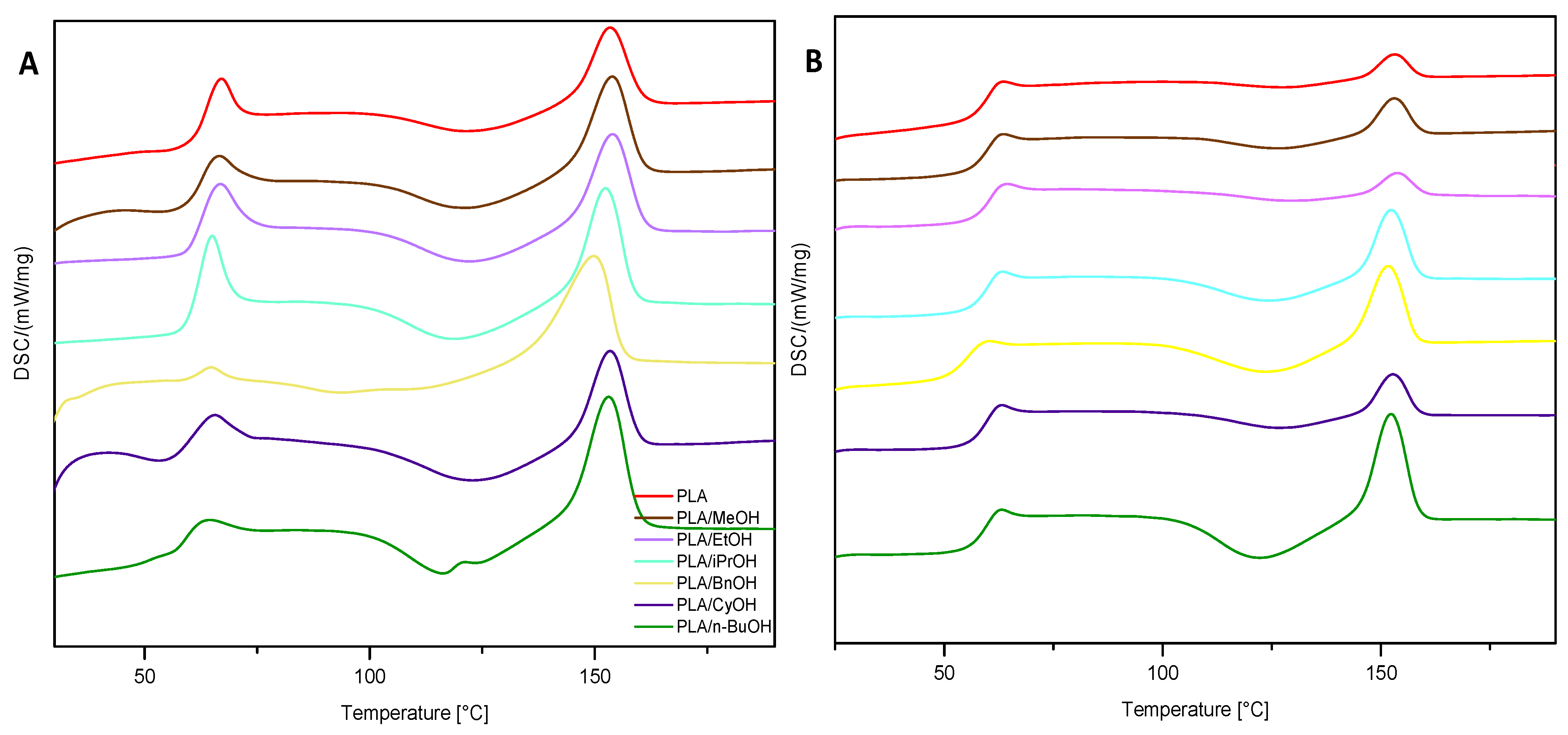
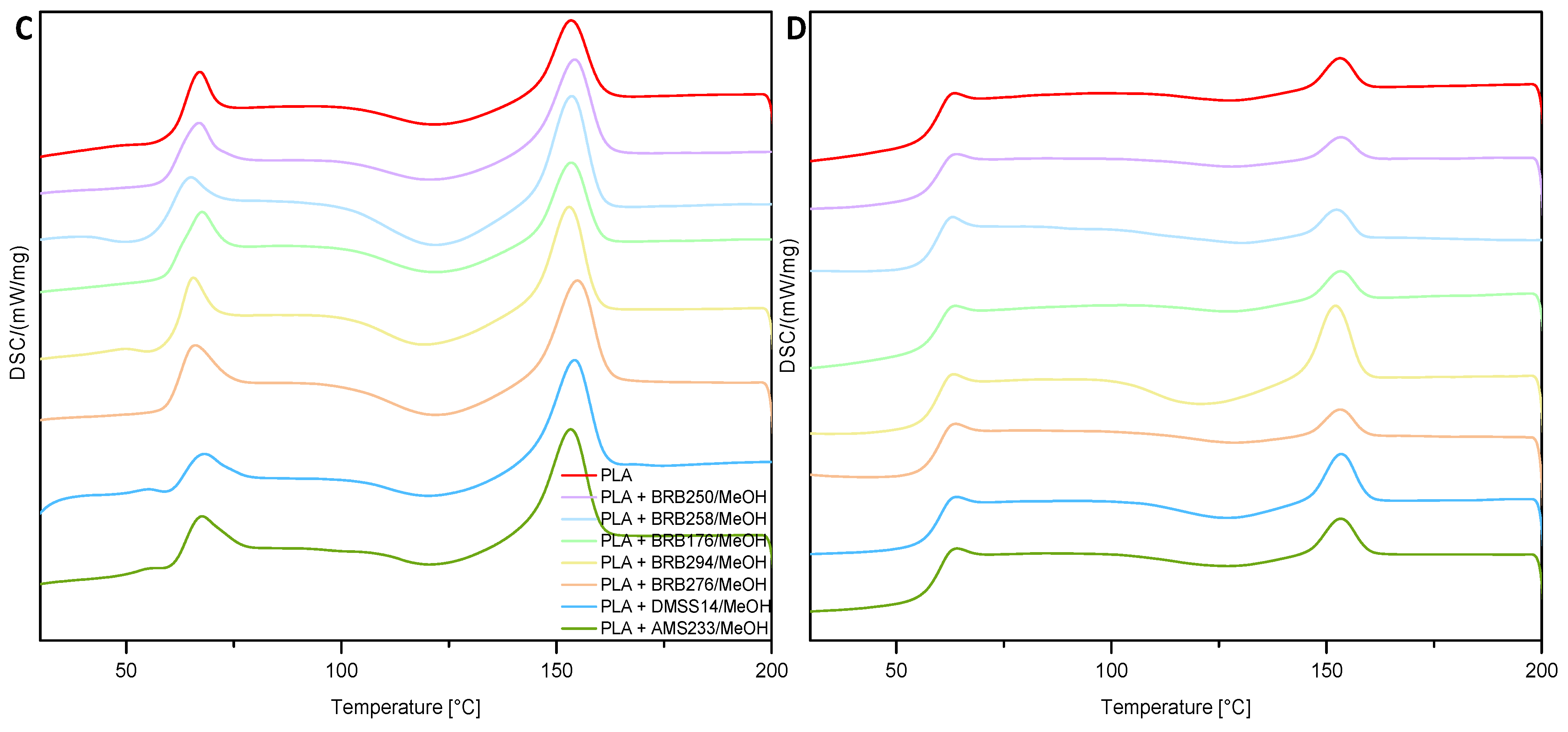
3.4. DSC
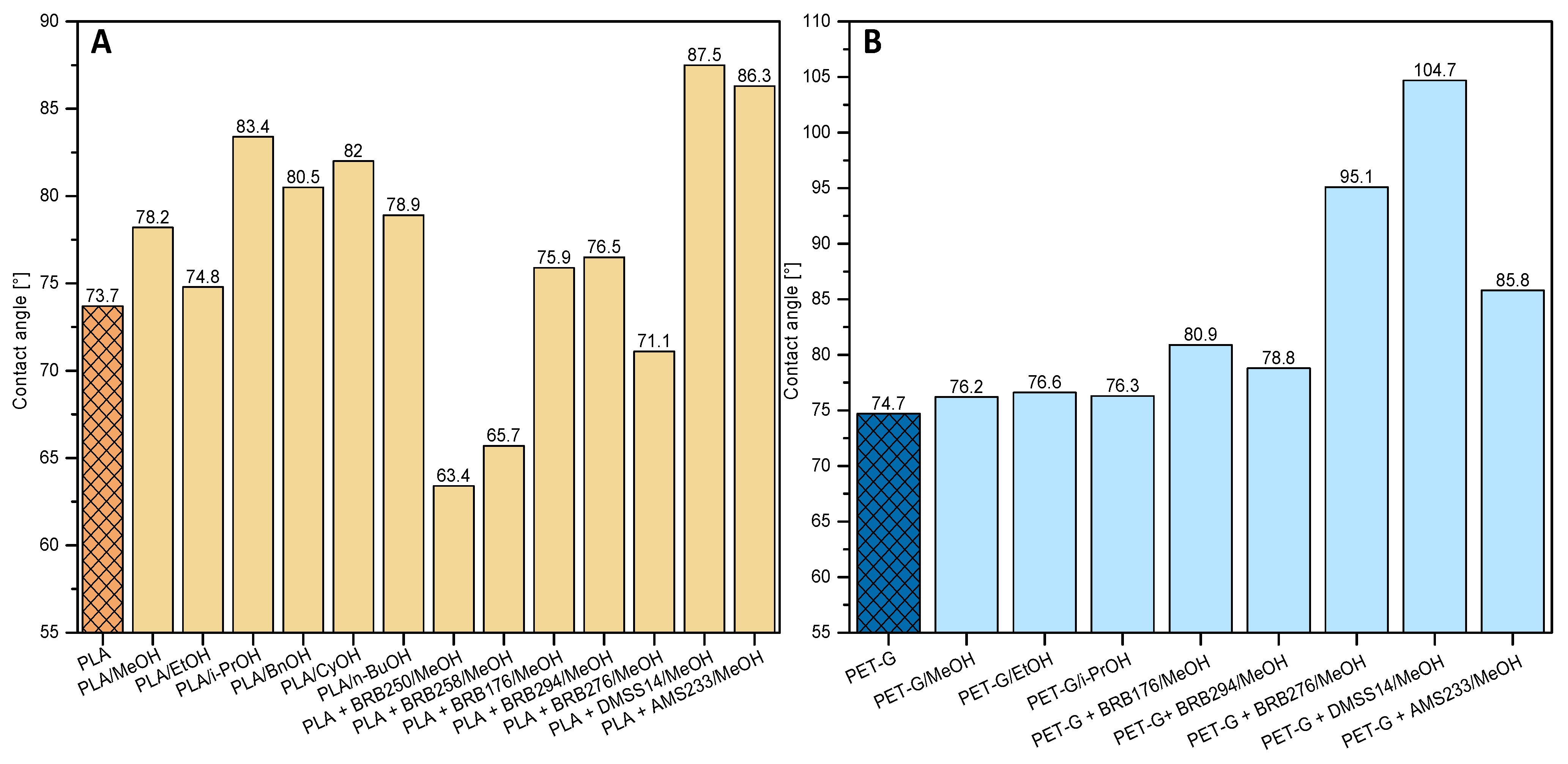
3.5. Water Contact Angle (WCA)
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Dhinakaran, V.; Kumar, K.M.; Ram, P.B.; Ravichandran, M.; Vinayagamoorthy, M. A review on recent advancements in fused deposition modeling. Mater. Today Proc. 2020, 27, 752–756. [Google Scholar] [CrossRef]
- Dizon, J.R.C.; Espera, A.H.; Chen, Q.; Advincula, R.C. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 2018, 20, 44–67. [Google Scholar] [CrossRef]
- Kristiawan, R.; Imaduddin, F.; Ariawan, D.; Ubaidillah Arifin, Z. A review on the fused deposition modeling (FDM) 3D printing: Filament processing, materials, and printing parameters. Open Eng. 2021, 11, 639–649. [Google Scholar] [CrossRef]
- Özen, A.; Auhl, D.; Völlmecke, C.; Kiendl, J.; Abali, B.E. Optimization of Manufacturing Parameters and Tensile Specimen Geometry for Fused Deposition Modeling (FDM) 3D-Printed PETG. Materials 2021, 14, 2556. [Google Scholar] [CrossRef]
- Arockiam, A.J.; Subramanian, K.; Padmanabhan, R.G.; Selvaraj, R.; Bagal, D.K.; Rajesh, S. A review on PLA with different fillers used as a filament in 3D printing. Mater. Today Proc. 2022, 50, 2057–2064. [Google Scholar] [CrossRef]
- Wang, J.; Yang, B.; Lin, X.; Gao, L.; Liu, T.; Lu, Y.; Wang, R. Research of TPU Materials for 3D Printing Aiming at Non-Pneumatic Tires by FDM Method. Polymers 2020, 12, 2492. [Google Scholar] [CrossRef]
- Wickramasinghe, S.; Do, T.; Tran, P. FDM-Based 3D Printing of Polymer and Associated Composite: A Review on Mechanical Properties, Defects and Treatments. Polymers 2020, 12, 1529. [Google Scholar] [CrossRef]
- Roberson, D.; Shemelya, C.M.; MacDonald, E.; Wicker, R. Expanding the applicability of FDM-type technologies through materials development. Rapid Prototyp. J. 2015, 21, 137–143. [Google Scholar] [CrossRef]
- Banjo, A.D.; Agrawal, V.; Auad, M.L.; Celestine, A.D.N. Moisture-induced changes in the mechanical behavior of 3D printed polymers. Compos. Part C Open Access 2022, 7, 100243. [Google Scholar] [CrossRef]
- Proikakis, C.S.; Mamouzelos, N.J.; Tarantili, P.A.; Andreopoulos, A.G. Swelling and hydrolytic degradation of poly(d,l-lactic acid) in aqueous solutions. Polym. Degrad. Stab. 2006, 91, 614–619. [Google Scholar] [CrossRef]
- Schott, H. Swelling kinetics of polymers. J. Macromol. Sci. 1992, 31, 1–9. [Google Scholar] [CrossRef]
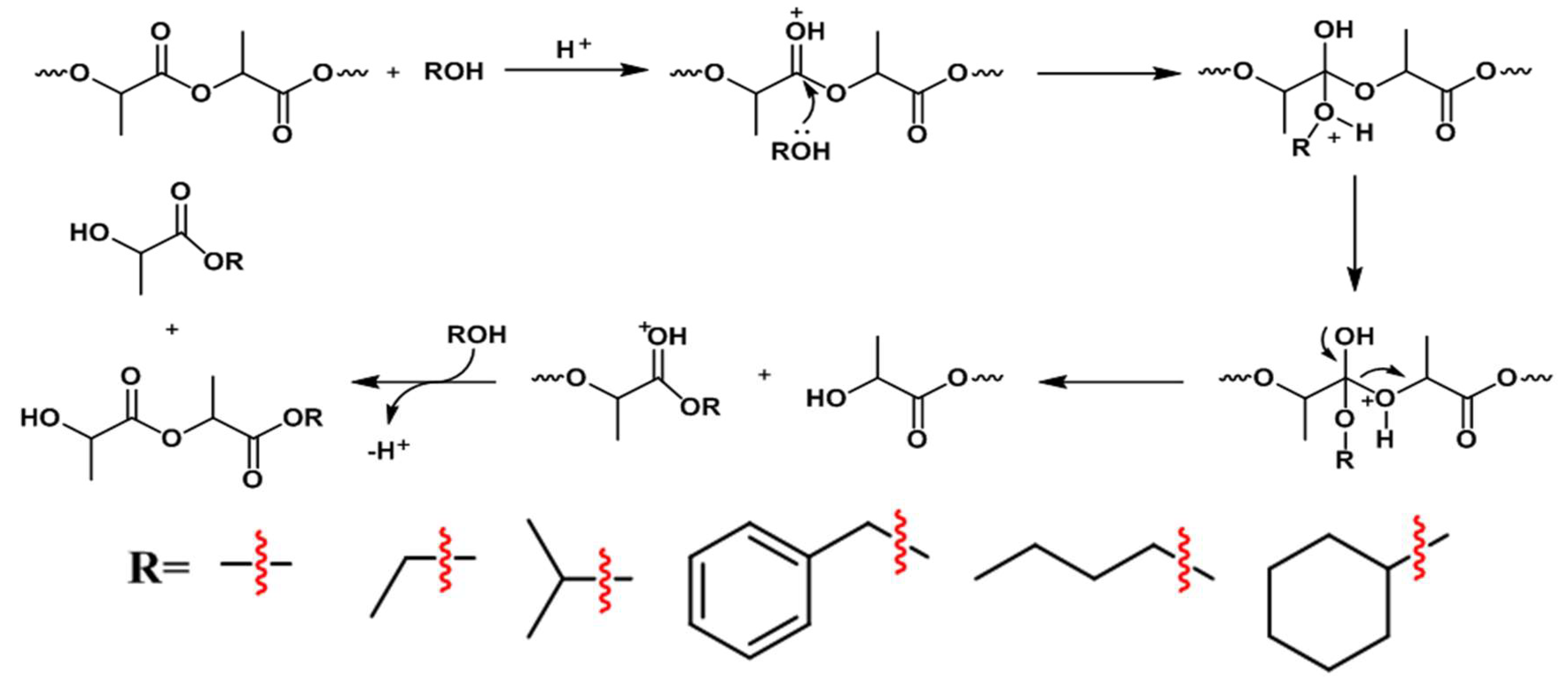
- Nim, B.; Opaprakasit, P. Quantitative analyses of products from chemical recycling of polylactide (PLA) by alcoholysis with various alcohols and their applications as healable lactide-based polyurethanes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 255, 119684. [Google Scholar] [CrossRef] [PubMed]
- Petrus, R.; Bykowski, D.; Sobota, P. Solvothermal Alcoholysis Routes for Recycling Polylactide Waste as Lactic Acid Esters. ACS Catal. 2016, 6, 5222–5235. [Google Scholar] [CrossRef]
- Rahmat, M.; Ghasemi, I.; Karrabi, M.; Azizi, H.; Zandi, M.; Riahinezhad, M. Silane crosslinking of poly(lactic acid): The effect of simultaneous hydrolytic degradation. Express Polym. Lett. 2015, 9, 1133–1141. [Google Scholar] [CrossRef]
- Sirisinha, K.; Kamphunthong, W. Rheological analysis as a means for determining the silane crosslink network structure and content in crosslinked polymer composites. Polym. Test. 2009, 28, 636–641. [Google Scholar] [CrossRef]
- Xu, D.; Auras, R.A.; Sonchaeng, U.; Rubino, M.; Lim, L.T. The effect of alcoholic solutions on the thermomechanical properties of immersed poly(lactic acid) films. J. Appl. Polym. Sci. 2023, 140, e53489. [Google Scholar] [CrossRef]
- Phongtamrug, S.; Makhon, R.; Wiriyosuttikul, T. Enhanced Performance of Polylactide Film via Simultaneous Biaxial Stretching and Silane Coupling Agent as a Thermal Shrinkable Film. Appl. Sci. Eng. Prog. 2023, 16, 5699. [Google Scholar] [CrossRef]
- Kong, Y.; Hay, J.N. The measurement of the crystallinity of polymers by DSC. Polymer 2002, 43, 3873–3878. [Google Scholar] [CrossRef]
- Khoo, R.Z.; Ismail, H.; Chow, W.S. Thermal and Morphological Properties of Poly (Lactic Acid)/Nanocellulose Nanocomposites. Procedia Chem. 2016, 19, 788–794. [Google Scholar] [CrossRef] [Green Version]
- Aliotta, L.; Sciara, L.M.; Cinelli, P.; Canesi, I.; Lazzeri, A. Improvement of the PLA Crystallinity and Heat Distortion Temperature Optimizing the Content of Nucleating Agents and the Injection Molding Cycle Time. Polymers 2022, 14, 977. [Google Scholar] [CrossRef]
- Schawe, J.E.K. Analysis of non-isothermal crystallization during cooling and reorganization during heating of isotactic polypropylene by fast scanning DSC. Thermochim. Acta 2015, 603, 85–93. [Google Scholar] [CrossRef]
- Lee, A.; Wynn, M.; Quigley, L.; Salviato, M.; Zobeiry, N. Effect of temperature history during additive manufacturing on crystalline morphology of PEEK. Adv. Ind. Manuf. Eng. 2022, 4, 100085. [Google Scholar] [CrossRef]
- Iñiguez-Franco, F.; Auras, R.; Burgess, G.; Holmes, D.; Fang, X.; Rubino, M.; Soto-Valdez, H. Concurrent solvent induced crystallization and hydrolytic degradation of PLA by water-ethanol solutions. Polymer 2016, 99, 315–323. [Google Scholar] [CrossRef] [Green Version]
- Zhou, D.; Xu, M.; Tan, R.; Sun, Y.; Ma, Z.; Li, J.; Dong, X.H. Quantitatively unravel the effect of chain length heterogeneity on polymer crystallization using discrete oligo l-lactide. Polymer 2021, 225, 123746. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Karapanagiotis, I.; Manoudis, P.N.; Zurba, A.; Lampakis, D. From Hydrophobic to Superhydrophobic and Superhydrophilic Siloxanes by Thermal Treatment. Langmuir 2014, 30, 13235–13243. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Z.; Ge, W.; Wang, Y.; Yin, X.; Zhang, L.; Yang, W.; Dai, J. Superhydrophobic sponge with the rod-spherical microstructure via palygorskite-catalyzed hydrolysis and condensation of vinyltriethoxysilane for oil-water separation. Appl. Clay Sci. 2020, 199, 105872. [Google Scholar] [CrossRef]
- Trofimchuk, E.S.; Nikonorova, N.I.; Moskvina, M.A.; Efimov, A.V.; Khavpachev, M.A.; Volynskii, A.L. Influence of liquid media on the craze initiation in amorphous polylactide. Polymer 2018, 142, 43–47. [Google Scholar] [CrossRef]
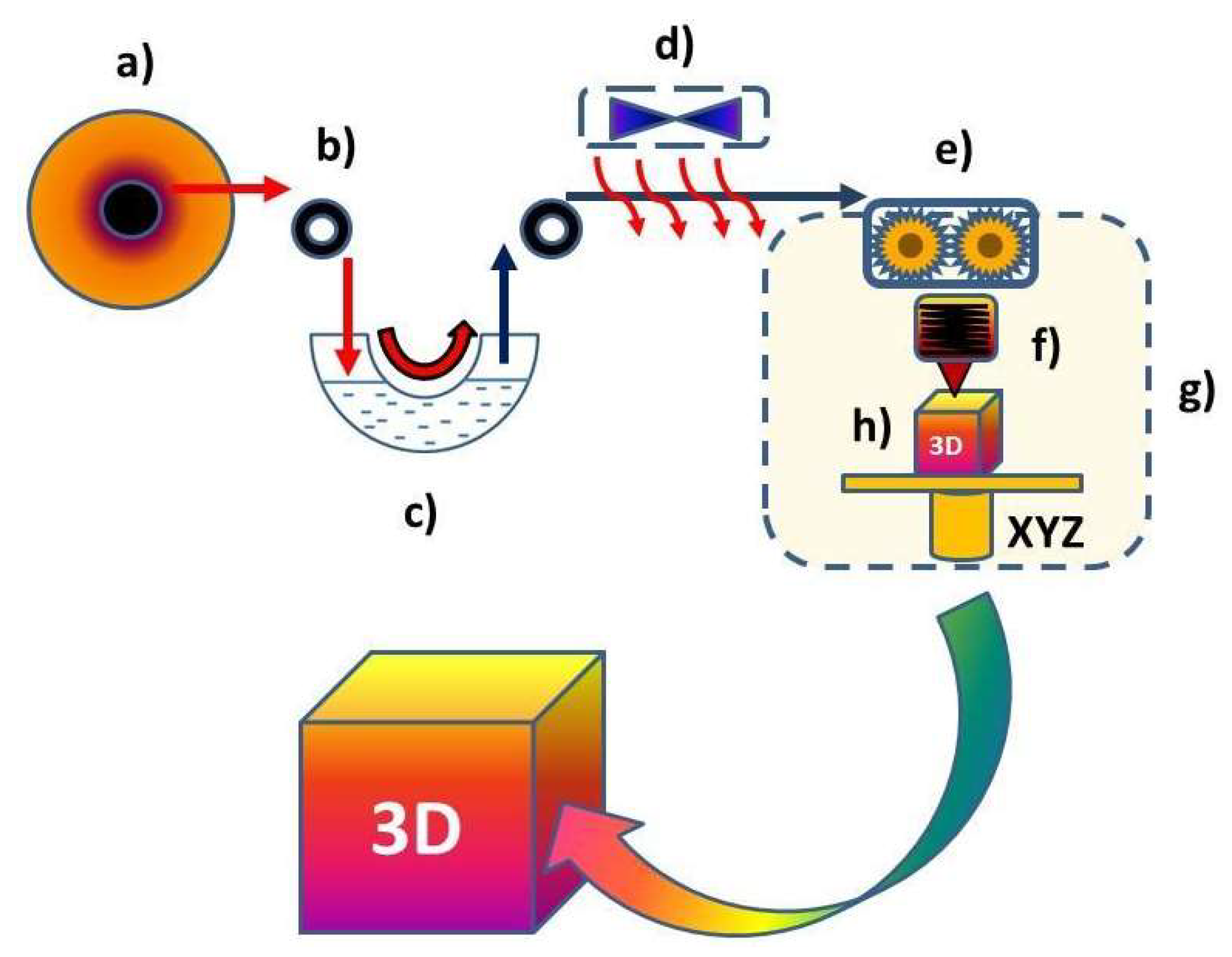



| Slicer | Creality 1.2.3. |
|---|---|
| layer height [mm] | 0.2 |
| first layer speed/printing speed [mm/s] | 20/50—horizontal, 20/30—vertical |
| print/bed temp. [°C] | 220/60 for PLA and 230/70 for PETG |
| number of shells | 2 |
| infill [%] | 100 |
| infill angle [°] | 45 |
| fan speed [%] | 100 |
| PLA/ | Alcohol Boiling Point [°C] | pKa Alcohol (from PuB Chem) | Tensile Strength [MPa] | Impact Strength [kJ/m2] | Crystallinity [%] 1 Cycle | Crystallinity [%] 2 Cycle |
|---|---|---|---|---|---|---|
| neat | - | - | 56.8 | 15.4 | 13.64 | 3.40 |
| MeOH | 65.0 | 15.3 (at 25 °C) | 67.22 | 17.9 | 20.61 | 6.39 |
| EtOH | 78.2 | 15.9 (at 25 °C) | 54.39 | 15.3 | 19.42 | 4.15 |
| i-PrOH | 83.0 | 17.1 | 45.07 | 14.9 | 21.55 | 4.44 |
| BnOH | 205.3 | 15.4 | 35.99 | 17.2 | 23.71 | 18.72 |
| CyOH | 161.0 | 16.0 | 50.43 | 14.8 | 19.06 | 6.49 |
| n-BuOH | 117.0 | 16.1 | 47.05 | 14.9 | 24.07 | 18.63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przekop, R.E.; Gabriel, E.; Pakuła, D.; Sztorch, B. Liquid to Fused Deposition Modeling (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology—Mechanical and Functional Properties. Appl. Sci. 2023, 13, 8462. https://doi.org/10.3390/app13148462
Przekop RE, Gabriel E, Pakuła D, Sztorch B. Liquid to Fused Deposition Modeling (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology—Mechanical and Functional Properties. Applied Sciences. 2023; 13(14):8462. https://doi.org/10.3390/app13148462
Chicago/Turabian StylePrzekop, Robert E., Ewa Gabriel, Daria Pakuła, and Bogna Sztorch. 2023. "Liquid to Fused Deposition Modeling (L-FDM)—A Revolution in Application Chemicals to 3D Printing Technology—Mechanical and Functional Properties" Applied Sciences 13, no. 14: 8462. https://doi.org/10.3390/app13148462







