Finite Element Method (FEM) Modeling of Laser-Tissue Interaction during Hair Removal
Abstract
:1. Introduction
2. Materials and Methods
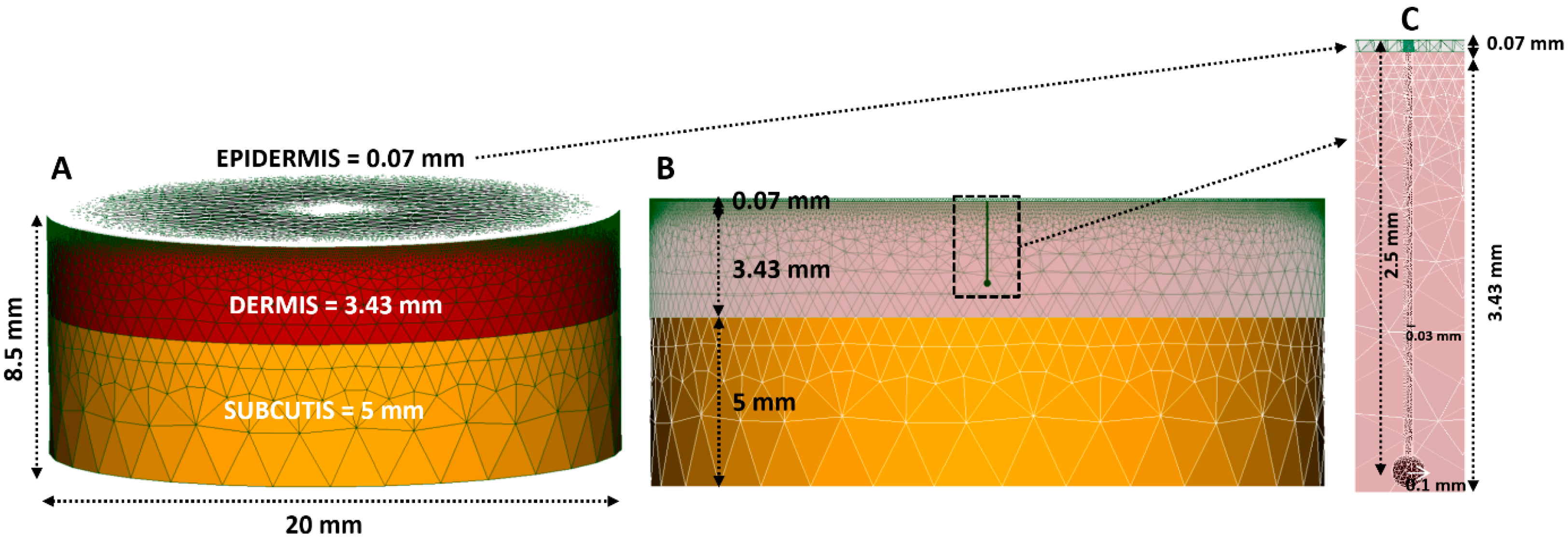
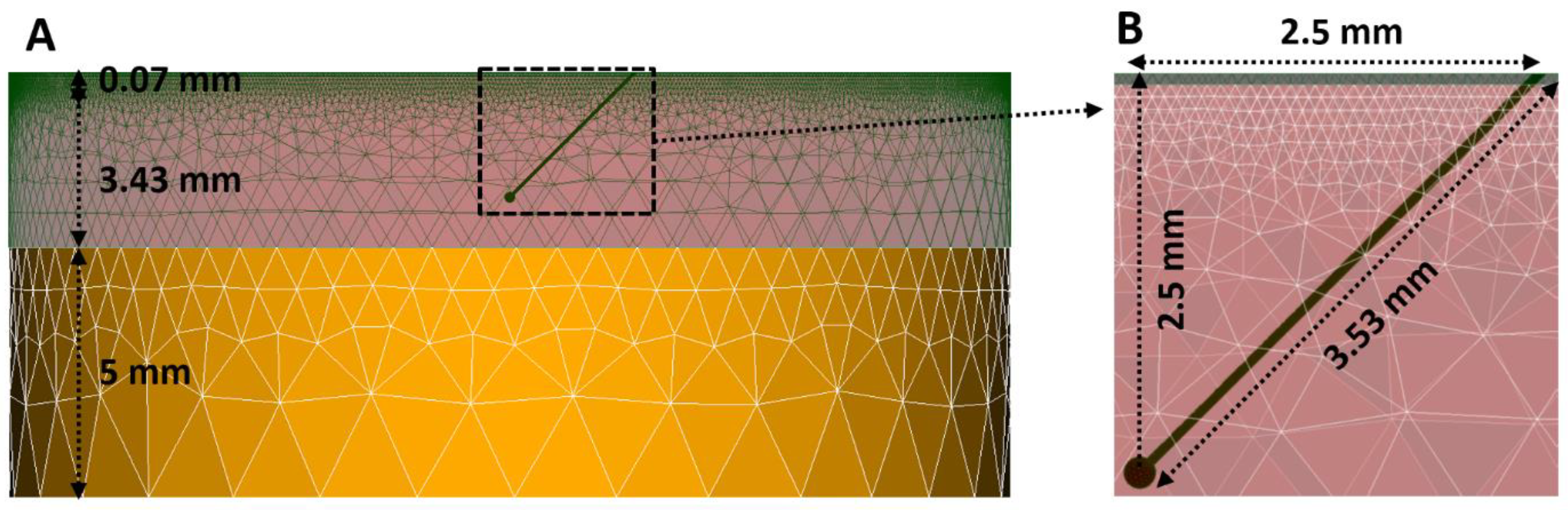
2.1. FEM (Finite Element Method) Modelling
2.2. Laser-Tissue Interaction
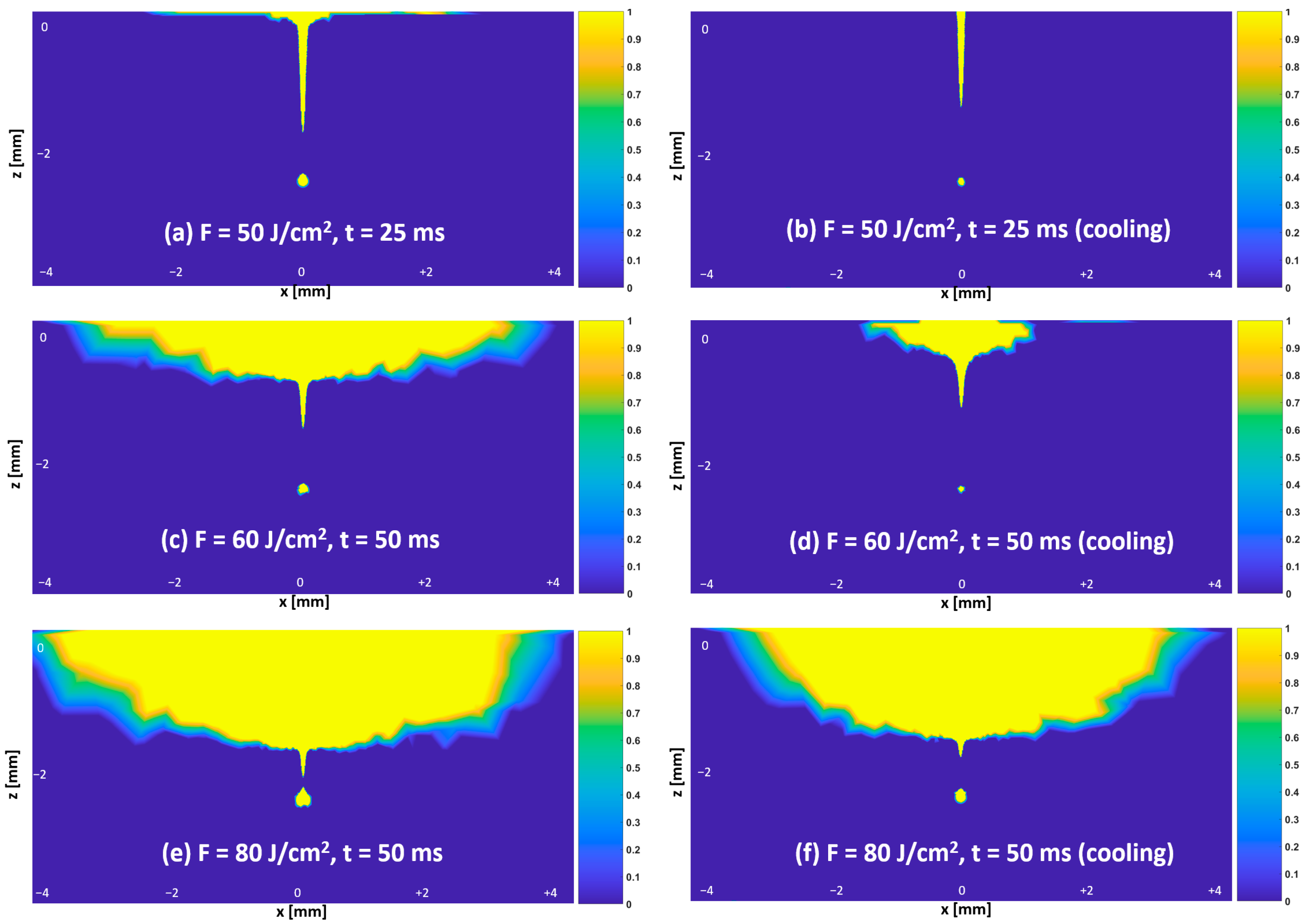
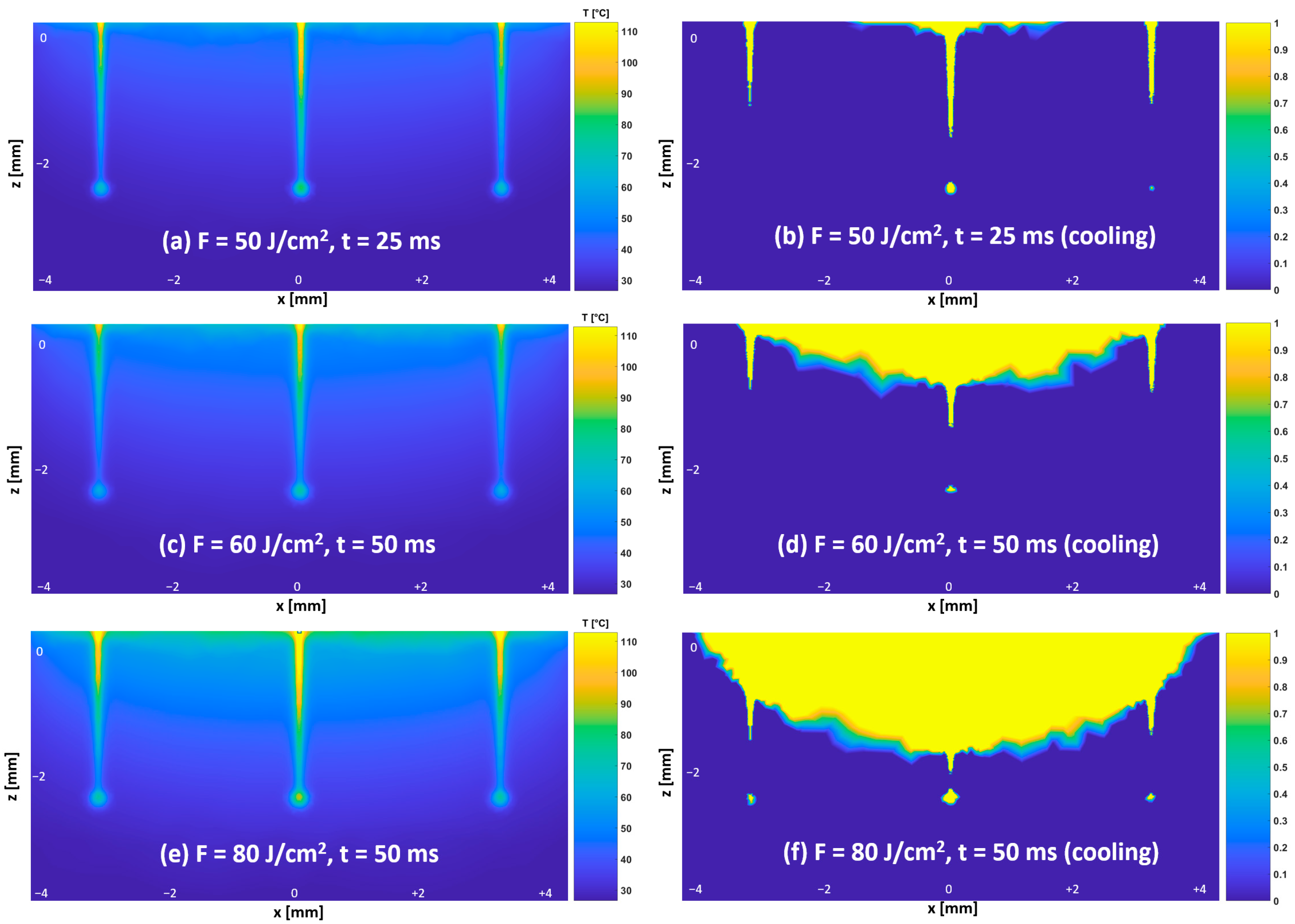
2.3. Thermal Damage Estimation in the Tissues
2.4. Simulation Protocols of Thermal Damage in the Tissue
- -
- The simulated laser beam, with a wavelength of 1064 nm and a disc-shaped radius of 4 mm, was directed perpendicularly to the short-axis center of FEM Model #1, FEM Model #2, and FEM Model #3.
- -
- The parametric study was undertaken by varying the fluence and pulse duration: we closely followed the clinical study [37] and accordingly used combinations of fluence and pulse duration of (i) 50 J/cm2 and 25 ms, (ii) 60 J/cm2 and 50 ms, and (iii) 80 J/cm2 and 50 ms.
- -
- We explicitly considered the (i) mode without forced cooling, with a value of the free convection coefficient h of 10 W/m2K [14,38] and ambient temperature T = 22 °C for all three FEM models, and the (ii) mode with forced cooling by means of a cryogenic spray with a convection coefficient h of 5000 W/m2K [39] and spray temperature T = −50 °C [39] for FEM Model #1 and FEM Model #2 only. The forced cooling by cryogenic spray consisted of 10 ms of preliminary cooling, with a 5-ms pause immediately after the pulse and 15 ms of cooling thereafter, as outlined in the clinical study [37].
- -
- The main outcomes of the simulations were temperature and thermal damage distributions after the completion of the irradiation.
3. Results
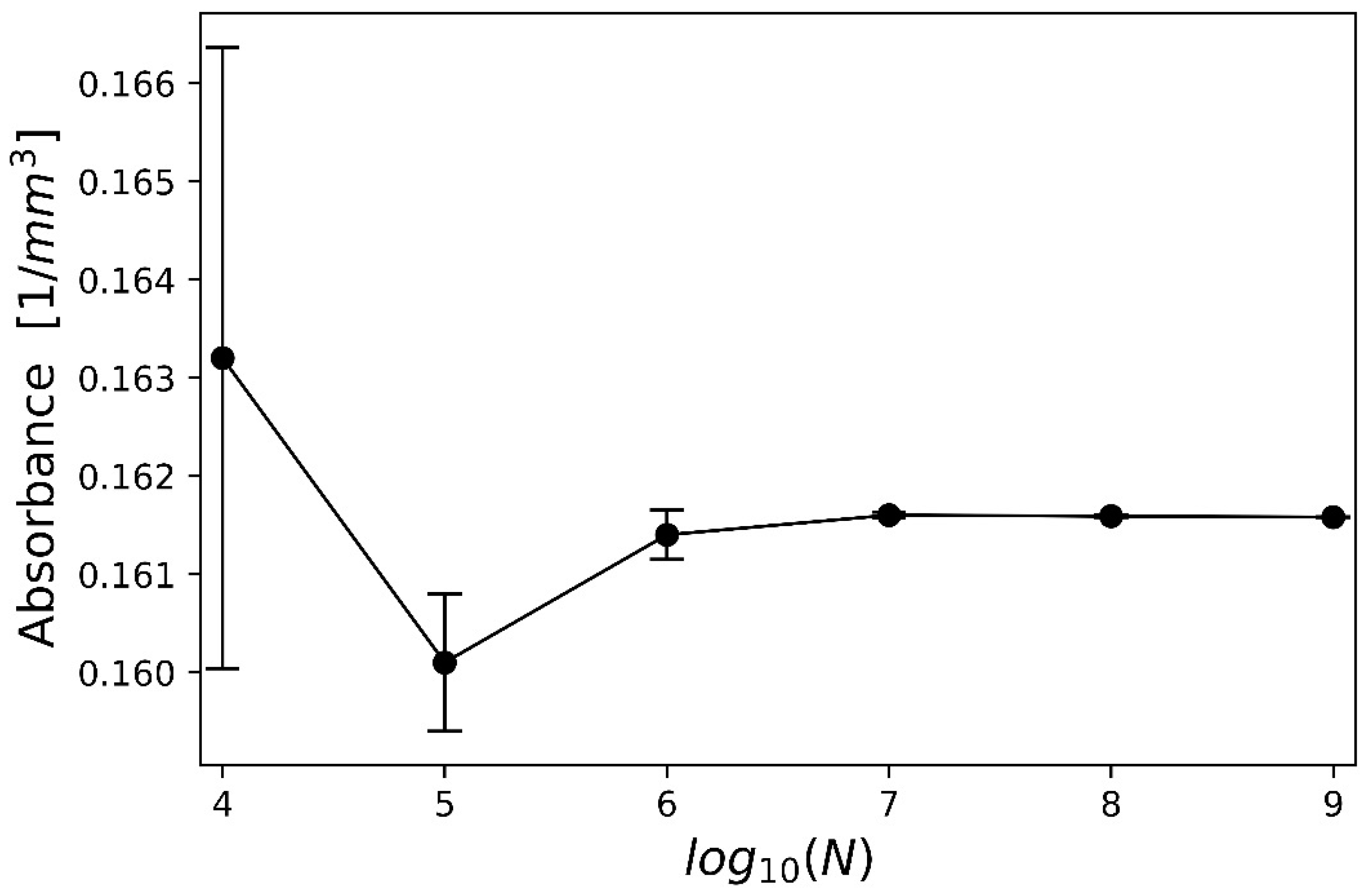
3.1. Optimization of the Laser Beam
3.2. Optimization of the FEM Model
3.3. Temperature and Thermal Damage Distributions—Protocol #1
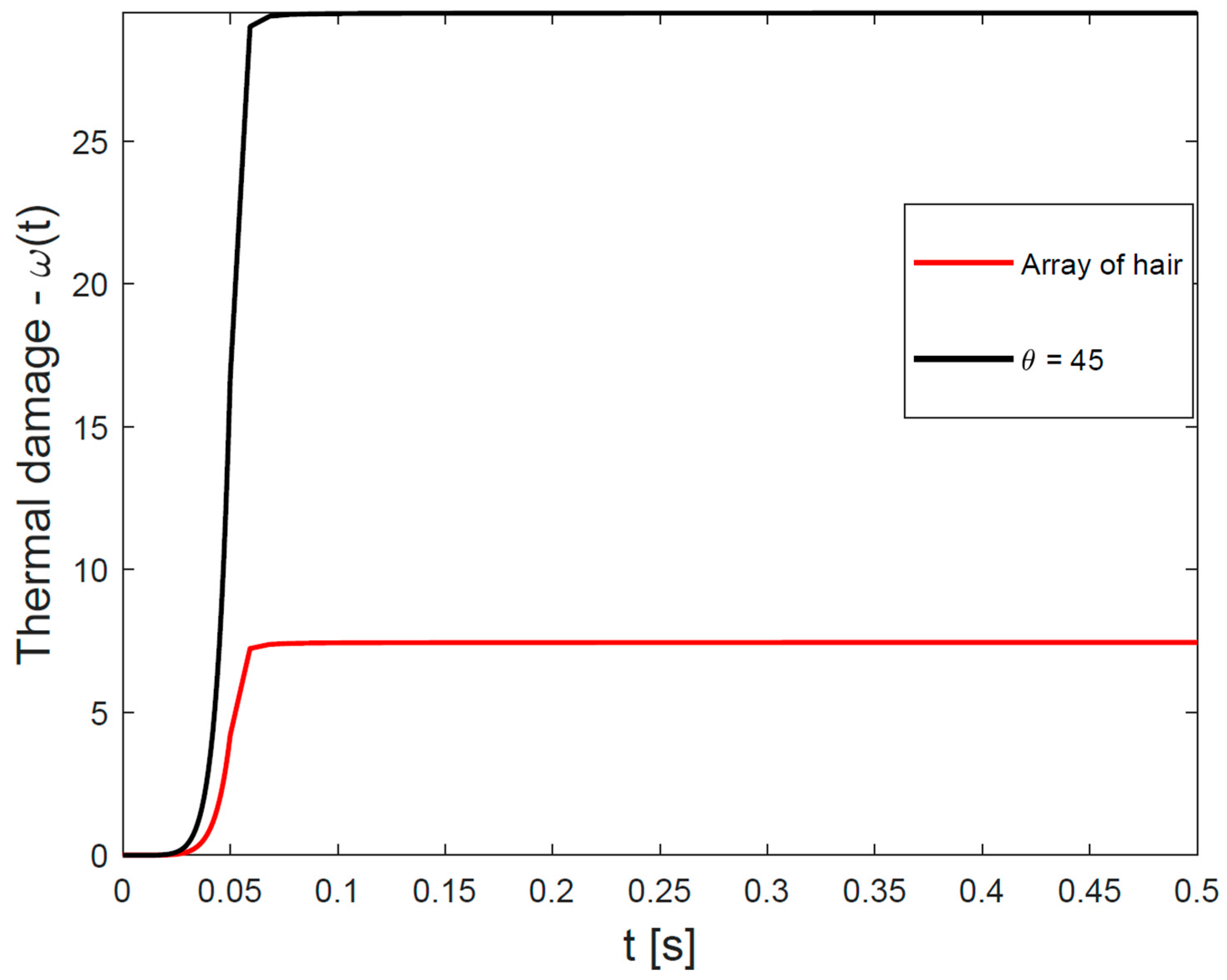
3.4. Time-Dependent Curve of Cumulative Hair-Follicle Damage—Protocol #2
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Protocol for In Vivo Measurement of Absorption Coefficients of Human Hair and the Epidermal Layer
Appendix A.1. Protocol
Appendix A.2. Measurements
Appendix A.3. Simulations

Appendix A.4. Skin Measurements
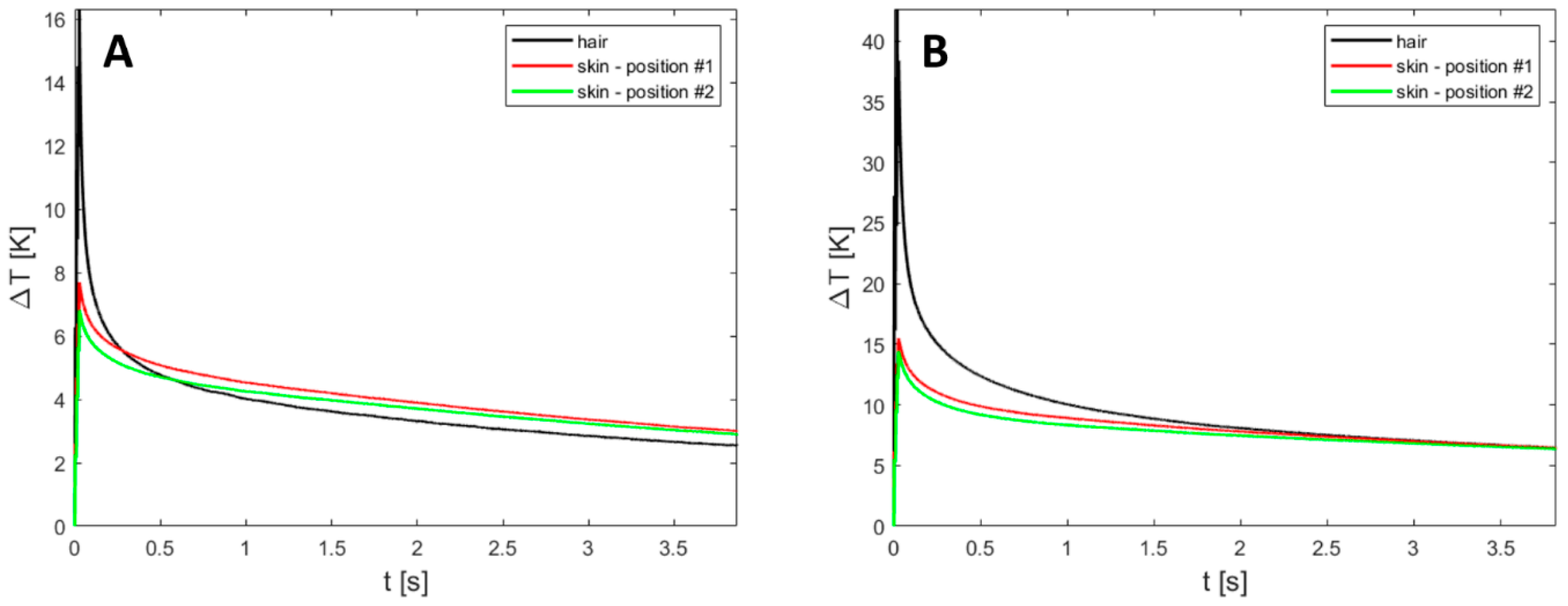
Appendix A.5. Simulations
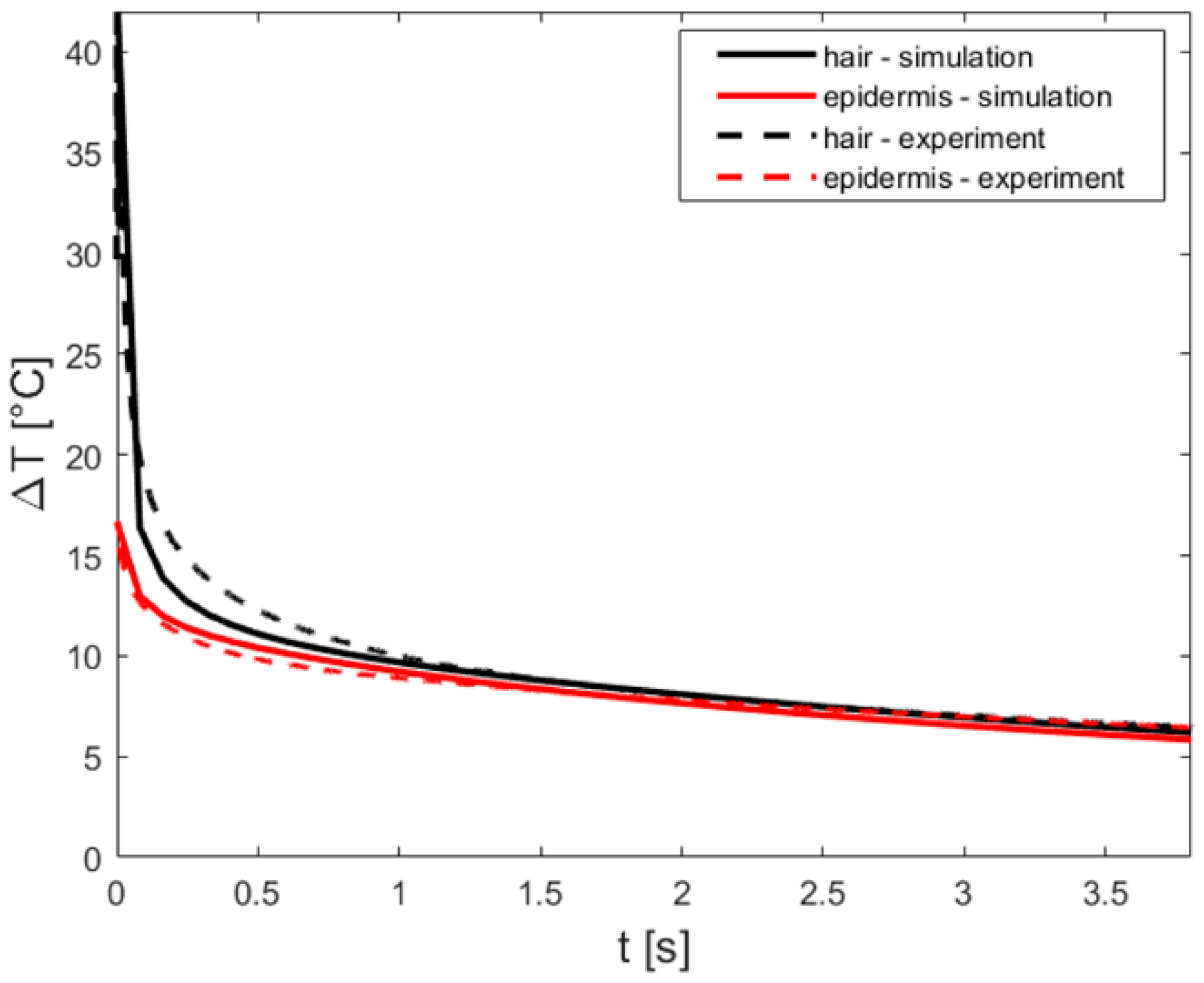
Appendix B. Heat Diffusion Equation
Appendix C. Validating a Finite-Element Method Model for Simulating Laser-Tissue Interaction in Hair Removal Systems
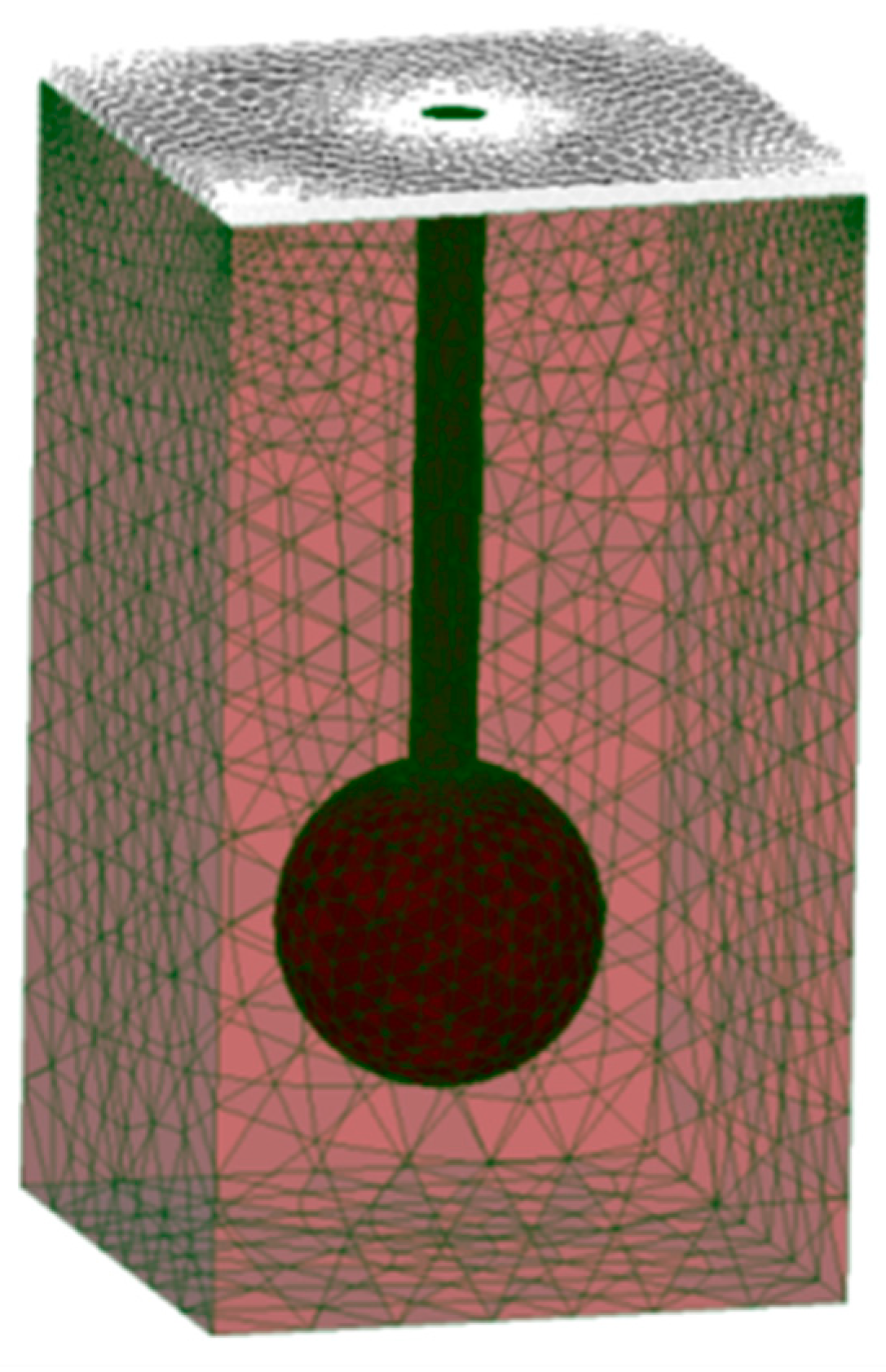
- -
- The simulated disc-shaped laser beam (1064 nm) with a radius of 1 mm was directed perpendicularly to the cross-section of the FEM model.
- -
- The main outcomes of simulations were temperature distribution maps and the average temperature of the follicle.
- -
- Simulations were undertaken by varying the fluence and pulse duration: we closely followed the published study and accordingly used the sequence of three pulses of fluence and pulse duration of 4.8 J/cm2 and 2.5 ms, respectively, with a pause of 200 ms between each pulse when analyzing temperature distribution maps; when analyzing average temperature of the follicle, the fluence was between 5.0 J/cm2 and 7.0 J/cm2 with 0.5 J/cm2 steps, each pulse duration of 2.5 ms, with a 200 ms pause between each pulse.

References
- Goldberg, D. Laser complications: Hair removal. J. Cosmet. Laser Ther. 2006, 8, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, A.A.; França, K.; Chacon, A.H.; Nouri, K. From flint razors to lasers: A timeline of hair removal methods. J. Cosmet. Dermatol. 2013, 12, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.D.; Graber, E.M. Laser Hair Removal: A Review. Dermatol. Surg. 2013, 39, 823–838. [Google Scholar] [CrossRef] [PubMed]
- Haedersdal, M.; Haak, C.S. Hair Removal. In Current Problems in Dermatology: Basics in Dermatological Laser Applications; Bogdan Allemann, I., Goldberg, D.J., Eds.; S. Karger AG: Basel, Switzerland, 2011; pp. 111–121. Available online: https://www.karger.com/Article/FullText/328272 (accessed on 18 July 2023).
- Haedersdal, M. Wulf H. Evidence-based review of hair removal using lasers and light sources. J. Eur. Acad. Dermatol. Venerol. 2006, 20, 9–20. [Google Scholar] [CrossRef]
- Russe, E.; Purschke, M.; Herold, M.; Sakamoto, F.H.; Wechselberger, G.; Russe-Wilflingseder, K. Evaluation of Safety and Efficacy of Laser Hair Removal with the Long-Pulsed 755 nm Wavelength Laser: A Two-Center Study with 948 Patients. Lasers Surg. Med. 2020, 52, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Vaidya, T.; Hohman, M.H.; Kumar, D.D. Laser Hair Removal; StatPearls: Treasure Island, FL, USA, 2023. Available online: https://www.ncbi.nlm.nih.gov/books/NBK507861/ (accessed on 18 July 2023).
- Lukač, M. Beyond Customary Paradigm: FRAC3 Nd:YAG Laser Hair Removal. LAHA (Online ed.). 2010. Available online: https://www.fotona.com/media/documents/97092_v1_hair_removal_leaflet_15_a4.pdf (accessed on 18 July 2023).
- Suzuki, H.; Anderson, R.R. Treatment of melanocytic nevi. Dermatol. Ther. 2005, 18, 217–226. [Google Scholar] [CrossRef]
- Majaron, B.; Kelly, K.M.; Park, H.B.; Verkruysse, W.; Nelson, J.S. Er:YAG laser skin resurfacing using repetitive long-pulse exposure and cryogen spray cooling: I. Histological study. Lasers Surg. Med. 2001, 28, 121–130. [Google Scholar] [CrossRef]
- Das, A.; Sarda, A.; De, A. Cooling devices in laser therapy. J. Cutan. Aesthet. Surg. 2016, 9, 215. [Google Scholar] [CrossRef]
- Grad, L.; Sult, T.; Sult, R. Scientic Evaluation of VSP Nd:YAG Lasers for Hair Removal. J. Laser Health Acad. 2007, 2, 1–11. [Google Scholar]
- Vella, D.; Jernejčič, U.; Kukovič, J.; Zorman, A.; Jezeršek, M.; Lukač, N.; Lukač, M. Enhanced Hair Removal based on the “Avalanche Effect” of the AvalancheLase® Hair Removal Laser System. J. Laser Health Acad. 2021, 1, 1–5. [Google Scholar]
- Sun, F.; Chaney, A.; Anderson, R.; Aguilar, G. Thermal modeling and experimental validation of human hair and skin heated by broadband light. Lasers Surg. Med. 2009, 41, 161–169. [Google Scholar] [CrossRef]
- Pfefer, T.J.; Kehlet Barton, J.; Chan, E.K.; Ducros, M.G.; Sorg, B.S.; Milner, T.E.; Nelson, J.S.; Welch, A.J. A three-dimensional modular adaptable grid numerical model for light propagation during laser irradiation of skin tissue. IEEE J Sel. Top. Quantum Electron. 1996, 2, 934–942. [Google Scholar] [CrossRef] [Green Version]
- Schöberl, J. NETGEN An advancing front 2D/3D-mesh generator based on abstract rules. Comput. Vis. Sci. 1997, 1, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Sandby-Møller, J.; Poulsen, T.; Wulf, H.C. Epidermal Thickness at Different Body Sites: Relationship to Age, Gender, Pigmentation, Blood Content, Skin Type and Smoking Habits. Acta Dermato-Venereol. 2003, 83, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Oltulu, P.; Ince, B.; Kokbudak, N.; Findik, S.; Kilinc, F. Measurement of epidermis, dermis, and total skin thicknesses from six different body regions with a new ethical histometric technique. Turk. J. Plast. Surg. 2018, 26, 56. [Google Scholar] [CrossRef]
- Abrahams, P. The Family Medical Encyclopedia: The Essential Guide to More than 120 Medical Conditions, Syndromes and Diseases; National Library: London, UK, 2005. [Google Scholar]
- Otberg, N.; Richter, H.; Schaefer, H.; Blume-Peytavi, U.; Sterry, W.; Lademann, J. Variations of Hair Follicle Size and Distribution in Different Body Sites. J. Investig. Dermatol. 2004, 122, 14–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Landthaler, M.; Brunner, R.; Braun-Falco, O.; Haina, D.; Waidelich, W. Effects of argon, dye, and Nd:YAG lasers on epidermis, dermis, and venous vessels. Lasers Surg. Med. 1986, 6, 87–93. [Google Scholar] [CrossRef]
- Fang, Q. Mesh-based Monte Carlo method using fast ray-tracing in Plücker coordinates. Biomed. Opt. Express 2010, 1, 165. [Google Scholar] [CrossRef]
- Alerstam, E.; Svensson, T.; Andersson-Engels, S. Parallel computing with graphics processing units for high-speed Monte Carlo simulation of photon migration. J. Biomed. Opt. 2008, 13, 060504. [Google Scholar] [CrossRef] [Green Version]
- Klaneček, Ž. Development of a Numerical Model of Laser Epilation. University of Ljubljana. Available online: https://repozitorij.uni-lj.si/IzpisGradiva.php?id=110385&lang=slv (accessed on 18 July 2023).
- Salomatina, E.; Jiang, B.; Novak, J.; Yaroslavsky, A.N. Optical properties of normal and cancerous human skin in the visible and near-infrared spectral range. J. Biomed. Opt. 2006, 11, 064026. [Google Scholar] [CrossRef] [Green Version]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, S.V.; Dhawan, A.P.; Relue, P.A. Monte Carlo Simulation of Light-Tissue Interaction: Three-Dimensional Simulation for Trans-Illumination-Based Imaging of Skin Lesions. IEEE Trans. Biomed. Eng. 2005, 52, 1227–1236. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Lu, J.Q.; Wooden, A.W.; Kragel, P.J.; Hu, X.-H. Refractive indices of human skin tissues at eight wavelengths and estimated dispersion relations between 300 and 1600 nm. Phys. Med. Biol. 2006, 51, 1479–1489. [Google Scholar] [CrossRef] [Green Version]
- Verdel, N.; Marin, A.; Milanič, M.; Majaron, B. Physiological and structural characterization of human skin in vivo using combined photothermal radiometry and diffuse reflectance spectroscopy. Biomed. Opt. Express 2019, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Nachabé, R.; Hendriks, B.H.W.; van der Voort, M.; Desjardins, A.E.; Sterenborg, H.J.C.M. Estimation of biological chromophores using diffuse optical spectroscopy: Benefit of extending the UV-VIS wavelength range to include 1000 to 1600 nm. Biomed. Opt. Express 2010, 1, 1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mordon, S.R.; Wassmer, B.; Reynaud, J.; Zemmouri, J. Mathematical modeling of laser lipolysis. BioMed Eng. OnLine 2008, 7, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bashkatov, A.N.; Genina, E.A.; Kochubei, V.I.; Tuchin, V.V. Estimate of the melanin content in human hairs by the inverse Monte-Carlo method using a system for digital image analysis. Quantum Electron. 2006, 36, 1111–1118. [Google Scholar] [CrossRef]
- Petrovicova, E.; Kamath, Y.K. Heat transfer in human hair: Heat transfer in human hair. Int. J. Cosmet. Sci. 2019, 41, 387–390. [Google Scholar] [CrossRef]
- Kim, T.-H.; Lee, G.-W.; Youn, J.-I. A comparison of temperature profile depending on skin types for laser hair removal therapy. Lasers Med. Sci. 2014, 29, 1829–1837. [Google Scholar] [CrossRef]
- Ataie-Fashtami, L.; Shirkavand, A.; Sarkar, S.; Alinaghizadeh, M.; Hejazi, M.; Fateh, M.; Djavid, G.E.; Zand, N.; Mohammadreza, H. Simulation of Heat Distribution and Thermal Damage Patterns of Diode Hair-Removal Lasers: An Applicable Method for Optimizing Treatment Parameters. Photomed. Laser Surg. 2011, 29, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Gaylor, D.C. Physical Mechanisms of Cellular Injury in Electrical Trauma; Massachusetts Institute of Technology: Boston, MA, USA, 1989. [Google Scholar]
- Rogachefsky, A.S.; Becker, K.; Weiss, G.; Goldberg, D.J. Evaluation of a Long-Pulsed Nd:YAG Laser at Different Parameters: An Analysis of Both Fluence and Pulse Duration. Dermatol. Surg. 2002, 28, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Milanic, M.; Muc, B.T.; Lukac, N.; Lukac, M. Numerical Study of Hyper-Thermic Laser Lipolysis With 1,064 nm Nd:YAG Laser in Human Subjects. Lasers Surg. Med. 2019, 51, 897–909. [Google Scholar] [CrossRef]
- Milanič, M.; Jia, W.; Nelson, J.S.; Majaron, B. Numerical optimization of sequential cryogen spray cooling and laser irradiation for improved therapy of port wine stain: Optimization of sequential laser irradiation. Lasers Surg. Med. 2011, 43, 164–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanni, C.A.; Alster, T.S. Optimizing treatment parameters for hair removal using a topical carbon-based solution and 1064-nm Q-switched neodymium:YAG laser energy. Arch. Dermatol. 1997, 133, 1546–1549. [Google Scholar] [CrossRef] [PubMed]
- Subodh, J.D.; Venkataram, M. Effectiveness of short-pulse width Nd:YAG in laser hair reduction. J. Cosmet. Dermatol. 2018, 17, 1046–1052. [Google Scholar] [CrossRef]
- Khatri, K.A.; Lee, R.A.; Goldberg, L.J.; Khatri, B.; Garcia, V. Efficacy and safety of a 0.65 millisecond pulsed portable ND:YAG laser for hair removal. J. Cosmet. Laser Ther. 2009, 11, 19–24. [Google Scholar] [CrossRef]
- Omland, S.H.; Wenande, E.C.; Svane, I.M.; Tam, J.; Olesen, U.H.; Hædersdal, M. Laser Immunotherapy: A Potential Treatment Modality for Keratinocyte Carcinoma. Cancers 2021, 13, 5405. [Google Scholar] [CrossRef]
- Chang, I.A. Considerations for Thermal Injury Analysis for RF Ablation Devices. Open Biomed. Eng. J. 2010, 4, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Lukač, M.; Lozar, A.; Perhavec, T.; Bajd, F. Variable heat shock response model for medical laser procedures. Lasers Med. Sci. 2019, 34, 1147–1158. [Google Scholar] [CrossRef]
- Vella, D.; Lukač, M.; Jernejčič, U.; Lukač, N.; Klaneček, Ž.; Milanič, M.; Jezeršek, M. Measurements of hair temperature avalanche effect with alexandrite and Nd:YAG hair removal lasers. Lasers Surg. Med. 2023. submitted. [Google Scholar] [CrossRef]
- Hren, R.; Sersa, G.; Simoncic, U.; Milanic, M. Imaging perfusion changes in oncological clinical applications by hyperspectral imaging: A literature review. Radiol. Oncol. 2022, 56, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Dorgham, N.A.; Dorgham, D.A. Lasers for reduction of unwanted hair in skin of colour: A systematic review and meta-analysis. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 948–955. [Google Scholar] [CrossRef] [PubMed]
- Sekar, S.K.V.; Pacheco, A.; Martella, P.; Li, H.; Lanka, P.; Pifferi, A.; Andersson-Engels, S. Solid phantom recipe for diffuse optics in biophotonics applications: A step towards anatomically correct 3D tissue phantoms. Biomed. Opt. Express 2019, 10, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Tissue | µa [mm−1] | µs [mm−1] | g | n |
|---|---|---|---|---|
| Epidermis | 0.07 [1] | 26.1 [2] | 0.89 [3] | 1.42 [4] |
| Dermis | 0.038 [5] | 5.25 [2] | 0.72 [3] | 1.37 [4] |
| Subcutis | 0.008 [6] | 2.27 [6] | 0.78 [3] | 1.44 [7] |
| Hair | 0.56 [1] | 79.7 [8] | 0.9 [8] | 1.37 [9] |
| Tissue | cp [J/kgK] | k [W/mK] | ρ [kg/m3] |
|---|---|---|---|
| Epidermis | 3200 [10] | 0.34 [10] | 1120 [10] |
| Dermis | 3500 [10] | 0.41 [10] | 1090 [10] |
| Subcutis | 2870 [6] | 0.3 [6] | 860 [6] |
| Hair | 2512 [11] | 0.63 [11] | 1300 [12] |
| Maximum Follicle Temperature after Pulse [°C] | |||||
|---|---|---|---|---|---|
| Fluence of 50 J/cm2 Pulse Duration of 25 ms | Fluence of 60 J/cm2 Pulse Duration of 50 ms | Fluence of 80 J/cm2 Pulse Duration of 50 ms | |||
| No Cooling | Cooling | No Cooling | Cooling | No Cooling | Cooling |
| 79.3 | 79.3 | 73.6 | 73.6 | 86.8 | 86.8 |
| Maximum Follicle Temperature after Pulse [°C] | |||||
|---|---|---|---|---|---|
| Fluence of 50 J/cm2 Pulse Duration of 25 ms | Fluence of 60 J/cm2 Pulse Duration of 50 ms | Fluence of 80 J/cm2 Pulse Duration of 50 ms | |||
| No Cooling | Cooling | NO COOLING | Cooling | No Cooling | Cooling |
| 77.9 | 77.9 | 72.6 | 72.6 | 85.5 | 85.5 |
| Maximum Follicle Temperature after Pulse [°C] | |||||
|---|---|---|---|---|---|
| Fluence of 50 J/cm2 Pulse Duration of 25 ms | Fluence of 60 J/cm2 Pulse Duration of 50 ms | Fluence of 80 J/cm2 Pulse Duration of 50 ms | |||
| Central Follicle | Non-Central Follicle | Central Follicle | Non-Central Follicle | Central Follicle | Non-Central Follicle |
| 73.0 | 63.0 | 68.8 | 60.0 | 80.4 | 68.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klanecek, Z.; Hren, R.; Simončič, U.; Muc, B.T.; Lukač, M.; Milanič, M. Finite Element Method (FEM) Modeling of Laser-Tissue Interaction during Hair Removal. Appl. Sci. 2023, 13, 8553. https://doi.org/10.3390/app13148553
Klanecek Z, Hren R, Simončič U, Muc BT, Lukač M, Milanič M. Finite Element Method (FEM) Modeling of Laser-Tissue Interaction during Hair Removal. Applied Sciences. 2023; 13(14):8553. https://doi.org/10.3390/app13148553
Chicago/Turabian StyleKlanecek, Zan, Rok Hren, Urban Simončič, Blaz Tasic Muc, Matjaž Lukač, and Matija Milanič. 2023. "Finite Element Method (FEM) Modeling of Laser-Tissue Interaction during Hair Removal" Applied Sciences 13, no. 14: 8553. https://doi.org/10.3390/app13148553
APA StyleKlanecek, Z., Hren, R., Simončič, U., Muc, B. T., Lukač, M., & Milanič, M. (2023). Finite Element Method (FEM) Modeling of Laser-Tissue Interaction during Hair Removal. Applied Sciences, 13(14), 8553. https://doi.org/10.3390/app13148553








