Porous Carbon Materials Based on Blue Shark Waste for Application in High-Performance Energy Storage Devices
Abstract
:1. Introduction
2. Materials and Methods
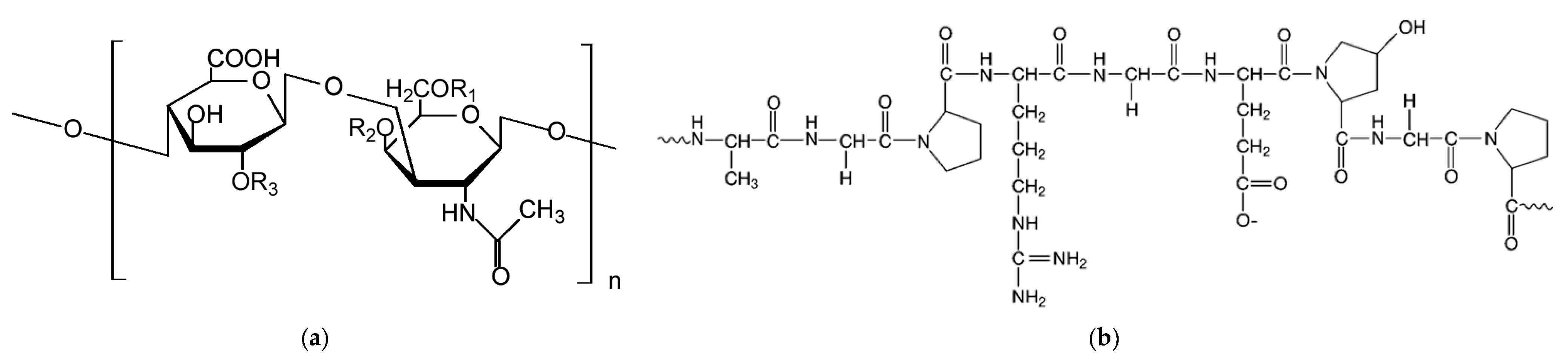
2.1. Preparation of Biocarbon from Fish Waste (Blue Shark Chondroitin Sulfate and Blue Shark Gelatine)
2.2. Ball-Milling
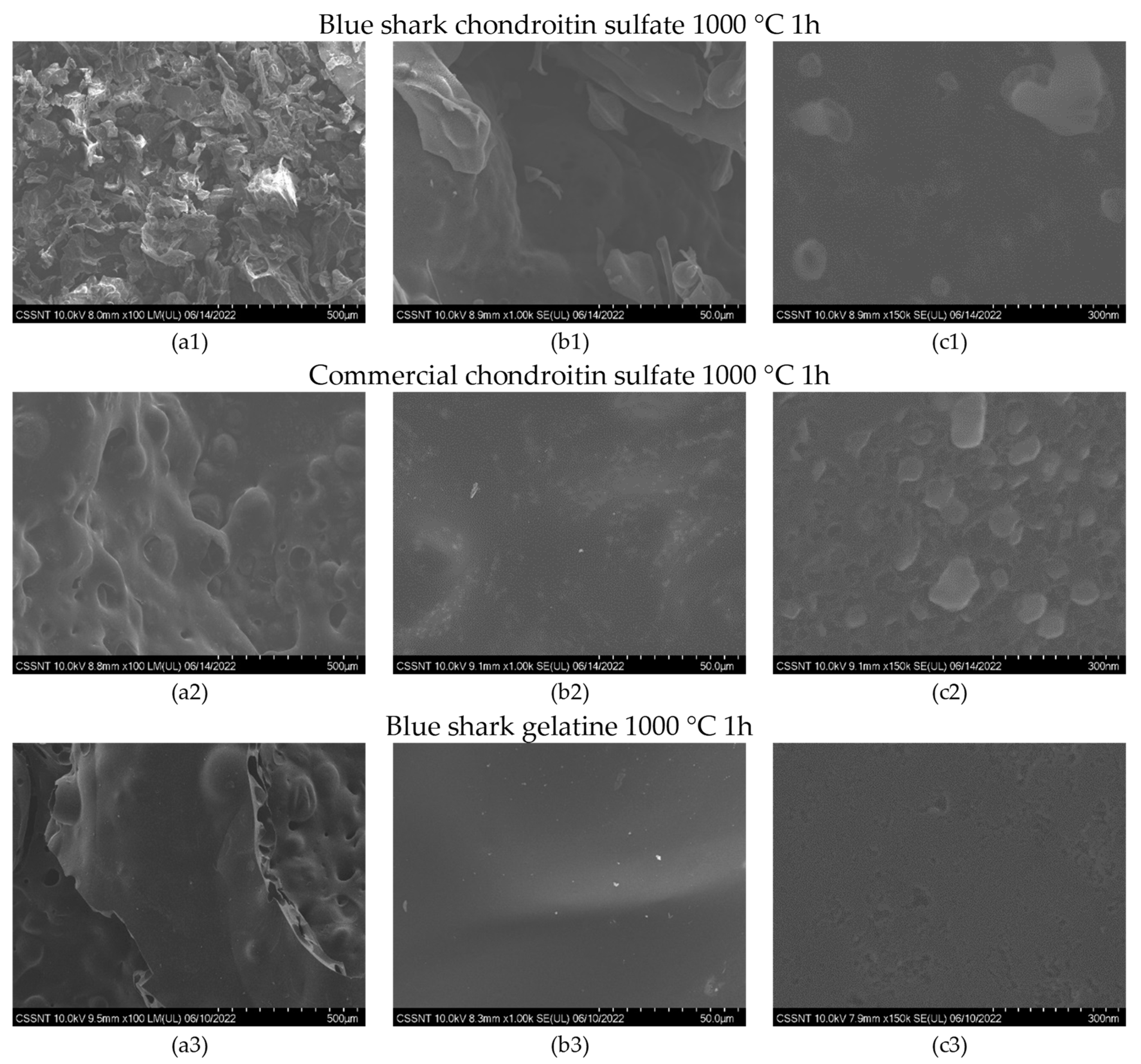
2.3. Morphological Characterization of the Carbon Materials
2.4. Electrochemical Studies
3. Results
3.1. Structural Characteristics
3.2. XPS (X-ray Photoelectron Spectroscopy)
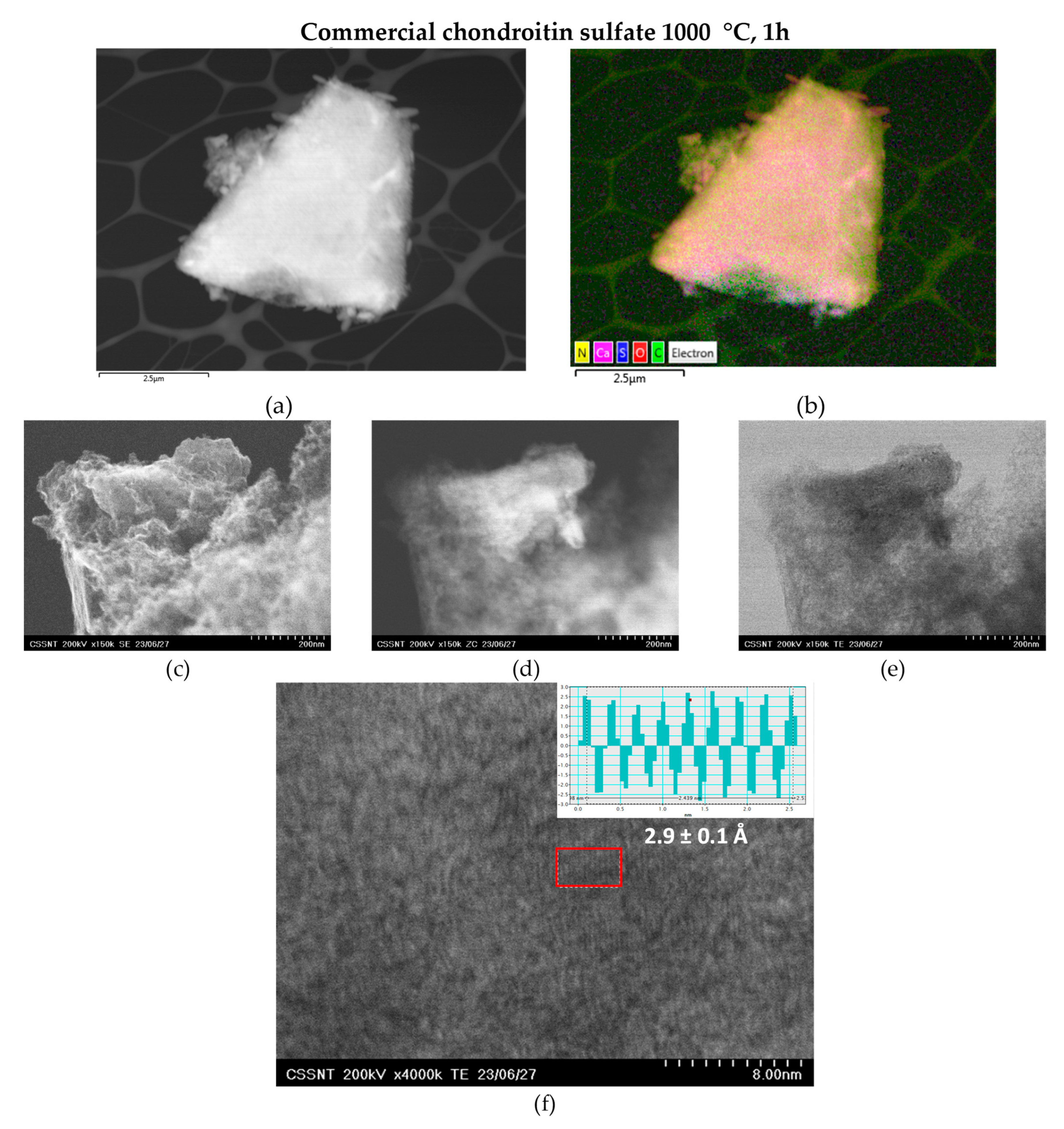
3.3. Scanning Transmission Electron Microscope (STEM)
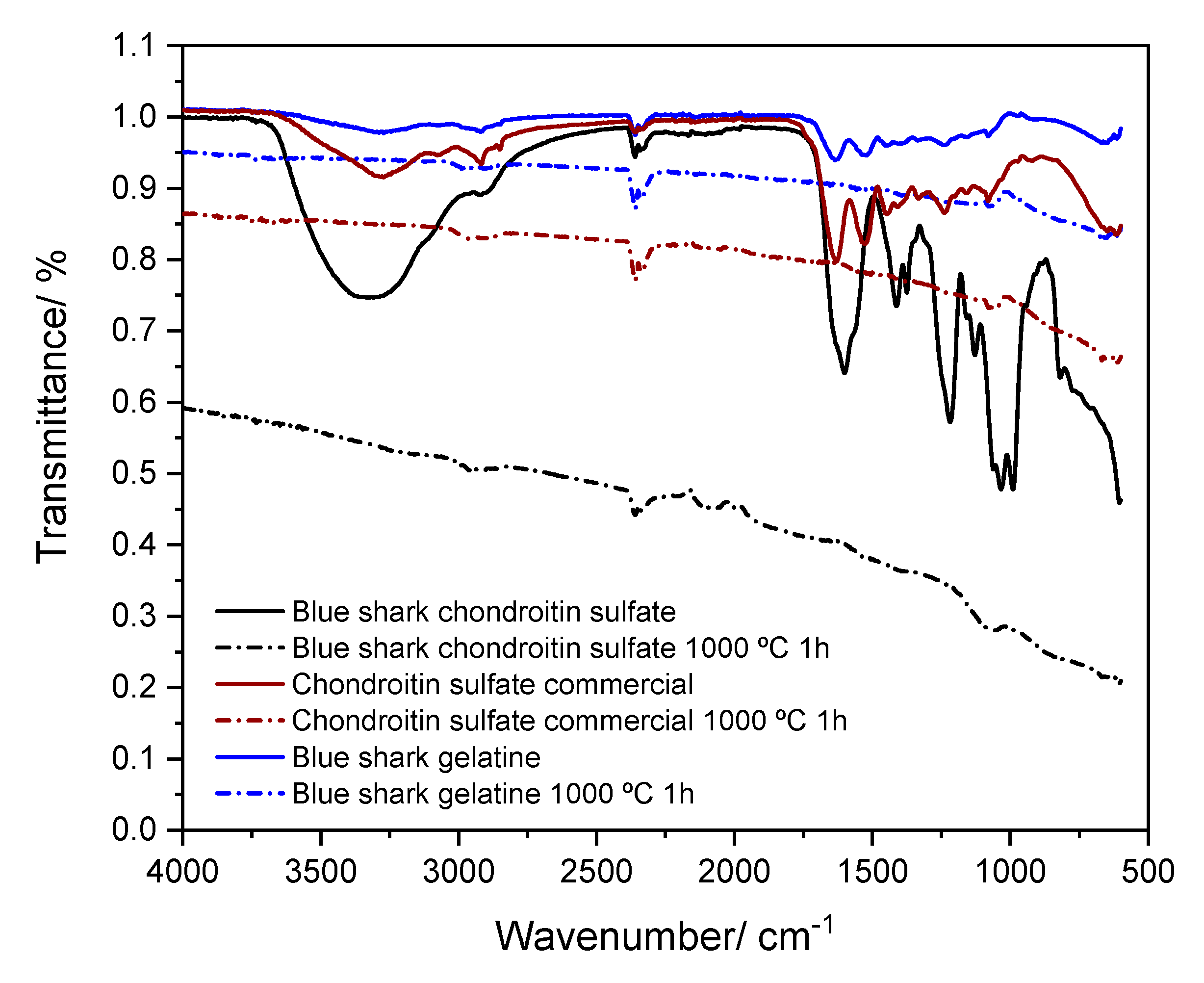
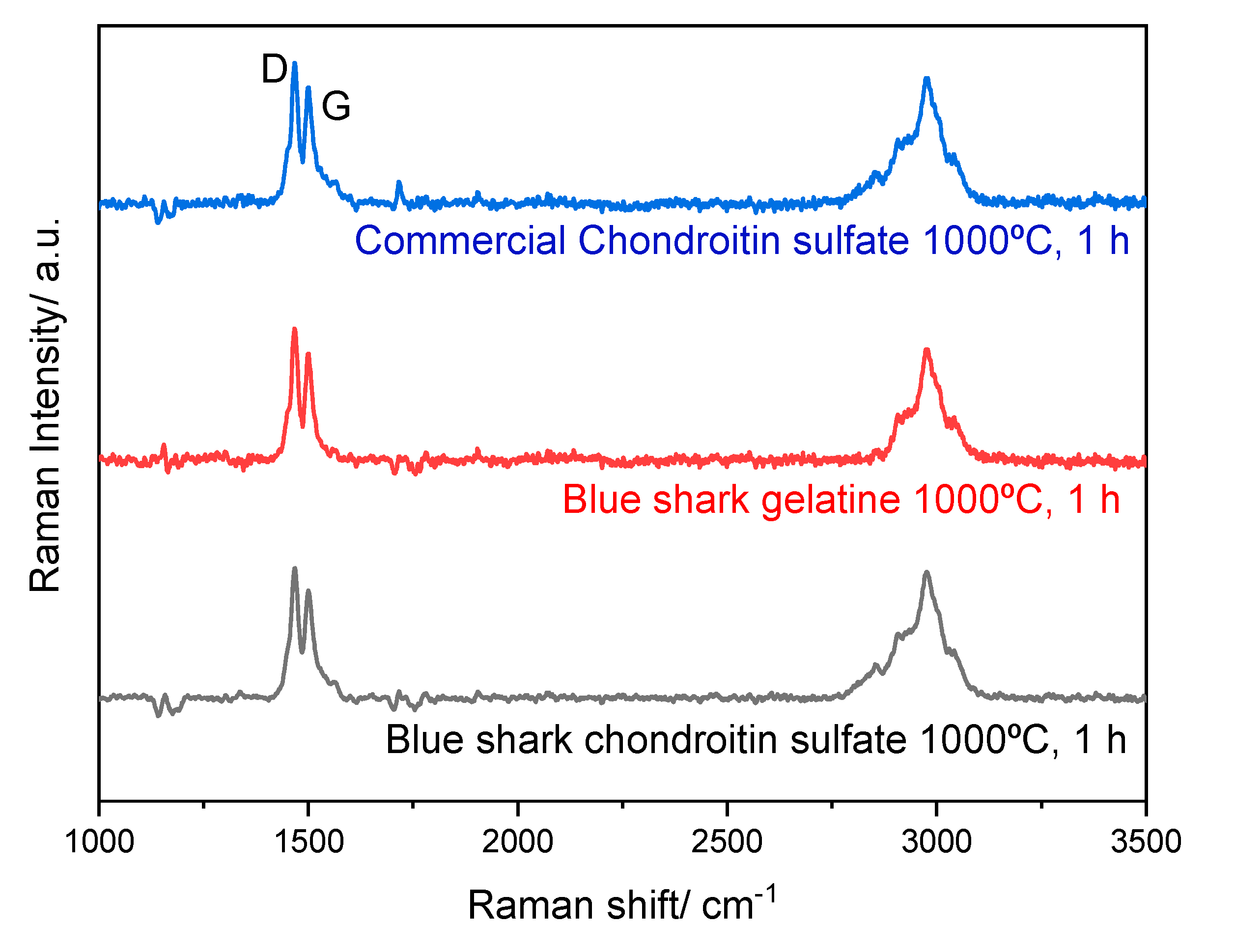
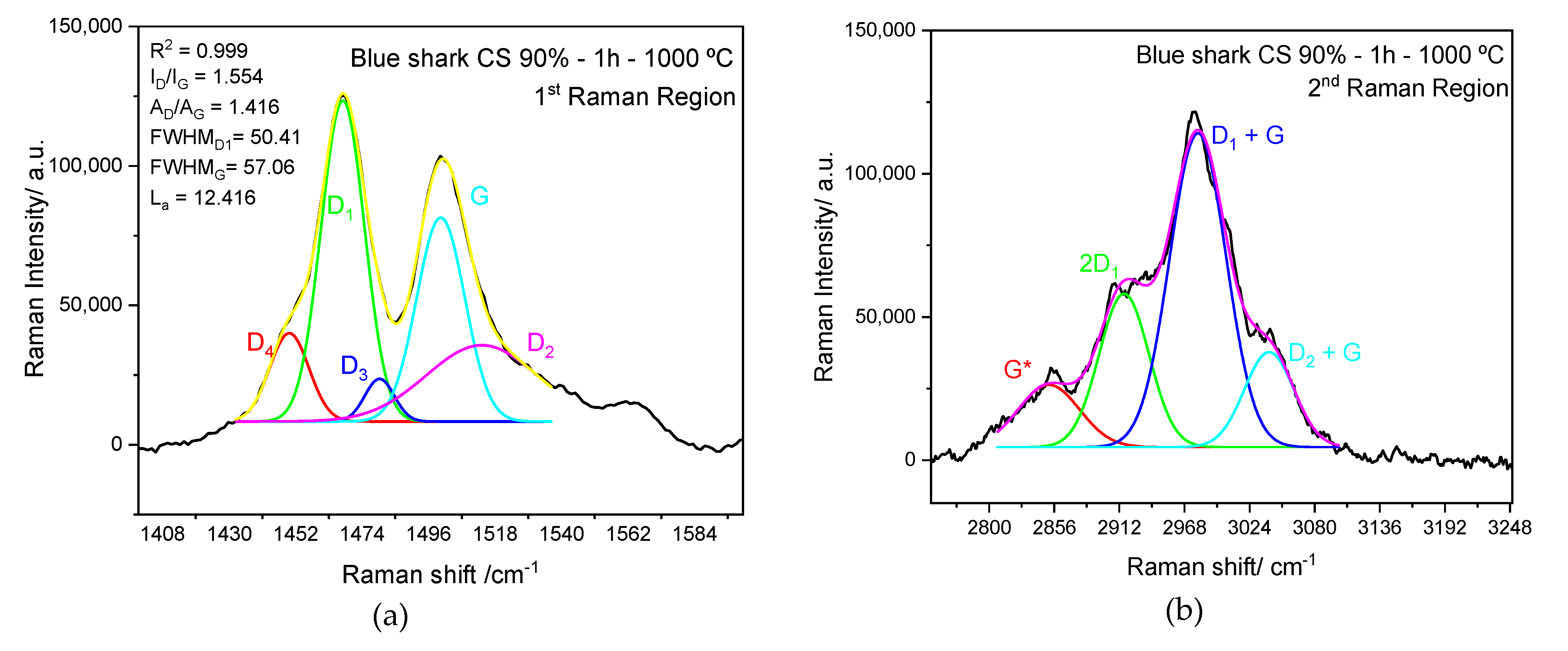
3.4. ATR-FTIR and Raman Spectra Analysis
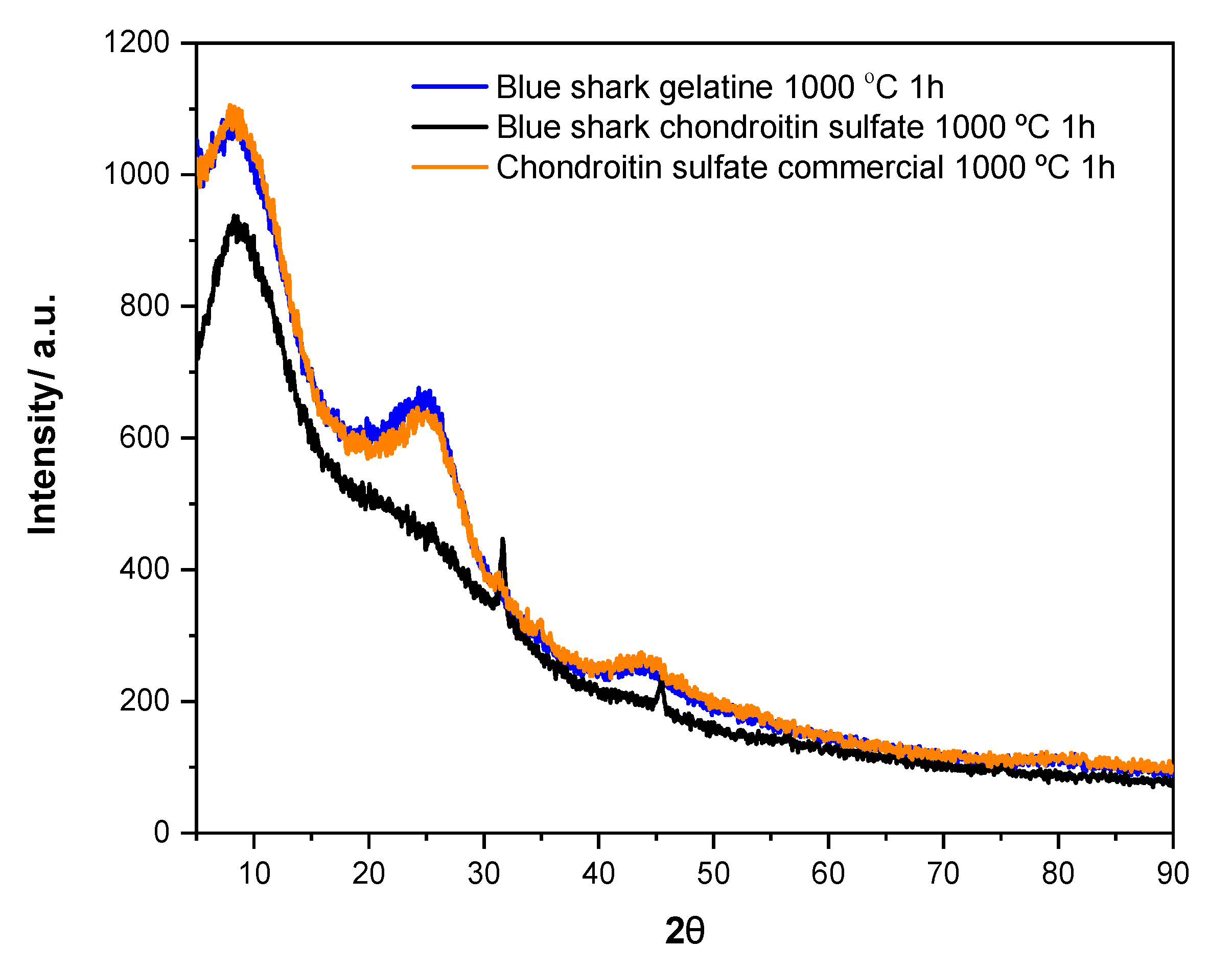
3.5. XRD Analysis
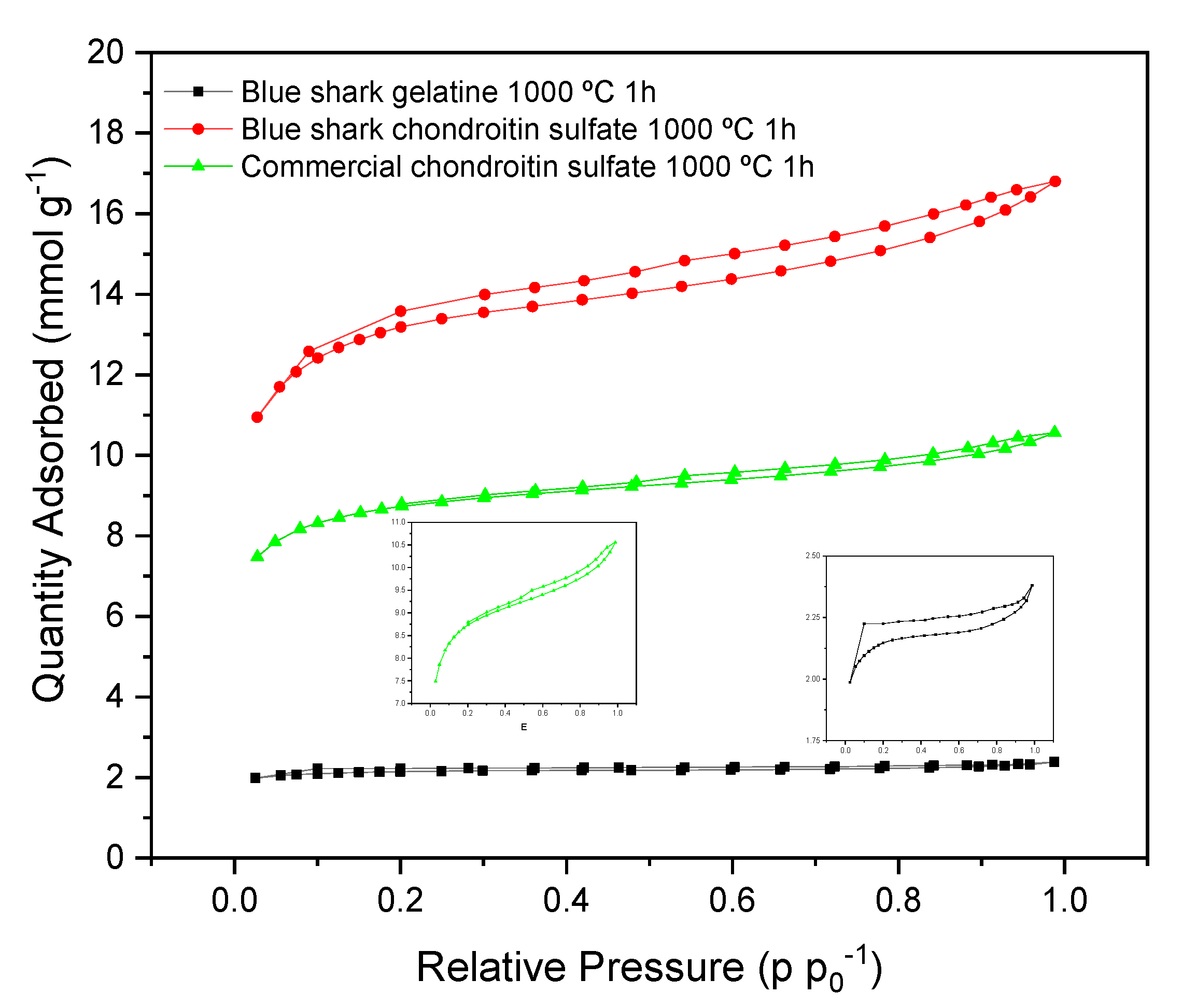
3.6. BET Analysis
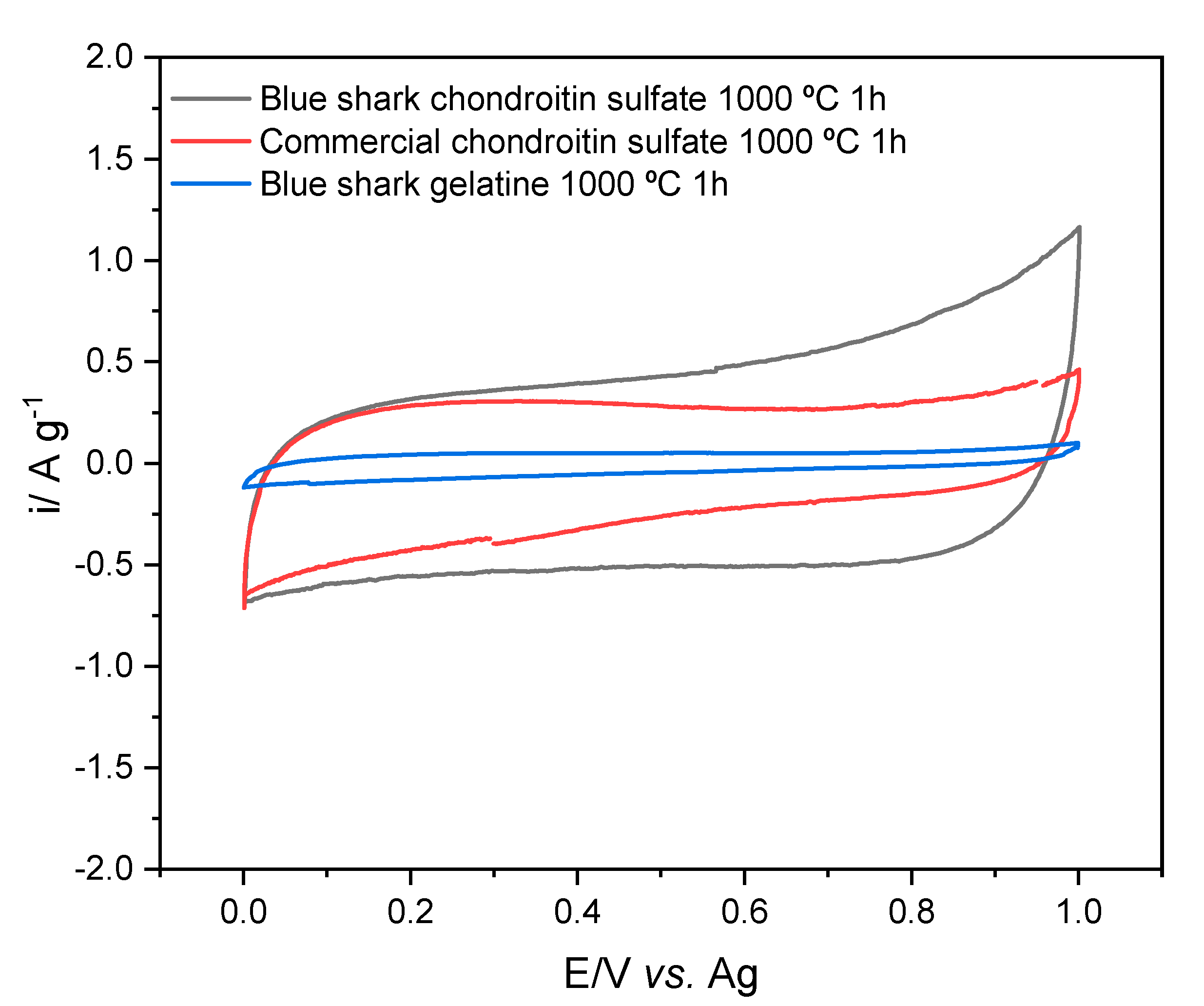
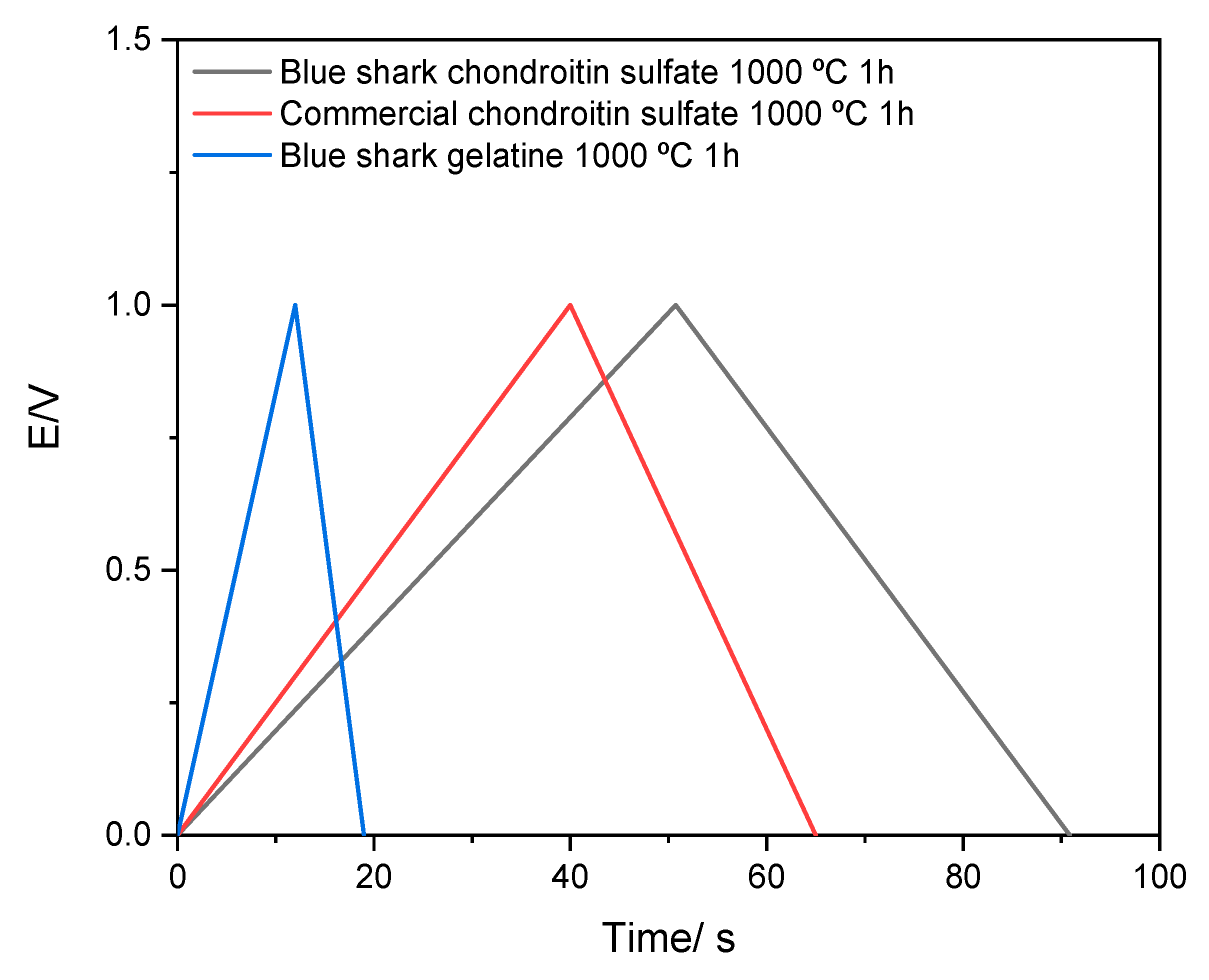
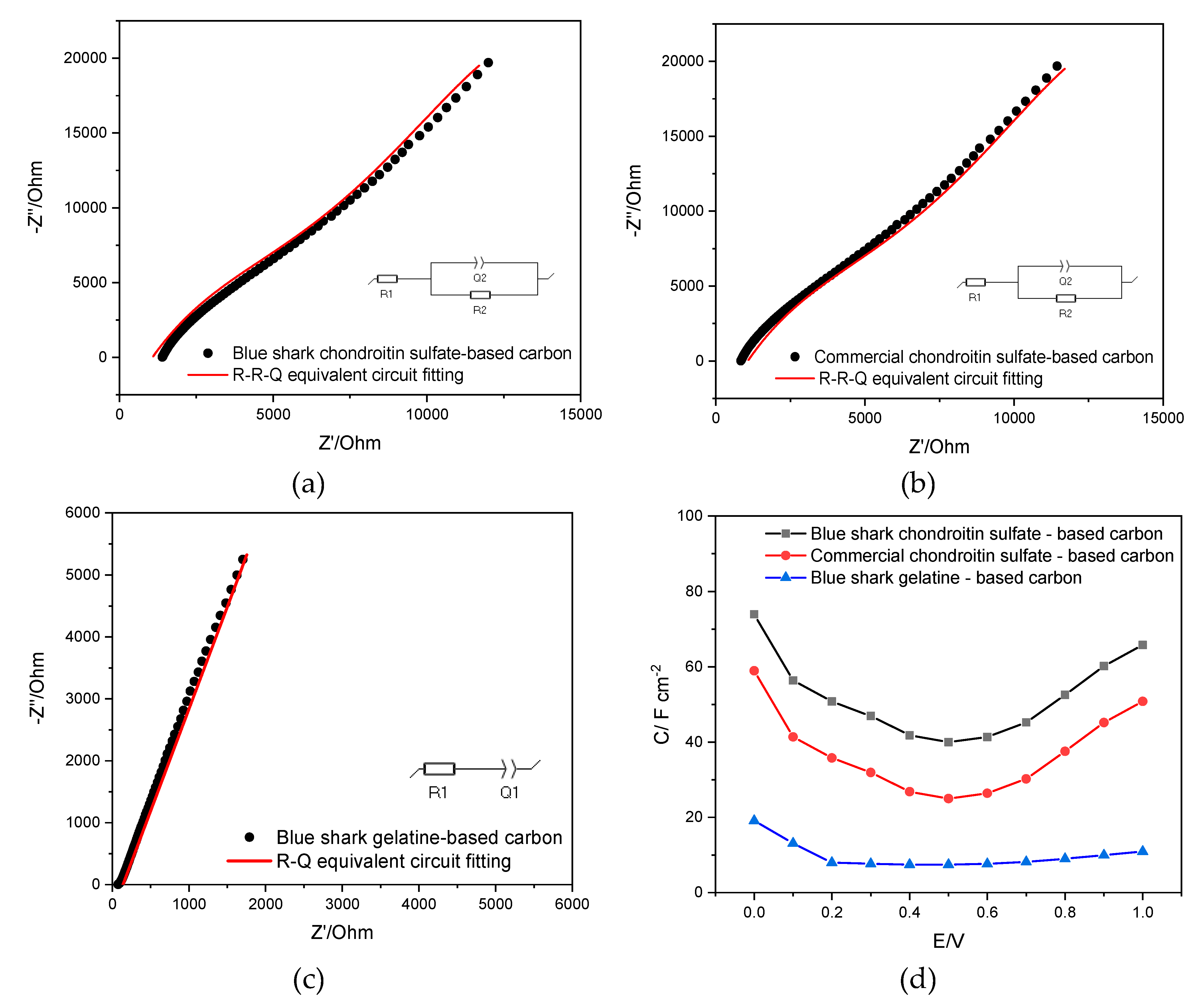
3.7. Electrochemical Characterization
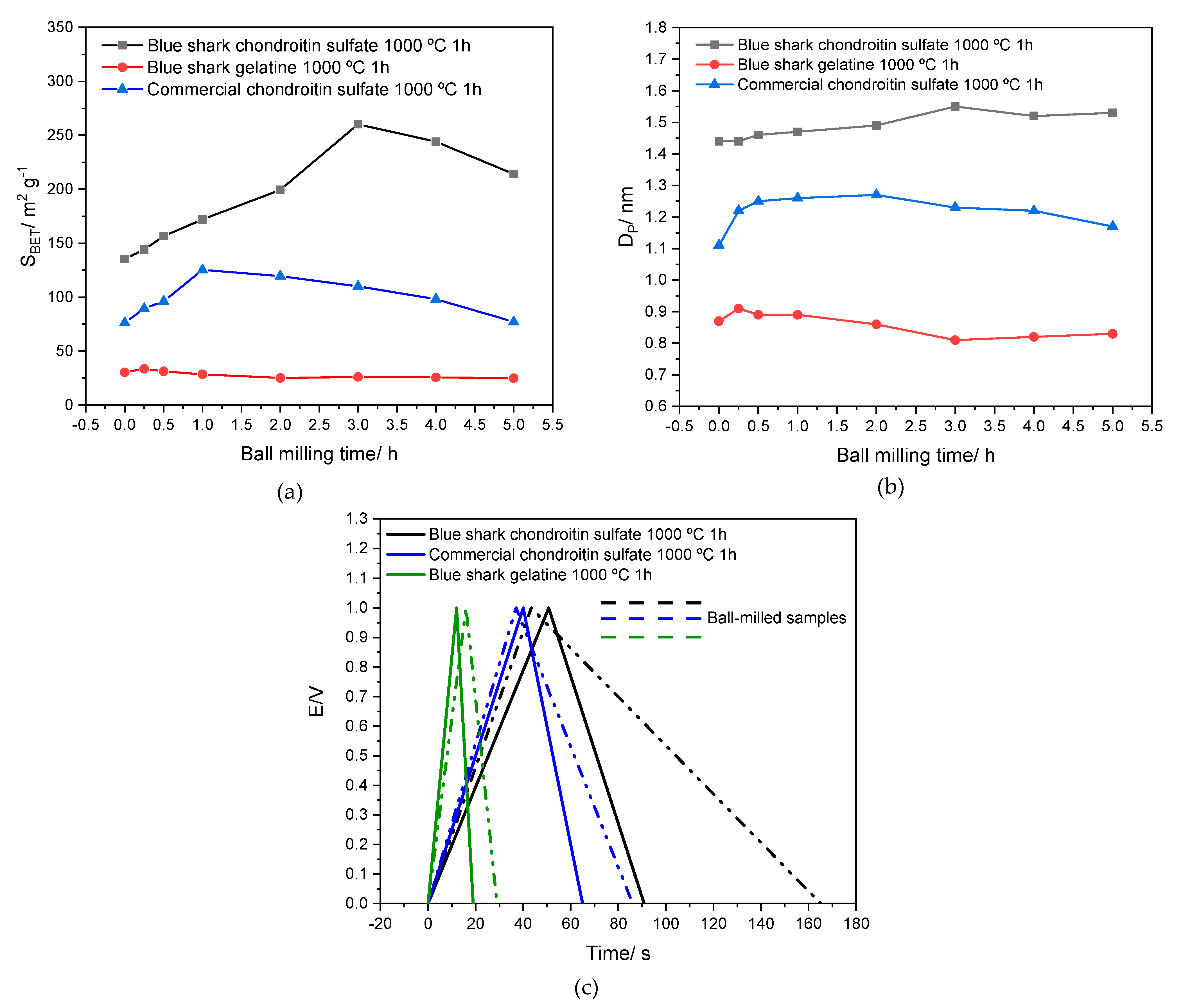
3.8. Preliminary Results: Ball-Milling Effect
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bhat, M.Y.; Hashmi, S.A.; Khan, M.; Choi, D.; Qurashi, A. Frontiers and recent developments on supercapacitor’s materials, design, and applications: Transport and power system applications. J. Energy Storage 2023, 58, 106104. [Google Scholar] [CrossRef]
- Mehdipour-Ataei, S.; Aram, E. Mesoporous Carbon-Based Materials: A Review of Synthesis, Modification, and Applications. Catalysts 2023, 13. [Google Scholar] [CrossRef]
- Soffian, M.S.; Abdul Halim, F.Z.; Aziz, F.; Rahman, M.A.; Mohamed Amin, M.A.; Awang Chee, D.N. Carbon-based material derived from biomass waste for wastewater treatment. Environ. Adv. 2022, 9, 100259. [Google Scholar] [CrossRef]
- FAO United Nations. The State of World Fisheries and Aquaculture 2022; FAO: Rome, Italy, 2022. [Google Scholar]
- Vázquez, J.A.; Fraguas, J.; González, P.; Serra, J.; Valcarcel, J. Optimal Recovery of Valuable Biomaterials, Chondroitin Sulfate and Bioapatites, from Central Skeleton Wastes of Blue Shark. Polymers 2020, 12, 2613. [Google Scholar] [CrossRef] [PubMed]
- De Iuliis, G.; Pulerà, D. Chapter 3—The Shark. In The Dissection of Vertebrates, 3rd ed.; De Iuliis, G., Pulerà, D., Eds.; Academic Press: Boston, MA, USA, 2019; pp. 53–109. ISBN 978-0-12-410460-0. [Google Scholar]
- López-Álvarez, M.; González, P.; Serra, J.; Fraguas, J.; Valcarcel, J.; Vázquez, J.A. Chondroitin sulfate and hydroxyapatite from Prionace glauca shark jaw: Physicochemical and structural characterization. Int. J. Biol. Macromol. 2020, 156, 329–339. [Google Scholar] [CrossRef]
- López-Álvarez, M.; Pérez-Davila, S.; Rodríguez-Valencia, C.; González, P.; Serra, J. The improved biological response of shark tooth bioapatites in a comparative in vitro study with synthetic and bovine bone grafts. Biomed. Mater. 2016, 11, 35011. [Google Scholar] [CrossRef]
- Valcarcel, J.; Novoa-Carballal, R.; Pérez-Martín, R.I.; Reis, R.L.; Vázquez, J.A. Glycosaminoglycans from marine sources as therapeutic agents. Biotechnol. Adv. 2017, 35, 711–725. [Google Scholar] [CrossRef]
- Taelman, S.E.; Tonini, D.; Wandl, A.; Dewulf, J. A Holistic Sustainability Framework for Waste Management in European Cities: Concept Development. Sustainability 2018, 10, 2184. [Google Scholar] [CrossRef] [Green Version]
- Brandão, A.T.S.C.; State, S.; Costa, R.; Potorac, P.; Vázquez, J.A.; Valcarcel, J.; Silva, A.F.; Anicai, L.; Enachescu, M.; Pereira, C.M. Renewable Carbon Materials as Electrodes for High-Performance Supercapacitors: From Marine Biowaste to High Specific Surface Area Porous Biocarbons. ACS Omega 2023, 8, 18782–18798. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Costa, R.; State, S.; Potorac, P.; Dias, C.; Vázquez, J.A.; Valcarcel, J.; Silva, A.F.; Enachescu, M.; Pereira, C.M. Chitins from Seafood Waste as Sustainable Porous Carbon Precursors for the Development of Eco-Friendly Supercapacitors. Materials 2023, 16, 2332. [Google Scholar] [CrossRef]
- Liu, H.-J.; Wang, X.-M.; Cui, W.-J.; Dou, Y.-Q.; Zhao, D.-Y.; Xia, Y.-Y. Highly ordered mesoporous carbon nanofiber arrays from a crab shell biological template and its application in supercapacitors and fuel cells. J. Mater. Chem. 2010, 20, 4223–4230. [Google Scholar] [CrossRef]
- Chen, W.; Zhang, H.; Huang, Y.; Wang, W. A fish scale based hierarchical lamellar porous carbon material obtained using a natural template for high performance electrochemical capacitors. J. Mater. Chem. 2010, 20, 4773–4775. [Google Scholar] [CrossRef]
- Wang, J.; Shen, L.; Xu, Y.; Dou, H.; Zhang, X. Lamellar-structured biomass-derived phosphorus- and nitrogen-co-doped porous carbon for high-performance supercapacitors. New J. Chem. 2015, 39, 9497–9503. [Google Scholar] [CrossRef]
- Zingare, P.A.; Dhoble, S.J.; Deshmukh, A.D. Highly stable fish-scale derived lamellar carbon for high performance supercapacitor application. Diam. Relat. Mater. 2022, 124, 108925. [Google Scholar] [CrossRef]
- Gao, F.; Qu, J.; Zhao, Z.; Wang, Z.; Qiu, J. Nitrogen-doped activated carbon derived from prawn shells for high-performance supercapacitors. Electrochim. Acta 2016, 190, 1134–1141. [Google Scholar] [CrossRef]
- Shan, B.; Cui, Y.; Liu, W.; Zhang, Y.; Liu, S.; Wang, H.; Sun, L.; Wang, Z.; Wu, R. Fibrous Bio-Carbon Foams: A New Material for Lithium-Ion Hybrid Supercapacitors with Ultrahigh Integrated Energy/Power Density and Ultralong Cycle Life. ACS Sustain. Chem. Eng. 2018, 6, 14989–15000. [Google Scholar] [CrossRef]
- Brandão, A.T.S.C.; Costa, R.; Silva, A.F.; Pereira, C.M. Sustainable Preparation of Nanoporous Carbons via Dry Ball Milling: Electrochemical Studies Using Nanocarbon Composite Electrodes and a Deep Eutectic Solvent as Electrolyte. Nanomaterials 2021, 11, 3258. [Google Scholar] [CrossRef]
- Vázquez, J.A.; Blanco, M.; Fraguas, J.; Pastrana, L.; Pérez-Martín, R. Optimisation of the extraction and purification of chondroitin sulphate from head by-products of Prionace glauca by environmental friendly processes. Food Chem. 2016, 198, 28–35. [Google Scholar] [CrossRef] [Green Version]
- Novoa-Carballal, R.; Pérez-Martín, R.; Blanco, M.; Sotelo, C.G.; Fassini, D.; Nunes, C.; Coimbra, M.A.; Silva, T.H.; Reis, R.L.; Vázquez, J.A. By-products of Scyliorhinus canicula, Prionace glauca and Raja clavata: A valuable source of predominantly 6S sulfated chondroitin sulfate. Carbohydr. Polym. 2017, 157, 31–37. [Google Scholar] [CrossRef] [Green Version]
- Sousa, S.C.; Vázquez, J.A.; Pérez-Martín, R.I.; Carvalho, A.P.; Gomes, A.M. Valorization of By-Products from Commercial Fish Species: Extraction and Chemical Properties of Skin Gelatins. Molecules 2017, 22, 1545. [Google Scholar] [CrossRef] [Green Version]
- Pimenta, M.A.; Dresselhaus, G.; Dresselhaus, M.S.; Cançado, L.G.; Jorio, A.; Saito, R. Studying disorder in graphite-based systems by Raman spectroscopy. Phys. Chem. Chem. Phys. 2007, 9, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Salomé, S.; Pereira, N.M.; Ferreira, E.S.; Pereira, C.M.; Silva, A.F. Tin electrodeposition from choline chloride based solvent: Influence of the hydrogen bond donors. J. Electroanal. Chem. 2013, 703, 80–87. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Neamtu, A.; Stoica, B.; Vasile, C. Cellulose/Chondroitin Sulphate Hydrogels As Carriers for Drug Delivery Applications; Analele Ştiinţifice ale Universităţii “Alexandru Ioan Cuza”, Secţiunea Genetică şi Biologie Moleculară: Iasi, Romania, 2009; Volume 10, pp. 85–92. [Google Scholar]
- Kommareddy, S.; Shenoy, D.; Amiji, M. Gelatin Nanoparticles and Their Biofunctionalization. In Nanotechnologies for the Life Sciences, Vol. 1 Biofunctionalization of Nanomaterials; Wiley: Hoboken, NJ, USA, 2007; ISBN 9783527610419. [Google Scholar]
- Yu, M.; Han, Y.; Li, J.; Wang, L. Magnetic N-doped carbon aerogel from sodium carboxymethyl cellulose/collagen composite aerogel for dye adsorption and electrochemical supercapacitor. Int. J. Biol. Macromol. 2018, 115, 185–193. [Google Scholar] [CrossRef]
- Ponce, A.; Mejía-Rosales, S.; José-Yacamán, M. Scanning transmission electron microscopy methods for the analysis of nanoparticles. Methods Mol. Biol. 2012, 906, 453–471. [Google Scholar] [PubMed]
- Qian, W.; Sun, F.; Xu, Y.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Human hair-derived carbon flakes for electrochemical supercapacitors. Energy Environ. Sci. 2014, 7, 379–386. [Google Scholar] [CrossRef]
- Guo, Y.; Peng, F.; Wang, H.; Huang, F.; Meng, F.; Hui, D.; Zhou, Z. Intercalation Polymerization Approach for Preparing Graphene/Polymer Composites. Polymers 2018, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Barroso-Bogeat, A.; Alexandre-Franco, M.; Fernández-González, C.; Macías-García, A.; Gómez-Serrano, V. Preparation of Activated Carbon-SnO2, TiO2, and WO3 Catalysts. Study by FT-IR Spectroscopy. Ind. Eng. Chem. Res. 2016, 55, 5200–5206. [Google Scholar] [CrossRef]
- Al Bahri, M.; Calvo, L.; Gilarranz, M.A.; Rodriguez, J.J. Activated carbon from grape seeds upon chemical activation with phosphoric acid: Application to the adsorption of diuron from water. Chem. Eng. J. 2012, 203, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, L.; Qu, H.; Jiao, Y.; Xie, J.; Xing, G. Preparation and characterization of activated carbon from reedy grass leaves by chemical activation with H 3 PO 4. Appl. Surf. Sci. 2014, 320, 674–680. [Google Scholar] [CrossRef]
- Luo, Y.; Li, D.; Chen, Y.; Sun, X.; Cao, Q.; Liu, X. The performance of phosphoric acid in the preparation of activated carbon-containing phosphorus species from rice husk residue. J. Mater. Sci. 2019, 54, 5008–5021. [Google Scholar] [CrossRef]
- Ilnicka, A.; Skorupska, M.; Tyc, M.; Kowalska, K.; Kamedulski, P.; Zielinski, W.; Lukaszewicz, J.P. Green algae and gelatine derived nitrogen rich carbon as an outstanding competitor to Pt loaded carbon catalysts. Sci. Rep. 2021, 11, 7084. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman spectroscopy of carbon materials and their composites: Graphene, nanotubes and fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar]
- Villa-Aleman, E.; Darvin, J.R.; Nielsen, M.H.; Willey, T.M. Raman signatures of detonation soot. J. Raman Spectrosc. 2022, 53, 1571–1579. [Google Scholar] [CrossRef]
- Henry, D.G.; Jarvis, I.; Gillmore, G.; Stephenson, M. Raman spectroscopy as a tool to determine the thermal maturity of organic matter: Application to sedimentary, metamorphic and structural geology. Earth-Sci. Rev. 2019, 198, 102936. [Google Scholar] [CrossRef]
- Zhang, S.; Su, Y.; Zhu, S.; Zhang, H.; Zhang, Q. Effects of pretreatment and FeCl3 preload of rice husk on synthesis of magnetic carbon composites by pyrolysis for supercapacitor application. J. Anal. Appl. Pyrolysis 2018, 135, 22–31. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, S.; Ruiz, B.; Martínez-Blanco, D.; Sánchez-Arenillas, M.; Diez, M.A.; Marco, J.F.; Gorria, P.; Fuente, E. Towards advanced industrial waste-based magnetic activated carbons with tunable chemical, textural and magnetic properties. Appl. Surf. Sci. 2021, 551, 149407. [Google Scholar] [CrossRef]
- Krishna, R.; Wade, J.; Jones, A.N.; Lasithiotakis, M.; Mummery, P.M.; Marsden, B.J. An understanding of lattice strain, defects and disorder in nuclear graphite. Carbon N. Y. 2017, 124, 314–333. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.C.; Cheng, J.; Wang, W.P.; Sun, G.T.; Hu, L.L.; Zhu, M.Q.; Huang, X.H. Structural changes and electrochemical properties of lacquer wood activated carbon prepared by phosphoric acid-chemical activation for supercapacitor applications. Renew. Energy 2021, 177, 82–94. [Google Scholar] [CrossRef]
- Flygare, M.; Svensson, K. Quantifying crystallinity in carbon nanotubes and its influence on mechanical behaviour. Mater. Today Commun. 2019, 18, 39–45. [Google Scholar] [CrossRef]
- Wang, L.; Mu, G.; Tian, C.; Sun, L.; Zhou, W.; Yu, P.; Yin, J.; Fu, H. Porous graphitic carbon nanosheets derived from cornstalk biomass for advanced supercapacitors. ChemSusChem 2013, 6, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Saravanan, S.; Ahmed, A.T.A.; Inamdar, A.I.; Im, H.; Lee, S.; Kim, D.Y. Spherical activated-carbon nanoparticles derived from biomass green tea wastes for anode material of lithium-ion battery. Mater. Lett. 2019, 240, 189–192. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Li, Z. Unusual interconnected graphitized carbon nanosheets as the electrode of high-rate ionic liquid-based supercapacitor. Carbon N. Y. 2017, 119, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Stephan, A.M.; Kumar, T.P.; Ramesh, R.; Thomas, S.; Jeong, S.K.; Nahm, K.S. Pyrolitic carbon from biomass precursors as anode materials for lithium batteries. Mater. Sci. Eng. A 2006, 430, 132–137. [Google Scholar] [CrossRef]
- Tian, W.; Gao, Q.; Tan, Y.; Yang, K.; Zhu, L.; Yang, C.; Zhang, H. Bio-inspired beehive-like hierarchical nanoporous carbon derived from bamboo-based industrial by-product as a high performance supercapacitor electrode material. J. Mater. Chem. A 2015, 3, 5656–5664. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Stoller, M.D.; Ganesh, K.J.; Cai, W.; Ferreira, P.J.; Pirkle, A.; Wallace, R.M.; Cychosz, K.A.; Thommes, M.; et al. Carbon-based supercapacitors produced by activation of graphene. Science 2011, 332, 1537–1541. [Google Scholar] [CrossRef] [Green Version]
- Kubicka, M.; Bakierska, M.; Chudzik, K.; Rutkowska, M.; Pacek, J.; Molenda, M. Electrochemical Properties and Structure Evolution of Starch-Based Carbon Nanomaterials as Li-Ion Anodes with Regard to Thermal Treatment. Polymers 2019, 11, 1527. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Chiang, Y.W.; Santos, R.M. X-ray Diffraction Techniques for Mineral Characterization: A Review for Engineers of the Fundamentals, Applications, and Research Directions. Minerals 2022, 12, 205. [Google Scholar] [CrossRef]
- Hallam, K.R.; Darnbrough, J.E.; Paraskevoulakos, C.; Heard, P.J.; Marrow, T.J.; Flewitt, P.E.J. Measurements by x-ray diffraction of the temperature dependence of lattice parameter and crystallite size for isostatically-pressed graphite. Carbon Trends 2021, 4, 100071. [Google Scholar] [CrossRef]
- Örkün, Y.; Karatepe, N.; Yavuz, R. Influence of temperature and impregnation ratio of H3PO 4 on the production of activated carbon from hazelnut shell. Acta Phys. Pol. A 2012, 121, 277–280. [Google Scholar] [CrossRef]
- Devi, M.; Rawat, S.; Sharma, S. A comprehensive review of the pyrolysis process: From carbon nanomaterial synthesis to waste treatment. Oxford Open Mater. Sci. 2021, 1, itab014. [Google Scholar] [CrossRef]
- Asif, F.C.; Saha, G.C. Graphene-like Carbon Structure Synthesis from Biomass Pyrolysis: A Critical Review on Feedstock–Process–Properties Relationship. C 2023, 9, 31. [Google Scholar] [CrossRef]
- Mruthunjayappa, M.H.; Kotrappanavar, N.S.; Mondal, D. New prospects on solvothermal carbonisation assisted by organic solvents, ionic liquids and eutectic mixtures—A critical review. Prog. Mater. Sci. 2022, 126, 100932. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Zularisam, A.W.; Nasrullah, M. A comprehensive assessment of the method for producing biochar, its characterization, stability, and potential applications in regenerative economic sustainability—A review. Clean. Mater. 2022, 3, 100045. [Google Scholar] [CrossRef]
- Wang, A.; Sun, K.; Xu, R.; Sun, Y.; Jiang, J. Cleanly synthesizing rotten potato-based activated carbon for supercapacitor by self-catalytic activation. J. Clean. Prod. 2021, 283, 125385. [Google Scholar] [CrossRef]
- Yakaboylu, G.A.; Jiang, C.; Yumak, T.; Zondlo, J.W.; Wang, J.; Sabolsky, E.M. Engineered hierarchical porous carbons for supercapacitor applications through chemical pretreatment and activation of biomass precursors. Renew. Energy 2021, 163, 276–287. [Google Scholar] [CrossRef]
- Heimböckel, R.; Hoffmann, F.; Fröba, M. Insights into the influence of the pore size and surface area of activated carbons on the energy storage of electric double layer capacitors with a new potentially universally applicable capacitor model. Phys. Chem. Chem. Phys. 2019, 21, 3122–3133. [Google Scholar] [CrossRef] [Green Version]
- Taer, E.; Agustino, A.; Farma, R.; Taslim, R.; Awitdrus; Paiszal, M.; Ira, A.; Yardi, S.D.; Sari, Y.P.; Yusra, H.; et al. The relationship of surface area to cell capacitance for monolith carbon electrode from biomass materials for supercapacitor aplication. J. Phys. Conf. Ser. 2018, 1116, 32040. [Google Scholar] [CrossRef]
- Lee, E.J.; Lee, L.; Abbas, M.A.; Bang, J.H. The influence of surface area, porous structure, and surface state on the supercapacitor performance of titanium oxynitride: Implications for a nanostructuring strategy. Phys. Chem. Chem. Phys. 2017, 19, 21140–21151. [Google Scholar] [CrossRef]
- Chen, T.; Dai, L. Carbon nanomaterials for high-performance supercapacitors. Mater. Today 2013, 16, 272–280. [Google Scholar] [CrossRef]
- Ab. Rahim, A.H.; Ramli, N.; Nordin, A.N.; Abd. Wahab, M.F. Supercapacitor performance with activated carbon and graphene nanoplatelets composite electrodes, and insights from the equivalent circuit model. Carbon Trends 2021, 5, 100101. [Google Scholar] [CrossRef]
- Lopez-Tenllado, F.J.; Motta, I.L.; Hill, J.M. Modification of biochar with high-energy ball milling: Development of porosity and surface acid functional groups. Bioresour. Technol. Reports 2021, 15, 100704. [Google Scholar] [CrossRef]
- Naghdi, M.; Taheran, M.; Brar, S.K.; Rouissi, T.; Verma, M.; Surampalli, R.Y.; Valero, J.R. A green method for production of nanobiochar by ball milling- optimization and characterization. J. Clean. Prod. 2017, 164, 1394–1405. [Google Scholar] [CrossRef] [Green Version]
- Welham, N.J.; Williams, J.S. Extended milling of graphite and activated carbon. Carbon N. Y. 1998, 36, 1309–1315. [Google Scholar] [CrossRef]
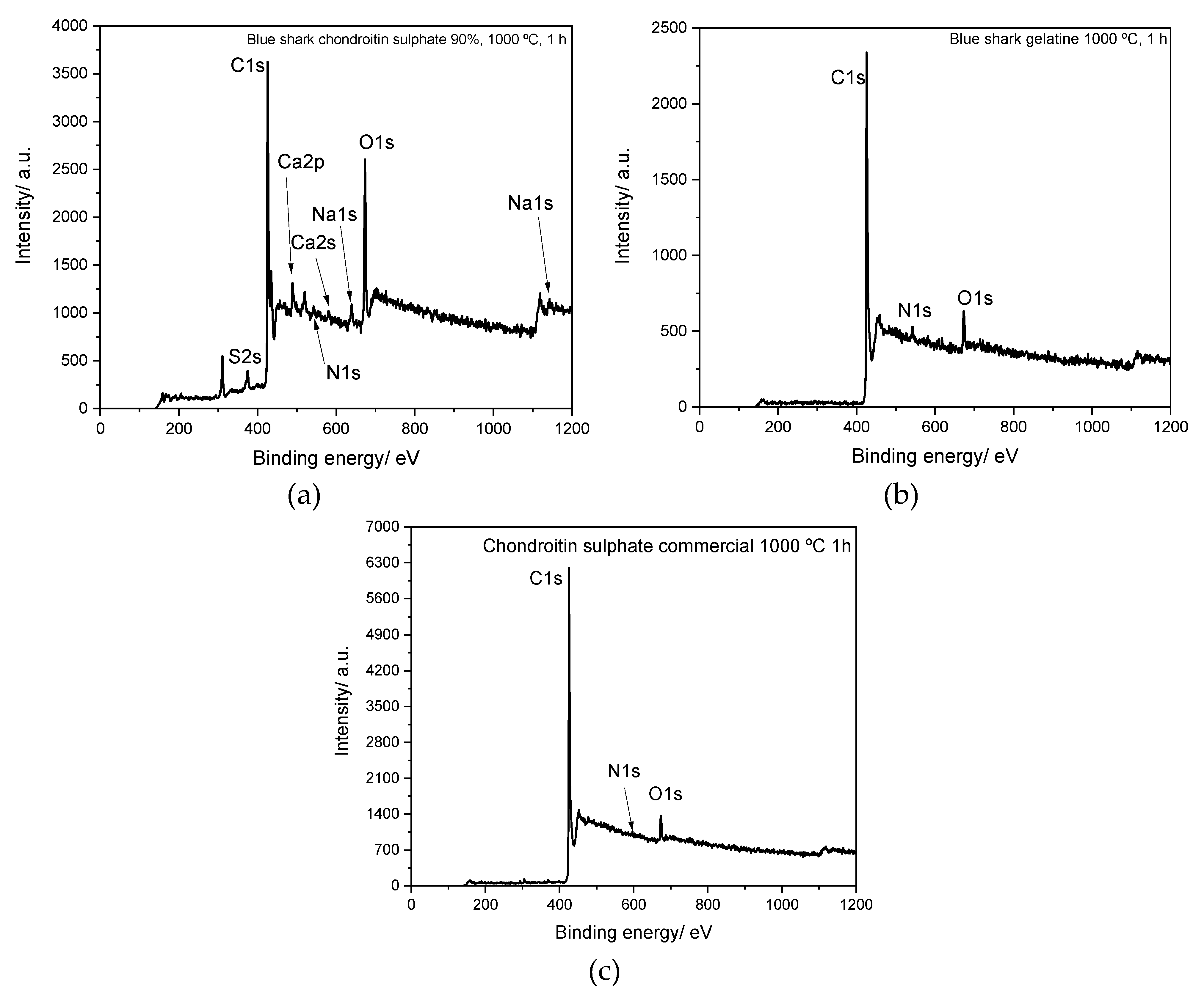



| At % | ||||||
|---|---|---|---|---|---|---|
| Element | Blue Shark Chondroitin Sulfate | Commercial Chondroitin Sulfate | Blue Shark Gelatine | |||
| Raw | 1000 °C 1 h | Raw | 1000 °C 1 h | Raw | 1000 °C 1 h | |
| C 1s | 49.1 | 75.4 | 45.6 | 71.2 | 68.1 | 81.4 |
| N 1s | 0.1 | 0.5 | 0.1 | 0.7 | 0.9 | 2.2 |
| O 1s | 38.1 | 15.7 | 54.3 | 28.1 | 14.1 | 4.1 |
| Na 1s | 2.0 | 1.0 | - | - | - | - |
| P 2p | - | - | - | - | - | - |
| S 2p | 5.1 | 3.2 | - | - | 9.1 | 4.3 |
| K 2p | 4.4 | 3.0 | - | - | 4.5 | 2.5 |
| Ca 2p | 1.2 | 1.2 | - | - | 6.3 | 2.5 |
| Carbon Source | R2 | ID/IG | La/nm |
|---|---|---|---|
| Blue shark chondroitin sulfate | 0.999 | 1.56 ± 0.02 | 12.40 |
| Commercial chondroitin sulfate | 0.998 | 1.84 ± 0.06 | 10.29 |
| Blue shark gelatine | 0.995 | 2.09 ± 0.04 | 9.13 |
| Carbon Source | Interplanar Distance (d) | |
|---|---|---|
| UHR-STEM | XRD | |
| Blue shark chondroitin sulfate | 2.6 ± 0.2 Å | 2.8 |
| Commercial chondroitin sulfate | 2.9 ± 0.1 Å | 3.5 |
| Blue shark gelatine | 3.41 ± 0.05 Å | 3.5 |
| BET Isotherm Analysis | |||||
|---|---|---|---|---|---|
| Carbon Source | SBET (m2 g−1) | Vmicro (cm3 g−1) | Vmeso (cm3 g−1) | Vtotal (cm3 g−1) | Dp (nm) |
| Blue shark chondroitin sulfate | 135.24 | 0.023 | 0.044 | 0.067 | 1.44 |
| Blue shark gelatine | 30.32 | 0.011 | 0.019 | 0.030 | 0.87 |
| Commercial chondroitin sulfate | 76.11 | 0.019 | 0.024 | 0.043 | 1.11 |
| Galvanostatic Charge–Discharge Analysis | |||||
|---|---|---|---|---|---|
| Source | Carbon Source | SBET (m2 g−1) | C (F g−1) | %C Retention | Reference |
| Blue shark | Chondroitin sulfate | 135.24 | 40 ± 2 | 71 @5000 cycles | This work |
| Gelatine | 30.32 | 7 ± 1 | 71 @5000 cycles | ||
| Commercial | Commercial chondroitin sulfate | 76.11 | 25 ± 3 | 86 @5000 cycles | |
| Squid | β-Chitin | 149.3 | 20 ± 1 | 96 @1000 cycles | [12] |
| Prawn | α-Chitin | 85.0 | 15 ± 2 | 92 @1000 cycles | |
| Mussel | Glycogen | 768.1 | 98 ± 2 | 99 @1000 cycles | [11] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brandão, A.T.S.C.; State, S.; Costa, R.; Enache, L.-B.; Potorac, P.; Vázquez, J.A.; Valcarcel, J.; Silva, A.F.; Enachescu, M.; Pereira, C.M. Porous Carbon Materials Based on Blue Shark Waste for Application in High-Performance Energy Storage Devices. Appl. Sci. 2023, 13, 8676. https://doi.org/10.3390/app13158676
Brandão ATSC, State S, Costa R, Enache L-B, Potorac P, Vázquez JA, Valcarcel J, Silva AF, Enachescu M, Pereira CM. Porous Carbon Materials Based on Blue Shark Waste for Application in High-Performance Energy Storage Devices. Applied Sciences. 2023; 13(15):8676. https://doi.org/10.3390/app13158676
Chicago/Turabian StyleBrandão, Ana T. S. C., Sabrina State, Renata Costa, Laura-Bianca Enache, Pavel Potorac, José A. Vázquez, Jesus Valcarcel, A. Fernando Silva, Marius Enachescu, and Carlos M. Pereira. 2023. "Porous Carbon Materials Based on Blue Shark Waste for Application in High-Performance Energy Storage Devices" Applied Sciences 13, no. 15: 8676. https://doi.org/10.3390/app13158676
APA StyleBrandão, A. T. S. C., State, S., Costa, R., Enache, L.-B., Potorac, P., Vázquez, J. A., Valcarcel, J., Silva, A. F., Enachescu, M., & Pereira, C. M. (2023). Porous Carbon Materials Based on Blue Shark Waste for Application in High-Performance Energy Storage Devices. Applied Sciences, 13(15), 8676. https://doi.org/10.3390/app13158676












