Utilization of Festuca arundinacea Schreb. Biomass with Different Salt Contents for Bioethanol and Biocomposite Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Plant Cultivation
2.3. Soil Salt Content
2.4. Sodium Content in F. arundinacea
2.5. Bioethanol Production Process
2.5.1. Pretreatment of Tall Fescue Biomass
2.5.2. Simultaneous Saccharification and Fermentation Process (SSF)
2.6. Biocomposites Production Process
2.6.1. Natural Fillers from Tall Fescue Biomass
2.6.2. Polymer Matrix
2.6.3. Composites Preparation
2.7. Analytical and Testing Methods
2.8. Calculations
2.9. Statistical Analysis
3. Results and Discussion
3.1. Effect of Soil Salinity on Biomass Yield and Sodium Content in F. arundinacea
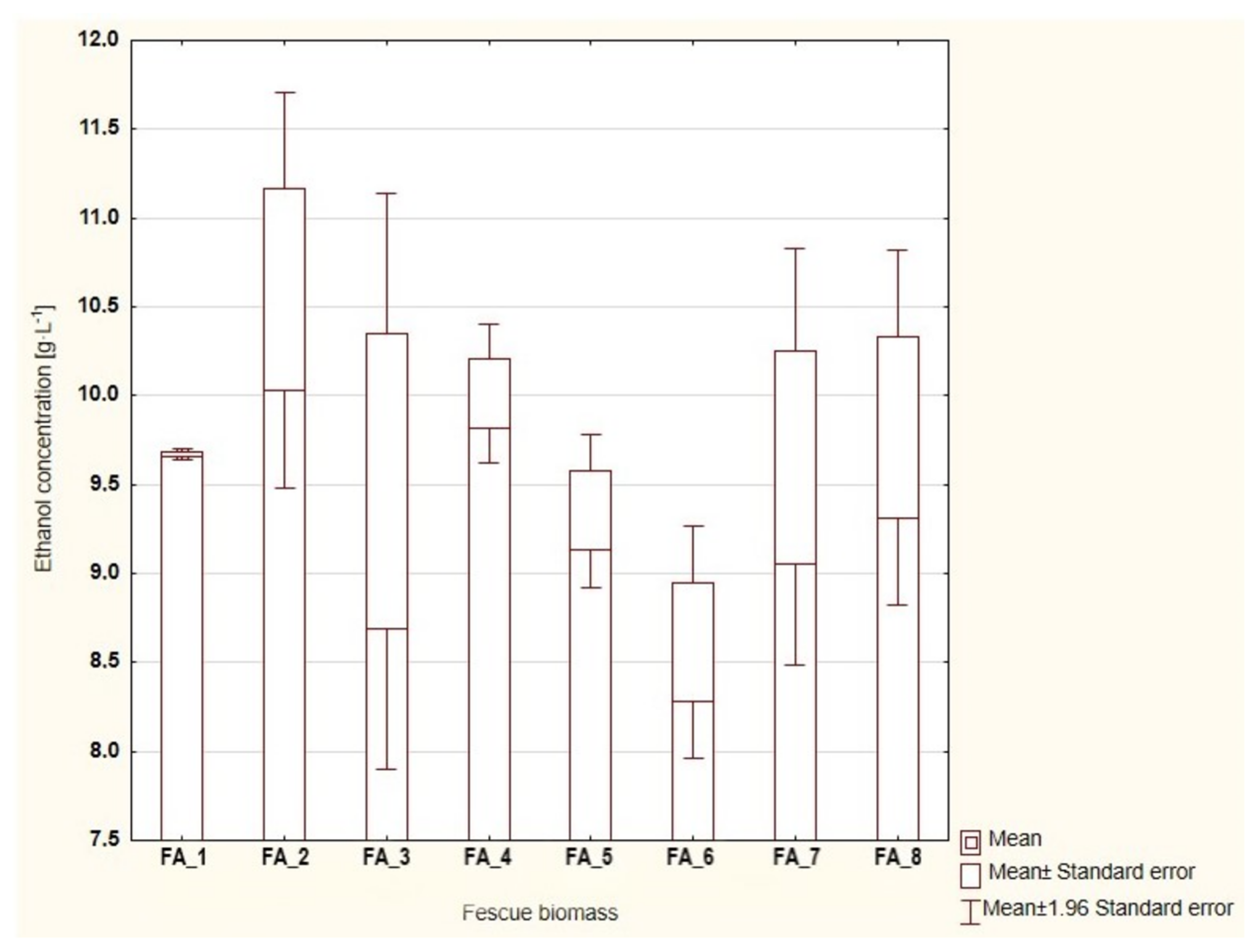
3.2. Bioethanol Production Process
3.3. Biocomposites Production Process
4. Conclusions
- Festuca arundinacea was cultivated on soil with a high concentration of NaCl in the top layer (up to 6 g∙L−1), and in the second year of cultivation, it formed a dense sod, produced three cuts and a medium high yield (14.8 Mg∙ha−1).
- In this work, the tall fescue biomass obtained from the soil with different salinities was used in the process of obtaining bioethanol and green composites based on the biodegradable polymer PLA.
- It was found that the content of Na+ in tall fescue biomass had no significant effect on the amount of ethanol obtained (average: 19.32 g∙100 g−1 of raw material), which may result from leaching of sodium ions during alkaline pretreatment.
- The possibility of using tall fescue biomass with the lowest Na+ content in biomass (approx. 2.5 mg∙g−1) from an area with high salinity (above 6 g NaCl∙L−1) as fillers for composites based on PLA was demonstrated. Moreover, for this biomass, the highest value of bioethanol concentration was obtained, i.e., 10.6 g∙L−1 (21.2 g∙100 g−1 of raw material).
- In summary, F. arundinacea can be successfully used for bioremediation of saline habitats and its biomass for industrial applications such as advanced biofuels and as natural fillers to improve the economics of using biodegradable polymers in composites.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharavdorj, K.; Jang, Y.; Byambadorj, S.-O.; Cho, J.-W. Understanding seed germination of forage crops under various salinity and temperature stress. J. Crop Sci. Biotechnol. 2021, 24, 545–554. [Google Scholar] [CrossRef]
- Shiade, S.R.G.; Boelt, B. Seed germination and seedling growth parameters in nine tall fescue varieties under salinity stress. Acta Agric. Scand. B Soil Plant Sci. 2020, 70, 485–494. [Google Scholar] [CrossRef]
- Gibson, D.J.; Newman, J.A. Festuca arundinacea Schreber (F. elatior L. ssp. arundinacea (Schreber) Hackel). J. Ecol. 2001, 89, 304–324. [Google Scholar] [CrossRef]
- Dierking, R.M.; Kallenbach, R.L. Mediterranean and Continental Tall Fescue: II. Effects of Cold, Nonfreezing Temperatures on Leaf Extension, Proline, Fructan, and Abscisic Acid. Crop Sci. 2012, 52, 460–469. [Google Scholar] [CrossRef]
- Amombo, E.; Li, X.; Wang, G.; Fan, S.; Shao, A.; Zhang, Y.; Fu, J. Screening of diverse tall fescue population for salinity tolerance based on SSR marker-physiological trait association. Euphytica 2018, 214, 220. [Google Scholar] [CrossRef]
- Menon-Martínez, F.E.; Grimoldi, A.A.; Striker, G.G.; Di Bella, C.E. Variability among Festuca arundinacea cultivars for tolerance to and recovery from waterlogging, salinity and their combination. Crop Pasture Sci. 2021, 72, 75–84. [Google Scholar] [CrossRef]
- Chandregowda, M.H.; Tjoelker, M.G.; Pendall, E.; Zhang, H.; Churchill, A.C.; Power, S.A. Belowground carbon allocation, root trait plasticity, and productivity during drought and warming in a pasture grass. J. Exp. Bot. 2023, 74, 2127–2145. [Google Scholar] [CrossRef]
- Liu, M.; Hulting, A.; Mallory-Smith, C. Comparison of growth and physiological characteristics between roughstalk bluegrass and tall fescue in response to simulated waterlogging. PLoS ONE 2017, 12, e0182035. [Google Scholar] [CrossRef]
- Lechowicz, K.; Pawłowicz, I.; Perlikowski, D.; Arasimowicz-Jelonek, M.; Majka, J.; Augustyniak, A.; Rapacz, M.; Kosmala, A. Two Festuca Species—F. arundinacea and F. glaucescens—Differ in the Molecular Response to Drought, While Their Physiological Response Is Similar. Int. J. Mol. Sci. 2020, 21, 3174. [Google Scholar] [CrossRef] [PubMed]
- Steliga, T.; Kluk, D. Application of Festuca arundinacea in phytoremediation of soils contaminated with Pb, Ni, Cd and petroleum hydrocarbons. Ecotoxicol. Environ. Saf. 2020, 194, 110409. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; He, W.; Wang, H.; Pan, H.; Yang, Q.; Lou, Y.; Zhuge, Y. Effect of phosphorus supplementation on growth, nutrient uptake, physiological responses, and cadmium absorption by tall fescue (Festuca arundinacea Schreb.) exposed to cadmium. Ecotoxicol. Environ. Saf. 2021, 213, 112021. [Google Scholar] [CrossRef] [PubMed]
- Pawłowicz, I.; Waśkiewicz, A.; Perlikowski, D.; Rapacz, M.; Ratajczak, D.; Kosmala, A. Remodeling of chloroplast proteome under salinity affects salt tolerance of Festuca arundinacea. Photosynth. Res. 2018, 137, 475–492. [Google Scholar] [CrossRef] [PubMed]
- Batog, J.; Bujnowicz, K.; Gieparda, W.; Wawro, A.; Rojewski, S. Effective Utilisation of Halophyte Biomass from Saline Soils for Biorefinering Processes. Molecules 2021, 26, 5393. [Google Scholar] [CrossRef]
- Inyang, V.; Laseinde, O.T.; Kanakana, G.M. Techniques and applications of lignocellulose biomass sources as transport fuels and other bioproducts. Int. J. Low-Carbon Technol. 2022, 17, 900–909. [Google Scholar] [CrossRef]
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, S.; Kuila, A. A review on current technological advancement of lignocellulosic bioethanol production. J. Appl. Biotechnol. Bioeng. 2016, 1, 61–66. [Google Scholar]
- Suttikul, S.; Srinorakutara, T.; Butivate, E.; Orasoon, K. Comparison of SHF and SSF processes for ethanol production from alkali-acid pretreated sugarcane trash. KKU Res. J. 2016, 21, 229–235. [Google Scholar]
- Abo, B.O.; Gao, M.; Wang, Y.; Wu, C.; Ma, H.; Wang, Q. Lignocellulosic biomass for bioethanol: An overview on pretreatment, hydrolysis and fermentation processes. Rev. Environ. Health 2019, 34, 57–68. [Google Scholar] [CrossRef]
- Vasić, K.; Knez, Ž.; Leitgeb, M. Bioethanol Production by Enzymatic Hydrolysis from Different Lignocellulosic Sources. Molecules 2021, 26, 753. [Google Scholar] [CrossRef]
- Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the Promotion of the Use of Energy from Renewable Sources and Amending and Subsequently Repealing Directives 2001/77/EC and 2003/30/EC. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX:32009L0028 (accessed on 8 April 2021).
- European Council. Directive (EU) 2019/904 of the European Parliament and of the Council of 5 June 2019 on the Reduction of the Impact of Certain Plastic Products on the Environment. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/HTML/?uri=CELEX:32019L0904&from=HU (accessed on 1 February 2021).
- Moore, C. Plastic Pollution. Encyclopedia Britannica. Available online: https://www.britannica.com/science/plastic-pollution (accessed on 18 June 2021).
- Getme, A.S.; Patel, B. A Review: Bio-fiber’s as reinforcement in composites of polylactic acid (PLA). Mater. Today Proc. 2019, 26, 2116–2122. [Google Scholar] [CrossRef]
- Das, P.P.; Chaudhary, V. Moving towards the era of bio fibre based polymer composites. Clean. Eng. Technol. 2021, 4, 100182. [Google Scholar] [CrossRef]
- Manral, A.; Ahmad, F.; Chaudhary, V. Static and dynamic mechanical properties of PLA bio-composite with hybrid reinforcement of flax and jute. Mater. Today Proc. 2020, 25, 577–580. [Google Scholar] [CrossRef]
- Papadopoulou, E.; Bikiaris, D.; Chrysafis, K.; Wladyka-Przybylak, M.; Wesolek, D.; Mankowski, J.; Kolodziej, J.; Baraniecki, P.; Bujnowicz, K.; Gronberg, V. Value-added industrial products from bast fiber crops. Ind. Crops Prod. 2015, 68, 116–125. [Google Scholar] [CrossRef]
- Sanivada, U.K.; Mármol, G.; Brito, F.P.; Fangueiro, R. PLA Composites Reinforced with Flax and Jute Fibers—A Review of Recent Trends, Processing Parameters and Mechanical Properties. Polymers 2020, 12, 2373. [Google Scholar] [CrossRef]
- Akter, M.; Uddin, H.; Tania, I.S. Biocomposites based on natural fibers and polymers: A review on properties and potential applications. J. Reinf. Plast. Compos. 2022, 41, 705–742. [Google Scholar] [CrossRef]
- Batog, J.; Wawro, A. Chemical and Biological Deconstruction in the Conversion Process of Sorghum Biomass for Bioethanol. J. Nat. Fibers 2021, 19, 5827–5838. [Google Scholar] [CrossRef]
- PN-EN ISO 3167:2014; Plastics—Multipurpose Test Specimens. Polish Committee for Standardization: Geneva, Switzerland, 2014.
- TAPPI 17 m-55. In Cellulose in Wood; TAPPI Press: Atlanta, GA, USA, 1955.
- Bagby, M.O.; Nelson, G.H.; Helman, E.G.; Clark, T.F. Determination of lignin non-wood plant fiber sources. TAPPI J. 1971, 54, 11. [Google Scholar]
- TAPPI T9 m-54. In Holocellulose in Wood; TAPPI Press: Atlanta, GA, USA, 1998.
- PN-EN ISO 527-1:2020; Plastics—Determination of Tensile Properties—Part 1: General Principles. Polish Committee for Standardization: Geneva, Switzerland, 2020.
- PN-EN ISO 527-2:2012; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. Polish Committee for Standardization: Geneva, Switzerland, 2012.
- PN-EN ISO 178:2019; Plastics—Determination of Flexural Properties. Polish Committee for Standardization: Geneva, Switzerland, 2019.
- Kawa-Rygielska, J.; Pietrzak, W. Zagospodarowanie odpadowe pieczywa do produkcji bioetanolu. Żywn. Nauka Technol. Jakość 2011, 79, 105–118. [Google Scholar]
- Głąb, T. Effect of soil compaction on root system development and yields of tall fescue. Int. Agrophys. 2007, 21, 233–239. [Google Scholar]
- Czyż, H.; Jänicke, H.; Kitczak, T.; Bury, M. Feasibility of Using Festuca Arundinacea for Regeneration of Grasslands by Means of Full Cultivation Method on Organic Soil. Folia Pomer. Univ. Technol. Stetin. Agric. Aliment. Pisc. Zootech. 2017, 336, 23–32. [Google Scholar] [CrossRef]
- Frydrych, J.; Jezerská, L.; Gerndtová, I.; Andert, D.; Bradáčová, L.; Sassmanová, V. Phytomass as a renewable energy source in conditions of the Czech Republic. Waste Forum 2021, 1, 126–136. [Google Scholar]
- Temel, S.; Keskin, B.; Simsek, U.; Yilmaz, I.H. Performance of Some Forage Grass Species in Halomorphic Soil. Turk. J. Field Crops 2015, 20, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Kaplan, M.; Baser, M.; Kale, H.; Irik, H.A.; Ulger, I.; Unlukara, A. Change in yield and chemical composition of tall fescue (festuca arundinacea schreb.) plants under salt stress. Turk. J. Field Crops 2017, 22, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Al-Ghumaiz, N.S.; Abd-Elmoniem, E.M.; Motawei, M.I. Salt Tolerance and K/Na Ratio of Some Introduced Forage Grass Species Under Salinity Stress in Irrigated Areas. Commun. Soil Sci. Plant Anal. 2017, 48, 1494–1502. [Google Scholar] [CrossRef]
- Jamaldheen, S.B.; Sharma, K.; Rani, A.; Moholkara, V.S.; Goyala, A. Comparative analysis of pretreatment methods on sorghum (Sorghum durra) stalk agrowaste for holocellulose content. Prep. Biochem. Biotechnol. 2018, 48, 457–464. [Google Scholar] [CrossRef]
- Zhao, J.; Xu, Y.; Zhang, M.; Wang, D. Integrating bran starch hydrolysates with alkaline pretreated soft wheat bran to boost sugar concentration. Bioresour. Technol. 2020, 302, 122826. [Google Scholar] [CrossRef]
- Kumar, D.; Murthy, G.S. Pretreatments and Enzymatic Hydrolysis of Grass Straws for Ethanol Production in the Pacific Northwest U.S. Biol. Eng. Trans. 2011, 3, 97–110. [Google Scholar] [CrossRef]
- Chang, S.; Li, W.; Zhang, Y. Impact of double alkaline peroxide pretreatment on enzymatic hydrolysis of palm fibre. Carbon Resour. Convers. 2018, 1, 147–152. [Google Scholar] [CrossRef]
- Lopez, L.; Alagna, F.; Bianco, L.; De Bari, I.; Fasano, C.; Panara, F.; Perrella, G. Plants: A sustainable platform for second-generation biofuels and biobased chemicals. In Handbook of Biofuels; Sahay, S., Ed.; Elsevier: London, UK, 2022; pp. 47–73. [Google Scholar]
- Horita, M.; Kiyoshi, T. Bioethanol Production from Various Plant Materials via Solid-state Fermentation. JARQ 2022, 56, 405–412. [Google Scholar] [CrossRef]
- Graupner, N.; Rößler, J.; Ziegmann, G.; Müssig, J. Fibre/matrix adhesion of cellulose fibres in PLA, PP and MAPP: A critical review of pull-out test, microbond test and single fibre fragmentation test results. Compos. Part A Appl. Sci. Manuf. 2014, 63, 133–148. [Google Scholar] [CrossRef]
- Asyraf, M.R.M.; Rafidah, M.; Azrina, A.; Razman, M.R. Dynamic mechanical behaviour of kenaf cellulosic fibre biocomposites: A comprehensive review on chemical treatments. Cellulose 2021, 28, 2675–2695. [Google Scholar] [CrossRef]
- Ilyas, R.A.; Zuhri, M.Y.M.; Aisyah, H.A.; Asyraf, M.R.M.; Hassan, S.A.; Zainudin, E.S.; Sapuan, S.M.; Sharma, S.; Bangar, S.P.; Jumaidin, R.; et al. Natural Fiber-Reinforced Polylactic Acid, Polylactic Acid Blends and Their Composites for Advanced Applications. Polymers 2022, 14, 202. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.A.; Joseph, K.; Mathew, G.G.; Pothen, L.A.; Thomas, S. Influence of polarity parameters on the mechanical properties of composites from polypropylene fiber and short banana fiber. Compos. Part A Appl. Sci. Manuf. 2010, 41, 1380–1387. [Google Scholar] [CrossRef]
- Yu, T.; Jiang, N.; Li, Y. Study on short ramie fiber/poly(lactic acid) composites compatibilized by maleic anhydride. Compos. Part A Appl. Sci. Manuf. 2014, 64, 139–146. [Google Scholar] [CrossRef]





| Plots | Fresh Biomass Yield (kg∙m−2) | Biomass Loss after Air-Drying (%) | Air-Dry Biomass Yield (g∙m−2) | Dry Biomass Yield (g∙m−2) | Moisture Content in Fresh Biomass (%) | Moisture Content in Air-Dry Biomass (%) |
|---|---|---|---|---|---|---|
| FA_1 | 2.05 | 68.73 | 641.10 | 578.37 | 71.79 | 9.79 |
| FA_2 | 1.75 | 69.84 | 527.85 | 474.11 | 72.91 | 10.18 |
| FA_3 | 2.05 | 70.56 | 603.53 | 541.16 | 73.60 | 10.34 |
| FA_4 | 2.75 | 74.09 | 712.50 | 636.76 | 76.85 | 10.63 |
| FA_5 | 2.25 | 71.20 | 648.08 | 576.76 | 74.37 | 11.01 |
| FA_6 | 2.55 | 71.15 | 735.57 | 655.43 | 74.30 | 10.90 |
| FA_7 | 2.55 | 70.71 | 746.95 | 663.96 | 73.96 | 11.11 |
| FA_8 | 2.90 | 68.88 | 902.48 | 807.67 | 72.15 | 10.51 |
| Mean ± SD | 2.36 ± 0.37 | 70.64 ± 1.58 | 689.76 ± 105.41 | 616.78 ± 93.35 | 73.74 ± 1.48 | 10.56 ± 0.42 |
| V [%] | 15.68 | 2.23 | 15.28 | 15.13 | 2.00 | 3.99 |
| Plots | Na+ Content in Dry Biomass (mg∙g−1) | Soil Salinity (g NaCl∙L−1) |
|---|---|---|
| FA_1 | 2.72 ± 0.30 | 6.85 ± 0.18 |
| FA_2 | 2.45 ± 0.11 | 6.24 ± 0.09 |
| FA_3 | 7.17 ± 1.18 | 5.76 ± 0.38 |
| FA_4 | 6.45 ± 0.27 | 4.75 ± 0.21 |
| FA_5 | 8.38 ± 0.21 | 4.02 ± 0.15 |
| FA_6 | 7.71 ± 0.73 | 2.82 ± 0.25 |
| FA_7 | 8.57 ± 0.48 | 1.49 ± 0.06 |
| FA_8 | 8.66 ± 0.88 | 0.70 ± 0.06 |
| Mean ± SD | 6.51 ± 2.37 | 4.08 ± 2.10 |
| V [%] | 36.44 | 51.55 |
| Plant Biomass | Humidity (%) | Particle Size Distribution (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 mm | 0.5 mm | 0.4 mm | 0.25 mm | 0.2 mm | 0.1 mm | Below 0.1 mm | ||
| FA_1 | 6.73 | 1.2 | 8.3 | 48.3 | 30.5 | 2.5 | 2.7 | 6.5 |
| FA_2 | 7.65 | 0.8 | 3.5 | 51.3 | 29.3 | 4.7 | 3.2 | 7.2 |
| FA_3 | 6.57 | 1.1 | 2.8 | 50.6 | 31.2 | 3.7 | 2.8 | 7.8 |
| FA_4 | 6.33 | 0.8 | 3.1 | 49.5 | 30.6 | 4.2 | 2.9 | 8.9 |
| FA_5 | 7.25 | 1.2 | 2.6 | 51.2 | 32.3 | 2.5 | 3.2 | 7.0 |
| FA_6 | 6.84 | 1.1 | 3.6 | 40.9 | 40.2 | 3.4 | 3.6 | 7.2 |
| FA_7 | 6.20 | 1.7 | 1.6 | 52.3 | 33.9 | 2.1 | 2.6 | 5.8 |
| FA_8 | 6.54 | 1.5 | 2.1 | 49.2 | 35.4 | 2.1 | 2.4 | 7.3 |
| Sample | Tensile Strength δM (MPa) | Tensile Modulus Et (GPa) | Flexural Strength δfM (MPa) | Flexural Modulus Ef (GPa) |
|---|---|---|---|---|
| PLA 3251D | 62.1 ± 0.5 | 2.34 ± 0.07 | 108.1 ± 0.4 | 2.21 ± 0.21 |
| PLA-FA_1 | 47.2 ± 0.6 | 3.00 ± 0.05 | 83.8 ± 0.5 | 2.80 ± 0.14 |
| PLA-FA_2 | 49.3 ± 0.9 | 3.14 ± 0.09 | 88.2 ± 0.9 | 2.92 ± 0.20 |
| PLA-FA_3 | 46.6 ± 1.1 | 2.97 ± 0.10 | 82.6 ± 0.8 | 2.77 ± 0.17 |
| PLA-FA_4 | 47.8 ± 0.9 | 3.06 ± 0.08 | 84.6 ± 0.8 | 2.85 ± 0.22 |
| PLA-FA_5 | 46.1 ± 0.7 | 2.93 ± 0.07 | 82.2 ± 0.8 | 2.74 ± 0.09 |
| PLA-FA_6 | 44.7 ± 0.6 | 2.85 ± 0.06 | 79.5 ± 1.1 | 2.66 ± 0.11 |
| PLA-FA_7 | 46.8 ± 0.9 | 2.98 ± 0.11 | 83.5 ± 1.0 | 2.79 ± 0.09 |
| PLA-FA_8 | 48.0 ± 0.6 | 3.06 ± 0.09 | 84.9 ± 0.9 | 2.83 ± 0.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batog, J.; Wawro, A.; Bujnowicz, K.; Gieparda, W.; Bilińska, E.; Pietrowiak, A.; Rojewski, S.; Adamczak, A. Utilization of Festuca arundinacea Schreb. Biomass with Different Salt Contents for Bioethanol and Biocomposite Production. Appl. Sci. 2023, 13, 8738. https://doi.org/10.3390/app13158738
Batog J, Wawro A, Bujnowicz K, Gieparda W, Bilińska E, Pietrowiak A, Rojewski S, Adamczak A. Utilization of Festuca arundinacea Schreb. Biomass with Different Salt Contents for Bioethanol and Biocomposite Production. Applied Sciences. 2023; 13(15):8738. https://doi.org/10.3390/app13158738
Chicago/Turabian StyleBatog, Jolanta, Aleksandra Wawro, Krzysztof Bujnowicz, Weronika Gieparda, Elżbieta Bilińska, Aurelia Pietrowiak, Szymon Rojewski, and Artur Adamczak. 2023. "Utilization of Festuca arundinacea Schreb. Biomass with Different Salt Contents for Bioethanol and Biocomposite Production" Applied Sciences 13, no. 15: 8738. https://doi.org/10.3390/app13158738





