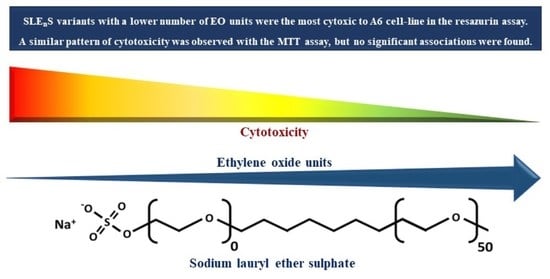
The Number of Ethylene Oxide Groups of Sulphate-Based Surfactants Influences the Cytotoxicity of Mixed Micelles to an Amphibian Cell Line
Abstract
:1. Introduction
2. Materials and Methods
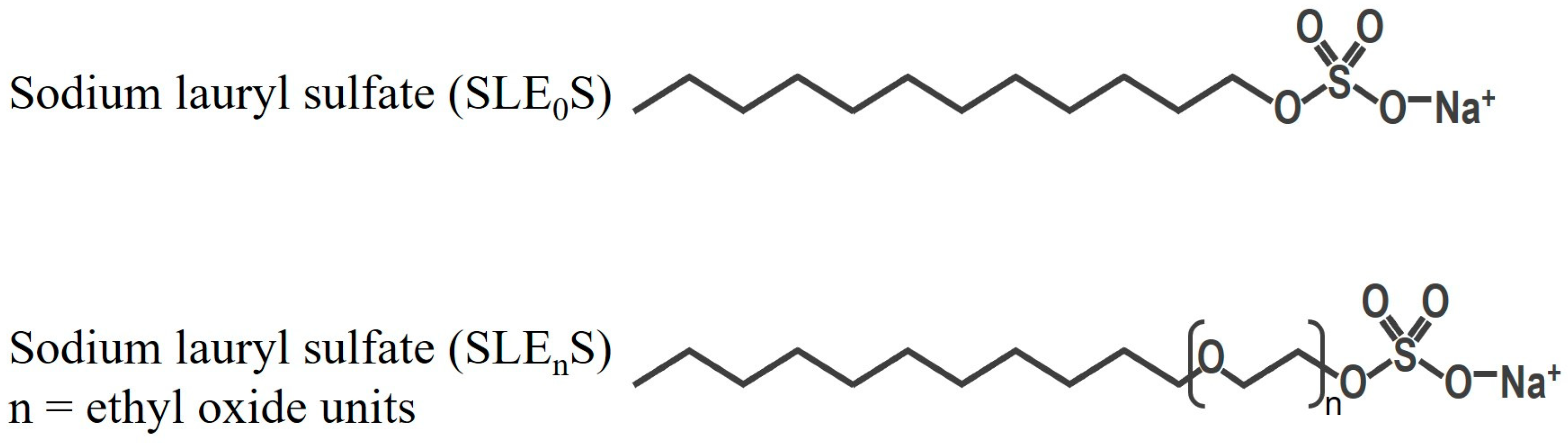
2.1. Test Substance
2.2. Biological Model
2.3. Cytotoxicity Assays
2.4. Data Analysis
3. Results
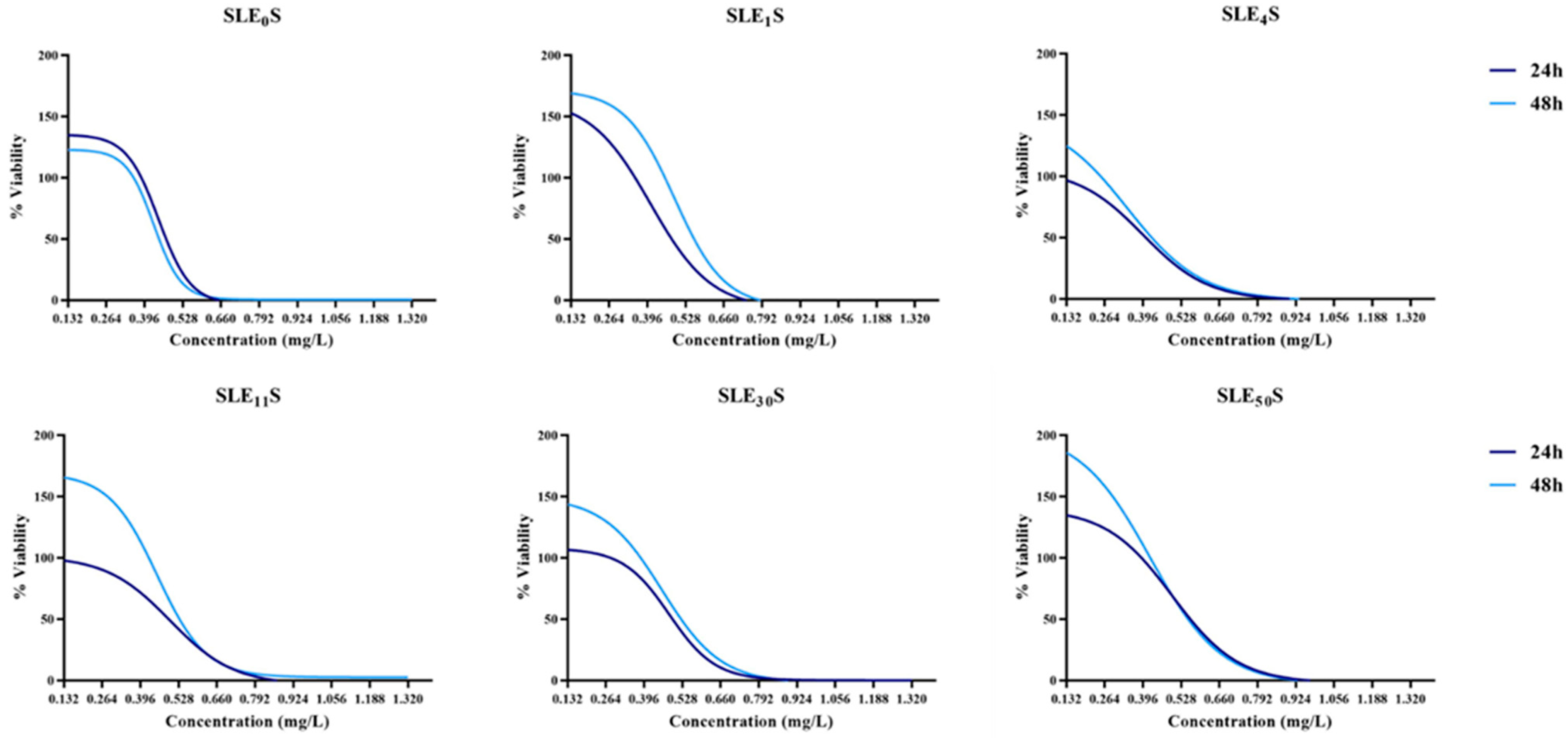
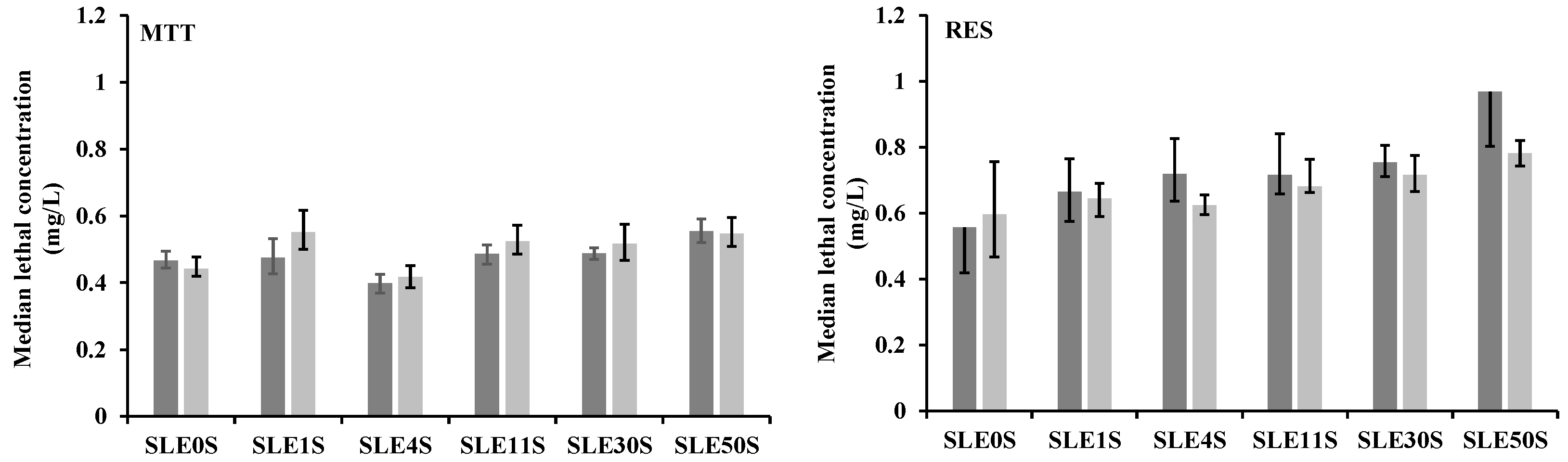
3.1. MTT Assay
3.2. Resazurin Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martins, N.; Pereira, J.L.; Antunes, F.E.; Melro, E.; Duarte, C.M.G.; Dias, L.; Soares, A.M.V.M.; Lopes, I. Role of surfactant headgroups on the toxicity of SLEnS-LAS mixed micelles: A case study using microtox test. Sci. Total Environ. 2018, 643, 1366–1372. [Google Scholar] [CrossRef] [PubMed]
- de Seixas-Junior, C.H.; de Carvalho, M.M.; Jacumazo, J.; Piazza, R.D.; Parchen, G.P.; de Freitas, R.A. Interaction of guar gum galactomannans with the anionic surfactant sodium lauryl ether sulphate. Int. J. Biol. Macromol. 2020, 165, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, S.F.S.; Mohamad, S.; Aziz, A.A. The susceptibility of aphids, Aphis gossypii Glover to lauric acid based natural pesticide. Procedia Eng. 2013, 53, 20–28. [Google Scholar] [CrossRef] [Green Version]
- Caracciolo, A.B.; Cardoni, M.; Pescatore, T.; Patrolecco, L. Characteristics and environmental fate of the anionic surfactant sodium lauryl ether sulphate (SLES) used as the main component in foaming agents for mechanized tunnelling. Environ. Pollut. 2017, 226, 94–103. [Google Scholar] [CrossRef]
- Sar, S.K.; Verma, C.; Pandey, P.K. Spectrophotometric determination of sodium lauryl sulphate as a prime anionic surfactant and contaminant in central East Chhattisgarh (Durg-Bhilai Region). Rasayan J. Chem. 2008, 1, 236–245. [Google Scholar]
- Bergé, A.; Wiest, L.; Baudot, R.; Giroud, B.; Vulliet, E. Occurrence of multi-class surfactants in urban wastewater: Contribution of a healthcare facility to the pollution transported into the sewerage system. Environ. Sci. Pollut. Res. 2018, 25, 9219–9229. [Google Scholar] [CrossRef]
- Bondi, C.A.; Marks, J.L.; Wroblewski, L.B.; Raatikainen, H.S.; Lenox, S.R.; Gebhardt, K.E. Human and environmental toxicity of sodium lauryl sulfate (SLS): Evidence for safe use in household cleaning products. Environ. Health Insights 2015, 9, 27–32. [Google Scholar] [CrossRef] [Green Version]
- Asio, J.R.G.; Garcia, J.S.; Antonatos, C.; Sevilla-Nastor, J.B.; Trinidad, L.C. Sodium lauryl sulfate and its potential impacts on organisms and the environment: A thematic analysis. Emerg. Contam. 2023, 9, 100205. [Google Scholar] [CrossRef]
- Mariani, L.; Grenni, P.; Barra Caracciolo, A.; Donati, E.; Rauseo, J.; Rolando, L.; Patrolecco, L. Toxic response of the bacterium Vibrio fischeri to sodium lauryl ether sulphate residues in excavated soils. Ecotoxicology 2020, 29, 815–824. [Google Scholar] [CrossRef]
- Lewis, M.A. The effects of mixtures and other environmental modifying factors on the toxicities of surfactants to freshwater and marine life. Water Res. 1992, 26, 1013–1023. [Google Scholar] [CrossRef]
- Wong, D.C.; Dorn, P.B.; Chai, E.Y. Acute toxicity and structure-activity relationships of nine alcohol ethoxylate surfactants to fathead minnow and Daphnia magna. Environ. Toxicol. Chem. 1997, 16, 1970–1976. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of animal studies for predicting toxicity in clinical trials: Is it time to rethink our current approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Ahyayauch, H.; Bennouna, M.; Alonso, A.; Goni, F.M. Detergent effects on membranes at subsolubilizing concentrations: Transmembrane lipid motion, bilayer permeabilization, and vesicle lysis/reassembly are independent phenomena. Langmuir 2010, 26, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Otzen, D. Protein–surfactant interactions: A tale of many states. BBA-Proteins Proteom. 2011, 1814, 562–591. [Google Scholar] [CrossRef] [PubMed]
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 8 April 2023).
- Wake, D.B.; Vredenburg, V.T. Are we in the midst of the sixth mass extinction? A view from the world of amphibians. Proc. Natl. Acad. Sci. USA 2008, 105 (Suppl. 1), 11466–11473. [Google Scholar] [CrossRef]
- Ivanković, T.; Hrenović, J. Surfactants in the environment. Arh. Hig. Rada Toksikol. 2010, 61, 95–109. [Google Scholar] [CrossRef] [Green Version]
- Shirriff, C.S.; Heikkila, J.J. Characterization of cadmium chloride-induced BiP accumulation in Xenopus laevis A6 kidney epithelial cells. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2017, 191, 117–128. [Google Scholar] [CrossRef]
- Khan, S.; Khamis, I.; Heikkila, J.J. The small heat shock protein, HSP30, is associated with aggresome-like inclusion bodies in proteasomal inhibitor-, arsenite-, and cadmium-treated Xenopus kidney cells. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2015, 189, 130–140. [Google Scholar] [CrossRef]
- Sun, H.; Niisato, N.; Nishio, K.; Hamilton, K.L.; Marunaka, Y. Distinct action of flavonoids, myricetin and quercetin, on epithelial Cl⁻ secretion: Useful tools as regulators of Cl⁻ secretion. BioMed Res. Int. 2014, 2014, 902735. [Google Scholar] [CrossRef]
- Thai, T.L.; Yu, L.; Eaton, D.C.; Duke, B.J.; Al-Khalili, O.; Lam, H.Y.; Ma, H.; Bao, H.F. Basolateral P2X4channels stimulate ENaC activity in Xenopus cortical collecting duct A6 cells. Am. J. Physiol. Renal. Physiol. 2014, 307, F806-13. [Google Scholar] [CrossRef] [Green Version]
- Tokuda, S.; Niisato, N.; Nakajima, K.; Marunaka, Y. Regulation of the paracellular Na+ and Cl− conductances by the NaCl− generated osmotic gradient in a manner dependent on the direction of osmotic gradients. Biochem. Biophys. Res. Commun. 2008, 366, 464–470, Erratum in Biochem. Biophys. Res. Commun. 2009, 384, 274. [Google Scholar] [CrossRef]
- Liley, J.R.; Penfold, J.; Thomas, R.K.; Tucker, I.M.; Petkov, J.T.; Stevenson, P.S.; Banat, I.M.; Marchant, R.; Rudden, M.; Terry, A.; et al. Self-assembly in dilute mixtures of non-ionic and anionic surfactants and rhamnolipd biosurfactants. J. Colloid Interface Sci. 2017, 487, 493–503. [Google Scholar] [CrossRef]
- Rafferty, K.A., Jr. Mass culture of amphibian cells: Methods and observations concerning stability of cell type. In Biology of Amphibian Tumors; Mizell, M., Ed.; Recent Results in Cancer Research; Springer: Berlin/Heidelberg, Germany, 1969; Volume 1969, pp. 52–81. [Google Scholar]
- Born, N.; Thiesen, H.J.; Lorenz, P. The B-subdomain of the Xenopus laevis XFIN KRAB-AB domain is responsible for its weaker transcriptional repressor activity compared to human ZNF10/Kox1. PLoS ONE 2014, 9, e87609. [Google Scholar] [CrossRef]
- Bui-Marinos, M.P.; Varga, J.F.; Vo, N.T.; Bols, N.C.; Katzenback, B.A. Xela DS2 and Xela VS2: Two novel skin epithelial-like cell lines from adult African clawed frog (Xenopus laevis) and their response to an extracellular viral dsRNA analogue. Dev. Comp. Immunol. 2020, 112, 103759. [Google Scholar] [CrossRef]
- Riss, T.L.; Moravec, R.A.; Niles, A.L.; Duellman, S.; Benink, H.A.; Worzella, T.J.; Minor, L. Cell Viability Assays. Assay Guidance Manual. Company and the National Center for Advancing Translational Sciences. 2016. Available online: https://www.ncbi.nlm.nih.gov/books/NBK144065/ (accessed on 6 April 2023).
- Löffler, H.; Happle, R. Profile of irritant patch testing with detergents: Sodium lauryl sulfate, sodium laureth sulfate and alkyl polyglucoside. Contact Dermat. 2003, 48, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lavogina, D.; Lust, H.; Tahk, M.J.; Laasfeld, T.; Vellama, H.; Nasirova, N.; Vardja, M.; Eskla, K.-L.; Salumets, A.; Rinken, A.; et al. Revisiting the Resazurin-Based Sensing of Cellular Viability: Widening the Application Horizon. Biosensors 2022, 12, 196. [Google Scholar] [CrossRef] [PubMed]
- Präbst, K.; Engelhardt, H.; Ringgeler, S.; Hübner, H. Basic colorimetric proliferation assays: MTT, WST, and resazurin. In Cell Viability Assays: Methods and Protocols; Gilbert, D., Friedrich, O., Eds.; Humana Press: New York, NY, USA, 2017; Volume 1601, pp. 1–17. [Google Scholar]
- Magalhães, S.S.; Alves, L.; Sebastiao, M.; Medronho, B.; Almeida, Z.L.; Faria, T.Q.; Brito, M.M.R.; Moreno, J.M.; Antunes, F.E. Effect of ethyleneoxide groups of anionic surfactants on lipase activity. Biotechnol. Prog. 2016, 32, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Nazari, M.; Kurdi, M.; Heerklotz, H. Classifying surfactants with respect to their effect on lipid membrane order. Biophys. J. 2012, 102, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.W.; Tettey, K.E.; Yarovoy, Y.; Lee, D. Effects of anionic surfactants on the water permeability of a model stratum corneum lipid membrane. Langmuir 2014, 30, 220–226. [Google Scholar] [CrossRef]
- Song, H.Y.; Kim, Y.H.; Seok, S.J.; Gil, H.W.; Yang, J.O.; Lee, E.Y.; Hong, S.Y. Cellular toxicity of surfactants used as herbicide additives. J. Korean Med. Sci. 2012, 27, 3–9. [Google Scholar] [CrossRef] [Green Version]
- El-Dossoki, F.I.; Gomaa, E.A.; Hamza, O.K. Solvation thermodynamic parameters for sodium dodecyl sulfate (SDS) and sodium lauryl ether sulfate (SLES) surfactants in aqueous and alcoholic-aqueous solvents. SN Appl. Sci. 2019, 1, 933. [Google Scholar] [CrossRef] [Green Version]
- Vater, C.; Adamovic, A.; Ruttensteiner, L.; Steiner, K.; Tajpara, P.; Klang, V.; Elbe-Burger, A.; Wirth, M.; Valenta, C. Cytotoxicity of lecithin-based nanoemulsions on human skin cells and ex vivo skin permeation: Comparison to conventional surfactant types. Int. J. Pharm. 2019, 566, 383–390. [Google Scholar] [CrossRef]
- Bjerregaard, H.F.; Stærmose, S.; Vang, J. Effect of linear alkylbenzene sulfonate (LAS) on ion transport and intracellular calcium in kidney distal epithelial cells (A6). Toxicol. In Vitro 2001, 15, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.; Shin, M.K.; Ahn, H.J.; Lee, T.R.; Son, Y.; Kim, K.S. Irritating effects of sodium lauryl sulfate on human primary keratinocytes at subtoxic levels of exposure. Microsc. Res. Tech. 2018, 81, 1339–1346. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Mori, R.; Hirayama, M.; Sagawa, Y.; Shimizu, K.; Okano, Y.; Masaki, H. Sodium lauryl sulfate stimulates the generation of reactive oxygen species through interactions with cell membranes. J. Oleo Sci. 2016, 65, 993–1001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apel-Paz, M.; Doncel, G.F.; Vanderlick, T.K. Impact of membrane cholesterol content on the resistance of vesicles to surfactant attack. Langmuir 2005, 21, 9843–9849. [Google Scholar] [CrossRef]
- Morris, S.A.; Bobbitt, J.R.; Ananthapadmanabhan, K.P.; Kasting, G.B. The effect of prolonged exposure on sodium dodecyl sulfate penetration into human skin. Toxicol. In Vitro 2021, 77, 105246. [Google Scholar] [CrossRef]
- Nigam, P. Thermodynamic quantification of sodium dodecyl sulfate penetration in cholesterol and phospholipid monolayers. Chem. Phys. Lipids 2020, 232, 104974. [Google Scholar] [CrossRef]
- Casares, D.; Escribá, P.V.; Rosselló, C.A. Membrane lipid composition: Effect on membrane and organelle structure, function and compartmentalization and therapeutic avenues. Int. J. Mol. Sci. 2019, 20, 2167. [Google Scholar] [CrossRef] [Green Version]
- Klose, C.; Surma, M.A.; Simons, K. Organellar lipidomics—Background and perspectives. Curr. Opin. Cell Biol. 2013, 25, 406–413. [Google Scholar] [CrossRef]
- Park, E.J.; Seong, E.; Kim, Y.; Lee, K. Ammonium lauryl sulfate-induced apoptotic cell death may be due to mitochondrial dysfunction triggered by caveolin-1. Toxicol. In Vitro 2019, 57, 132–142. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topliceanu, S.; Almeida, M.; Oliveira, M.; Cogălniceanu, D.; Lopes, I. The Number of Ethylene Oxide Groups of Sulphate-Based Surfactants Influences the Cytotoxicity of Mixed Micelles to an Amphibian Cell Line. Appl. Sci. 2023, 13, 8745. https://doi.org/10.3390/app13158745
Topliceanu S, Almeida M, Oliveira M, Cogălniceanu D, Lopes I. The Number of Ethylene Oxide Groups of Sulphate-Based Surfactants Influences the Cytotoxicity of Mixed Micelles to an Amphibian Cell Line. Applied Sciences. 2023; 13(15):8745. https://doi.org/10.3390/app13158745
Chicago/Turabian StyleTopliceanu, Sebastian, Monica Almeida, Miguel Oliveira, Dan Cogălniceanu, and Isabel Lopes. 2023. "The Number of Ethylene Oxide Groups of Sulphate-Based Surfactants Influences the Cytotoxicity of Mixed Micelles to an Amphibian Cell Line" Applied Sciences 13, no. 15: 8745. https://doi.org/10.3390/app13158745
APA StyleTopliceanu, S., Almeida, M., Oliveira, M., Cogălniceanu, D., & Lopes, I. (2023). The Number of Ethylene Oxide Groups of Sulphate-Based Surfactants Influences the Cytotoxicity of Mixed Micelles to an Amphibian Cell Line. Applied Sciences, 13(15), 8745. https://doi.org/10.3390/app13158745