Optimization of Image Capture Distance for Facial Thermograms in Dentistry
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. Study Population
2.3. Thermography Procedure and the Thermograms
2.3.1. Thermal Camera
2.3.2. Imaging Procedure
Participant Positioning
Camera Positioning
Image Views
Composing the Frame
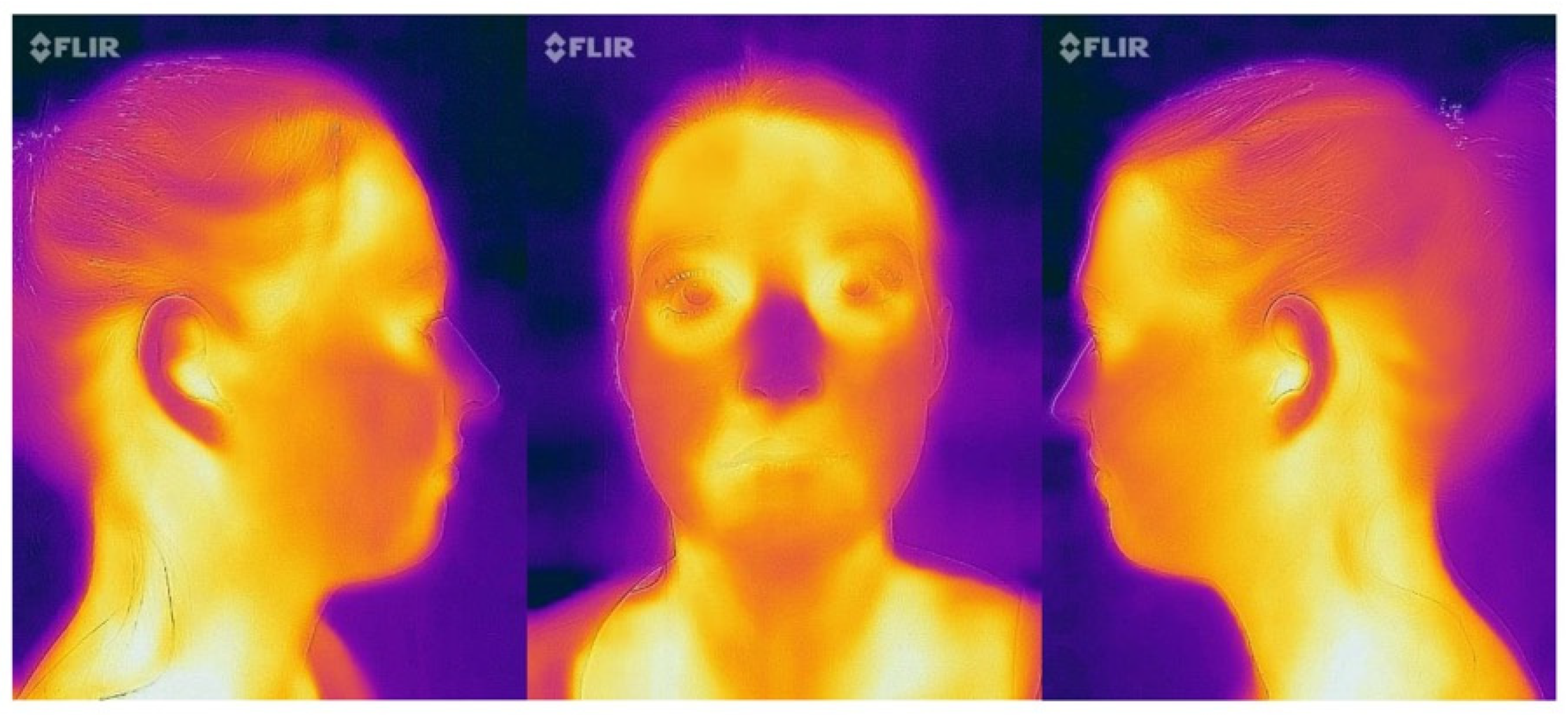
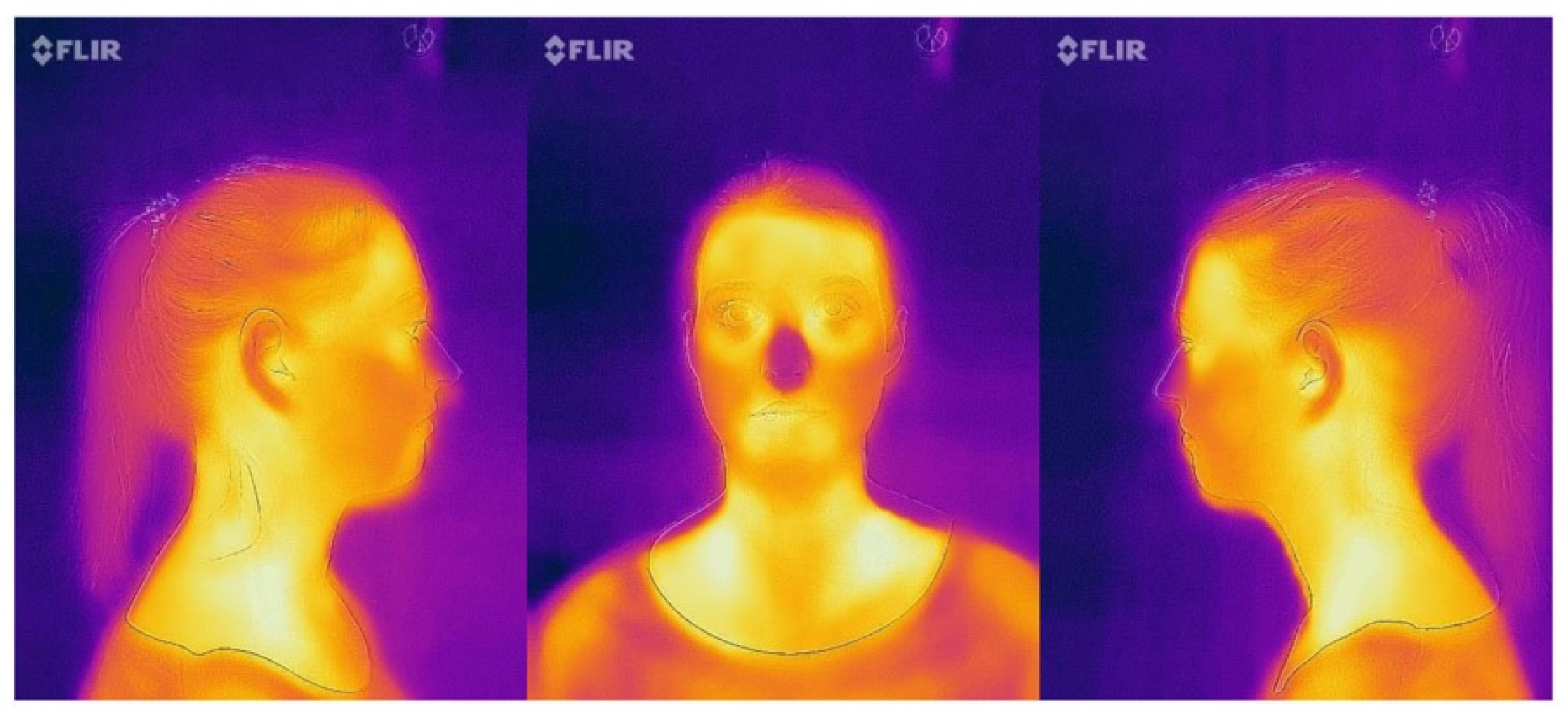
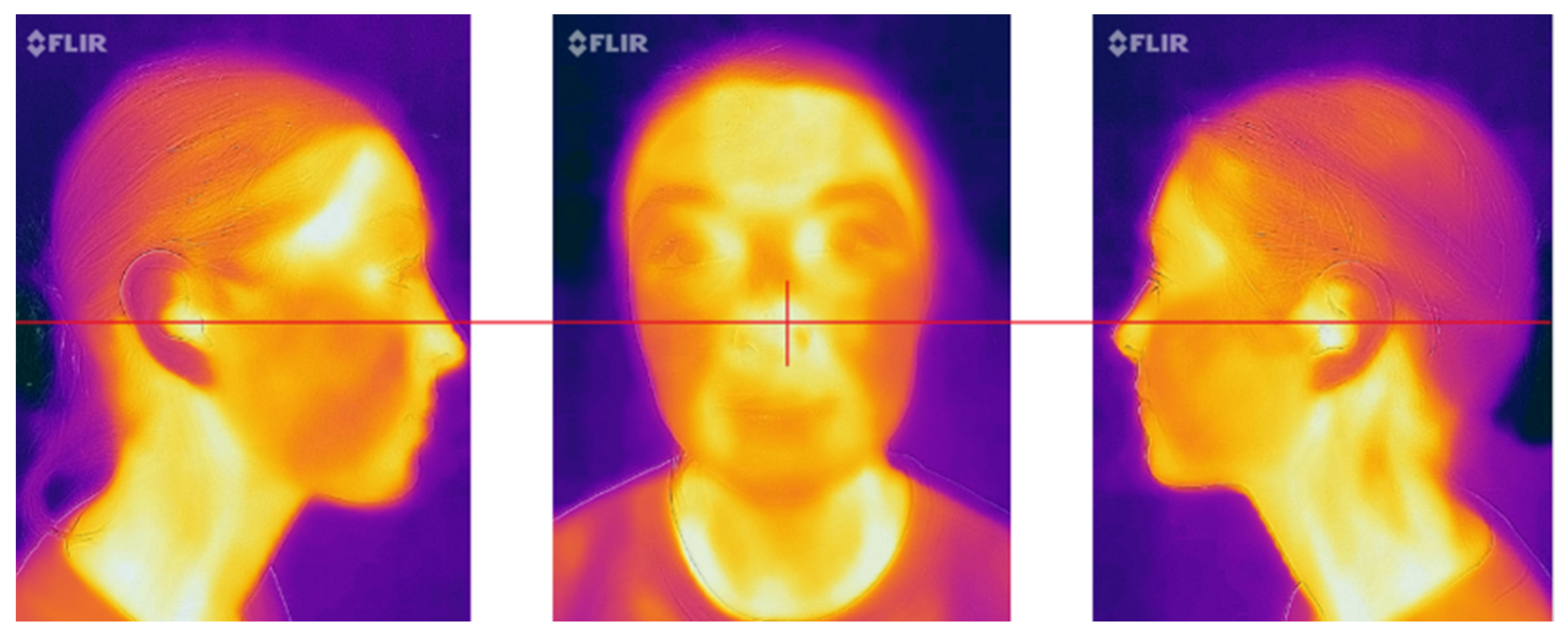
- Frontal view: the nose was placed in the center of the frame (Figure 4);
- Profile images (15 cm and 30 cm): the tip of the nose was positioned at the border of the frame with the ears maintained at the middle of the picture (Figure 4);
- Profile image (50 cm): the ears were positioned at the center of the image (Figure 5).
Object Parameters
2.3.3. Region of Interest
2.4. Endpoints/Outcome Measures
- Mean overall temperature observed in the thermogram;
- Mean temperature observed in the region of interest (ROI).
2.5. Sample Size
2.6. Statistical Methods
2.7. Study Procedure
3. Results
3.1. Overall Temperature of the Image
3.2. Temperature of the Region of Interest (ROI) in the Image
3.3. Correlations of the Mean Overall Temperature
3.4. Correlations of the Mean Temperature at ROI
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- UNDESA (United Nations Department of Economic and Social Affairs, Population Division). World Population Ageing 2020 Highlights: Living Arrangements of Older Persons (ST/ESA/SER.A/451); United Nations: New York, NY, USA, 2020. [Google Scholar]
- UNDESA. Ageing Populations: We Are Living Longer Lives, but Are We Healthier? UNDESA/POP/2021/TP/NO. 2; Department of Economics and Social Affairs, Population Division, United Nations: New York, NY, USA, 2021; p. 116. [Google Scholar]
- United-Nations; World Health Organization. Aging and Health. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/ageing-and-health (accessed on 7 January 2022).
- Inouye, S.K.; Studenski, S.; Tinetti, M.E.; Kuchel, G.A. Geriatric syndromes: Clinical, research, and policy implications of a core geriatric concept. J. Am. Geriatr. Soc. 2007, 55, 780–791. [Google Scholar] [CrossRef] [Green Version]
- Jaul, E.; Barron, J. Age-Related Diseases and Clinical and Public Health Implications for the 85 Years Old and Over Population. Front. Public Health 2017, 5, 335. [Google Scholar] [CrossRef] [Green Version]
- Wallace, E.; Salisbury, C.; Guthrie, B.; Lewis, C.; Fahey, T.; Smith, S.M. Managing patients with multimorbidity in primary care. BMJ 2015, 350, h176. [Google Scholar] [CrossRef] [Green Version]
- Fortin, M.; Bravo, G.; Hudon, C.; Vanasse, A.; Lapointe, L. Prevalence of multimorbidity among adults seen in family practice. Ann. Fam. Med. 2005, 3, 223–228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cockburn, N.; Pradhan, A.; Taing, M.W.; Kisely, S.; Ford, P.J. Oral health impacts of medications used to treat mental illness. J. Affect. Disord. 2017, 223, 184–193. [Google Scholar] [CrossRef]
- Schneider, C.; Zemp, E.; Zitzmann, N.U. Oral health improvements in Switzerland over 20 years. Eur. J. Oral Sci. 2017, 125, 55–62. [Google Scholar] [CrossRef]
- Slade, G.D.; Akinkugbe, A.A.; Sanders, A.E. Projections of U.S. edentulism prevalence following 5 decades of decline. J. Dent. Res. 2014, 93, 959–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peltola, P.; Vehkalahti, M.M.; Wuolijoki-Saaristo, K. Oral health and treatment needs of the long-term hospitalised elderly. Gerodontology 2004, 21, 93–99. [Google Scholar] [CrossRef]
- Holmen, A.; Stromberg, E.; Hagman-Gustafsson, M.L.; Wardh, I.; Gabre, P. Oral status in home-dwelling elderly dependent on moderate or substantial supportive care for daily living: Prevalence of edentulous subjects, caries and periodontal disease. Gerodontology 2012, 29, e503–e511. [Google Scholar] [CrossRef] [Green Version]
- Imsand, M.; Janssens, J.P.; Auckenthaler, R.; Mojon, P.; Budtz-Jorgensen, E. Bronchopneumonia and oral health in hospitalized older patients. A pilot study. Gerodontology 2002, 19, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, E.; Hagman-Gustafsson, M.L.; Holmen, A.; Wardh, I.; Gabre, P. Oral status, oral hygiene habits and caries risk factors in home-dwelling elderly dependent on moderate or substantial supportive care for daily living. Community Dent. Oral Epidemiol. 2012, 40, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Chalmers, J.M.; Carter, K.D.; Spencer, A.J. Oral diseases and conditions in community-living older adults with and without dementia. Spec. Care Dent. 2003, 23, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Schwendicke, F.; Krois, J.; Kocher, T.; Hoffmann, T.; Micheelis, W.; Jordan, R.A. More teeth in more elderly: Periodontal treatment needs in Germany 1997–2030. J. Clin. Periodontol. 2018, 45, 1400–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scannapieco, F.A.; Cantos, A. Oral inflammation and infection, and chronic medical diseases: Implications for the elderly. Periodontology 2000 2016, 72, 153–175. [Google Scholar] [CrossRef]
- Loesche, W.J.; Lopatin, D.E. Interactions between periodontal disease, medical diseases and immunity in the older individual. Periodontology 2000 1998, 16, 80–105. [Google Scholar] [CrossRef]
- Hayes, M.; Brady, P.; Burke, F.M.; Allen, P.F. Failure rates of class V restorations in the management of root caries in adults—A systematic review. Gerodontology 2016, 33, 299–307. [Google Scholar] [CrossRef]
- Zhang, J.; Leung, K.C.M.; Sardana, D.; Wong, M.C.M.; Lo, E.C.M. Risk predictors of dental root caries: A systematic review. J. Dent. 2019, 89, 103166. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Bottenberg, P.; Conrads, G.; Eickholz, P.; Heasman, P.; Huysmans, M.C.; Lopez, R.; Madianos, P.; Muller, F.; Needleman, I.; et al. Dental caries and periodontal diseases in the ageing population: Call to action to protect and enhance oral health and well-being as an essential component of healthy ageing—Consensus report of group 4 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal diseases. J Clin. Periodontol. 2017, 44 (Suppl. S18), S135–S144. [Google Scholar] [CrossRef] [Green Version]
- Tonetti, M.S.; Eickholz, P.; Loos, B.G.; Papapanou, P.; van der Velden, U.; Armitage, G.; Bouchard, P.; Deinzer, R.; Dietrich, T.; Hughes, F.; et al. Principles in prevention of periodontal diseases: Consensus report of group 1 of the 11th European Workshop on Periodontology on effective prevention of periodontal and peri-implant diseases. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S5–S11. [Google Scholar] [CrossRef]
- Ervin, R.B.; Dye, B.A. Number of natural and prosthetic teeth impact nutrient intakes of older adults in the United States. Gerodontology 2012, 29, e693–e702. [Google Scholar] [CrossRef]
- Gil-Montoya, J.A.; de Mello, A.L.; Barrios, R.; Gonzalez-Moles, M.A.; Bravo, M. Oral health in the elderly patient and its impact on general well-being: A nonsystematic review. Clin. Interv. Aging 2015, 10, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Stenman, U.; Ahlqwist, M.; Bjorkelund, C.; Hakeberg, M. Oral health-related quality of life—Associations with oral health and conditions in Swedish 70-year-old individuals. Gerodontology 2012, 29, e440–e446. [Google Scholar] [CrossRef]
- Wright, T.; McGechan, A. Breast cancer: New technologies for risk assessment and diagnosis. Mol. Diagn. 2003, 7, 49–55. [Google Scholar] [CrossRef]
- Pawl, R.P. Thermography in the diagnosis of low back pain. Neurosurg. Clin. N. Am. 1991, 2, 839–850. [Google Scholar] [CrossRef]
- Chiu, W.T.; Lin, P.W.; Chiou, H.Y.; Lee, W.S.; Lee, C.N.; Yang, Y.Y.; Lee, H.M.; Hsieh, M.S.; Hu, C.J.; Ho, Y.S.; et al. Infrared thermography to mass-screen suspected SARS patients with fever. Asia Pac. J. Public Health 2005, 17, 26–28. [Google Scholar] [CrossRef]
- Spiegel, S.; Spiegel, F.; Luepke, M.; Wendt, M.; von Altrock, A. Ultrasonography and Infrared Thermography as a Comparative Diagnostic Tool to Clinical Examination to Determine Udder Health in Sows. Animals 2022, 12, 2713. [Google Scholar] [CrossRef]
- Arora, N.; Martins, D.; Ruggerio, D.; Tousimis, E.; Swistel, A.J.; Osborne, M.P.; Simmons, R.M. Effectiveness of a noninvasive digital infrared thermal imaging system in the detection of breast cancer. Am. J. Surg. 2008, 196, 523–526. [Google Scholar] [CrossRef]
- Bagavathiappan, S.; Saravanan, T.; Philip, J.; Jayakumar, T.; Raj, B.; Karunanithi, R.; Panicker, T.M.; Korath, M.P.; Jagadeesan, K. Infrared thermal imaging for detection of peripheral vascular disorders. J. Med. Phys. 2009, 34, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Cherkas, L.F.; Carter, L.; Spector, T.D.; Howell, K.J.; Black, C.M.; MacGregor, A.J. Use of thermographic criteria to identify Raynaud’s phenomenon in a population setting. J. Rheumatol. 2003, 30, 720–722. [Google Scholar] [PubMed]
- Hewlett, A.L.; Kalil, A.C.; Strum, R.A.; Zeger, W.G.; Smith, P.W. Evaluation of an infrared thermal detection system for fever recognition during the H1N1 influenza pandemic. Infect. Control Hosp. Epidemiol. 2011, 32, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Kosus, N.; Kosus, A.; Duran, M.; Simavli, S.; Turhan, N. Comparison of standard mammography with digital mammography and digital infrared thermal imaging for breast cancer screening. J. Turk. Ger. Gynecol. Assoc. 2010, 11, 152–157. [Google Scholar] [CrossRef]
- Lee, C.H.; Dershaw, D.D.; Kopans, D.; Evans, P.; Monsees, B.; Monticciolo, D.; Brenner, R.J.; Bassett, L.; Berg, W.; Feig, S.; et al. Breast cancer screening with imaging: Recommendations from the Society of Breast Imaging and the ACR on the use of mammography, breast MRI, breast ultrasound, and other technologies for the detection of clinically occult breast cancer. J. Am. Coll. Radiol. 2010, 7, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.C.; Chang, R.E.; Chang, W.C. Limitations of forehead infrared body temperature detection for fever screening for severe acute respiratory syndrome. Infect. Control Hosp. Epidemiol. 2004, 25, 1109–1111. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y. Is thermal scanner losing its bite in mass screening of fever due to SARS? Med. Phys. 2005, 32, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.; Acharya, R.U. Remote-sensing infrared thermography. IEEE Eng. Med. Biol. Mag. 2009, 28, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.; Kaw, G.J.; Chang, W.M. Analysis of IR thermal imager for mass blind fever screening. Microvasc. Res. 2004, 68, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.Y.; Muljo, W.; Wong, B.S. Study of facial skin and aural temperature. IEEE Eng. Med. Biol. Mag. 2006, 25, 68–74. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Cohen, N.J.; Lipman, H.; Brown, C.M.; Molinari, N.A.; Jackson, W.L.; Kirking, H.; Szymanowski, P.; Wilson, T.W.; Salhi, B.A.; et al. Comparison of 3 infrared thermal detection systems and self-report for mass fever screening. Emerg. Infect. Dis. 2010, 16, 1710–1717. [Google Scholar] [CrossRef]
- Renkielska, A.; Kaczmarek, M.; Nowakowski, A.; Grudzinski, J.; Czapiewski, P.; Krajewski, A.; Grobelny, I. Active dynamic infrared thermal imaging in burn depth evaluation. J. Burn. Care Res. 2014, 35, e294–e303. [Google Scholar] [CrossRef]
- Ring, F. Thermal imaging today and its relevance to diabetes. J. Diabetes Sci. Technol. 2010, 4, 857–862. [Google Scholar] [CrossRef] [Green Version]
- Wishart, G.C.; Campisi, M.; Boswell, M.; Chapman, D.; Shackleton, V.; Iddles, S.; Hallett, A.; Britton, P.D. The accuracy of digital infrared imaging for breast cancer detection in women undergoing breast biopsy. Eur. J. Surg. Oncol. 2010, 36, 535–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagioni, P.A.; Longmore, R.B.; McGimpsey, J.G.; Lamey, P.J. Infrared thermography. Its role in dental research with particular reference to craniomandibular disorders. Dento Maxillo Facial Radiol. 1996, 25, 119–124. [Google Scholar] [CrossRef]
- Clemente, M.; Coimbra, D.; Silva, A.; Aguiar Branco, C.; Pinho, J.C. Application of Infrared Thermal Imaging in a Violinist with Temporomandibular Disorder. Med. Probl. Perform. Art 2015, 30, 251–254. [Google Scholar] [CrossRef] [Green Version]
- Dibai Filho, A.V.; Packer, A.C.; Costa, A.C.; Rodrigues-Bigaton, D. Accuracy of infrared thermography of the masticatory muscles for the diagnosis of myogenous temporomandibular disorder. J. Manip. Physiol. Ther. 2013, 36, 245–252. [Google Scholar] [CrossRef]
- Rodrigues-Bigaton, D.; Dibai-Filho, A.V.; Packer, A.C.; Costa, A.C.; de Castro, E.M. Accuracy of two forms of infrared image analysis of the masticatory muscles in the diagnosis of myogenous temporomandibular disorder. J. Bodyw. Mov. Ther. 2014, 18, 49–55. [Google Scholar] [CrossRef]
- Gratt, B.M.; Sickles, E.A. Electronic facial thermography: An analysis of asymptomatic adult subjects. J. Orofac. Pain 1995, 9, 255–265. [Google Scholar]
- Gratt, B.M.; Shetty, V.; Saiar, M.; Sickles, E.A. Electronic thermography for the assessment of inferior alveolar nerve deficit. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 80, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Gratt, B.M.; Anbar, M. Thermology and facial telethermography: Part II. Current and future clinical applications in dentistry. Dento Maxillo Facial Radiol. 1998, 27, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Gratt, B.M.; Sickles, E.A.; Shetty, V. Thermography for the clinical assessment of inferior alveolar nerve deficit: A pilot study. J. Orofac. Pain 1994, 8, 369–374. [Google Scholar]
- Shetty, V.; Gratt, B.M.; Flack, V. Thermographic assessment of reversible inferior alveolar nerve deficit. J. Orofac. Pain 1994, 8, 375–383. [Google Scholar] [PubMed]
- Friedlander, A.H.; Gratt, B.M. Panoramic dental radiography as an aid in detecting patients at risk for stroke. J. Oral Maxillofac. Surg. 1994, 52, 1257–1262. [Google Scholar] [CrossRef]
- Nicolas-Rodriguez, E.; Garcia-Martinez, A.; Molino-Pagan, D.; Marin-Martinez, L.; Pons-Fuster, E.; Lopez-Jornet, P. Thermography as a Non-Ionizing Quantitative Tool for Diagnosing Burning Mouth Syndrome: Case-Control Study. Int. J. Environ. Res. Public Health 2022, 19, 8903. [Google Scholar] [CrossRef] [PubMed]
- Wolowski, A.; Schwarzbach, N.; Horning, H. Thermal quantitative sensory testing in burning mouth syndrome. Clin. Oral Investig. 2021, 25, 3059–3066. [Google Scholar] [CrossRef]
- Mo, X.; Zhang, J.; Fan, Y.; Svensson, P.; Wang, K. Thermal and mechanical quantitative sensory testing in Chinese patients with burning mouth syndrome—A probable neuropathic pain condition? J. Headache Pain 2015, 16, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aboushady, M.A.; Talaat, W.; Hamdoon, Z.; Elshazly, T.E.; Ragy, N.; Bourauel, C.; Talaat, S. Thermography as a non-ionizing quantitative tool for diagnosing periapical inflammatory lesions. BMC Oral Health 2021, 21, 260. [Google Scholar] [CrossRef]
- Kasprzyk-Kucewicz, T.; Cholewka, A.; Bałamut, K.; Kownacki, P.; Kaszuba, N.; Kaszuba, M.; Stanek, A.; Sieroń, K.; Stransky, J.; Pasz, A.; et al. The applications of infrared thermography in surgical removal of retained teeth effects assessment. J. Therm. Anal. Calorim. 2021, 144, 139–144. [Google Scholar] [CrossRef] [Green Version]
- Komoriyama, M.; Nomoto, R.; Tanaka, R.; Hosoya, N.; Gomi, K.; Iino, F.; Yashima, A.; Takayama, Y.; Tsuruta, M.; Tokiwa, H.; et al. Application of thermography in dentistry—Visualization of temperature distribution on oral tissues. Dent. Mater. J. 2003, 22, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, G.M.; Feinn, R. Using Effect Size-or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [Green Version]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Tkáčova, M.; Hudak, R.; Foffová, P.; Živčák, J. An importance of camera—Subject distance and angle in musculoskeletal application of medical thermography. Acta Electrotech. Et Inform. 2010, 10, 57–60. [Google Scholar]
- Liu, G.; Xia, Y.; Yang, C.; Zhang, L. The review of the major entropy methods and applications in biomedical signal research. In Bioinformatics Research and Applications; Zhang, F., Cai, Z., Skums, P., Zhang, S., Eds.; Springer International Publishing: Cham, Switzerland; University of Hong Kong: Hong Kong, China, 2018; pp. 87–100. [Google Scholar]
- Ghassemi, P.; Pfefer, T.J.; Casamento, J.P.; Simpson, R.; Wang, Q. Best practices for standardized performance testing of infrared thermographs intended for fever screening. PLoS ONE 2018, 13, e0203302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, A.S.; Cañada-Soriano, M.; Jimenez-Perez, I.; Gil-Calvo, M.; Carpes, F.P.; Perez-Soriano, P.; Priego-Quesada, J.I. Distance and camera features measurements affect the detection of temperature asymmetries using infrared thermography. Quant. Infrared Thermogr. J. 2022, 2022, 2143227. [Google Scholar] [CrossRef]
- Clark, R.P.; de Calcina-Goff, M.L. International standardisation in medical thermography-draft proposals. In Proceedings of the 18th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Amsterdam, The Netherlands, 31 October–3 November 1996; Volume 5, pp. 2089–2090. [Google Scholar]




| n (%) | Mean | SD | Minimum | Maximum | |
|---|---|---|---|---|---|
| Participants | 30 (100.0) | ||||
| Age [in years] | 25.8 | 6.0 | 21.0 | 45.0 | |
| Sex (n) | |||||
| Men | 9 (30.0) | ||||
| Women | 21 (70.0) | ||||
| Body Temperature | |||||
| Right ear | 37.00 | 0.27 | 36.5 | 37.5 | |
| Left ear | 37.05 | 0.32 | 36.5 | 37.6 | |
| Average of right and left ear | 37.03 | 0.28 | 36.6 | 37.5 | |
| No. of teeth present | 28.53 | 1.20 | 27.0 | 32.0 | |
| DMF (T) | 2.97 | 2.86 | 0.0 | 11.0 | |
| D | 0.03 | 0.18 | 0.0 | 1.0 | |
| F | 2.93 | 2.85 | 0.0 | 11.0 | |
| M | 0.00 | 0.00 | 0.0 | 0.0 | |
| Gingival index | 0.004 | 0.01 | 0.0 | 0.1 | |
| Plaque index | 0.16 | 0.52 | 0.0 | 2.9 | |
| Presence of pain | |||||
| No | 0 (0.0) | ||||
| Yes | 30 (100.0) | ||||
| Presence of any intra-oral findings | |||||
| No | 29 (96.7) | ||||
| Yes | 1 (3.3) | ||||
| Room temperature | 22.07 | 0.36 | 21.3 | 22.6 | |
| Room relative humidity (%) | 36 | 5 | 30 | 50 |
| Influencing factors | No | Yes |
| n (%) | n (%) | |
| Personal effects | ||
| Jewelry | 26 (86.7) | 4 (13.3) |
| Facial cosmetics present | 7 (23.3) | 23 (76.7) |
| Spectacles * | 12 (40.0) | 18 (60.0) |
| Tight clothes * | 27 (90.0) | 3 (10.0) |
| Pressure stockings * | 28 (93.3) | 2 (6.7) |
| Scarf over head, neck and mouth * | 28 (93.3) | 2 (6.7) |
| Use of scarf/headphones/cap/hat/shawl *** | 22 (73.3) | 8 (26.7) |
| Object around the neck *** | 19 (63.3) | 11 (36.7) |
| Mask *** | 0 (0.0) | 30 (100.0) |
| Medications | ||
| Sympathetic blockers ** | 29 (96.7) | 1 (3.3) |
| Neurological Blockers ** | 30 (100) | 0 (0.0) |
| Steroids ** | 26 (86.7) | 4 (13.3) |
| Vasoactive drugs ** | 29 (96.7) | 1 (3.3) |
| Treatments | ||
| Dental treatments ** | 23 (76.7) | 7 (23.3) |
| Physiotherapy ** | 29 (96.7) | 1 (3.3) |
| Oral care | ||
| Tooth brushing * | 9 (30.0) | 21 (70.0) |
| Inter-dental cleaning * | 26 (86.7) | 4 (13.3) |
| Mouthwash use * | 24 (80.0) | 6 (20.0) |
| Food & beverages | ||
| Bonbons * | 28 (93.3) | 2 (6.7) |
| Eating * | 14 (46.7) | 16 (53.3) |
| Drinking * | 7 (23.3) | 23 (76.7) |
| Coffee * | 27 (90.0) | 3 (10.0) |
| Smoking * | 28 (93.3) | 2 (6.7) |
| Energy drinks ** | 28 (93.3) | 2 (6.7) |
| Eating *** | 23 (76.7) | 7 (23.3) |
| Drinking *** | 11 (36.7) | 19 (63.3) |
| Miscellaneous | ||
| Hot-water * | 20 (66.7) | 10 (33.3) |
| Sauna ** | 29 (96.7) | 1 (3.3) |
| Shower **** | 10 (33.3) | 20 (66.7) |
| Shaving **** | 27 (90.0) | 3 (10.0) |
| Hairdryer use * | 27 (90.0) | 3 (10.0) |
| Views | Temperature (in °C) | Measured Temperatures at Different Shooting Distances for the Different Profiles (in °C) | Temperature Differences between Shooting Distances (in °C) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 15 cm | 30 cm | 50 cm | 15–30 cm | 30–50 cm | 15–50 cm | ||||||
| Front | N | Mean | SD (SE) | Mean | SD (SE) | Mean | SD (SE) | Mean (SE) | Mean (SE) | Mean (SE) | |
| Overall | 150 | 31.80 | 1.43 (0.12) | 29.62 | 0.73 (0.06) | 27.25 | 0.74 (0.06) | 2.19 (0.12) | 2.36 (0.12) | 4.55 (0.12) | |
| ROI | 150 | 32.93 | 1.05 (0.09) | 34.07 | 0.86 (0.07) | 33.62 | 1.06 (0.09) | −1.14 (0.11) | 0.45 (0.11) | −0.69 (0.11) | |
| Left | |||||||||||
| Overall | 150 | 31.90 | 0.85 (0.07) | 29.72 | 0.93 (0.08) | 26.86 | 0.71 (0.06) | 2.18 (0.10) | 2.86 (0.10) | 5.04 (0.10) | |
| ROI | 150 | 32.83 | 2.55 (0.21) | 32.83 | 1.15 (0.09) | 32.57 | 0.87 (0.07) | 0.00 (0.20) | 0.26 (0.20) | 0.26 (0.20) | |
| Right | |||||||||||
| Overall | 150 | 31.67 | 0.79 (0.06) | 29.86 | 1.08 (0.09) | 26.67 | 0.70 (0.06) | 1.81 (0.10) | 3.19 (0.10) | 5.00 (0.10) | |
| ROI | 150 | 33.06 | 0.80 (0.07) | 32.85 | 0.96 (0.08) | 32.35 | 0.93 (0.04) | 0.21 (0.10) | 0.50 (0.10) | 0.71 (0.10) | |
| Profile Views | Mean Overall Temperature | Mean ROI | |
|---|---|---|---|
| Front | Total n | 450.00 | 450.00 |
| Test Statistic | 368.018 a | 80.176 a | |
| df | 2.00 | 2.00 | |
| p-value | <0.001 | <0.001 | |
| Left | Total n | 450.00 | 450.00 |
| Test Statistic | 384.353 a | 21.399 a | |
| df | 2.00 | 2.00 | |
| p-value | <0.001 | <0.001 | |
| Right | Total n | 450.00 | 450.00 |
| Test Statistic | 364.811 a | 49.451 a | |
| df | 2.00 | 2.00 | |
| p-value | <0.001 | <0.001 |
| Front | |||||
| Mean overall temp. | Test Statistic | Std. Error | Std. Test Statistic | Sig. | p-value |
| 50 cm–30 cm | 152.38 | 15.02 | 10.15 | <0.001 | <0.001 |
| 50 cm–15 cm | 287.91 | 15.02 | 19.17 | <0.001 | <0.001 |
| 30 cm–15 cm | 135.53 | 15.02 | 9.03 | <0.001 | <0.001 |
| Mean ROI | |||||
| 50 cm–30 cm | −78.55 | 15.02 | −5.23 | <0.001 | <0.001 |
| 50 cm–15 cm | −133.78 | 15.02 | −8.91 | <0.001 | <0.001 |
| 30 cm–15 cm | 55.24 | 15.02 | 3.68 | <0.001 | <0.001 |
| Left | |||||
| Mean overall temp | |||||
| 50 cm–30 cm | 155.56 | 15.02 | 10.36 | <0.001 | <0.001 |
| 50 cm–15 cm | 294.24 | 15.02 | 19.59 | <0.001 | <0.001 |
| 30 cm–15 cm | 138.67 | 15.02 | 9.24 | <0.001 | <0.001 |
| Mean ROI | |||||
| 50 cm–30 cm | 49.97 | 15.02 | 3.33 | <0.001 | <0.001 |
| 50 cm–15 cm | 66.77 | 15.02 | 4.45 | <0.001 | <0.001 |
| 30 cm–15 cm | 16.81 | 15.02 | 1.12 | 0.26 | 0.789 |
| Right | |||||
| Mean overall temp | |||||
| 50 cm–30 cm | 161.32 | 15.02 | 10.74 | <0.001 | <0.001 |
| 50 cm–15 cm | 286.04 | 15.02 | 19.05 | <0.001 | <0.001 |
| 30 cm–15 cm | 124.71 | 15.02 | 8.31 | <0.001 | <0.001 |
| Mean ROI | |||||
| 50 cm–30 cm | 72.50 | 15.02 | 4.83 | <0.001 | <0.001 |
| 50 cm–15 cm | 102.74 | 15.02 | 6.84 | <0.001 | <0.001 |
| 30 cm–15 cm | 30.24 | 15.02 | 2.01 | 0.04 | 0.132 |
| Parameters | Mean Overall Temp. | Mean ROI | |||
|---|---|---|---|---|---|
| Demographic factors | n | CC | p-value | CC | p-value |
| Sex | 1350.00 | −0.033 | 0.223 | −0.040 | 0.146 |
| Age | 1350.00 | 0.031 | 0.249 | 0.009 | 0.744 |
| Room temperature | 1350.00 | −0.014 | 0.603 | −0.150 ** | <0.001 |
| Relative humidity | 1350.00 | −0.012 | 0.670 | −0.058 * | 0.033 |
| Personal effects | |||||
| Jewelry | 1350.00 | −0.042 | 0.127 | −0.001 | 0.979 |
| Facial cosmetics present | 1350.00 | 0.042 | 0.126 | 0.206 ** | <0.001 |
| Spectacles * | 1350.00 | 0.070 * | 0.011 | 0.136 ** | <0.001 |
| Tight clothes * | 1350.00 | −0.001 | 0.971 | −0.013 | 0.639 |
| Pressure stockings * | 1350.00 | 0.006 | 0.839 | 0.023 | 0.406 |
| Scarf over head, neck, and mouth * | 1350.00 | −0.043 | 0.118 | 0.022 | 0.424 |
| Use of scarf/headphones/cap/hat/shawl *** | 1350.00 | −0.084 ** | 0.002 | −0.163 ** | <0.001 |
| Object around the neck *** | 1350.00 | −0.099 ** | <0.001 | −0.243 ** | <0.001 |
| Mask *** | 1350.00 | . | . | . | . |
| Medications | |||||
| Sympathetic blockers ** | 1350.00 | 0.010 | 0.711 | −0.058 * | 0.034 |
| Neurological Blockers ** | 1350.00 | . | . | . | . |
| Steroids ** | 1350.00 | −0.031 | 0.259 | −0.123 ** | <0.001 |
| Vasoactive drugs ** | 1350.00 | 0.010 | 0.711 | −0.058 * | 0.034 |
| Treatments | |||||
| Dental treatments ** | 1350.00 | 0.079 ** | 0.004 | 0.160 ** | <0.001 |
| Physiotherapy ** | 1350.00 | −0.071 ** | 0.009 | −0.179 ** | <0.001 |
| Oral care | |||||
| Tooth brushing * | 1350.00 | −0.041 | 0.134 | −0.183 ** | <0.001 |
| Inter-dental cleaning * | 1350.00 | 0.095 ** | <0.001 | 0.214 ** | <0.001 |
| Mouthwash use * | 1350.00 | 0.077 ** | 0.005 | 0.228 ** | <0.001 |
| Food & beverages | |||||
| Bonbons * | 1350.00 | −0.018 | 0.519 | 0.090 ** | <0.001 |
| Eating * | 1350.00 | 0.011 | 0.685 | 0.056 * | 0.040 |
| Drinking * | 1350.00 | 0.002 | 0.956 | 0.056 * | 0.041 |
| Coffee * | 1350.00 | 0.039 | 0.147 | −0.119 ** | <0.001 |
| Smoking * | 1350.00 | −0.022 | 0.411 | −0.219 ** | <0.001 |
| Energy drinks ** | 1350.00 | 0.043 | 0.113 | 0.132 ** | <0.001 |
| Eating *** | 1350.00 | 0.021 | 0.436 | 0.025 | 0.352 |
| Drinking *** | 1350.00 | 0.008 | 0.783 | −0.005 | 0.845 |
| Miscellaneous | |||||
| Hot-water use * | 1350.00 | 0.043 | 0.115 | −0.098 ** | <0.001 |
| Sauna ** | 1350.00 | −0.036 | 0.181 | −0.009 | 0.749 |
| Shower **** | 1350.00 | 0.040 | 0.138 | 0.034 | 0.209 |
| Shaving **** | 1350.00 | −0.034 | 0.208 | −0.076 ** | 0.005 |
| Hairdryer use * | 1350.00 | 0.082 ** | 0.003 | 0.132 ** | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schöffauer, M.; Angst, L.; Stillhart, A.; Srinivasan, M. Optimization of Image Capture Distance for Facial Thermograms in Dentistry. Appl. Sci. 2023, 13, 8851. https://doi.org/10.3390/app13158851
Schöffauer M, Angst L, Stillhart A, Srinivasan M. Optimization of Image Capture Distance for Facial Thermograms in Dentistry. Applied Sciences. 2023; 13(15):8851. https://doi.org/10.3390/app13158851
Chicago/Turabian StyleSchöffauer, Mona, Lea Angst, Angela Stillhart, and Murali Srinivasan. 2023. "Optimization of Image Capture Distance for Facial Thermograms in Dentistry" Applied Sciences 13, no. 15: 8851. https://doi.org/10.3390/app13158851






