Effect of Textural Properties on the Degradation of Bisphenol from Industrial Wastewater Effluent in a Photocatalytic Reactor: A Modeling Approach
Abstract
:1. Introduction
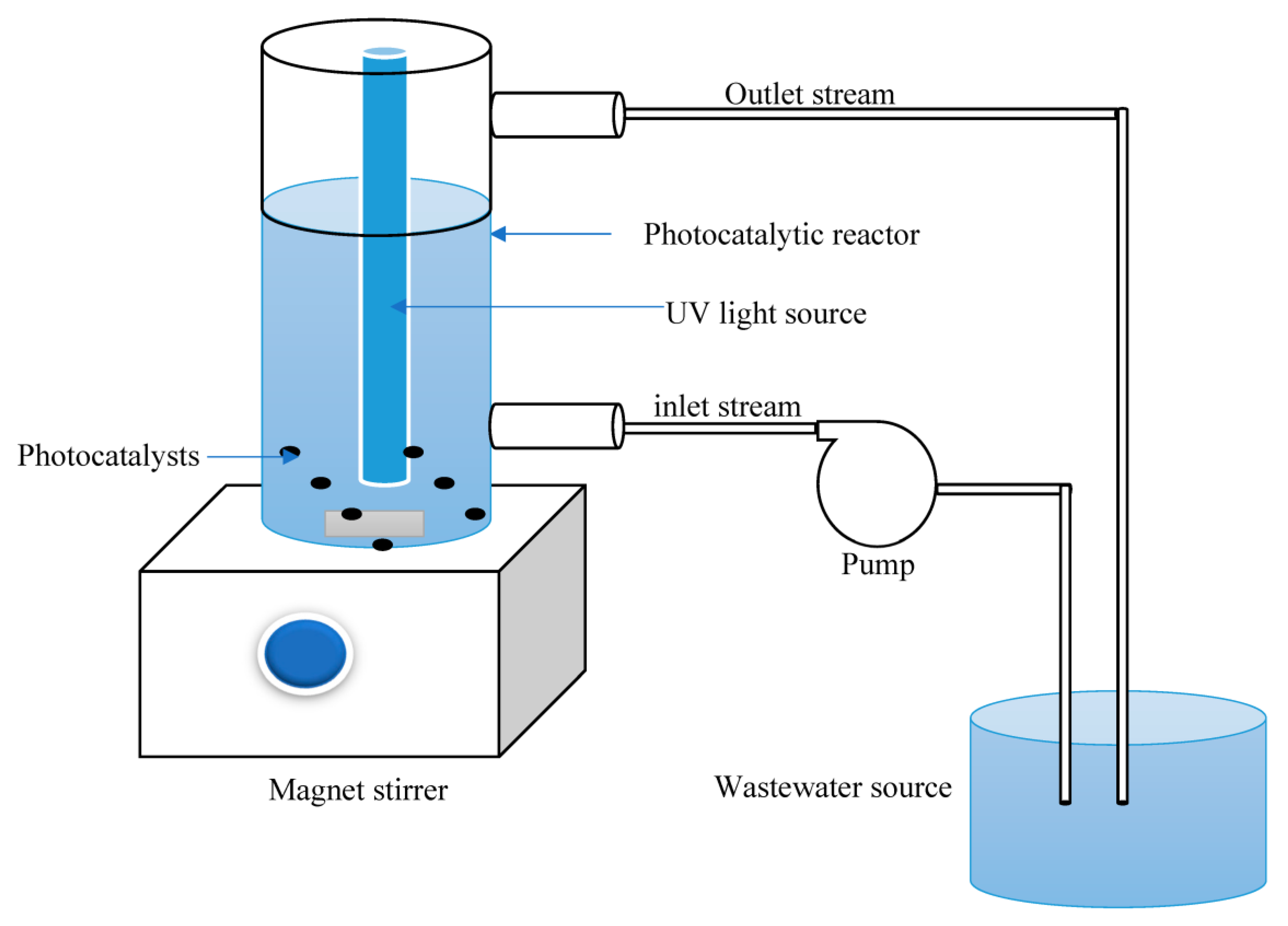
2. Data Acquisition and Model Configuration
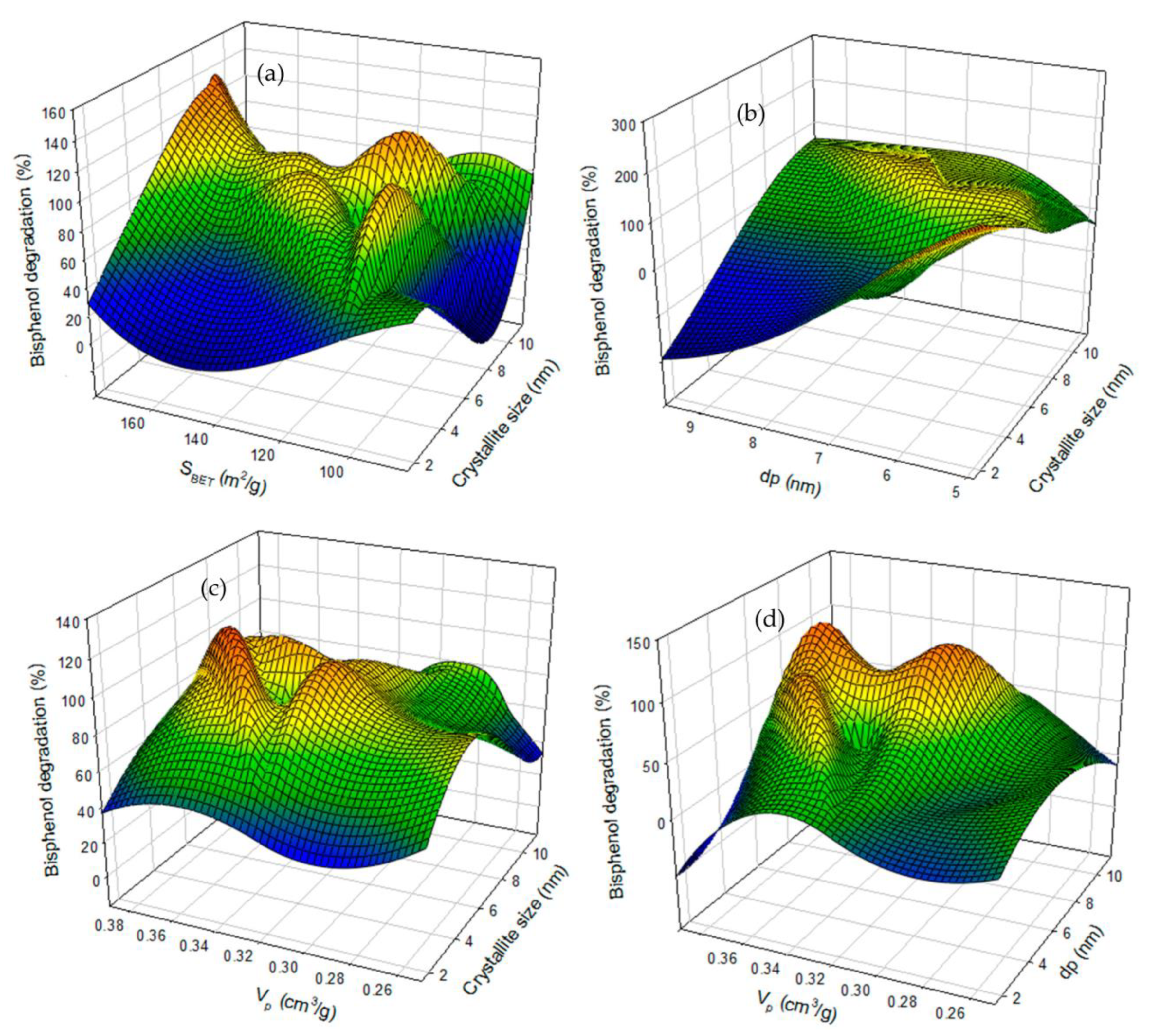
Data Visualization and Trend Analysis
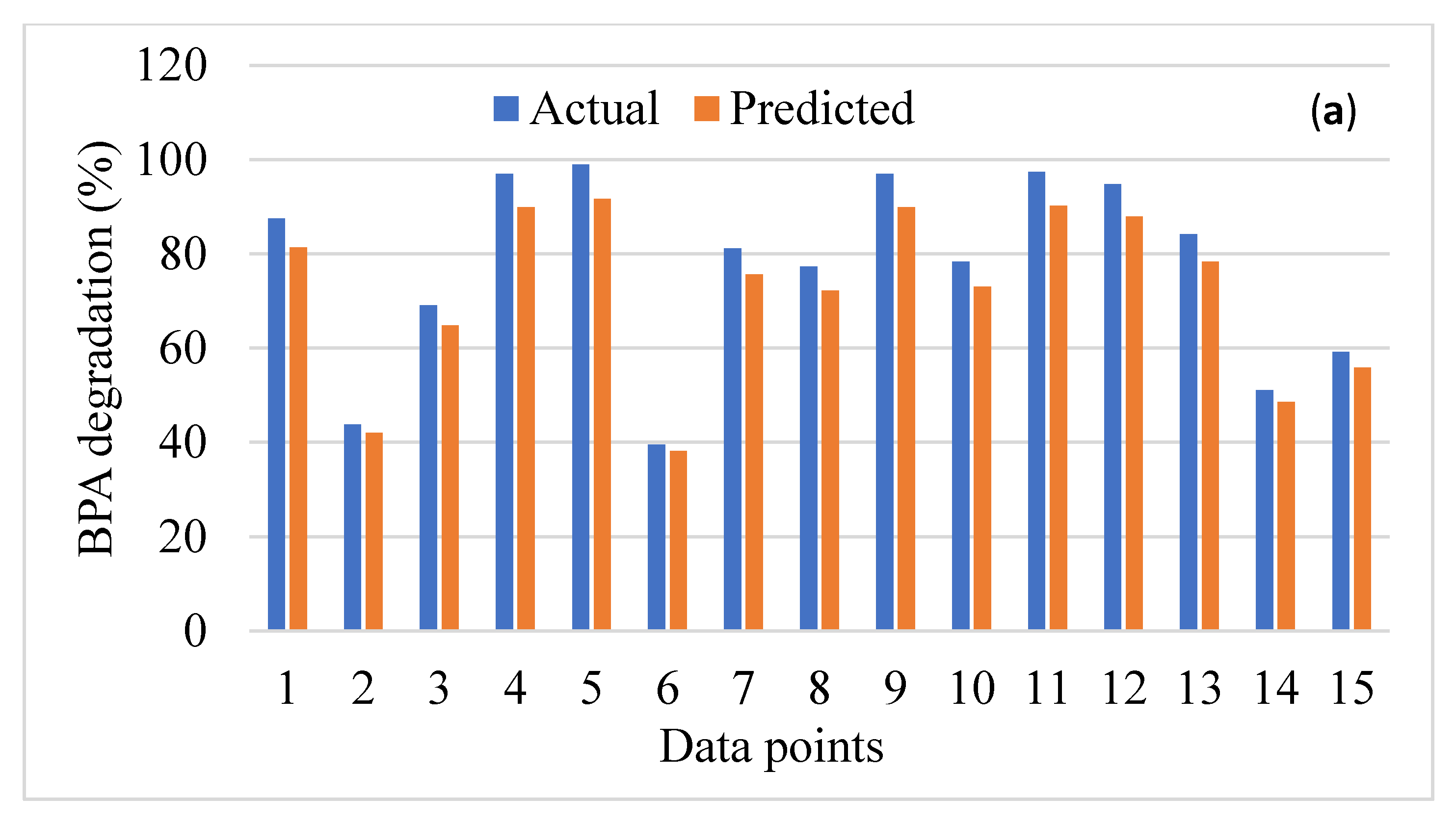
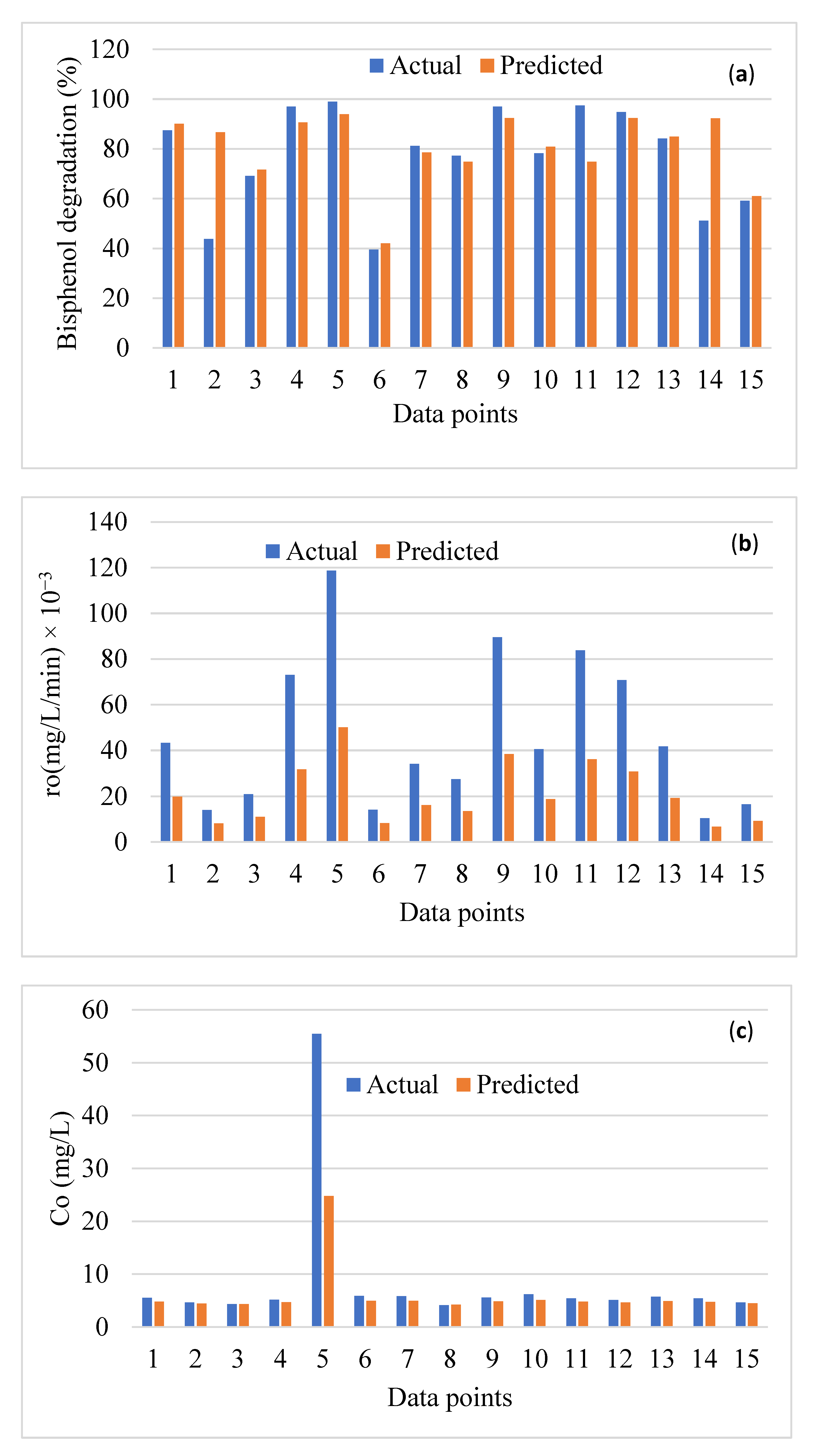
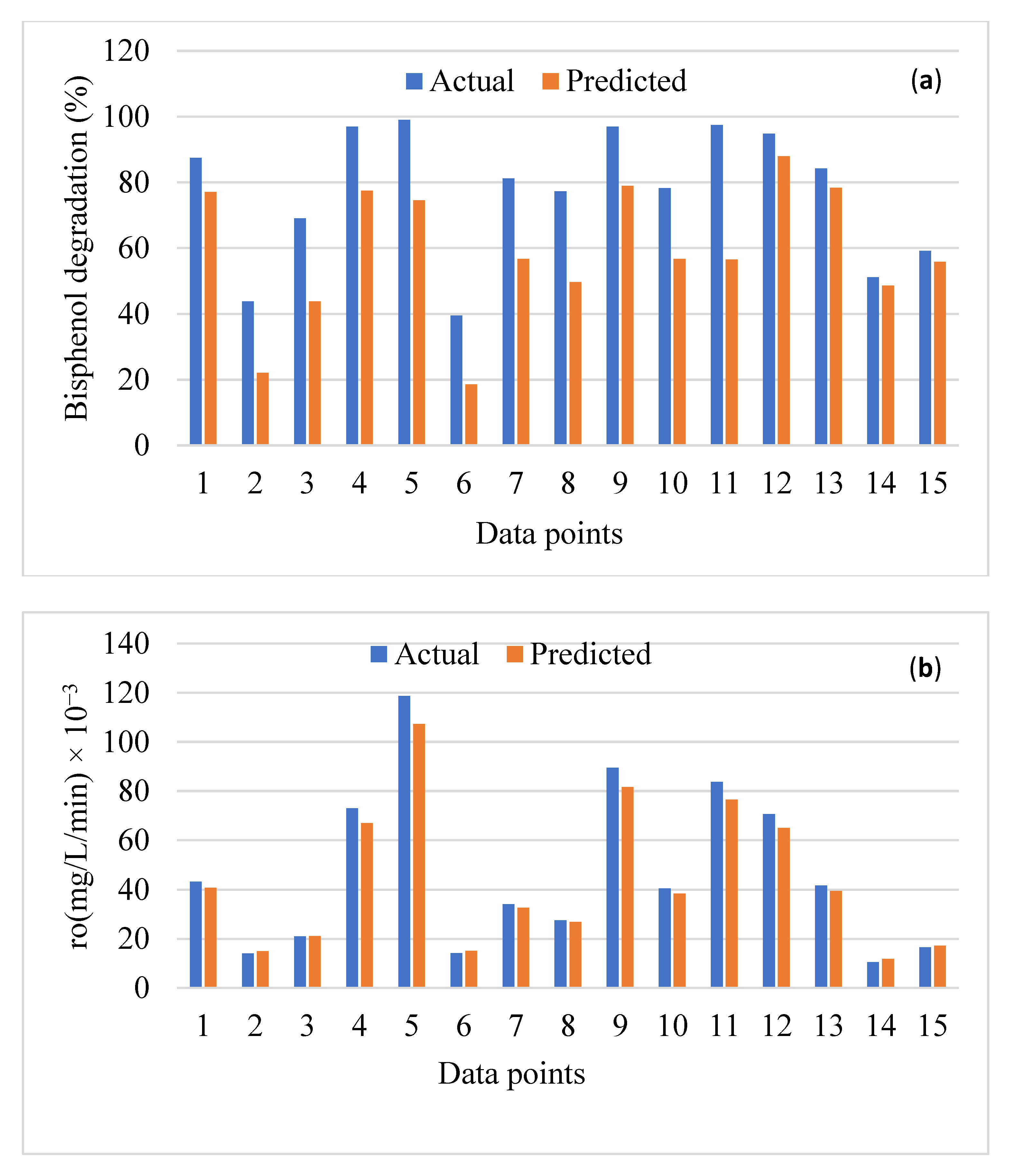
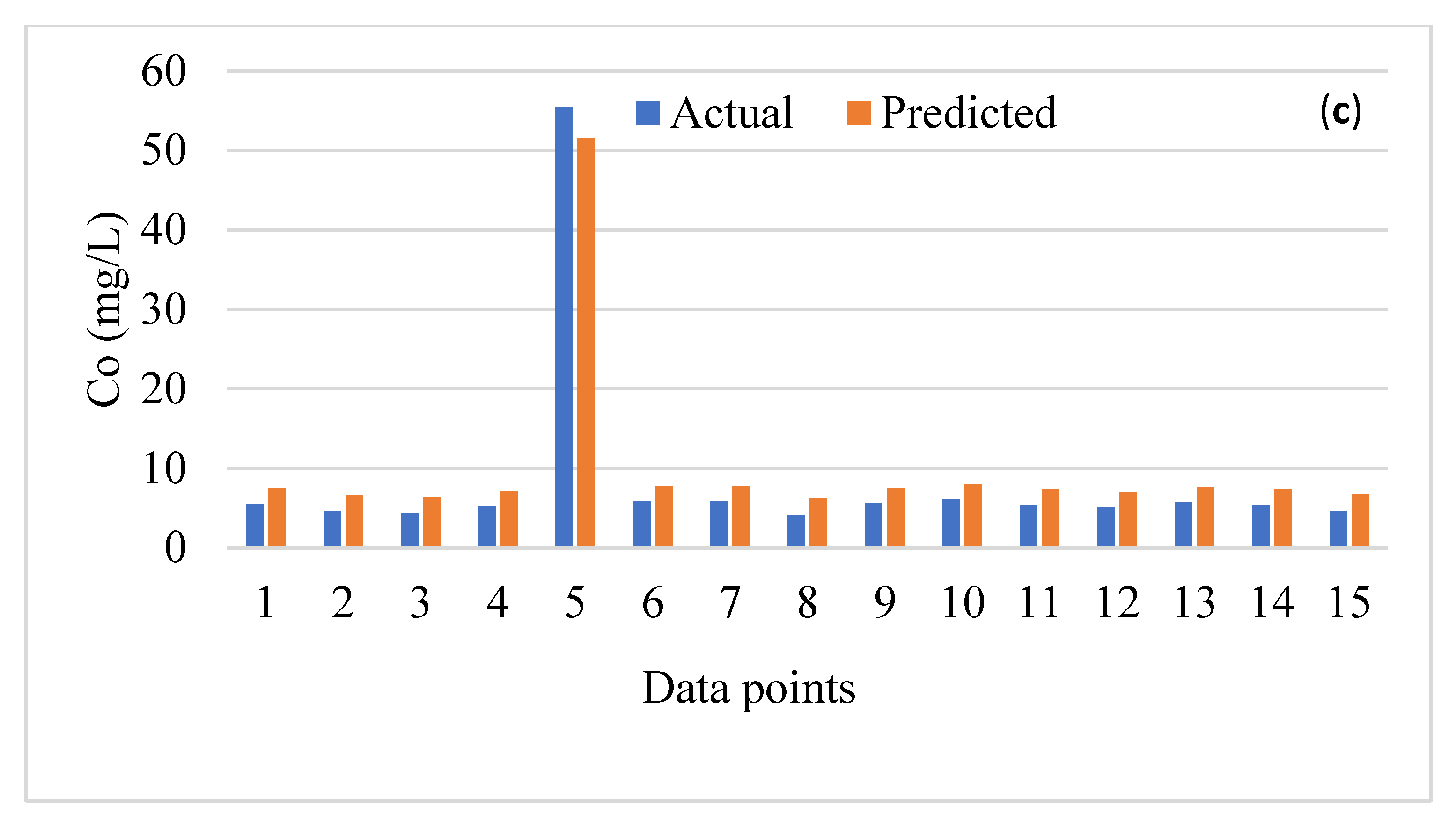
3. Performance Analysis of the Data-Driven Models
4. Comparison of the Best Model with the Literature
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Diao, P.; Chen, Q.; Wang, R.; Sun, D.; Cai, Z.; Wu, H.; Duan, S. Phenolic endocrine-disrupting compounds in the Pearl River Estuary: Occurrence, bioaccumulation and risk assessment. Sci. Total Environ. 2017, 584–585, 1100–1107. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gao, W. Photodegradation of Endocrine Disrupting Chemicals by ZnO Nanorod Arrays. Mol. Cryst. Liq. Cryst. 2014, 603, 194–201. [Google Scholar] [CrossRef]
- Sánchez-Avila, J.; Bonet, J.; Velasco, G.; Lacorte, S. Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a Municipal Wastewater Treatment Plant. Sci. Total Environ. 2009, 407, 4157–4167. [Google Scholar] [CrossRef] [PubMed]
- Chiang, L.-F.; Doong, R.-A. Cu–TiO2 nanorods with enhanced ultraviolet- and visible-light photoactivity for bisphenol A degradation. J. Hazard. Mater. 2014, 277, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Azizullah, A.; Khan, S.; Gao, G.; Gao, K. The interplay between bisphenol A and algae—A review. J. King Saud Univ.-Sci. 2022, 34, 102050. [Google Scholar] [CrossRef]
- Al-Qadri, A.A.Q.; Drmosh, Q.A.; Onaizi, S.A. Enhancement of bisphenol a removal from wastewater via the covalent functionalization of graphene oxide with short amine molecules. Case Stud. Chem. Environ. Eng. 2022, 6, 100233. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Yang, D.; Zhang, J.; Guo, Y.; Zhong, X.; Kong, J.; Gu, J. High-efficiency improvement of thermal conductivities for epoxy composites from synthesized liquid crystal epoxy followed by doping BN fillers. Compos. Part B Eng. 2020, 185, 107784. [Google Scholar] [CrossRef]
- Sheikh Asadi, A.M.S.; Malakootian, M.; Kowsari, E.; Alidadi, H. Ionic liquid-assisted sol-gel synthesis of Fe2O3-TiO2 for enhanced photocatalytic degradation of bisphenol a under UV illumination: Modeling and optimization using response surface methodology. Optik 2020, 204, 164229. [Google Scholar] [CrossRef]
- Ncube, A.; Mtetwa, S.; Bukhari, M.; Fiorentino, G.; Passaro, R. Circular Economy and Green Chemistry: The Need for Radical Innovative Approaches in the Design for New Products. Energies 2023, 16, 1752. [Google Scholar] [CrossRef]
- Dueñas-Moreno, J.; Mora, A.; Cervantes-Avilés, P.; Mahlknecht, J. Groundwater contamination pathways of phthalates and bisphenol A: Origin, characteristics, transport, and fate—A review. Environ. Int. 2022, 170, 107550. [Google Scholar] [CrossRef]
- Wang, X.; Nag, R.; Brunton, N.P.; Siddique, M.A.B.; Harrison, S.M.; Monahan, F.J.; Cummins, E. Human health risk assessment of bisphenol A (BPA) through meat products. Environ. Res. 2022, 213, 113734. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, B.; Cappello Riguzzi, A.; Turolla, A.; Antonelli, M. Bisphenol A leaching from epoxy resins in the drinking water distribution networks as human health risk determinant. Sci. Total Environ. 2021, 783, 146908. [Google Scholar] [CrossRef]
- Mohammadi, A.; Dobaradaran, S.; Schmidt, T.C.; Malakootian, M.; Spitz, J. Emerging contaminants migration from pipes used in drinking water distribution systems: A review of the scientific literature. Environ. Sci. Pollut. Res. 2022, 29, 75134–75160. [Google Scholar] [CrossRef] [PubMed]
- Penserini, L.; Cantoni, B.; Gabrielli, M.; Sezenna, E.; Saponaro, S.; Antonelli, M. An integrated human health risk assessment framework for alkylphenols due to drinking water and crops’ food consumption. Chemosphere 2023, 325, 138259. [Google Scholar] [CrossRef]
- Mustieles, V.; Arrebola, J.-P.; Porta, M. From old pollutants to the regulation of bisphenol A: Lessons learned for health promotion and disease prevention. Prev. Med. 2023, 169, 107460. [Google Scholar] [CrossRef] [PubMed]
- Postnikova, L.A.; Patkin, E.L. The possible effect of lactoferrin on the epigenetic characteristics of early mammalian embryos exposed to bisphenol A. Birth Defects Res. 2022, 114, 1199–1209. [Google Scholar] [CrossRef]
- He, H.; Li, X.; Shen, J.; Bai, S.; Li, C.; Shi, H. Bisphenol A exposure causes testicular toxicity by targeting DPY30-mediated post-translational modification of PI3K/AKT signaling in mice. Ecotoxicol. Environ. Saf. 2022, 243, 113996. [Google Scholar] [CrossRef]
- Li, Z.; Ren, Y.; Li, X.; Wang, W. KDM2A interacts with estrogen receptor α to promote bisphenol A and S-induced breast cancer cell proliferation by repressing TET2 expression. Ecotoxicol. Environ. Saf. 2023, 262, 115132. [Google Scholar] [CrossRef]
- Casas, M.; Forns, J.; Martínez, D.; Avella-García, C.; Valvi, D.; Ballesteros-Gómez, A.; Luque, N.; Rubio, S.; Julvez, J.; Sunyer, J.; et al. Exposure to bisphenol A during pregnancy and child neuropsychological development in the INMA-Sabadell cohort. Environ. Res. 2015, 142, 671–679. [Google Scholar] [CrossRef]
- Shirdel, I.; Kalbassi, M.R.; Esmaeilbeigi, M.; Tinoush, B. Disruptive effects of nonylphenol on reproductive hormones, antioxidant enzymes, and histology of liver, kidney and gonads in Caspian trout smolts. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 232, 108756. [Google Scholar] [CrossRef]
- Liang, J.; Li, Y.; Xie, P.; Liu, C.; Yu, L.; Ma, X. Dualistic effects of bisphenol A on growth, photosynthetic and oxidative stress of duckweed (Lemna minor). Environ. Sci. Pollut. Res. 2022, 29, 87717–87729. [Google Scholar] [CrossRef] [PubMed]
- Mg, R.; Girigoswami, A. Bisphenol A-an Overview on its Effect on Health and Environment. Biointerface Res. Appl. Chem. 2021, 12, 105–119. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Anastopoulos, I. Adsorptive removal of bisphenol A (BPA) from aqueous solution: A review. Chemosphere 2017, 168, 885–902. [Google Scholar] [CrossRef] [PubMed]
- Ohore, O.E.; Zhang, S. Endocrine disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems. A review. Sci. Afr. 2019, 5, e00135. [Google Scholar] [CrossRef]
- Kataria, N.; Bhushan, D.; Gupta, R.; Rajendran, S.; Teo, M.Y.M.; Khoo, K.S. Current progress in treatment technologies for plastic waste (bisphenol A) in aquatic environment: Occurrence, toxicity and remediation mechanisms. Environ. Pollut. 2022, 315, 120319. [Google Scholar] [CrossRef]
- Tai, X.H.; Lai, C.W.; Yang, T.C.K.; Johan, M.R.; Lee, K.M.; Chen, C.-Y.; Juan, J.C. Highly effective removal of volatile organic pollutants with p-n heterojunction photoreduced graphene oxide-TiO2 photocatalyst. J. Environ. Chem. Eng. 2022, 10, 107304. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Zhou, Q.; Huang, X. Hazards of bisphenol A (BPA) exposure: A systematic review of plant toxicology studies. J. Hazard. Mater. 2020, 384, 121488. [Google Scholar] [CrossRef]
- Bassim, S.; Mageed, A.K.; AbdulRazak, A.A.; Al-Sheikh, F. Photodegradation of Methylene Blue with Aid of Green Synthesis of CuO/TiO2 Nanoparticles from Extract of Citrus Aurantium Juice. Bull. Chem. React. Eng. Catal. 2023, 18, 1–16. [Google Scholar] [CrossRef]
- Nur, A.S.M.; Sultana, M.; Mondal, A.; Islam, S.; Robel, F.N.; Islam, A.; Sumi, M.S.A. A review on the development of elemental and codoped TiO2 photocatalysts for enhanced dye degradation under UV–vis irradiation. J. Water Process. Eng. 2022, 47, 102728. [Google Scholar] [CrossRef]
- Hou, D.; Feng, L.; Zhang, J.; Dong, S.; Zhou, D.; Lim, T.-T. Preparation, characterization and performance of a novel visible light responsive spherical activated carbon-supported and Er3+:YFeO3-doped TiO2 photocatalyst. J. Hazard. Mater. 2012, 199–200, 301–308. [Google Scholar] [CrossRef]
- Singh, K.; Harish, S.; Kristy, A.P.; Shivani, V.; Archana, J.; Navaneethan, M.; Shimomura, M.; Hayakawa, Y. Erbium doped TiO2 interconnected mesoporous spheres as an efficient visible light catalyst for photocatalytic applications. Appl. Surf. Sci. 2018, 449, 755–763. [Google Scholar] [CrossRef]
- Martins, P.M.; Gomez, V.; Lopes, A.C.; Tavares, C.J.; Botelho, G.; Irusta, S.; Lanceros-Mendez, S. Improving Photocatalytic Performance and Recyclability by Development of Er-Doped and Er/Pr-Codoped TiO2/Poly(vinylidene difluoride)–Trifluoroethylene Composite Membranes. J. Phys. Chem. C 2014, 118, 27944–27953. [Google Scholar] [CrossRef]
- Parida, K.; Satpathy, M.; Mohapatra, L. Incorporation of Fe3+ into Mg/Al layered double hydroxide framework: Effects on textural properties and photocatalytic activity for H2 generation. J. Mater. Chem. 2012, 22, 7350–7357. [Google Scholar] [CrossRef]
- Parayil, S.K.; Psota, R.J.; Koodali, R.T. Modulating the textural properties and photocatalytic hydrogen production activity of TiO2 by high temperature supercritical drying. Int. J. Hydrogen Energy 2013, 38, 10215–10225. [Google Scholar] [CrossRef]
- Mageed, A.K.; Abdel Ghany, M.A.; Alsaffar, M.A.; Ali, J.M. Modeling the Effect of Magnesia Nanoparticles on CO Hydrogenation to Light Olefins in a Continuous Flow Reactor Using Fine Gaussian Support Vector Machine. Asian J. Water Environ. Pollut. 2022, 19, 73–79. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I.; Cheng, C.K.; Witoon, T. Modeling the effect of process parameters on the photocatalytic degradation of organic pollutants using artificial neural networks. Process. Saf. Environ. Prot. 2021, 145, 120–132. [Google Scholar] [CrossRef]
- Liu, Q.; Pan, K.; Lu, Y.; Wei, W.; Wang, S.; Du, W.; Ding, Z.; Zhou, Y. Data-driven for accelerated design strategy of photocatalytic degradation activity prediction of doped TiO2 photocatalyst. J. Water Process. Eng. 2022, 49, 103126. [Google Scholar] [CrossRef]
- Bhagat, S.K.; Pilario, K.E.; Babalola, O.E.; Tiyasha, T.; Yaqub, M.; Onu, C.E.; Pyrgaki, K.; Falah, M.W.; Jawad, A.H.; Yaseen, D.A.; et al. Comprehensive review on machine learning methodologies for modeling dye removal processes in wastewater. J. Clean. Prod. 2023, 385, 135522. [Google Scholar] [CrossRef]
- Hou, D.; Goei, R.; Wang, X.; Wang, P.; Lim, T.-T. Preparation of carbon-sensitized and Fe–Er codoped TiO2 with response surface methodology for bisphenol A photocatalytic degradation under visible-light irradiation. Appl. Catal. B Environ. 2012, 126, 121–133. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Ghazali, A.A.; Yassin, M.Y.M.; Abdullah, S. Optimization of hydrogen production by photocatalytic steam methane reforming over lanthanum modified Titanium (IV) oxide using response surface methodology. Int. J. Hydrogen Energy 2019, 44, 20700–20710. [Google Scholar] [CrossRef]
- Benimam, H.; Si-Moussa, C.; Laidi, M.; Hanini, S. Modeling the activity coefficient at infinite dilution of water in ionic liquids using artificial neural networks and support vector machines. Neural Comput. Appl. 2020, 32, 8635–8653. [Google Scholar] [CrossRef]
- Mageed, A.K.; Shnain, Z.Y.; Mahdi, G.S. Modeling the effect of non-linear process parameters on the prediction of hydrogen production by steam reforming of bio-oil and glycerol using artificial neural network. Int. J. Energy Res. 2020, 44, 10523–10537. [Google Scholar] [CrossRef]
- Alwasiti, A.A.; Shnain, Z.Y.; Abid, M.F.; Abdul Razak, A.A.; Abdulhussein, B.A.; Mahdi, G.S. Experimental and numerical study on the degradation of mefenamic acid in a synthetic wastewater. IOP Conf. Ser. Earth Environ. Sci. 2021, 779, 012073. [Google Scholar] [CrossRef]
- Shnain, Z.Y.; Alwaiti, A.A.; Rashed, M.K.; Shakor, Z.M. Experimental and Data-driven approach of investigating the effect of parameters on the fluid flow characteristic of nanosilica enhanced two phase flow in pipeline. Alex. Eng. J. 2022, 61, 1159–1170. [Google Scholar] [CrossRef]
- Katsumata, H.; Kawabe, S.; Kaneco, S.; Suzuki, T.; Ohta, K. Degradation of bisphenol A in water by the photo-Fenton reaction. J. Photochem. Photobiol. A Chem. 2004, 162, 297–305. [Google Scholar] [CrossRef]
- Cai, C.; Kang, S.; Xie, X.; Liao, C.; Duan, X.; Dionysiou, D.D. Efficient degradation of bisphenol A in water by heterogeneous activation of peroxymonosulfate using highly active cobalt ferrite nanoparticles. J. Hazard. Mater. 2020, 399, 122979. [Google Scholar] [CrossRef]
- Ahmed, W.; Noor El-Din, M.R.; Aboul-Enein, A.A.; Awadallah, A.E. Effect of textural properties of alumina support on the catalytic performance of Ni/Al2O3 catalysts for hydrogen production via methane decomposition. J. Nat. Gas Sci. Eng. 2015, 25, 359–366. [Google Scholar] [CrossRef]
- Ahmed, Y.; Yaakob, Z.; Akhtar, P.; Sopian, K. Production of biogas and performance evaluation of existing treatment processes in palm oil mill effluent (POME). Renew. Sustain. Energy Rev. 2015, 42, 1260–1278. [Google Scholar] [CrossRef]
- González-Castaño, M.; de Miguel, J.C.N.; Penkova, A.; Centeno, M.A.; Odriozola, J.A.; Arellano-Garcia, H. Ni/YMnO3 perovskite catalyst for CO2 methanation. Appl. Mater. Today 2021, 23, 101055. [Google Scholar] [CrossRef]
- Zeng, Y.-R.; Zeng, Y.; Choi, B.; Wang, L. Multifactor-influenced energy consumption forecasting using enhanced back-propagation neural network. Energy 2017, 127, 381–396. [Google Scholar] [CrossRef]
- Sahu, S.; Shera, S.S.; Banik, R.M. Artificial Neural Network Modeling to Predict the Non-Linearity in Reaction Conditions of Cholesterol Oxidase from Streptomyces olivaceus MTCC 6820. J. Biosci. Med. 2019, 7, 14–24. [Google Scholar] [CrossRef] [Green Version]
- Goldt, S.; Advani, M.S.; Saxe, A.M.; Krzakala, F.; Zdeborová, L. Dynamics of stochastic gradient descent for two-layer neural networks in the teacher–student setup*. J. Stat. Mech. Theory Exp. 2020, 2020, 124010. [Google Scholar] [CrossRef] [PubMed]
- Alsaffar, M.A.; Ayodele, B.V.; Mustapa, S.I. Scavenging carbon deposition on alumina supported cobalt catalyst during renewable hydrogen-rich syngas production by methane dry reforming using artificial intelligence modeling technique. J. Clean. Prod. 2020, 247, 119168. [Google Scholar] [CrossRef]
- Bahadar, A.; Kanthasamy, R.; Sait, H.H.; Zwawi, M.; Algarni, M.; Ayodele, B.V.; Cheng, C.K.; Wei, L.J. Elucidating the effect of process parameters on the production of hydrogen-rich syngas by biomass and coal Co-gasification techniques: A multi-criteria modeling approach. Chemosphere 2022, 287, 132052. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Mustapa, S.I.; Kanthasamy, R.; Zwawi, M.; Cheng, C.K. Modeling the prediction of hydrogen production by co-gasification of plastic and rubber wastes using machine learning algorithms. Int. J. Energy Res. 2021, 45, 9580–9594. [Google Scholar] [CrossRef]
- Al Haiqi, O.; Nour, A.H.; Ayodele, B.V.; Bargaa, R. Bayesian Regularization-Trained Multi-layer Perceptron Neural Network Predictive Modelling of Phenol Degradation using ZnO/Fe2O3 photocatalyst. J. Phys. Conf. Ser. 2020, 1529, 052058. [Google Scholar] [CrossRef]
- Zeng, A.; Ho, H.; Yu, Y. Prediction of building electricity usage using Gaussian Process Regression. J. Build. Eng. 2020, 28, 101054. [Google Scholar] [CrossRef]
- Huang, J.; Jin, T.; Liang, M.; Chen, H. Prediction of heat exchanger performance in cryogenic oscillating flow conditions by support vector machine. Appl. Therm. Eng. 2021, 182, 116053. [Google Scholar] [CrossRef]
- Jaffari, Z.H.; Abbas, A.; Lam, S.-M.; Park, S.; Chon, K.; Kim, E.-S.; Cho, K.H. Machine learning approaches to predict the photocatalytic performance of bismuth ferrite-based materials in the removal of malachite green. J. Hazard. Mater. 2023, 442, 130031. [Google Scholar] [CrossRef]
- Ayodele, B.V.; Alsaffar, M.A.; Mustapa, S.I.; Vo, D.-V.N. Back-propagation neural networks modeling of photocatalytic degradation of organic pollutants using TiO2-based photocatalysts. J. Chem. Technol. Biotechnol. 2020, 95, 2730–2749. [Google Scholar] [CrossRef]
- Jiang, Z.; Hu, J.; Tong, M.; Samia, A.C.; Zhang, H.; Yu, X. A Novel Machine Learning Model to Predict the Photo-Degradation Performance of Different Photocatalysts on a Variety of Water Contaminants. Catalysts 2021, 11, 1107. [Google Scholar] [CrossRef]
- Navidpour, A.H.; Hosseinzadeh, A.; Huang, Z.; Li, D.; Zhou, J.L. Application of machine learning algorithms in predicting the photocatalytic degradation of perfluorooctanoic acid. Catal. Rev. 2022, 1–26. [Google Scholar] [CrossRef]


| Process | Best Model | Performance | Reference |
|---|---|---|---|
| Photodegradation of BPA | MLPNN | R2 = 0.992 RMSE = 4.21 | This study |
| Photodegradation of malachite green | CatBoost | R2 = 0.990, RMSE = 1.34 | [59] |
| Photodegradation of organic pollutants | LightGBM | R2 = 0.928, RMSE = 0.194 | [37] |
| Photodegradation of organic pollutants | BPANN | R2 = 0.999, RMSE = N. R | [60] |
| Photodegradation of organic pollutants | CGCCN-MF-ANN | R2 = 0.746 and RMSE of 0.293 | [61] |
| Photodegradation of perfluorooctanoic acid | AdaBoost | R2 = 0.878 and RMSE = 10.33 | [62] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsaffar, M.A.; Ghany, M.A.R.A.; Mageed, A.K.; AbdulRazak, A.A.; Ali, J.M.; Sukkar, K.A.; Ayodele, B.V. Effect of Textural Properties on the Degradation of Bisphenol from Industrial Wastewater Effluent in a Photocatalytic Reactor: A Modeling Approach. Appl. Sci. 2023, 13, 8966. https://doi.org/10.3390/app13158966
Alsaffar MA, Ghany MARA, Mageed AK, AbdulRazak AA, Ali JM, Sukkar KA, Ayodele BV. Effect of Textural Properties on the Degradation of Bisphenol from Industrial Wastewater Effluent in a Photocatalytic Reactor: A Modeling Approach. Applied Sciences. 2023; 13(15):8966. https://doi.org/10.3390/app13158966
Chicago/Turabian StyleAlsaffar, May Ali, Mohamed Abdel Rahman Abdel Ghany, Alyaa K. Mageed, Adnan A. AbdulRazak, Jamal Manee Ali, Khalid A. Sukkar, and Bamidele Victor Ayodele. 2023. "Effect of Textural Properties on the Degradation of Bisphenol from Industrial Wastewater Effluent in a Photocatalytic Reactor: A Modeling Approach" Applied Sciences 13, no. 15: 8966. https://doi.org/10.3390/app13158966
APA StyleAlsaffar, M. A., Ghany, M. A. R. A., Mageed, A. K., AbdulRazak, A. A., Ali, J. M., Sukkar, K. A., & Ayodele, B. V. (2023). Effect of Textural Properties on the Degradation of Bisphenol from Industrial Wastewater Effluent in a Photocatalytic Reactor: A Modeling Approach. Applied Sciences, 13(15), 8966. https://doi.org/10.3390/app13158966







