Lose Your Grip: Challenging Varroa destructor Host Attachment with Tartaric, Lactic, Formic, and Citric Acids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mites and Honeybees Sampling
2.2. Acid Preparation and Control
2.3. Grip on Wood (Rotavar)
2.4. Grip on Host Experiment (Honeybees)
2.5. Statistical Analyses
3. Results
3.1. Organic Acids Impact on V. destructor Grip Skill on Wood
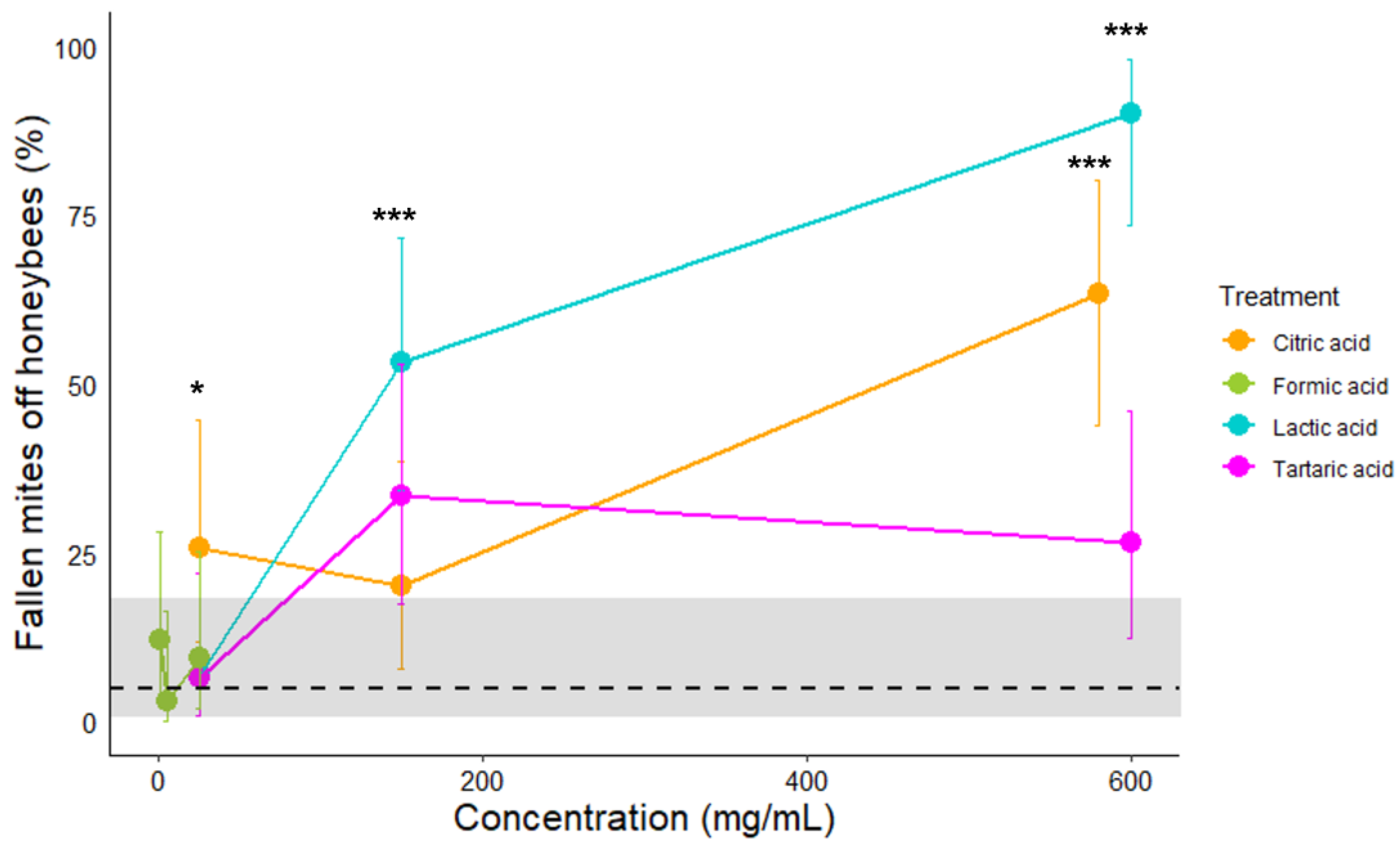
3.2. Effect of Organic Acids on V. destructor Fall off Honeybees
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Parameters | Estimate | SE | p-Value |
|---|---|---|---|
| Lactic acid—24 h | |||
| Intercept | 3.367 | 1.017 | 0.0009 |
| 25 mg/mL | 3.367 | 1.083 | 0.0004 |
| 150 mg/mL | 5.565 | 1.185 | <0.0001 |
| 600 mg/mL | 6.006 | 1.253 | <0.0001 |
| Formic acid—24 h | |||
| Intercept | −3.367 | 1.017 | 0.0009 |
| 1 mg/mL | 2.962 | 1.083 | 0.0062 |
| 5 mg/mL | 4.557 | 1.105 | <0.0001 |
| 25 mg/mL | 4.178 | 1.102 | 0.0001 |
| Tartaric acid—24 h | |||
| Intercept | −1.8718 | 0.5371 | 0.0004 |
| 25 mg/mL | 0.8602 | 0.6774 | 0.2041 |
| 150 mg/mL | 3.0169 | 0.6905 | <0.0001 |
| 600 mg/mL | 2.7368 | 0.6827 | <0.0001 |
| Citric acid—1 h 30 | |||
| Intercept | −2.7408 | 0.7296 | 0.0001 |
| 25 mg/mL | 1.5513 | 0.8477 | 0.0672 |
| 150 mg/mL | 1.1314 | 0.8788 | 0.1979 |
| 580 mg/mL | 2.4532 | 0.8056 | 0.0023 |
| Citric acid—24 h | |||
| Intercept | −3.434 | 1.016 | 0.0007 |
| 25 mg/mL | 3.029 | 1.082 | 0.0051 |
| 150 mg/mL | 2.741 | 1.087 | 0.0117 |
| 580 mg/mL | 2.828 | 1.078 | 0.0086 |
| Parameters | Estimate | SE | p-Value |
|---|---|---|---|
| Lactic acid | |||
| Intercept | −3.5835 | 1.0138 | 0.0004 |
| 25 mg/mL | 0.9445 | 1.2504 | 0.45 |
| 150 mg/mL | 3.7171 | 1.0778 | 0.0005 |
| 600 mg/mL | 5.7807 | 1.1824 | <0.0001 |
| Formic acid | |||
| Intercept | −3.4965 | 1.015 | 0.0005 |
| 1 mg/mL | 1.5155 | 1.1466 | 0.1862 |
| 5 mg/mL | 0.0625 | 1.4361 | 0.9652 |
| 25 mg/mL | 1.2278 | 1.1824 | 0.299 |
| Tartaric acid | |||
| Intercept | −2.1972 | 0.6086 | 0.0003 |
| 25 mg/mL | −0.4418 | 0.9519 | 0.6425 |
| 150 mg/mL | 1.5041 | 0.7214 | 0.037 |
| 600 mg/mL | 1.1856 | 0.7354 | 0.1069 |
| Citric acid | |||
| Intercept | −3.367 | 1.017 | 0.0009 |
| 25 mg/mL | 2.311 | 1.097 | 0.035 |
| 150 mg/mL | 1.981 | 1.115 | 0.0755 |
| 580 mg/mL | 3.914 | 1.085 | 0.0003 |
Appendix B

References
- Brodschneider, R.; Schlagbauer, J.; Arakelyan, I.; Ballis, A.; Brus, J.; Brusbardis, V.; Cadahía, L.; Charrière, J.-D.; Chlebo, R.; Coffey, M.F.; et al. Spatial Clusters of Varroa destructor Control Strategies in Europe. J. Pest Sci. 2023, 96, 759–783. [Google Scholar] [CrossRef]
- Gisder, S.; Aumeier, P.; Genersch, E. Deformed Wing Virus: Replication and Viral Load in Mites (Varroa destructor). J. Gen. Virol. 2009, 90, 463–467. [Google Scholar] [CrossRef]
- Wilfert, L.; Long, G.; Leggett, H.C.; Schmid-Hempel, P.; Butlin, R.; Martin, S.J.M.; Boots, M. Deformed Wing Virus Is a Recent Global Epidemic in Honeybees Driven by Varroa Mites. Science 2016, 351, 594–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, S.J.; Brettell, L.E. Deformed Wing Virus in Honeybees and Other Insects. Annu. Rev. Virol. 2019, 6, 49–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boecking, O.; Genersch, E. Varroosis—The Ongoing Crisis in Bee Keeping. J. Verbr. Lebensm. 2008, 3, 221–228. [Google Scholar] [CrossRef]
- Dainat, B.; Evans, J.D.; Chen, Y.P.; Gauthier, L.; Neumann, P. Dead or Alive: Deformed Wing Virus and Varroa Destructor Reduce the Life Span of Winter Honeybees. Appl. Environ. Microbiol. 2012, 78, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, S.D.; Ochoa, R.; Bauchan, G.; Gulbronson, C.; Mowery, J.D.; Cohen, A.; Lim, D.; Joklik, J.; Cicero, J.M.; Ellis, J.D.; et al. Varroa destructor Feeds Primarily on Honey Bee Fat Body Tissue and Not Hemolymph. Proc. Natl. Acad. Sci. USA 2019, 116, 1792–1801. [Google Scholar] [CrossRef] [Green Version]
- Piou, V.; Vilarem, C.; Blanchard, S.; Strub, J.; Bertile, F.; Bocquet, M.; Arafah, K.; Bulet, P.; Vétillard, A. Honey Bee Larval Hemolymph as a Source of Key Nutrients and Proteins Offers a Promising Medium for Varroa destructor Artificial Rearing. Int. J. Mol. Sci. 2023, 24, 12443. [Google Scholar] [CrossRef]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and Control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Vilarem, C.; Piou, V.; Vogelweith, F.; Vétillard, A. Varroa destructor from the Laboratory to the Field: Control, Biocontrol and IPM Perspectives—A Review. Insects 2021, 12, 800. [Google Scholar] [CrossRef]
- Sanchez-Bayo, F.; Goka, K. Pesticide Residues and Bees—A Risk Assessment. PLoS ONE 2014, 9, e94482. [Google Scholar] [CrossRef] [Green Version]
- Rinkevich, F.D. Detection of Amitraz Resistance and Reduced Treatment Efficacy in the Varroa Mite, Varroa destructor, within Commercial Beekeeping Operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maggi, M.; Tourn, E.; Negri, P.; Szawarski, N.; Marconi, A.; Gallez, L.; Medici, S.; Ruffinengo, S.; Brasesco, C.; De Feudis, L.; et al. A New Formulation of Oxalic Acid for Varroa destructor Control Applied in Apis mellifera Colonies in the Presence of Brood. Apidologie 2016, 47, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Steube, X.; Beinert, P.; Kirchner, W.H. Efficacy and Temperature Dependence of 60% and 85% Formic Acid Treatment against Varroa destructor. Apidologie 2021, 52, 720–729. [Google Scholar] [CrossRef]
- Căuia, E.; Căuia, D. Improving the Varroa (Varroa destructor) Control Strategy by Brood Treatment with Formic Acid—A Pilot Study on Spring Applications. Insects 2022, 13, 149. [Google Scholar] [CrossRef] [PubMed]
- Nozal, M.J.; Bernal, J.L.; Gomez, L.A.; Higes, M.; Meana, A. Determination of Oxalic Acid and Other Organic Acids in Honey and in Some Anatomic Structures of Bees. Apidologie 2003, 34, 181–188. [Google Scholar] [CrossRef] [Green Version]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Analytical Methods for the Determination of Organic Acids in Honey. Crit. Rev. Anal. Chem. 2006, 36, 3–11. [Google Scholar] [CrossRef]
- Gregorc, A.; Planinc, I. Acaricidal Effect of Oxalic Acid in Honeybee (Apis mellifera) Colonies. Apidologie 2001, 32, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Charriére, J.-D.; Imdorf, A. Oxalic Acid Treatment by Trickling against Varroa Destructor: Recommendations for Use in Central Europe and under Temperate Climate Conditions. Bee World 2002, 83, 51–60. [Google Scholar] [CrossRef]
- Jack, C.J.; van Santen, E.; Ellis, J.D. Evaluating the Efficacy of Oxalic Acid Vaporization and Brood Interruption in Controlling the Honey Bee Pest Varroa destructor (Acari: Varroidae). J. Econ. Entomol. 2020, 113, 582–588. [Google Scholar] [CrossRef]
- Papežíková, I.; Palíková, M.; Kremserová, S.; Zachová, A.; Peterová, H.; Babák, V.; Navrátil, S. Effect of Oxalic Acid on the Mite Varroa destructor and Its Host the Honey Bee Apis mellifera. J. Apic. Res. 2017, 56, 400–408. [Google Scholar] [CrossRef]
- Evans, J.D.; Cook, S.C. Genetics and Physiology of Varroa Mites. Curr. Opin. Insect Sci. 2018, 26, 130–135. [Google Scholar] [CrossRef]
- Schimpf, D.J.; Ewert, M.M.; Lai, V.K.; Clarke, B.L. Responses of Ticks to Immersion in Hot Bathing Water: Effect of Surface Type, Water Temperature, and Soap on Tick Motor Control. PLoS ONE 2021, 16, e0261592. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Gorb, S. Functional Morphology of Tarsal Adhesive Pads and Attachment Ability in Ticks Ixodes ricinus (Arachnida, Acari, Ixodidae). J. Exp. Biol. 2017, 220, 1984–1996. [Google Scholar] [CrossRef] [Green Version]
- van den Boogaart, L.M.; Langowski, J.K.A.; Amador, G.J. Studying Stickiness: Methods, Trade-Offs, and Perspectives in Measuring Reversible Biological Adhesion and Friction. Biomimetics 2022, 7, 134. [Google Scholar] [CrossRef] [PubMed]
- Dietemann, V.; Pflugfelder, J.; Anderson, D.; Charrière, J.; Chejanovsky, N.; Dainat, B.; de Miranda, J.; Delaplane, K.; Dillier, F.; Fuch, S.; et al. Varroa destructor: Research Avenues towards Sustainable Control. J. Apic. Res. 2012, 51, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Dhooria, M.S. Morphology and Anatomy of Acari. In Fundamentals of Applied Acarology; Springer: Singapore, 2016; pp. 41–61. ISBN 978-981-10-1592-2. [Google Scholar]
- Plettner, E.; Eliash, N.; Singh, N.K.; Pinnelli, G.R.; Soroker, V. The Chemical Ecology of Host-Parasite Interaction as a Target of Varroa destructor Control Agents. Apidologie 2017, 48, 78–92. [Google Scholar] [CrossRef] [Green Version]
- Wolff, J.O.; Gorb, S.N. Attachment Structures and Adhesive Secretions in Arachnids; Biologically-Inspired Systems; Springer International Publishing: Cham, Switzerland, 2016; Volume 7, ISBN 978-3-319-45712-3. [Google Scholar]
- Mitton, G.A.; Meroi Arcerito, F.; Cooley, H.; Fernández de Landa, G.; Eguaras, M.J.; Ruffinengo, S.R.; Maggi, M.D. More than Sixty Years Living with Varroa destructor: A Review of Acaricide Resistance. Int. J. Pest Manag. 2022, 68, 1–18. [Google Scholar] [CrossRef]
- Kraus, B.; Berg, S. Effect of a Lactic Acid Treatment during Winter in Temperate Climate upon Varroa jacobsoni Oud. and the Bee (Apis mellifera L.) Colony. Exp. Appl. Acarol. 1994, 18, 459–468. [Google Scholar] [CrossRef]
- Smodiš Škerl, M.I.; Rivera-Gomis, J.; Tlak Gajger, I.; Bubnič, J.; Talakić, G.; Formato, G.; Baggio, A.; Mutinelli, F.; Tollenaers, W.; Laget, D.; et al. Efficacy and Toxicity of VarroMed® Used for Controlling Varroa Destructor Infestation in Different Seasons and Geographical Areas. Appl. Sci. 2021, 11, 8564. [Google Scholar] [CrossRef]
- Milani, N. Activity of Oxalic and Citric Acids on the Mite Varroa destructor in Laboratory Assays. Apidologie 2001, 32, 127–138. [Google Scholar] [CrossRef] [Green Version]
- Dietemann, V.; Nazzi, F.; Martin, S.J.; Anderson, D.L.; Locke, B.; Delaplane, K.S.; Wauquiez, Q.; Tannahill, C.; Frey, E.; Ziegelmann, B.; et al. Standard Methods for Varroa Research. J. Apic. Res. 2013, 52, 1–54. [Google Scholar] [CrossRef] [Green Version]
- Piou, V.; Tabart, J.; Urrutia, V.; Hemptinne, J.; Vétillard, A. Impact of the Phoretic Phase on Reproduction and Damage Caused by Varroa destructor (Anderson and Trueman) to Its Host, the European Honey Bee (Apis mellifera L.). PLoS ONE 2016, 11, e0153482. [Google Scholar] [CrossRef] [Green Version]
- Vilarem, C.; Piou, V.; Blanchard, S.; Armengaud, C.; Vogelweith, F.; Vétillard, A. Lactic Acid Impairs Varroa destructor Grip Skill: Effects on Its Biology and Behaviour under Artificial Conditions. 2023; under review. [Google Scholar]
- Dunham, N.W.; Miya, T.S. A Note on a Simple Apparatus for Detecting Neurological Deficit in Rats and Mice** College of Pharmacy, University of Nebraska, Lincoln 8. J. Am. Pharm. Assoc. 1957, 46, 208–209. [Google Scholar] [CrossRef] [PubMed]
- Gashout, H.A.; Guzmán-Novoa, E. Acute Toxicity of Essential Oils and Other Natural Compounds to the Parasitic Mite, Varroa destructor, and to Larval and Adult Worker Honey Bees (Apis mellifera L.). J. Apic. Res. 2009, 48, 263–269. [Google Scholar] [CrossRef]
- Evans, J.; Chen, Y.; di Prisco, G.; Pettis, J.; Williams, V. Bee Cups: Single-Use Cages for Honey Bee Experiments. J. Apic. Res. 2009, 48, 300–302. [Google Scholar] [CrossRef]
- Huang, S.K.; Csaki, T.; Doublet, V.; Dussaubat, C.; Evans, J.D.; Gajda, A.M.; Gregorc, A.; Hamilton, M.C.; Kamler, M.; Lecocq, A.; et al. Evaluation of Cage Designs and Feeding Regimes for Honey Bee (Hymenoptera: Apidae) Laboratory Experiments. J. Econ. Entom. 2014, 107, 54–62. [Google Scholar] [CrossRef]
- Hervé, M. RVAideMemoire: Testing and Plotting Procedures for Biostatistics. 2020. Available online: https://rdrr.io/cran/RVAideMemoire/ (accessed on 5 July 2023).
- Alboukadel, K.; Marcin, K.; Przemyslaw, B.; Scheipl, F. Survminer: Drawing Survival Curves Using “Ggplot2”. 2022. Available online: https://rdrr.io/cran/survminer/ (accessed on 5 July 2023).
- Therneau, T. Survival: Survival Analysis 2023; Agroscope: Bern, Switzerland, 2004. [Google Scholar]
- Lupo, A.; Gerling, D. A Comparison between the Efficiency of Summer Treatments Using Formic Acid and Taktic® against Varroa jacobsoni in Beehives. Apidologie 1990, 21, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Charrière, J.; Imdorf, A.; Kuhn, R. Bienenverträglichkeit von Varroabehandlungen Im Winter. Schweiz. Bienen-Ztg. 2004, 14, 19–23. [Google Scholar]
- Evans, P.D.; Gee, J.D. Action of Formamidine Pesticides on Octopamine Receptors. Nature 1980, 287, 60–62. [Google Scholar] [CrossRef]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant Essential Oils and Formamidines as Insecticides/Acaricides: What Are the Molecular Targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef] [Green Version]
- Federle, W. An Integrative Study of Insect Adhesion: Mechanics and Wet Adhesion of Pretarsal Pads in Ants. Integr. Comp. Biol. 2002, 42, 1100–1106. [Google Scholar] [CrossRef] [PubMed]
- Gladun, D.; Gorb, S.N.; Frantsevich, L.I. Alternative Tasks of the Insect Arolium with Special Reference to Hymenoptera. In Functional Surfaces in Biology; Springer: Dordrecht, The Netherlands, 2009; pp. 67–103. ISBN 978-1-4020-6694-8. [Google Scholar]
- Peattie, A.M.; Dirks, J.; Henriques, S.; Federle, W. Arachnids Secrete a Fluid over Their Adhesive Pads. PLoS ONE 2011, 6, e20485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolff, J.O.; Huber, S.J.; Gorb, S.N. How to Stay on Mummy’s Back: Morphological and Functional Changes of the Pretarsus in Arachnid Postembryonic Stages. Arthropod Struct. Dev. 2015, 44, 301–312. [Google Scholar] [CrossRef]
- Vötsch, W.; Nicholson, G.; Müller, R.; Stierhof, Y.-D.; Gorb, S.; Schwarz, U. Chemical Composition of the Attachment Pad Secretion of the Locust Locusta Migratoria. Insect Biochem. Mol. Biol. 2002, 32, 1605–1613. [Google Scholar] [CrossRef]
- Genath, A.; Petruschke, H.; von Bergen, M.; Einspanier, R. Influence of Formic Acid Treatment on the Proteome of the Ectoparasite Varroa destructor. PLoS ONE 2021, 16, e0258845. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Suo, Y.; Zhao, J.; Zhu, P.; Tan, J.; Wang, B.; Lu, H. Effect of Demulsification for Crude Oil-in-Water Emulsion: Comparing CO2 and Organic Acids. Energy Fuels 2018, 32, 757–764. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Fakhru’l-Razi, A.; Abdullah, L.C.; Elnashaie, S.S.E.H.; Pendashteh, A. Demulsification Techniques of Water-in-Oil and Oil-in-Water Emulsions in Petroleum Industry. Sep. Purif. Technol. 2016, 170, 377–407. [Google Scholar] [CrossRef]
- Yao, L.; Selmi, A.; Esmaeili, H. A Review Study on New Aspects of Biodemulsifiers: Production, Features and Their Application in Wastewater Treatment. Chemosphere 2021, 284, 131364. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ge, L.; Friberg, S.E.; Guo, R. Equilibration in a Tartaric Acid Emulsion System. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 135–141. [Google Scholar] [CrossRef]
- Yesiltas, B.; García-Moreno, P.J.; Sørensen, A.D.M.; Anankanbil, S.; Guo, Z.; Jacobsen, C. Effects of Modified DATEMs with Different Alkyl Chain Lengths on Improving Oxidative and Physical Stability of 70% Fish Oil-in-Water Emulsions. J. Agric. Food Chem. 2018, 66, 12512–12520. [Google Scholar] [CrossRef] [Green Version]
- Bohn, H.F.; Federle, W. Insect Aquaplaning: Nepenthes Pitcher Plants Capture Prey with the Peristome, a Fully Wettable Water-Lubricated Anisotropic Surface. Proc. Natl. Acad. Sci. USA 2004, 101, 14138–14143. [Google Scholar] [CrossRef]
- Dirks, J.; Clemente, C.J.; Federle, W. Insect Tricks: Two-Phasic Foot Pad Secretion Prevents Slipping. J. R. Soc. Interface. 2010, 7, 587–593. [Google Scholar] [CrossRef] [Green Version]
- Aliano, N.P.; Ellis, M.D. Bee-to-Bee Contact Drives Oxalic Acid Distribution in Honey Bee Colonies. Apidologie 2008, 39, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Rademacher, E.; Harz, M.; Schneider, S. Effects of Oxalic Acid on Apis mellifera (Hymenoptera: Apidae). Insects 2017, 8, 84. [Google Scholar] [CrossRef] [Green Version]
- Pritchard, D.J. Grooming by Honey Bees as a Component of Varroa Resistant Behavior. J. Apic. Res. 2016, 55, 38–48. [Google Scholar] [CrossRef]
- Büscher, T.H.; Petersen, D.S.; Bijma, N.N.; Bäumler, F.; Pirk, C.W.W.; Büsse, S.; Heepe, L.; Gorb, S.N. The Exceptional Attachment Ability of the Ectoparasitic Bee Louse Braula coeca (Diptera, Braulidae) on the Honeybee. Physiol. Entomol. 2022, 47, 83–95. [Google Scholar] [CrossRef]
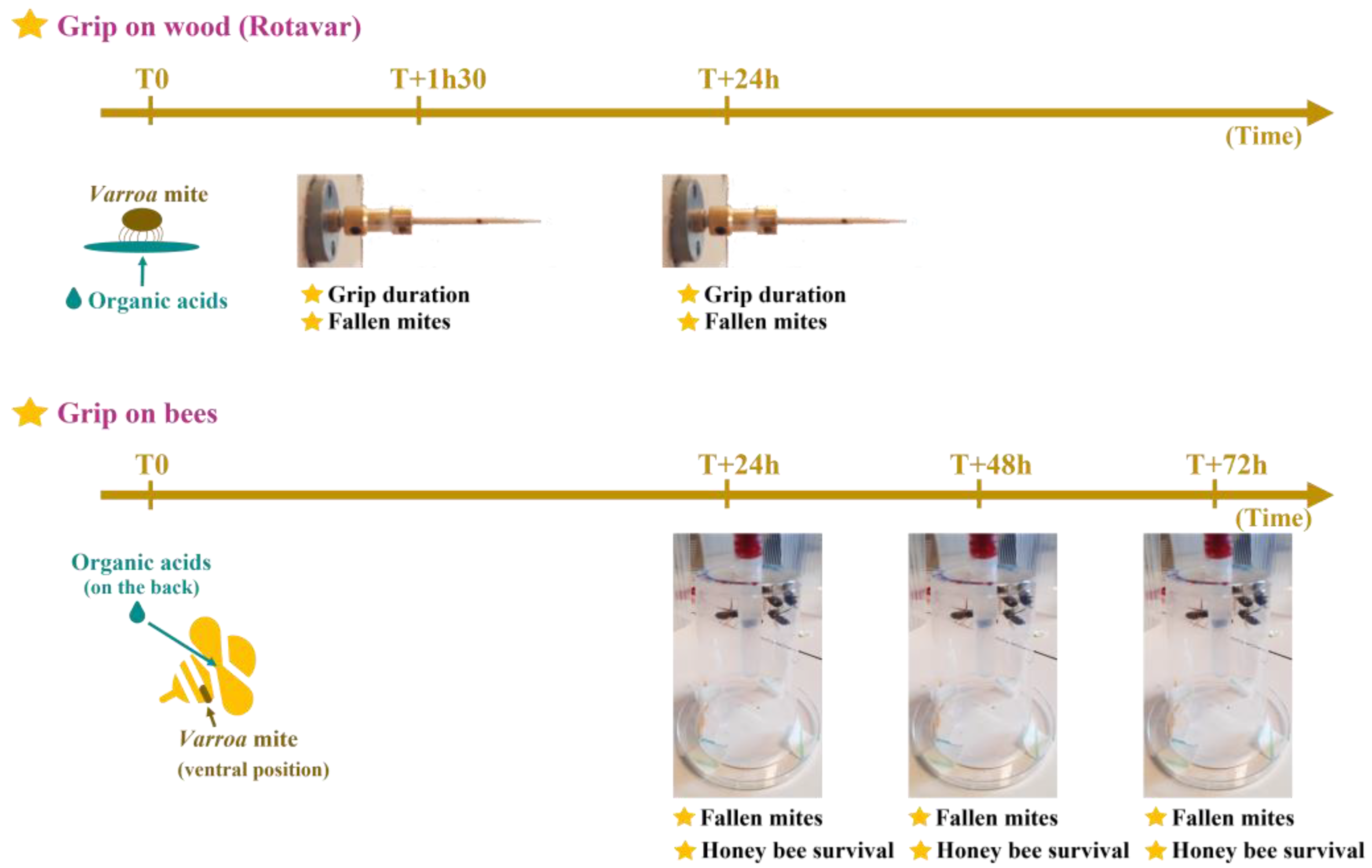




| Experiment | Parameters | Control | Citric Acid | Formic Acid | Lactic Acid | Tartaric Acid |
|---|---|---|---|---|---|---|
| Grip on wood | Concentrations tested (mg/mL) | 0 | 25 | 1 | 25 | 25 |
| 150 | 5 | 150 | 150 | |||
| 580 | 25 | 600 | 600 | |||
| Sample sizes (n) per concentration ([C]) | 30 mites | 30 mites/[C] | 30 mites/[C] | 30 mites/[C] | 30 mites/[C] | |
| Grip on bees | Concentrations tested (mg/mL) | 0 | 25 | 1 | 25 | 25 |
| 150 | 5 | 150 | 150 | |||
| 580 | 25 | 600 | 600 | |||
| Sample sizes (n) per concentration ([C]) | 30 mites | 30 mites/[C] | 30 mites/[C] | 30 mites/[C] | 30 mites/[C] |
| Acids | Conditions | (A) Percentages of Fallen Mites | (B) Median Grip Duration | ||
|---|---|---|---|---|---|
| 1 h 30 Post-Treatment p-Value Adjusted | 24 h Post-Treatment p-Value Adjusted | 1 h 30 Post-Treatment p-Value Adjusted | 24 h Post-Treatment p-Value Adjusted | ||
| Citric | Ctrl vs. 25 mg/mL | 0.1468 | 0.0023 | 0.5266 | 0.124 |
| Citric | Ctrl vs. 150 mg/mL | 0.2914 | 0.0045 | 1 | 0.482 |
| Citric | Ctrl vs. 580 mg/mL | 0.0033 | 0.0038 | 0.1468 | 0.1036 |
| Formic | Ctrl vs. 1 mg/mL | 0.4915 | 0.0021 | 1 | 0.0661 |
| Formic | Ctrl vs. 5 mg/mL | 0.1474 | <0.0001 | 0.2 | <0.0001 |
| Formic | Ctrl vs. 25 mg/mL | 0.0047 | <0.0001 | 0.1011 | <0.0001 |
| Lactic | Ctrl vs. 25 mg/mL | 0.0002 | <0.0001 | 0.0358 | 0.0081 |
| Lactic | Ctrl vs. 150 mg/mL | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Lactic | Ctrl vs. 600 mg/mL | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
| Tartaric | Ctrl vs. 25 mg/mL | 0.4745 | 0.4168 | 1 | 1 |
| Tartaric | Ctrl vs. 150 mg/mL | 0.0457 | <0.0001 | 0.1071 | <0.0001 |
| Tartaric | Ctrl vs. 600 mg/mL | 0.3746 | <0.0001 | 0.696 | 0.0001 |
| Acids | Conditions | 72 h Post-Treatment p-Value Adjusted |
|---|---|---|
| Citric | Control vs. 25 mg/mL | 0.0391 |
| Citric | Control vs. 150 mg/mL | 0.1027 |
| Citric | Control vs. 580 mg/mL | <0.0001 |
| Formic | Control vs. 1 mg/mL | 0.1212 |
| Formic | Control vs. 5 mg/mL | 0.3893 |
| Formic | Control vs. 25 mg/mL | 0.093 |
| Lactic | Control vs. 25 mg/mL | 0.5829 |
| Lactic | Control vs. 150 mg/mL | <0.0001 |
| Lactic | Control vs. 600 mg/mL | <0.0001 |
| Tartaric | Control vs. 25 mg/mL | 1 |
| Tartaric | Control vs. 150 mg/mL | 0.2657 |
| Tartaric | Control vs. 600 mg/mL | 0.4515 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilarem, C.; Piou, V.; Blanchard, S.; Vogelweith, F.; Vétillard, A. Lose Your Grip: Challenging Varroa destructor Host Attachment with Tartaric, Lactic, Formic, and Citric Acids. Appl. Sci. 2023, 13, 9085. https://doi.org/10.3390/app13169085
Vilarem C, Piou V, Blanchard S, Vogelweith F, Vétillard A. Lose Your Grip: Challenging Varroa destructor Host Attachment with Tartaric, Lactic, Formic, and Citric Acids. Applied Sciences. 2023; 13(16):9085. https://doi.org/10.3390/app13169085
Chicago/Turabian StyleVilarem, Caroline, Vincent Piou, Solène Blanchard, Fanny Vogelweith, and Angélique Vétillard. 2023. "Lose Your Grip: Challenging Varroa destructor Host Attachment with Tartaric, Lactic, Formic, and Citric Acids" Applied Sciences 13, no. 16: 9085. https://doi.org/10.3390/app13169085
APA StyleVilarem, C., Piou, V., Blanchard, S., Vogelweith, F., & Vétillard, A. (2023). Lose Your Grip: Challenging Varroa destructor Host Attachment with Tartaric, Lactic, Formic, and Citric Acids. Applied Sciences, 13(16), 9085. https://doi.org/10.3390/app13169085






