Integrating Thermal Indices and Phenotypic Traits for Assessing Tree Health: A Comprehensive Framework for Conservation and Monitoring of Urban, Agricultural, and Forest Ecosystems
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
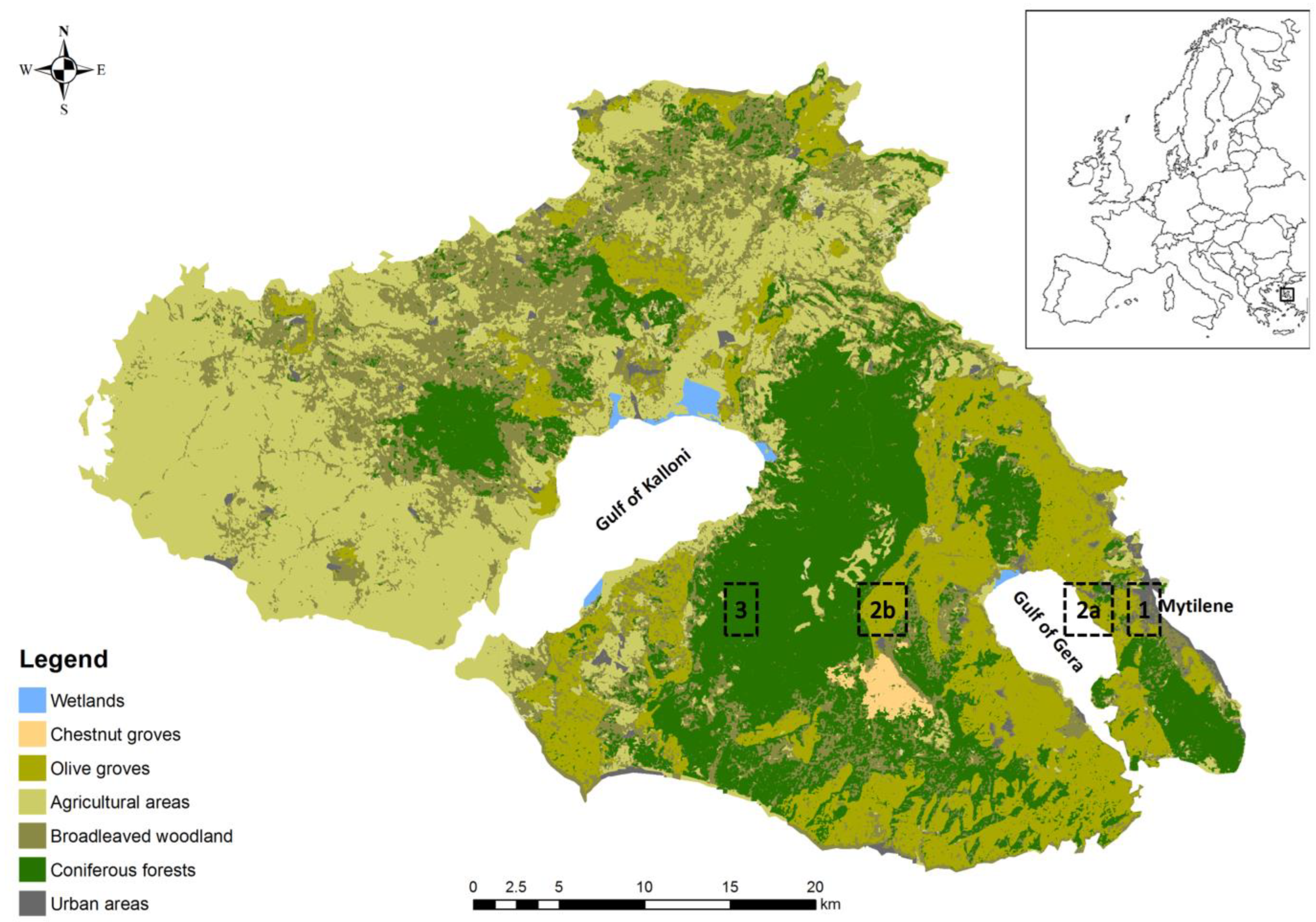
2.1. Study Area
2.2. Site Selection and Metrics of Trees’ Phenotypic Traits
2.3. Estimation of Wood-Decay Fungi Presence
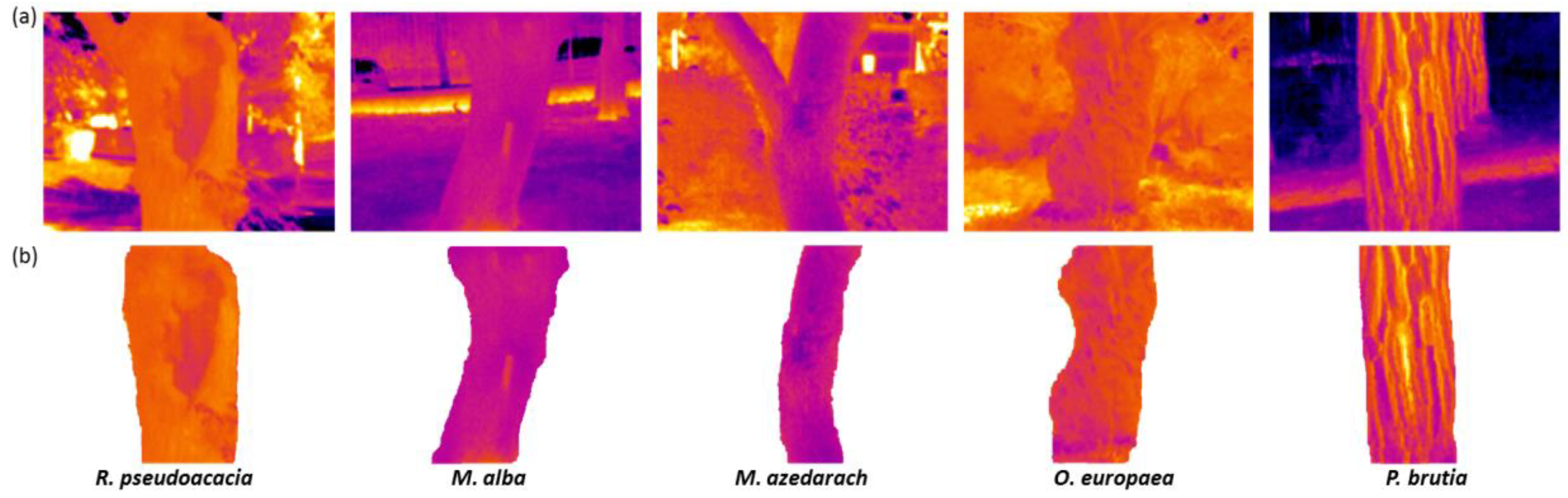
2.4. Creation of Trees’ Thermal Profile and Indices Using IRT
2.5. Statistical Analysis
3. Results
3.1. Trees’ Architectural and Vitality Traits
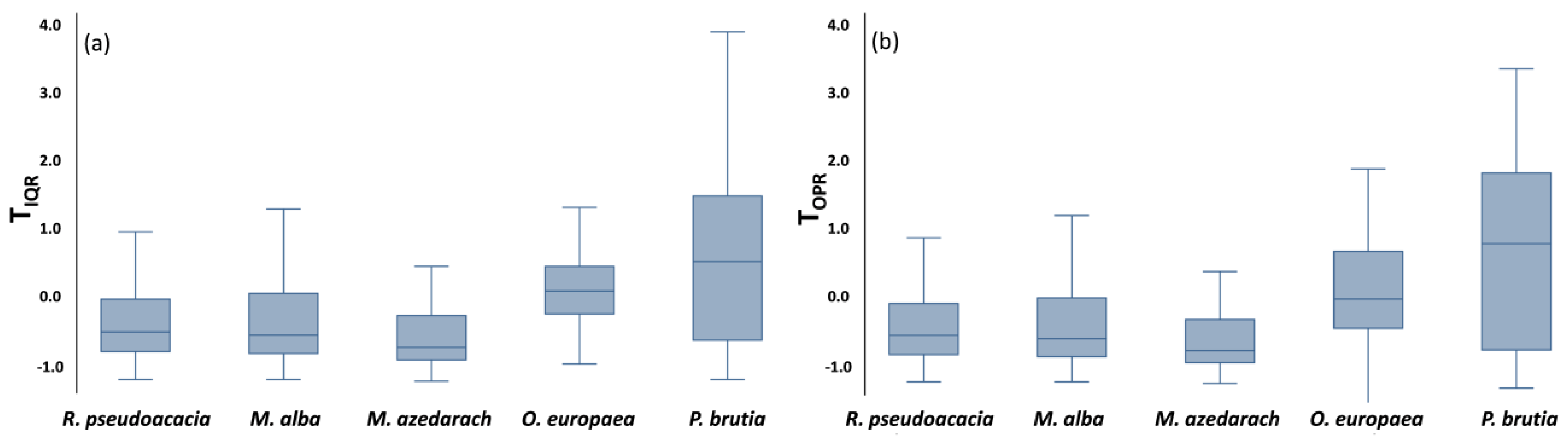
3.2. Trees’ Thermal and LAI Patterns
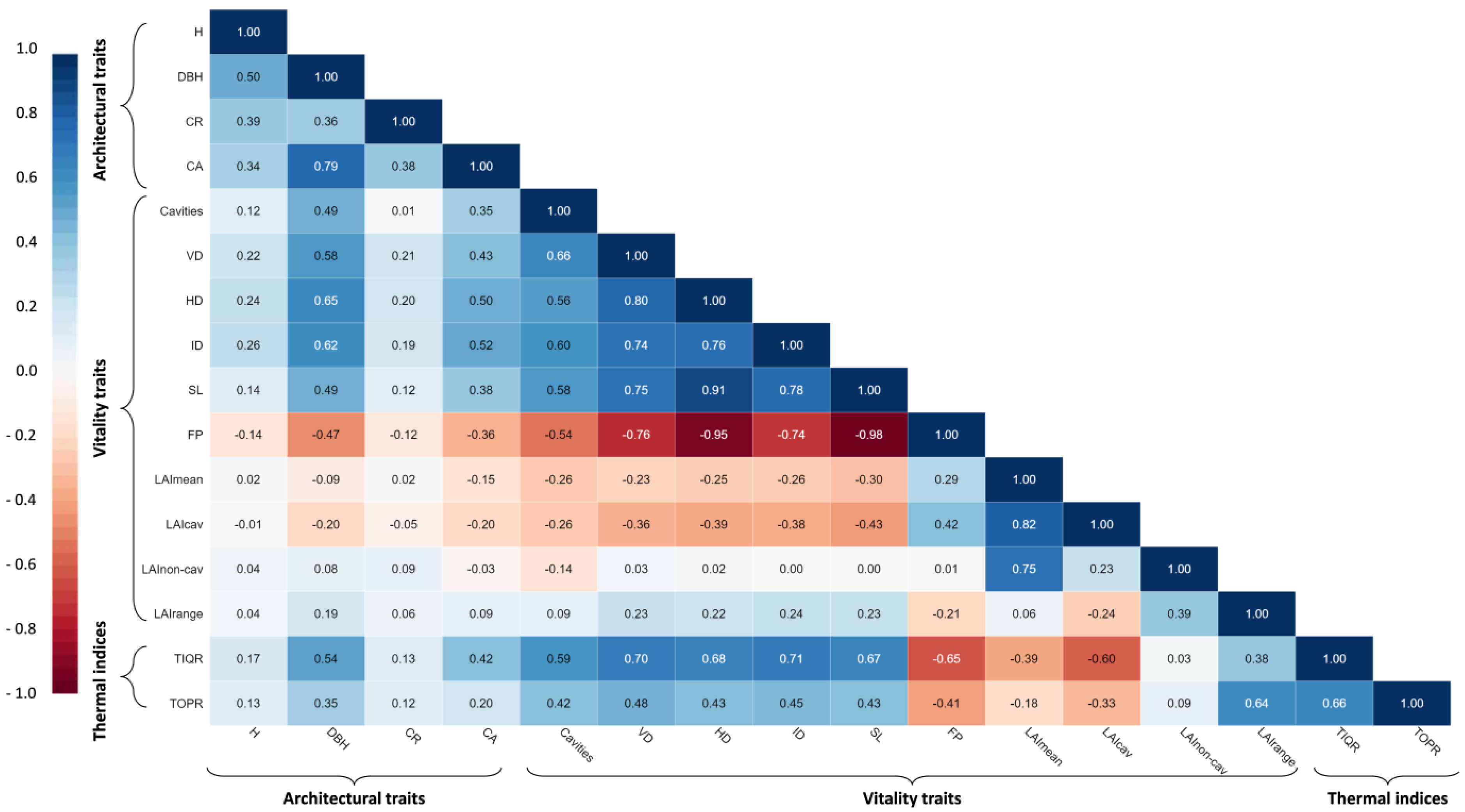
3.3. Relationships among Trees’ Phenotypic Traits and Trunk Thermal Indices and Their Effects on LAI
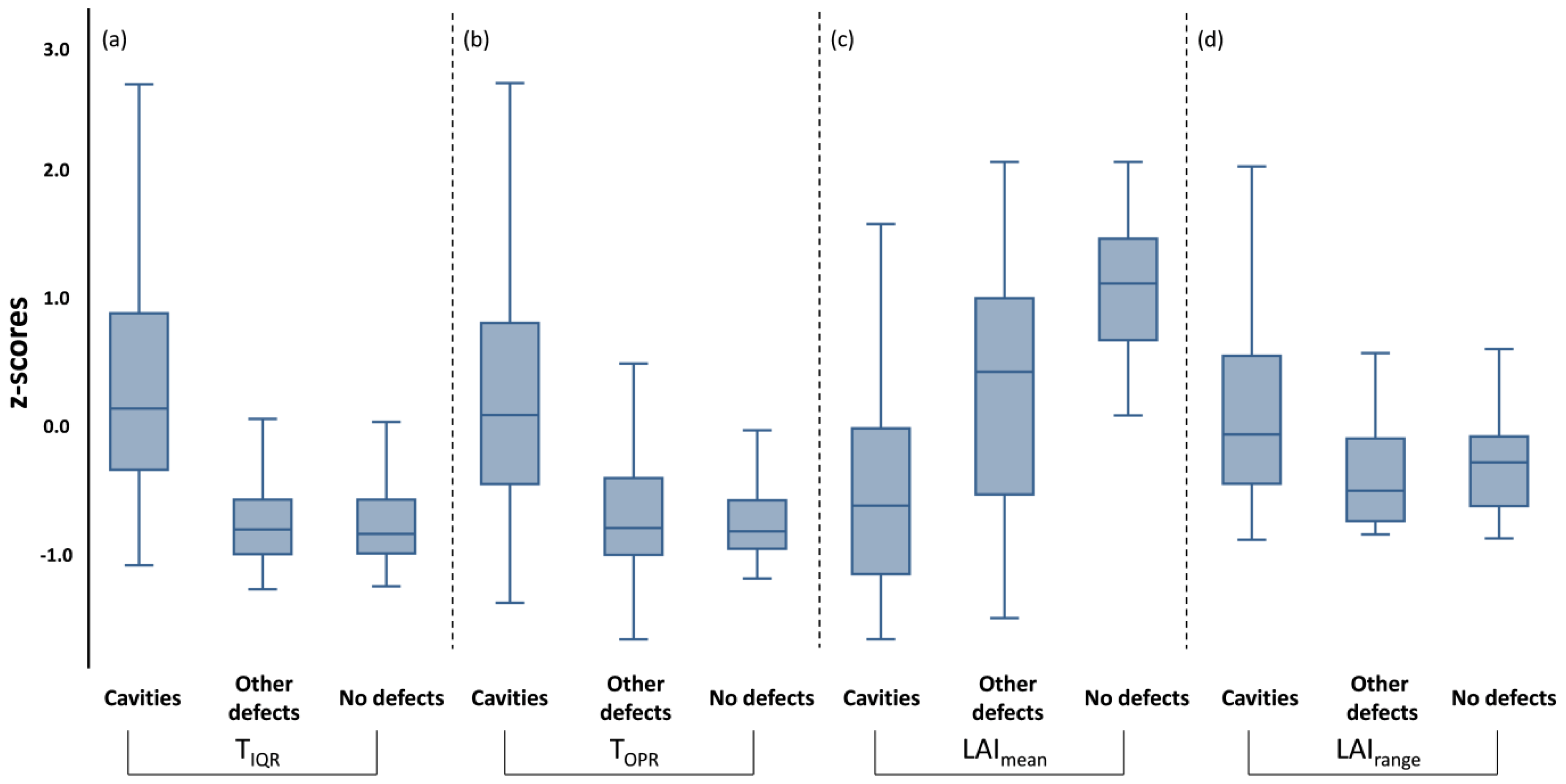
3.4. Modelling the Incidence of Defects, Cavities, and Fungal Infestation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Folke, C.; Polasky, S.; Rockström, J.; Galaz, V.; Westley, F.; Lamont, M.; Scheffer, M.; Österblom, H.; Carpenter, S.R.; Chapin, F.S.; et al. Our future in the Anthropocene biosphere. Ambio 2021, 50, 834–869. [Google Scholar] [CrossRef]
- Tortell, P.D. Earth 2020: Science, society, and sustainability in the Anthropocene. Proc. Natl. Acad. Sci. USA 2020, 117, 8683–8691. [Google Scholar] [CrossRef] [PubMed]
- Lucash, M.S.; Scheller, R.M.; Sturtevant, B.R.; Gustafson, E.J.; Kretchun, A.M.; Foster, J.R. More than the sum of its parts: How disturbance interactions shape forest dynamics under climate change. Ecosphere 2018, 9, e02293. [Google Scholar] [CrossRef]
- Trumbore, S.; Brando, P.; Hartmann, H. Forest health and global change. Science 2015, 349, 814–818. [Google Scholar] [CrossRef] [PubMed]
- Cobb, R.; Metz, M. Tree Diseases as a Cause and Consequence of Interacting Forest Disturbances. Forests 2017, 8, 147. [Google Scholar] [CrossRef]
- Hong, P.; Schmid, B.; De Laender, F.; Eisenhauer, N.; Zhang, X.; Chen, H.; Craven, D.; De Boeck, H.J.; Hautier, Y.; Petchey, O.L.; et al. Biodiversity promotes ecosystem functioning despite environmental change. Ecol. Lett. 2022, 25, 555–569. [Google Scholar] [CrossRef]
- Rapport, D.J. What constitutes ecosystem health? Perspect. Biol. Med. 1989, 33, 120–132. [Google Scholar] [CrossRef]
- Rapport, D. Assessing ecosystem health. Trends Ecol. Evol. 1998, 13, 397–402. [Google Scholar] [CrossRef]
- Su, M.; Fath, B.D.; Yang, Z. Urban ecosystem health assessment: A review. Sci. Total Environ. 2010, 408, 2425–2434. [Google Scholar] [CrossRef]
- Walker, B.; Carpenter, S.R.; Kinzing, A. Resilience, Adaptability and Transformability in Social–ecological Systems. Ecol. Soc. 2004, 9, 5. [Google Scholar] [CrossRef]
- Cumming, G.S.; Collier, J. Change and Identity in Complex Systems. Ecol. Soc. 1967, 10, 29. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, R.; Zhang, Y.; Su, H.; Wang, P.; Jenkins, A.; Ferrier, R.C.; Bailey, M.; Squire, G. Ecosystem health towards sustainability. Ecosyst. Health Sustain. 2015, 1, 1–15. [Google Scholar] [CrossRef]
- Boa, E. An Illustrated Guide to the State of Health of Trees Recognition and Interpretation; FAO, Ed.; CABI Bioscience: London, UK, 2003; ISBN 9251050201. [Google Scholar]
- Cavender, N.; Donnelly, G. Intersecting urban forestry and botanical gardens to address big challenges for healthier trees, people, and cities. Plants People Planet 2019, 1, 315–322. [Google Scholar] [CrossRef]
- Brockerhoff, E.G.; Barbaro, L.; Castagneyrol, B.; Forrester, D.I.; Gardiner, B.; González-Olabarria, J.R.; Lyver, P.O.B.; Meurisse, N.; Oxbrough, A.; Taki, H.; et al. Forest biodiversity, ecosystem functioning and the provision of ecosystem services. Biodivers. Conserv. 2017, 26, 3005–3035. [Google Scholar] [CrossRef]
- Rashidi, F.; Jalili, A.; Kafaki, S.B.; Sagheb-Talebi, K.; Hodgson, J. Anatomical responses of leaves of Black Locust (Robinia pseudoacacia L.) to urban pollutant gases and climatic factors. Trees Struct. Funct. 2012, 26, 363–375. [Google Scholar] [CrossRef]
- Samecka-Cymerman, A.; Kolon, K.; Kempers, A.J. Short shoots of Betula pendula Roth. as bioindicators of urban environmental pollution in Wrocław (Poland). Trees Struct. Funct. 2009, 23, 923–929. [Google Scholar] [CrossRef]
- Chen, Z.; He, X.; Cui, M.; Davi, N.; Zhang, X.; Chen, W.; Sun, Y. The effect of anthropogenic activities on the reduction of urban tree sensitivity to climatic change: Dendrochronological evidence from Chinese pine in Shenyang city. Trees Struct. Funct. 2011, 25, 393–405. [Google Scholar] [CrossRef]
- Scharenbroch, B.C.; Carter, D.; Bialecki, M.; Fahey, R.; Scheberl, L.; Catania, M.; Roman, L.A.; Bassuk, N.; Harper, R.W.; Werner, L.; et al. A rapid urban site index for assessing the quality of street tree planting sites. Urban For. Urban Green. 2017, 27, 279–286. [Google Scholar] [CrossRef]
- Mullaney, J. Using Permeable Pavements to Promote Street Tree Growth; University of the Sunshine Coast: Sippy Downs, Austria, 2015; ISBN 1312698837. [Google Scholar]
- Ghosh, S.; Scharenbroch, B.C.; Burcham, D.; Ow, L.F.; Shenbagavalli, S.; Mahimairaja, S. Influence of soil properties on street tree attributes in Singapore. Urban Ecosyst. 2016, 19, 949–967. [Google Scholar] [CrossRef]
- Dahlhausen, J.; Biber, P.; Rötzer, T.; Uhl, E.; Pretzsch, H. Tree species and their space requirements in six urban environments worldwide. Forests 2016, 7, 111. [Google Scholar] [CrossRef]
- Savi, T.; Bertuzzi, S.; Branca, S.; Tretiach, M.; Nardini, A. Drought-induced xylem cavitation and hydraulic deterioration: Risk factors for urban trees under climate change? New Phytol. 2015, 205, 1106–1116. [Google Scholar] [CrossRef] [PubMed]
- Hilbert, D.R.; Roman, L.A.; Koeser, A.K.; Vogt, J.; van Doorn, N.S. Urban tree mortality: A literature review. Arboric. Urban For. 2019, 45, 167–200. [Google Scholar] [CrossRef]
- Zhang, B.; Brack, C.L. Urban forest responses to climate change: A case study in Canberra. Urban For. Urban Green. 2021, 57, 126910. [Google Scholar] [CrossRef]
- Ruiz-Martinez, I.; Marraccini, E.; Debolini, M.; Bonari, E. Indicators of agricultural intensity and intensification: A review of the literature. Ital. J. Agron. 2015, 10, 74–84. [Google Scholar] [CrossRef]
- Martin, A.R.; Cadotte, M.W.; Isaac, M.E.; Milla, R.; Vile, D.; Violle, C. Regional and global shifts in crop diversity through the Anthropocene. PLoS ONE 2019, 14, e0209788. [Google Scholar] [CrossRef]
- Florence, A.M.; McGuire, A.M. Do diverse cover crop mixtures perform better than monocultures? A systematic review. Agron. J. 2020, 112, 3513–3534. [Google Scholar] [CrossRef]
- Salvati, L.; Ferrara, C. The local-scale impact of soil salinization on the socioeconomic context: An exploratory analysis in Italy. Catena 2015, 127, 312–322. [Google Scholar] [CrossRef]
- Iwu, C.D.; Korsten, L.; Okoh, A.I. The incidence of antibiotic resistance within and beyond the agricultural ecosystem: A concern for public health. Microbiologyopen 2020, 9, e1035. [Google Scholar] [CrossRef]
- Bennett, E.M.; Baird, J.; Baulch, H.; Chaplin-Kramer, R.; Fraser, E.; Loring, P.; Morrison, P.; Parrott, L.; Sherren, K.; Winkler, K.J.; et al. Ecosystem services and the resilience of agricultural landscapes. In Advances in Ecological Research; Academic Press: Cambridge, MA, USA, 2021; Volume 64, pp. 1–43. ISBN 9780128229798. [Google Scholar]
- Teshome, D.T.; Zharare, G.E.; Naidoo, S. The Threat of the Combined Effect of Biotic and Abiotic Stress Factors in Forestry under a Changing Climate. Front. Plant Sci. 2020, 11, 1874. [Google Scholar] [CrossRef]
- Anderegg, W.R.L.; Hicke, J.A.; Fisher, R.A.; Allen, C.D.; Aukema, J.; Bentz, B.; Hood, S.; Lichstein, J.W.; Macalady, A.K.; Mcdowell, N.; et al. Tree mortality from drought, insects, and their interactions in a changing climate. New Phytol. 2015, 208, 674–683. [Google Scholar] [CrossRef]
- IPCC. IPCC Special Report on the Impacts of Global Warming of 1.5 °C; Intergovernmental Panel on Climate Change (IPCC): Geneva, Switzerland, 2018; Volume 2. [Google Scholar]
- Siry, J.P.; Cubbage, F.W.; Potter, K.M.; McGinley, K. Current Perspectives on Sustainable Forest Management: North America. Curr. For. Rep. 2018, 4, 138–149. [Google Scholar] [CrossRef]
- Nerfa, L.; Rhemtulla, J.M.; Zerriffi, H. Forest dependence is more than forest income: Development of a new index of forest product collection and livelihood resources. World Dev. 2020, 125, 104689. [Google Scholar] [CrossRef]
- Pretzsch, H.; Biber, P.; Uhl, E.; Dauber, E. Long-term stand dynamics of managed spruce-fir-beech mountain forests in Central Europe: Structure, productivity and regeneration success. Forestry 2015, 88, 407–428. [Google Scholar] [CrossRef]
- Gardner, C.J.; Bicknell, J.E.; Baldwin-Cantello, W.; Struebig, M.J.; Davies, Z.G. Quantifying the impacts of defaunation on natural forest regeneration in a global meta-analysis. Nat. Commun. 2019, 10, 4590. [Google Scholar] [CrossRef] [PubMed]
- Escobedo, F.J.; Palmas-Perez, S.; Dobbs, C.; Gezan, S.; Hernandez, J. Spatio-temporal changes in structure for a mediterranean urban forest: Santiago, Chile 2002 to 2014. Forests 2016, 7, 121. [Google Scholar] [CrossRef]
- Miesbauer, J.W.; Gilman, E.F.; Masters, F.J.; Nitesh, S. Impact of branch reorientation on breaking stress in Liriodendron tulipifera L. Urban For. Urban Green. 2014, 13, 526–533. [Google Scholar] [CrossRef]
- Shigo, A.L.; Marx, H.G. Compartmentalization of Decay in Trees, Department of Agriculture, Forest Service: Washington, DC, USA, 1977; Volume 252.
- Boddy, L. Fungal Community Ecology and Wood Decomposition Processes in Angiosperms: From Standing Tree to Complete Decay of Coarse Woody Debris. Ecol. Bull. 2001, 49, 43–56. [Google Scholar]
- Paoletti, A.; Rosati, A.; Famiani, F. Effects of cultivar, fruit presence and tree age on whole-plant dry matter partitioning in young olive trees. Heliyon 2021, 7, e06949. [Google Scholar] [CrossRef]
- Suvanto, S.; Henttonen, H.M.; Nöjd, P.; Mäkinen, H. Forest susceptibility to storm damage is affected by similar factors regardless of storm type: Comparison of thunder storms and autumn extra-tropical cyclones in Finland. For. Ecol. Manag. 2016, 381, 17–28. [Google Scholar] [CrossRef]
- Morel, M.; Meux, E.; Mathieu, Y.; Thuillier, A.; Chibani, K.; Harvengt, L.; Jacquot, J.-P.; Gelhaye, E. Xenomic networks variability and adaptation traits in wood decaying fungi. Microb. Biotechnol. 2013, 6, 248–263. [Google Scholar] [CrossRef]
- Fay, N.; de Berker, N. A review of the theory and practice of tree coring on live ancient and veteran trees. Scott. Nat. Herit. 2018, 789, 843. [Google Scholar]
- Huang, Y.; Ren, Z.; Li, D.; Liu, X. Phenotypic techniques and applications in fruit trees: A review. Plant Methods 2020, 16, 107. [Google Scholar] [CrossRef] [PubMed]
- Leong, E.C.; Burcham, D.C.; Fong, Y.K. A purposeful classification of tree decay detection tools. Arboric. J. 2012, 34, 91–115. [Google Scholar] [CrossRef]
- Goh, C.L.; Abdul Rahim, R.; Fazalul Rahiman, M.H.; Mohamad Talib, M.T.; Tee, Z.C. Sensing wood decay in standing trees: A review. Sens. Actuators A Phys. 2018, 269, 276–282. [Google Scholar] [CrossRef]
- Pitarma, R.; Crisóstomo, J.; Ferreira, M.E. Contribution to trees health assessment using infrared thermography. Agriculture 2019, 9, 171. [Google Scholar] [CrossRef]
- Allison, R.B.; Wang, X. Chapter 7 Nondestructive Testing in the Urban Forest. USDA For. Serv. 2015, 238, 77–86. [Google Scholar]
- Giannakis, I.; Tosti, F.; Lantini, L.; Alani, A.M. Diagnosing Emerging Infectious Diseases of Trees Using Ground Penetrating Radar. IEEE Trans. Geosci. Remote Sens. 2020, 58, 1146–1155. [Google Scholar] [CrossRef]
- Espinosa, L.; Prieto, F.; Brancheriau, L.; Lasaygues, P. Ultrasonic imaging of standing trees: Factors influencing the decay detection. In Proceedings of the XXII Symposium on Image, Signal Processing and Artificial Vision (STSIVA), Bucaramanga, Colombia, 24–26 April 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Soge, A.O.; Popoola, O.I.; Adetoyinbo, A.A. A four-point electrical resistivity method for detecting wood decay and hollows in living trees. Eur. J. Wood Wood Prod. 2019, 77, 465–474. [Google Scholar] [CrossRef]
- Ishimwe, R.; Abutaleb, K.; Ahmed, F. Applications of Thermal Imaging in Agriculture—A Review. Adv. Remote Sens. 2014, 3, 128–140. [Google Scholar] [CrossRef]
- Dragavtsev, V.; Nartov, V.P. Application of Thermal Imaging in Agriculture and Forestry. Eur. Agrophys. J. 2015, 2, 15. [Google Scholar] [CrossRef]
- Still, C.; Powell, R.; Aubrecht, D.; Kim, Y.; Helliker, B.; Roberts, D.; Richardson, A.D.; Goulden, M. Thermal imaging in plant and ecosystem ecology: Applications and challenges. Ecosphere 2019, 10, e02768. [Google Scholar] [CrossRef]
- Al-doski, J.; Shattri, B.M.; Helmi-Zulhai, B.M.-S. Thermal Imaging for Pests Detecting—A Review. Int. J. Agric. For. Plant. 2016, 2, 10–30. [Google Scholar]
- Asner, G.P.; Martin, R.E.; Keith, L.M.; Heller, W.P.; Hughes, M.A.; Vaughn, N.R.; Hughes, R.F.; Balzotti, C. A spectral mapping signature for the Rapid Ohia Death (ROD) pathogen in Hawaiian forests. Remote Sens. 2018, 10, 404. [Google Scholar] [CrossRef]
- Lenthe, J.H.; Oerke, E.C.; Dehne, H.W. Digital infrared thermography for monitoring canopy health of wheat. Precis. Agric. 2007, 8, 15–26. [Google Scholar] [CrossRef]
- Chaerle, L.; De Boever, F.; Van Montagu, M.; Van der Straeten, D. Thermographic visualization of cell death in tobacco and Arabidopsis. Plant Cell Environ. 2001, 24, 15–25. [Google Scholar] [CrossRef]
- Agam, N.; Cohen, Y.; Berni, J.A.J.; Alchanatis, V.; Kool, D.; Dag, A.; Yermiyahu, U.; Ben-Gal, A. An insight to the performance of crop water stress index for olive trees. Agric. Water Manag. 2013, 118, 79–86. [Google Scholar] [CrossRef]
- Struthers, R.; Ivanova, A.; Tits, L.; Swennen, R.; Coppin, P. Thermal infrared imaging of the temporal variability in stomatal conductance for fruit trees. Int. J. Appl. Earth Obs. Geoinf. 2015, 39, 9–17. [Google Scholar] [CrossRef]
- Egea, G.; Padilla-Díaz, C.M.; Martinez-Guanter, J.; Fernández, J.E.; Pérez-Ruiz, M. Assessing a crop water stress index derived from aerial thermal imaging and infrared thermometry in super-high density olive orchards. Agric. Water Manag. 2017, 187, 210–221. [Google Scholar] [CrossRef]
- Ouledali, S.; Ennajeh, M.; Zrig, A.; Gianinazzi, S.; Khemira, H. Estimating the contribution of arbuscular mycorrhizal fungi to drought tolerance of potted olive trees (Olea europaea). Acta Physiol. Plant. 2018, 40, 81. [Google Scholar] [CrossRef]
- Oerke, E.C.; Fröhling, P.; Steiner, U. Thermographic assessment of scab disease on apple leaves. Precis. Agric. 2011, 12, 699–715. [Google Scholar] [CrossRef]
- Catena, A.; Catena, G.; Lugaresi, D.; Gasperoni, R.L. Termografia rivela la presenza di danni anche nell’apparato radicale degli alberi. Agric. Ric. 2002, 81–100. [Google Scholar]
- Catena, A.; Catena, G. Overview of thermal imaging for tree assessment. Arboric. J. 2008, 30, 259–270. [Google Scholar] [CrossRef]
- Bellett-Travers, M.; Morris, S. The relationship between surface temperature and radial wood thickness of twelve trees harvested in nottinghamshire. Arboric. J. 2010, 33, 15–26. [Google Scholar] [CrossRef]
- Vidal, D.; Pitarma, R. Infrared thermography applied to tree health assessment: A review. Agriculture 2019, 9, 156. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Kamatsos, E.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Estimating Productivity, Detecting Biotic Disturbances, and Assessing the Health State of Traditional Olive Groves, Using Nondestructive Phenotypic Techniques. Sustainability 2021, 14, 391. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Alsamail, M.Z.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Detecting, quantifying, and mapping urban trees’ structural defects using infrared thermography: Implications for tree risk assessment and management. Urban For. Urban Green. 2022, 75, 127691. [Google Scholar] [CrossRef]
- Zevgolis, Y.G.; Sazeides, C.I.; Zannetos, S.P.; Grammenou, V.; Fyllas, N.M.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Investigating the effect of resin collection and detecting fungal infection in resin-tapped and non-tapped pine trees, using minimally invasive and non-invasive diagnostics. For. Ecol. Manag. 2022, 524, 120498. [Google Scholar] [CrossRef]
- Loumou, A.; Giourga, C. Olive groves: “The life and identity of the Mediterranean”. Agric. Hum. Values 2003, 20, 87–95. [Google Scholar] [CrossRef]
- Palaiologou, P.; Kalabokidis, K.; Ager, A.A.; Day, M.A. Development of comprehensive fuel management strategies for reducing wildfire risk in Greece. Forests 2020, 11, 789. [Google Scholar] [CrossRef]
- Kosmas, C.; Danalatos, N.G.; Poesen, J.; Van Wesemael, B. The effect of water vapour adsorption on soil moisture content under Mediterranean climatic conditions. Agric. Water Manag. 1998, 36, 157–168. [Google Scholar] [CrossRef]
- Blozan, W. Tree measuring guidelines of the eastern native tree society. Featur. Artic. Bull. East. Nativ. Tree Soc. 2006, 1, 1–10. [Google Scholar]
- Assmann, E. The Principles of Forest Yield Study: Studies in the Organic Production, Structure, Increment and Yield of Forest Stands; Pergamon: Oxford, UK, 1970; ISBN 9781483150932. [Google Scholar]
- Kontogianni, A.; Tsitsoni, T.; Goudelis, G. An index based on silvicultural knowledge for tree stability assessment and improved ecological function in urban ecosystems. Ecol. Eng. 2011, 37, 914–919. [Google Scholar] [CrossRef]
- Soares, P.; Tomé, M. GLOBTREE, an individual tree growth model for Eucalyptus globulus in Portugal. In Modelling Forest Systems; Amaro, A., Reed, D., Soares, P., Eds.; CAB International: Wallingford, UK, 2003; pp. 97–110. ISBN 0851996930. [Google Scholar]
- Smiley, E.T.; Fraedrich, B.R. Determining strength loss from decay. J. Arboric. 1992, 18, 201–204. [Google Scholar] [CrossRef]
- Bréda, N.J.J. Ground-based measurements of leaf area index: A review of methods, instruments and current controversies. J. Exp. Bot. 2003, 54, 2403–2417. [Google Scholar] [CrossRef]
- Mezghani, M.A.; Gouta, H.; Laaribi, I.; Labidi, F. Leaf area index and light distribution in olive tree canopies (Olea europaea L.). Int. J. Agron. Agric. Res. 2016, 8, 60–65. [Google Scholar] [CrossRef]
- Nicolotti, G.; Gonthier, P.; Guglielmo, F. Advances in Detection and Identification of Wood Rotting Fungi in Timber and Standing Trees. In Molecular Identification of Fungi; Springer: Berlin/Heidelberg, Germany, 2010; pp. 251–276. ISBN 9783642050411. [Google Scholar]
- Slippers, B.; Wingfield, M.J. Botryosphaeriaceae as endophytes and latent pathogens of woody plants: Diversity, ecology and impact. Fungal Biol. Rev. 2007, 21, 90–106. [Google Scholar] [CrossRef]
- Salman, M. Biological control of Spilocaea oleagina, the causal agent of olive leaf spot disease, using antagonistic bacteria. J. Plant Pathol. 2017, 99, 741–744. [Google Scholar] [CrossRef]
- Minkina, W.; Dudzik, S. Algorithm of Infrared Camera Measurement Processing Path. In Infrared Thermography; John Wiley & Sons, Ltd.: Chichester, UK, 2009; pp. 41–60. ISBN 9780470747186. [Google Scholar]
- Faye, E.; Dangles, O.; Pincebourde, S. Distance makes the difference in thermography for ecological studies. J. Therm. Biol. 2016, 56, 1–9. [Google Scholar] [CrossRef]
- López-Bernal, Á.; Alcántara, E.; Testi, L.; Villalobos, F.J. Spatial sap flow and xylem anatomical characteristics in olive trees under different irrigation regimes. Tree Physiol. 2010, 30, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- Burcham, D.C.; Leong, E.C.; Fong, Y.K.; Tan, P.Y. An evaluation of internal defects and their effect on trunk surface temperature in Casuarina equisetifolia L. (Casuarinaceae). Arboric. Urban For. 2012, 38, 277–286. [Google Scholar] [CrossRef]
- Schönauer, M.; Hietz, P.; Schuldt, B.; Rewald, B. Root and branch hydraulic functioning and trait coordination across organs in drought-deciduous and evergreen tree species of a subtropical highland forest. Front. Plant Sci. 2023, 14, 1127292. [Google Scholar] [CrossRef] [PubMed]
- Catena, G. A new application of thermography. Atti Fond. Giorgio Ronchi 1990, 45, 947–952. [Google Scholar]
- Wong, M.; Tang, H.; Lam, L. Introduction to the Applications of Remote Sensing Techniques on the Tree Health Monitoring; HKIS: Hong Kong, China, 2020. [Google Scholar]
- Leverenz, J.W.; Hinckley, T.M. Shoot structure, leaf area index and productivity of evergreen conifer stands. Tree Physiol. 1990, 6, 135–149. [Google Scholar] [CrossRef]
- Zarate-valdez, J.L.; Whiting, M.L.; Lampinen, B.D.; Metcalf, S.; Ustin, S.L.; Brown, P.H. Prediction of leaf area index in almonds by vegetation indexes. Comput. Electron. Agric. 2012, 85, 24–32. [Google Scholar] [CrossRef]
- Musau, J.; Patil, S.; Sheffield, J.; Marshall, M. Spatio-temporal vegetation dynamics and relationship with climate over East Africa. Hydrol. Earth Syst. Sci. Discuss. 2016, 1–30. [Google Scholar] [CrossRef]
- Jonckheere, I.; Fleck, S.; Nackaerts, K.; Muys, B.; Coppin, P.; Weiss, M.; Baret, F. Review of methods for in situ leaf area index determination. Agric. For. Meteorol. 2004, 121, 19–35. [Google Scholar] [CrossRef]
- Parker, G.G. Tamm review: Leaf Area Index (LAI) is both a determinant and a consequence of important processes in vegetation canopies. For. Ecol. Manag. 2020, 477, 118496. [Google Scholar] [CrossRef]
- Liu, Y.; Ji, D.; Turgeon, R.; Chen, J.; Lin, T.; Huang, J.; Luo, J.; Zhu, Y.; Zhang, C.; Lv, Z. Physiological and proteomic responses of mulberry trees (Morus alba. L.) to combined salt and drought stress. Int. J. Mol. Sci. 2019, 20, 2486. [Google Scholar] [CrossRef] [PubMed]
- Van Hees, A.F.M. Growth and morphology of pedunculate oak (Quercus robur L.) and beech (Fagus sylvatica L.) seedlings in relation to shading and drought. Ann. Des Sci. For. 1997, 54, 9–18. [Google Scholar] [CrossRef]
- Johnson, D.M.; Wortemann, R.; McCulloh, K.A.; Jordan-Meille, L.; Ward, E.; Warren, J.M.; Palmroth, S.; Domec, J.C. A test of the hydraulic vulnerability segmentation hypothesis in angiosperm and conifer tree species. Tree Physiol. 2016, 36, 983–993. [Google Scholar] [CrossRef]
- Kim, Y.; Rahardjo, H.; Tsen-Tieng, D.L. Stability analysis of laterally loaded trees based on tree-root-soil interaction. Urban For. Urban Green. 2020, 49, 126639. [Google Scholar] [CrossRef]
- Kim, Y.; Rahardjo, H.; Tsen-Tieng, D.L. Mechanical behavior of trees with structural defects under lateral load: A numerical modeling approach. Urban For. Urban Green. 2021, 59, 126987. [Google Scholar] [CrossRef]
- Kopaczyk, J.M.; Warguła, J.; Jelonek, T. The variability of terpenes in conifers under developmental and environmental stimuli. Environ. Exp. Bot. 2020, 180, 104197. [Google Scholar] [CrossRef]
- Dias, M.C.; Azevedo, C.; Costa, M.; Pinto, G.; Santos, C. Melia azedarach plants show tolerance properties to water shortage treatment: An ecophysiological study. Plant Physiol. Biochem. 2014, 75, 123–127. [Google Scholar] [CrossRef] [PubMed]
| Phenotypic Traits | R. pseudoacacia (N =174) | M. alba (N = 65) | M. azedarach (N = 48) | O. europaea (N =80) | P. brutia (N = 176) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| H (m) | 6.15 | 1.65 | 7.57 | 3.06 | 8.22 | 3.12 | 7.25 | 2.74 | 14.04 | 4.18 |
| DBH (cm) | 41.01 | 9.70 | 40.23 | 11.33 | 28.68 | 10.67 | 74.41 | 15.45 | 37.15 | 9.94 |
| CR | 0.66 | 0.12 | 0.74 | 0.08 | 0.71 | 0.12 | 0.65 | 0.09 | 0.35 | 0.10 |
| CA (m2) | 33.47 | 13.76 | 25.45 | 11.37 | 23.72 | 14.55 | 51.78 | 24.69 | 46.36 | 33.8 |
| Cavities (ratio) | 0.02 | 0.03 | 0.03 | 0.03 | 0.03 | 0.06 | 0.05 | 0.04 | 0.03 | 0.04 |
| VD (cm) | 15.14 | 13.38 | 13.41 | 11.15 | 6.82 | 8.50 | 12.88 | 11.23 | 36.07 | 39.56 |
| HD (cm) | 37.91 | 44.56 | 21.84 | 22.59 | 21.12 | 30.48 | 18.56 | 13.31 | 9.06 | 8.64 |
| ID (cm) | 14.25 | 13.89 | 13.00 | 13.82 | 6.87 | 9.77 | 14.15 | 9.33 | 4.56 | 4.61 |
| SL (%) | 18.99 | 27.65 | 29.83 | 42.95 | 24.48 | 47.10 | 18.92 | 9.51 | 6.37 | 5.582 |
| FP (%) | −30.49 | 37.39 | −17.93 | 16.24 | −24.66 | 34.53 | −37.12 | 18.23 | −6.96 | 6.42 |
| LAImean | 2.21 | 0.76 | 1.85 | 0.83 | 2.01 | 0.72 | 2.14 | 1.01 | 1.05 | 0.43 |
| LAIcav | 2.06 | 0.9 | 1.71 | 0.95 | 1.85 | 0.77 | 1.8 | 0.86 | 0.92 | 0.59 |
| LAInon-cav | 2.36 | 0.72 | 1.98 | 0.82 | 2.18 | 0.75 | 2.45 | 1.47 | 1.18 | 0.51 |
| LAIrange | 0.46 | 0.47 | 0.49 | 0.47 | 0.36 | 0.46 | 2.44 | 1.12 | 0.55 | 0.48 |
| Tree Species | Indices | t-Value | df | p-Value |
|---|---|---|---|---|
| R. pseudoacacia | TIQR | −4.98 | 63.79 | <0.05 |
| TOPR | −4.99 | 63.87 | <0.05 | |
| LAImean | 7.65 | 73.64 | <0.05 | |
| LAIrange | −3.39 | 46.22 | <0.05 | |
| M. alba | TIQR | −6.13 | 60.50 | <0.05 |
| TOPR | −6.14 | 60.46 | <0.05 | |
| LAImean | 5.83 | 29.27 | <0.05 | |
| LAIrange | −1.88 | 16.65 | <0.05 | |
| M. azedarach | TIQR | −3.38 | 43.06 | <0.05 |
| TOPR | −3.37 | 43.06 | <0.05 | |
| LAImean | 3.70 | 45.10 | <0.05 | |
| LAIrange | −2.33 | 45.98 | <0.05 | |
| O. europaea | TIQR | −3.77 | 8.48 | <0.05 |
| TOPR | −2.79 | 6.08 | <0.05 | |
| LAImean | 3.73 | 6.27 | <0.05 | |
| LAIrange | −1.63 | 7.01 | >0.05 | |
| P. brutia | TIQR | −13.99 | 172.24 | <0.05 |
| TOPR | −6.48 | 137.41 | <0.05 | |
| LAImean | 5.83 | 132.05 | <0.05 | |
| LAIrange | −3.52 | 152.92 | <0.05 |
| Trees Classification | Response Variable | Predictor Variables | B | SE B | β | t | p-Value | R2 adj. | F |
|---|---|---|---|---|---|---|---|---|---|
| All five species | LAImean | (constant) | −0.13 | 0.04 | −3.26 | 0.001 | 0.45 | 128.24 | |
| TIQR | −0.5 | 0.03 | −0.52 | −14.16 | 0.001 | ||||
| LAIrange | −0.23 | 0.07 | −0.12 | −3.37 | 0.001 | ||||
| SL | −0.23 | 0.03 | −0.23 | −6.65 | 0.001 | ||||
| Deciduous | LAImean | (constant) | 0.22 | 0.06 | 3.57 | 0.001 | 0.54 | 115.44 | |
| TIQR | −0.25 | 0.09 | −0.15 | −2.65 | 0.008 | ||||
| LAIrange | −0.41 | 0.07 | −0.21 | −5.36 | 0.001 | ||||
| SL | −0.44 | 0.04 | −0.55 | −9.66 | 0.001 | ||||
| Evergreen | LAImean | (constant) | −0.55 | 0.04 | −13.21 | 0.001 | 0.35 | 97.15 | |
| TIQR | −0.29 | 0.03 | −0.59 | −9.85 | 0.001 |
| (a) Defects | |||||
|---|---|---|---|---|---|
| Predictor | Β | S.E. | Wald’s χ2 | df | p-Value |
| TIQR | 3.03 | 0.47 | 40.75 | 1 | 0.001 |
| LAIcav | −1.29 | 0.32 | 15.65 | 1 | 0.001 |
| CA | 2.67 | 0.52 | 25.52 | 1 | 0.001 |
| Constant | 2.09 | 0.36 | 32.35 | 1 | 0.001 |
| (b) Cavities | |||||
| TIQR | 2.09 | 0.31 | 43.70 | 1 | 0.001 |
| TOPR | −0.57 | 0.21 | 6.87 | 1 | 0.001 |
| LAIcav | −1.00 | 0.15 | 40.34 | 1 | 0.001 |
| LAIrange | 1.49 | 0.21 | 47.32 | 1 | 0.001 |
| Constant | 1.13 | 0.17 | 43.13 | 1 | 0.001 |
| (c) Fungal infestation | |||||
| TIQR | 2.09 | 0.27 | 58.62 | 1 | 0.001 |
| LAIcav | −1.35 | 0.21 | 38.21 | 1 | 0.001 |
| LAIrange | 0.88 | 0.20 | 19.37 | 1 | 0.001 |
| Constant | −0.64 | 0.16 | 15.34 | 1 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zevgolis, Y.G.; Akriotis, T.; Dimitrakopoulos, P.G.; Troumbis, A.Y. Integrating Thermal Indices and Phenotypic Traits for Assessing Tree Health: A Comprehensive Framework for Conservation and Monitoring of Urban, Agricultural, and Forest Ecosystems. Appl. Sci. 2023, 13, 9493. https://doi.org/10.3390/app13179493
Zevgolis YG, Akriotis T, Dimitrakopoulos PG, Troumbis AY. Integrating Thermal Indices and Phenotypic Traits for Assessing Tree Health: A Comprehensive Framework for Conservation and Monitoring of Urban, Agricultural, and Forest Ecosystems. Applied Sciences. 2023; 13(17):9493. https://doi.org/10.3390/app13179493
Chicago/Turabian StyleZevgolis, Yiannis G., Triantaphyllos Akriotis, Panayiotis G. Dimitrakopoulos, and Andreas Y. Troumbis. 2023. "Integrating Thermal Indices and Phenotypic Traits for Assessing Tree Health: A Comprehensive Framework for Conservation and Monitoring of Urban, Agricultural, and Forest Ecosystems" Applied Sciences 13, no. 17: 9493. https://doi.org/10.3390/app13179493
APA StyleZevgolis, Y. G., Akriotis, T., Dimitrakopoulos, P. G., & Troumbis, A. Y. (2023). Integrating Thermal Indices and Phenotypic Traits for Assessing Tree Health: A Comprehensive Framework for Conservation and Monitoring of Urban, Agricultural, and Forest Ecosystems. Applied Sciences, 13(17), 9493. https://doi.org/10.3390/app13179493










