Impact of Bird Cherry (Prunus padus) Extracts on the Oxidative Stability of a Model O/W Linoleic Acid Emulsion
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Extracts
2.3. Preparation of Emulsions
2.4. Measurement of Mean Particle Size (Z-Ave), Zeta Potential (ZP), and Polydispersity Index (PDI)
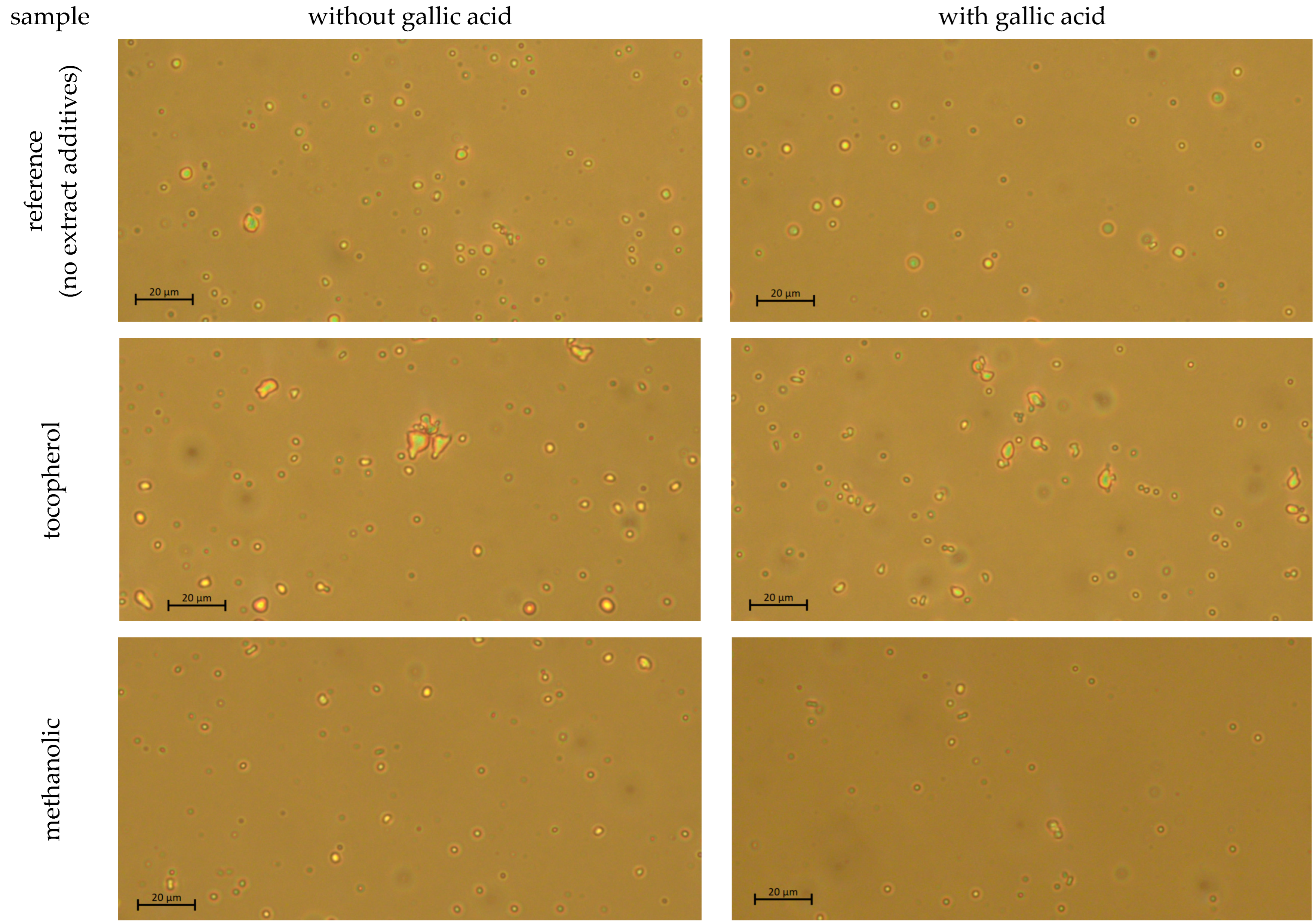
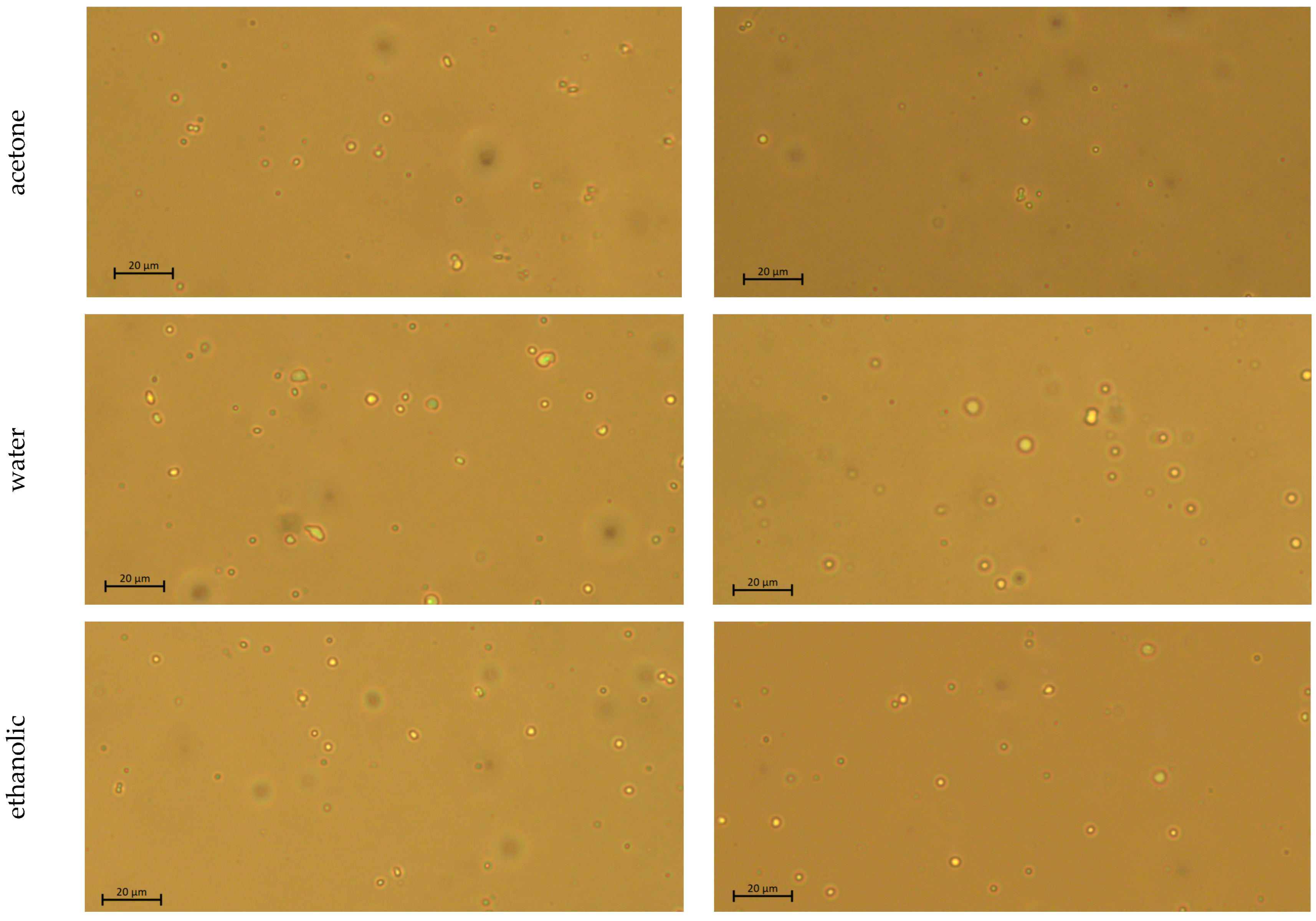
2.5. Microscopic Observation
2.6. Storage Stability of Emulsions
2.7. Oxidation Tests
2.8. Data Evaluation and Statistical Analysis
3. Results and Discussion
3.1. Physiochemical Properties of Emulsions
3.1.1. Freshly Prepared Emulsion Systems
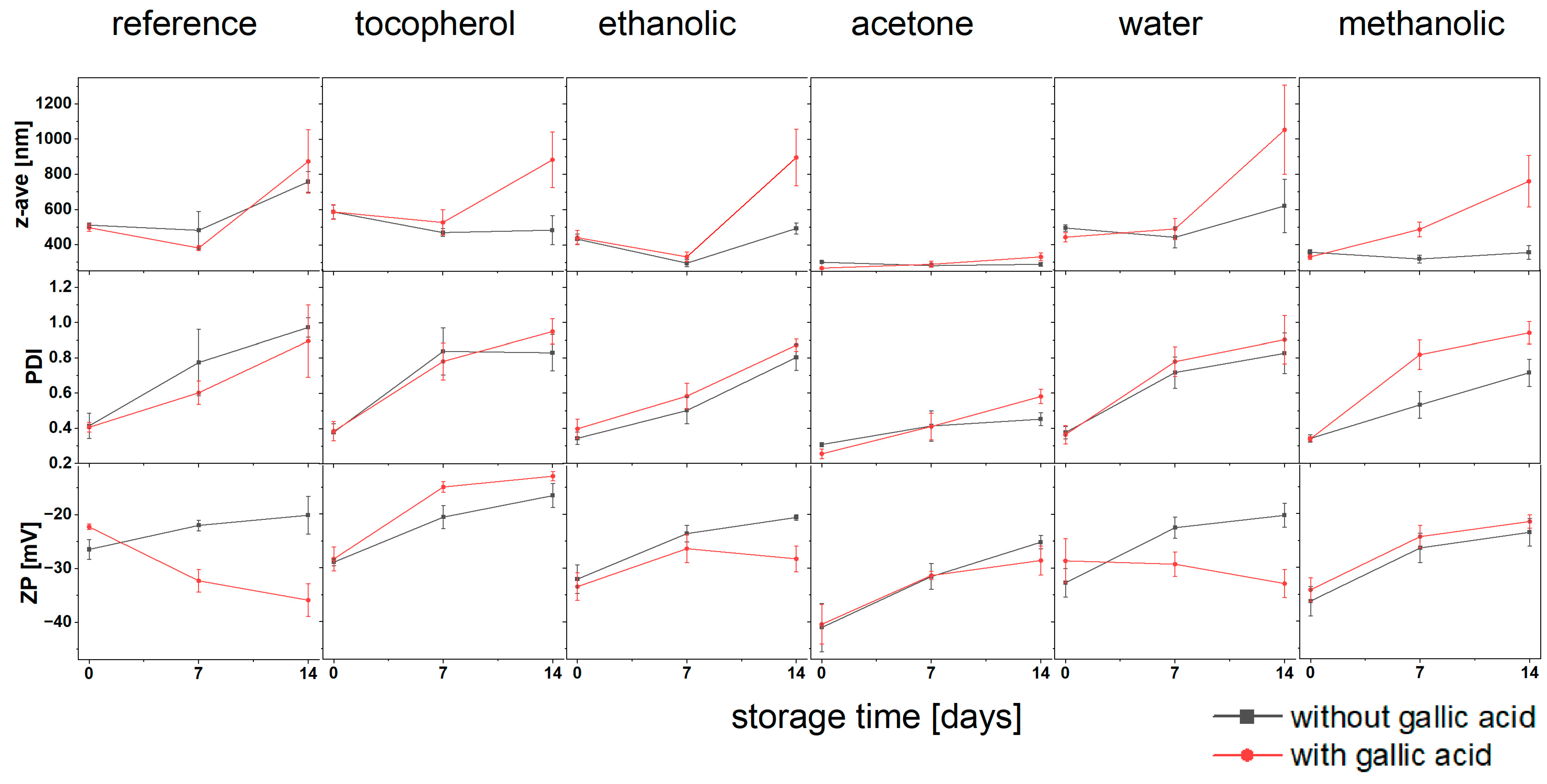
3.1.2. Storage Stability of Produced Formulations
3.2. Oxidation Tests
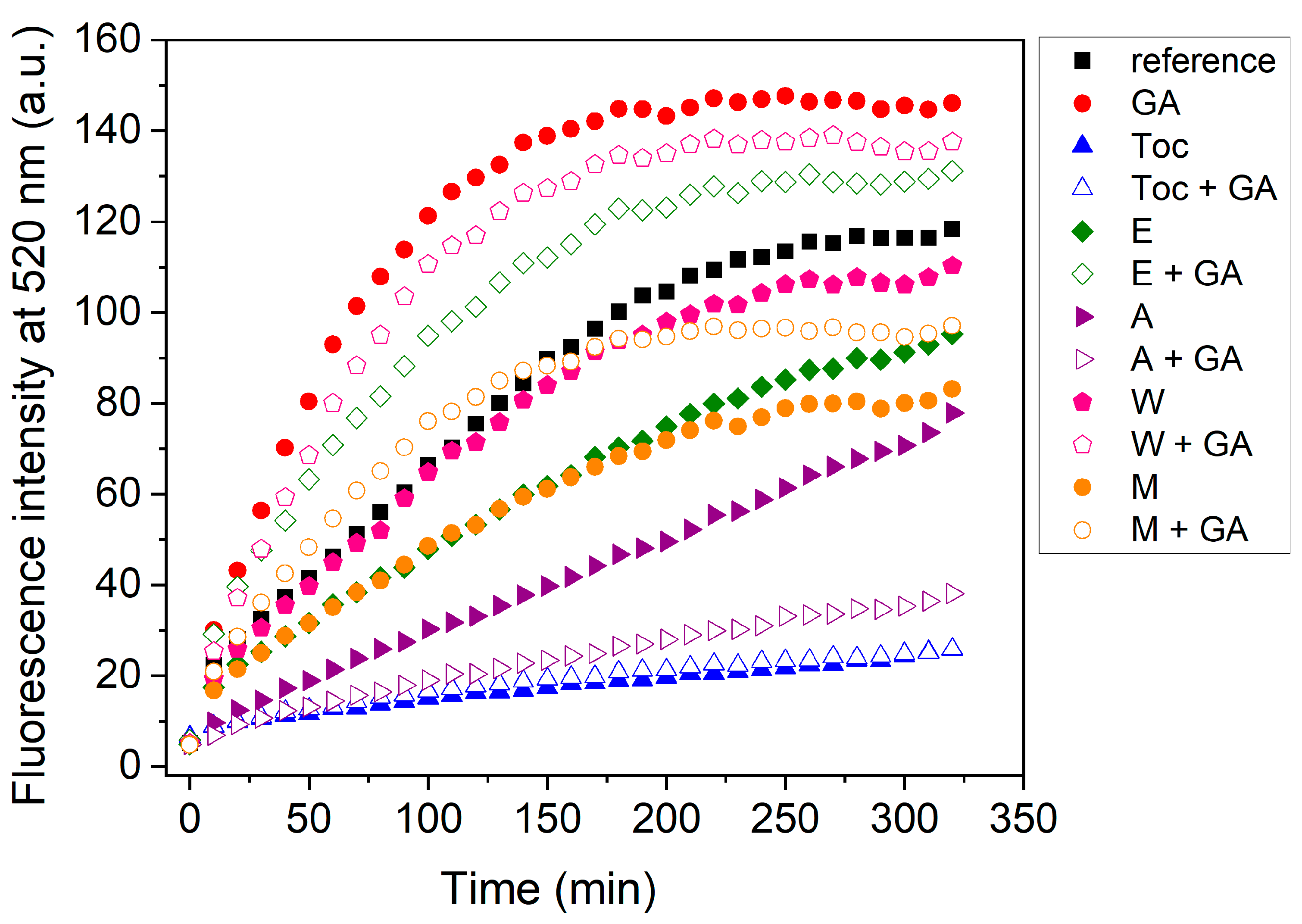
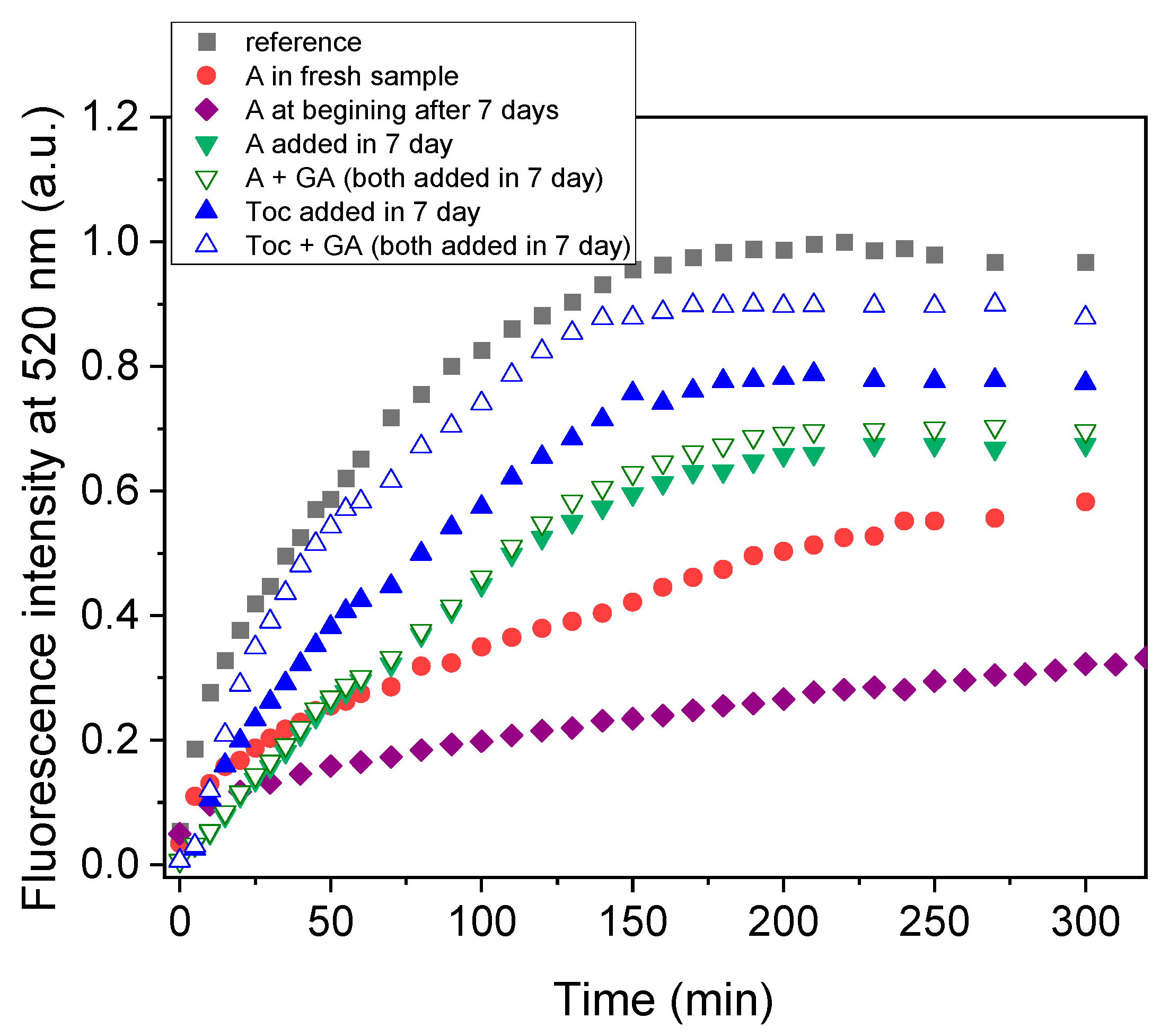
3.2.1. Oxidative Stability of Extract-Loaded Emulsions
3.2.2. Storage Stability of Extract-Loaded Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Felix, M.; Cermeño, M.; FitzGerald, R.J. Structure and in vitro bioactive properties of O/W emulsions generated with fava bean protein hydrolysates. Food Res. Int. 2021, 150, 110780. [Google Scholar] [CrossRef] [PubMed]
- Pintado, T.; Herrero, A.; Ruiz-Capillas, C.; Triki, M.; Carmona, P.; Jiménez-Colmenero, F. Effects of emulsion gels containing bioactive compounds on sensorial, technological, and structural properties of frankfurters. Food Sci. Technol. Int. 2016, 22, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Yu, C.; Guo, S.; Chiou, B.-S.; Jia, M.; Xu, F.; Chen, M.; Zhong, F. Extending shelf life of chilled pork meat by cinnamaldehyde nano emulsion at non-contact mode. Food Packag. Shelf Life 2023, 37, 101067. [Google Scholar] [CrossRef]
- Nejadmansouri, M.; Hosseini, S.M.H.; Niakosari, M.; Yousefi, G.H.; Golmakani, M.T. Physicochemical properties and storage stability of ultrasound-mediated WPI-stabilized fish oil nanoemulsions. Food Hydrocoll. 2016, 61, 801–811. [Google Scholar] [CrossRef]
- Fan, H.; Zhu, P.; Hui, G.; Shen, Y.; Yong, Z.; Xie, Q.; Wang, M. Mechanism of synergistic stabilization of emulsions by amorphous taro starch and protein and emulsion stability. Food Chem. 2023, 424, 136342. [Google Scholar] [CrossRef]
- Zhang, S.; Tian, L.; Yi, J.; Zhu, Z.; Decker, E.A.; McClements, D.J. Mixed plant-based emulsifiers inhibit the oxidation of proteins and lipids in walnut oil-in-water emulsions: Almond protein isolate-camellia saponin. Food Hydrocoll. 2020, 109, 106136. [Google Scholar] [CrossRef]
- Rajasekaran, B.; Singh, A.; Benjakul, S. Combined effect of chitosan and bovine serum albumin/whey protein isolate on the characteristics and stability of shrimp oil-in-water emulsion. J. Food Sci. 2022, 87, 2879–2893. [Google Scholar] [CrossRef]
- Komaiko, J.S.; McClements, D.J. Formation of Food-Grade Nanoemulsions Using Low-Energy Preparation Methods: A Review of Available Methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–352. [Google Scholar] [CrossRef]
- McClements, D.J. Food Emulsions; CRC Press: Boca Raton, FL, USA, 2015; ISBN 9780429154034. [Google Scholar]
- Blondeau, N.; Lipsky, R.H.; Bourourou, M.; Duncan, M.W.; Gorelick, P.B.; Marini, A.M. Alpha-Linolenic Acid: An Omega-3 Fatty Acid with Neuroprotective Properties—Ready for Use in the Stroke Clinic? Biomed. Res. Int. 2015, 2015, 519830. [Google Scholar] [CrossRef]
- Rodriguez-Leyva, D.; Bassett, C.M.C.; McCullough, R.; Pierce, G.N. The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can. J. Cardiol. 2010, 26, 489–496. [Google Scholar] [CrossRef]
- Bourre, J.M. Where to find omega-3 fatty acids and how feeding animals with diet enriched in omega-3 fatty acids to increase nutritional value of derived products for human: What is actually useful? J. Nutr. Health Aging 2005, 9, 232–242. [Google Scholar] [PubMed]
- Yamamoto, Y.; Hiyama, S.; Takase, Y.; Kadowaki, A.; Hara, S. Effects of Antioxidants and Additional Emulsifiers on the Stability of Emulsified Milk Fat in the Photo/Radical Oxidation System. J. Oleo Sci. 2014, 63, 893–901. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Berton-Carabin, C.; Schroën, K. Towards new food emulsions: Designing the interface and beyond. Curr. Opin. Food Sci. 2019, 27, 74–81. [Google Scholar] [CrossRef]
- Mcclements, D.J.; Decker, E.A. Lipid Oxidation in Oil-in-Water Emulsions: Impact of Molecular Environment on Chemical Reactions in Heterogeneous Food Systems. J. Food Sci. 2000, 65, 1270–1282. [Google Scholar] [CrossRef]
- Niknam, S.M.; Kashaninejad, M.; Escudero, I.; Sanz, M.T.; Beltrán, S.; Benito, J.M. Preparation of Water-in-Oil Nanoemulsions Loaded with Phenolic-Rich Olive Cake Extract Using Response Surface Methodology Approach. Foods 2022, 11, 279. [Google Scholar] [CrossRef]
- Martins, C.; Higaki, N.T.F.; Montrucchio, D.P.; de Oliveira, C.F.; Gomes, M.L.S.; Miguel, M.D.; Miguel, O.G.; Zanin, S.M.W.; de Dias, J.F.G. Development of W1/O/W2 emulsion with gallic acid in the internal aqueous phase. Food Chem. 2020, 314, 126174. [Google Scholar] [CrossRef]
- Gomes, A.; Costa, A.L.R.; de Assis Perrechil, F.; da Cunha, R.L. Role of the phases composition on the incorporation of gallic acid in O/W and W/O emulsions. J. Food Eng. 2016, 168, 205–214. [Google Scholar] [CrossRef]
- Kaczmarek-Szczepańska, B.; Kozak, A.; Zasada, L. The Preparation and Characterization of Emulsions with the Addition of Tannic Acid and Gallic Acid. Curr. Cosmet. Sci. 2022, 1, e120422203422. [Google Scholar] [CrossRef]
- Shahidi, F.; Zhong, Y. Revisiting the Polar Paradox Theory: A Critical Overview. J. Agric. Food Chem. 2011, 59, 3499–3504. [Google Scholar] [CrossRef]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Relationship between Hydrophobicity and Antioxidant Ability of “Phenolipids” in Emulsion: A Parabolic Effect of the Chain Length of Rosmarinate Esters. J. Agric. Food Chem. 2010, 58, 2869–2876. [Google Scholar] [CrossRef]
- Laguerre, M.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Baréa, B.; Weiss, J.; Decker, E.A.; Villeneuve, P. Chain Length Affects Antioxidant Properties of Chlorogenate Esters in Emulsion: The Cutoff Theory behind the Polar Paradox. J. Agric. Food Chem. 2009, 57, 11335–11342. [Google Scholar] [CrossRef] [PubMed]
- Farooq, S.; Abdullah; Zhang, H.; Weiss, J. A comprehensive review on polarity, partitioning, and interactions of phenolic antioxidants at oil–water interface of food emulsions. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4250–4277. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Comelles, F.; Alcántara, D.; Maldonado, O.S.; Curcuroze, M.; Parra, J.L.; Morales, J.C. Surface-Active Properties of Lipophilic Antioxidants Tyrosol and Hydroxytyrosol Fatty Acid Esters: A Potential Explanation for the Nonlinear Hypothesis of the Antioxidant Activity in Oil-in-Water Emulsions. J. Agric. Food Chem. 2010, 58, 8021–8026. [Google Scholar] [CrossRef] [PubMed]
- Laguerre, M.; Bayrasy, C.; Panya, A.; Weiss, J.; McClements, D.J.; Lecomte, J.; Decker, E.A.; Villeneuve, P. What Makes Good Antioxidants in Lipid-Based Systems? The Next Theories Beyond the Polar Paradox. Crit. Rev. Food Sci. Nutr. 2015, 55, 183–201. [Google Scholar] [CrossRef]
- Siejak, P.; Smułek, W.; Karnowska, J.N.; Dembska, A.; Neunert, G.; Polewski, K. Bird Cherry (Prunus padus) Fruit Extracts Inhibit Lipid Peroxidation in PC Liposomes: Spectroscopic, HPLC, and GC–MS Studies. Appl. Sci. 2022, 12, 7820. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Misawa, R. Effect of Emulsifier Concentration on the Oxidation of an O/W Emulsion Prepared from Canola Oil. Food Nutr. Sci. 2018, 9, 683–692. [Google Scholar] [CrossRef][Green Version]
- Rehman, A.; Jafari, S.M.; Tong, Q.; Karim, A.; Mahdi, A.A.; Iqbal, M.W.; Aadil, R.M.; Ali, A.; Manzoor, M.F. Role of peppermint oil in improving the oxidative stability and antioxidant capacity of borage seed oil-loaded nanoemulsions fabricated by modified starch. Int. J. Biol. Macromol. 2020, 153, 697–707. [Google Scholar] [CrossRef]
- Honary, S.; Zahir, F. Effect of Zeta Potential on the Properties of Nano-Drug Delivery Systems—A Review (Part 2). Trop. J. Pharm. Res. 2013, 12, 2. [Google Scholar] [CrossRef]
- Abbas, S.; Bashari, M.; Akhtar, W.; Li, W.W.; Zhang, X. Process optimization of ultrasound-assisted curcumin nanoemulsions stabilized by OSA-modified starch. Ultrason. Sonochem. 2014, 21, 1265–1274. [Google Scholar] [CrossRef]
- Cabrera-Trujillo, M.A.; Filomena-Ambrosio, A.; Quintanilla-Carvajal, M.X.; Sotelo-Díaz, L.I. Stability of low-fat oil in water emulsions obtained by ultra turrax, rotor-stator and ultrasound homogenization methods. Int. J. Gastron. Food Sci. 2018, 13, 58–64. [Google Scholar] [CrossRef]
- Marques, C.; Sotiles, A.R.; Farias, F.O.; Oliveira, G.; Mitterer-Daltoé, M.L.; Masson, M.L. Full physicochemical characterization of malic acid: Emphasis in the potential as food ingredient and application in pectin gels. Arab. J. Chem. 2020, 13, 9118–9129. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, J.; Zhou, Y.; Cheng, Y.; Nirasawa, S. Improving solubility and emulsifying property of wheat gluten by deamidation with four different acids: Effect of replacement of folded conformation by extended structure. Food Hydrocoll. 2017, 72, 105–114. [Google Scholar] [CrossRef]
- Teo, B.S.X.; Basri, M.; Zakaria, M.R.S.; Salleh, A.B.; Rahman, R.N.Z.R.A.; Rahman, M.B.A. A potential tocopherol acetate loaded palm oil esters-in-water nanoemulsions for nanocosmeceuticals. J. Nanobiotechnology 2010, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Relkin, P.; Jung, J.M.; Ollivon, M. Factors affecting vitamin degradation in oil-in-water nano-emulsions. J. Therm. Anal. Calorim. 2009, 98, 13–18. [Google Scholar] [CrossRef]
- Liu, K.; Liu, Y.; Chen, F. Effect of storage temperature on lipid oxidation and changes in nutrient contents in peanuts. Food Sci. Nutr. 2019, 7, 2280–2290. [Google Scholar] [CrossRef]
- Ghelichi, S.; Hajfathalian, M.; Yesiltas, B.; Sørensen, A.M.; García-Moreno, P.J.; Jacobsen, C. Oxidation and oxidative stability in emulsions. Compr. Rev. Food Sci. Food Saf. 2023, 22, 1864–1901. [Google Scholar] [CrossRef]
- Drummen, G.P.C.; Van Liebergen, L.C.M.; Op den Kamp, J.A.F.; Post, J.A. C11-BODIPY581/591, an oxidation-sensitive fluorescent lipid peroxidation probe: (Micro)spectroscopic characterization and validation of methodology. Free Radic. Biol. Med. 2002, 33, 473–490. [Google Scholar] [CrossRef]
- Pap, E.H.W.; Drummen, G.P.C.; Winter, V.J.; Kooij, T.W.A.; Rijken, P.; Wirtz, K.W.A.; Op den Kamp, J.A.F.; Hage, W.J.; Post, J.A. Ratio-fluorescence microscopy of lipid oxidation in living cells using C11-BODIPY581/591. FEBS Lett. 1999, 453, 278–282. [Google Scholar] [CrossRef]
- Neunert, G.; Górnaś, P.; Dwiecki, K.; Siger, A.; Polewski, K. Synergistic and antagonistic effects between alpha-tocopherol and phenolic acids in liposome system: Spectroscopic study. Eur. Food Res. Technol. 2015, 241, 749–757. [Google Scholar] [CrossRef]
- Wang, F.; Naowarojna, N.; Zou, Y. Stratifying ferroptosis sensitivity in cells and mouse tissues by photochemical activation of lipid peroxidation and fluorescent imaging. STAR Protoc. 2022, 3, 101189. [Google Scholar] [CrossRef]
- Viglianisi, C.; Menichetti, S. Chain Breaking Antioxidant Activity of Heavy (S, Se, Te) Chalcogens Substituted Polyphenols. Antioxidants 2019, 8, 487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Long, Q.; Zhou, B.; Prenzler, P.D.; Zhong, H. Antioxidant Activity of Phenolic Compounds in Bulk Camellia Oil and Corresponding Oil in Water (O/W) Emulsions. Adv. J. Food Sci. Technol. 2013, 5, 1238–1243. [Google Scholar] [CrossRef]
- Kampa, J.; Frazier, R.; Rodriguez-Garcia, J. Physical and Chemical Characterisation of Conventional and Nano/Emulsions: Influence of Vegetable Oils from Different Origins. Foods 2022, 11, 681. [Google Scholar] [CrossRef] [PubMed]
- Telichowska, A.; Kobus-Cisowska, J.; Stuper-Szablewska, K.; Ligaj, M.; Tichoniuk, M.; Szymanowska, D.; Szulc, P. Exploring antimicrobial and antioxidant properties of phytocomponents from different anatomical parts of Prunus padus L. Int. J. Food Prop. 2020, 23, 2097–2109. [Google Scholar] [CrossRef]
- Donno, D.; Mellano, M.G.; De Biaggi, M.; Riondato, I.; Rakotoniaina, E.N.; Beccaro, G.L. New findings in prunus padus l. Fruits as a source of natural compounds: Characterization of metabolite profiles and preliminary evaluation of antioxidant activity. Molecules 2018, 23, 725. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; McClements, D.J.; Decker, E.A. Lipid Oxidation in Corn Oil-in-Water Emulsions Stabilized by Casein, Whey Protein Isolate, and Soy Protein Isolate. J. Agric. Food Chem. 2003, 51, 1696–1700. [Google Scholar] [CrossRef]

| Agent/Extract Added | Mean Droplet Size by Intensity Z-Ave (nm) | Dominant Droplet Diameter by Number (nm) | PDI | ZP (mV) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Without Gallic Acid | With Gallic Acid | Without Gallic Acid | With Gallic Acid | Without Gallic Acid | With Gallic Acid | Without Gallic Acid | With Gallic Acid | ||
| No addition (reference sample) | 511.0 ± 13.1 b | 496.5 ± 21.4 b,c | 260.6 ± 74.7 a | 268.4 ± 27.4 a | 0.414 ± 0.072 a | 0.406 ± 0.028 a | −26.5 ± 3.1 e,f | −22.3 ± 1.8 f | |
| tocopherol | 587.5 ± 38.2 a | 587.8 ± 41.5 a | 280.9 ± 44.1 a | 294.8 ± 120.1 a | 0.379 ± 0.048 a,b | 0.385 ± 0.055 a,b | −28.8 ± 1.9 c,d,e | −28.4 ± 3.6 d,e | |
| Bird Cherry Extracts | Ethanolic | 433.2 ± 28.2 e | 441.5 ± 40.2 d,e | 208.4 ± 83.9 a | 225.1 ± 27.9 a | 0.343 ± 0.035 a,b,c | 0.397 ± 0.055 a,b | −32.1 ± 4.0 b,c,d | −33.5 ± 3.9 b,c |
| Acetone | 302.2 ± 5.2 f,g | 269.0 ± 6.4 g | 187.1 ± 27.4 a | 203.2 ± 43.2 a | 0.308 ± 0.012 b,c | 0.255 ± 0.027 c | −41.1 ± 5.8 a | −40.5 ± 5.0 a | |
| Water | 494.8 ± 17.6 b,c,d | 442.3 ± 26.4 c,d,e | 230.8 ± 70.8 a | 181.9 ± 55.9 a | 0.376 ± 0.035 a,b | 0.364 ± 0.052 a,b | −32.8 ± 4.0 b,c,d | −28.7 ± 5.4 c,d,e | |
| Methanolic | 357.0 ± 14.3 f | 330.3 ± 12.5 f | 214.9 ± 44.4 a | 216.1 ± 30.3 a | 0.342 ± 0.020 a,b,c | 0.339 ± 0.013 a,b,c | −36.3 ± 4.0 a,b | −34.2 ± 3.5 b | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siejak, P.; Neunert, G.; Smułek, W.; Polewski, K. Impact of Bird Cherry (Prunus padus) Extracts on the Oxidative Stability of a Model O/W Linoleic Acid Emulsion. Appl. Sci. 2023, 13, 9560. https://doi.org/10.3390/app13179560
Siejak P, Neunert G, Smułek W, Polewski K. Impact of Bird Cherry (Prunus padus) Extracts on the Oxidative Stability of a Model O/W Linoleic Acid Emulsion. Applied Sciences. 2023; 13(17):9560. https://doi.org/10.3390/app13179560
Chicago/Turabian StyleSiejak, Przemysław, Grażyna Neunert, Wojciech Smułek, and Krzysztof Polewski. 2023. "Impact of Bird Cherry (Prunus padus) Extracts on the Oxidative Stability of a Model O/W Linoleic Acid Emulsion" Applied Sciences 13, no. 17: 9560. https://doi.org/10.3390/app13179560
APA StyleSiejak, P., Neunert, G., Smułek, W., & Polewski, K. (2023). Impact of Bird Cherry (Prunus padus) Extracts on the Oxidative Stability of a Model O/W Linoleic Acid Emulsion. Applied Sciences, 13(17), 9560. https://doi.org/10.3390/app13179560









