Study of Wettability Alteration of Hydrophobic Carbonate Rock by Surfactant-Containing Chelating Agent Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Rock Hydrophobization
2.2.2. Solutions Preparation
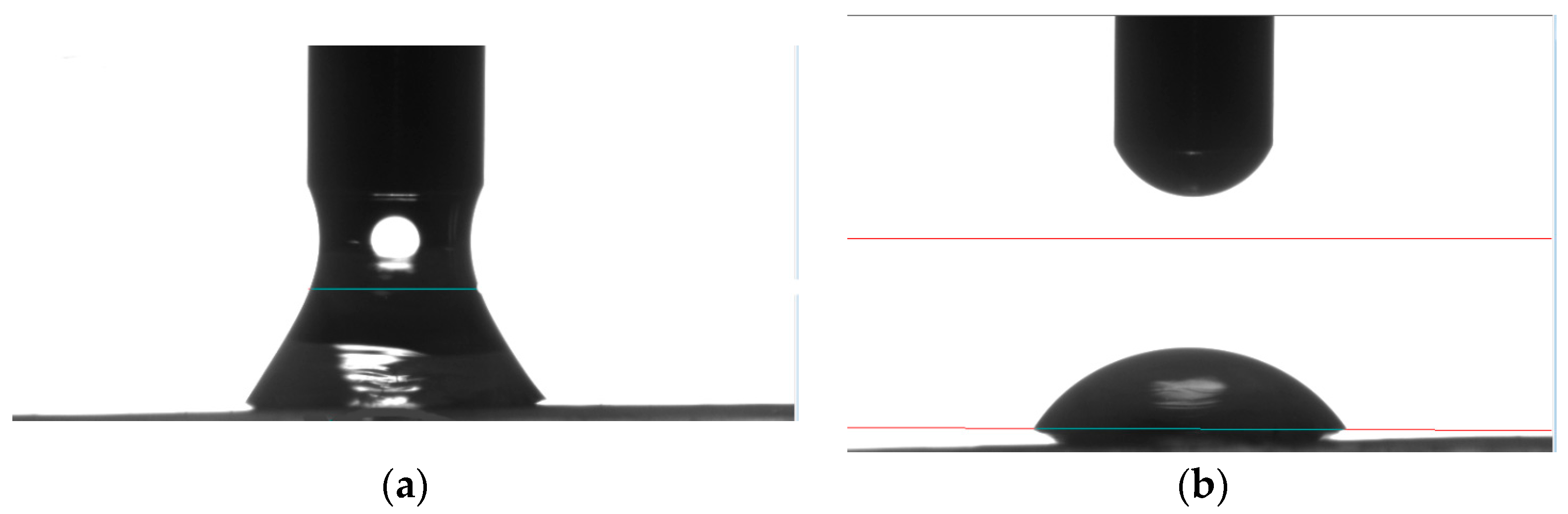
2.2.3. IFT Measurement
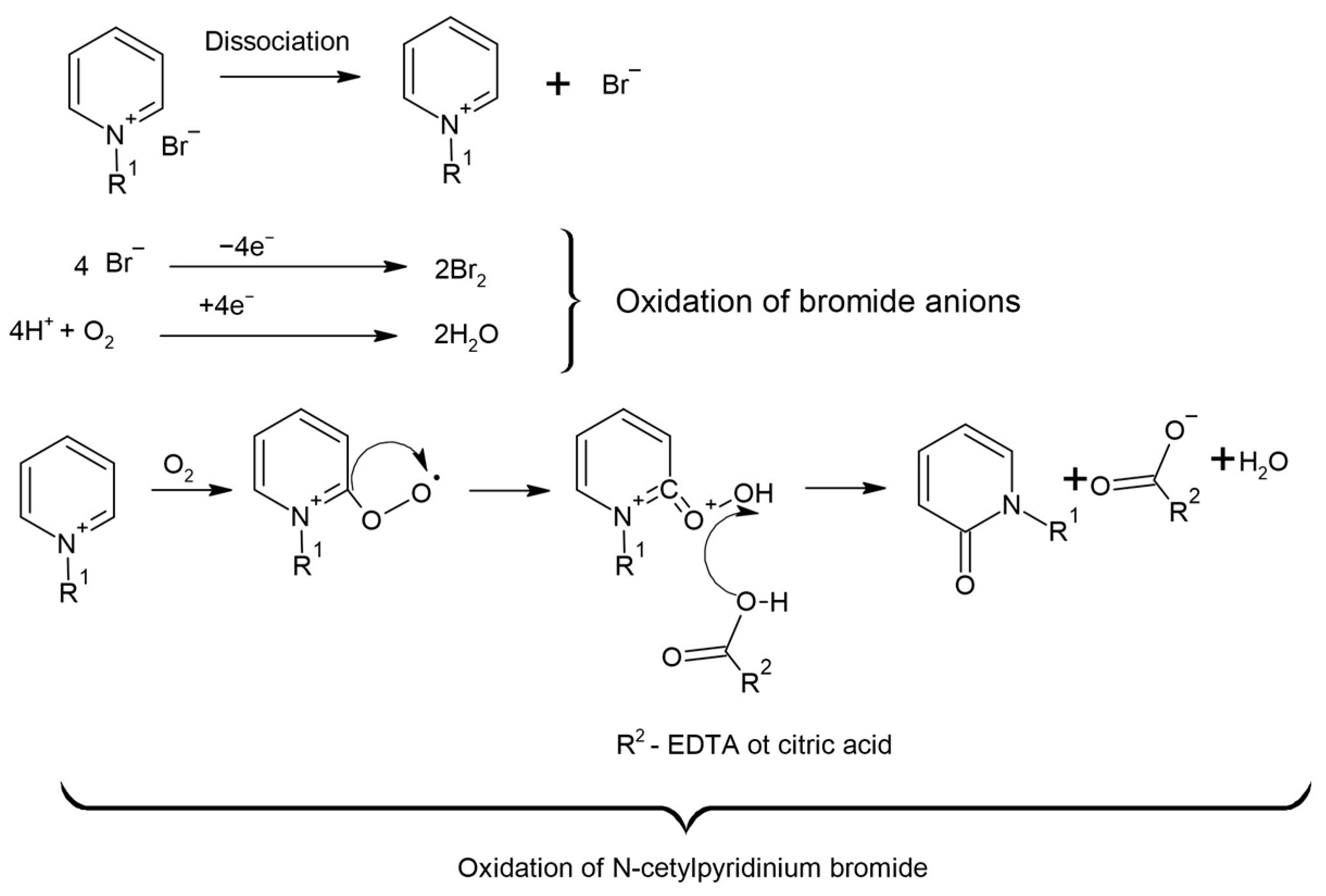
2.2.4. Rock Treatment
2.2.5. Contact Angle Measurement
2.2.6. Washburn Capillary Rise Method
2.2.7. Dissolution Capacity Measurement
3. Results and Discussion
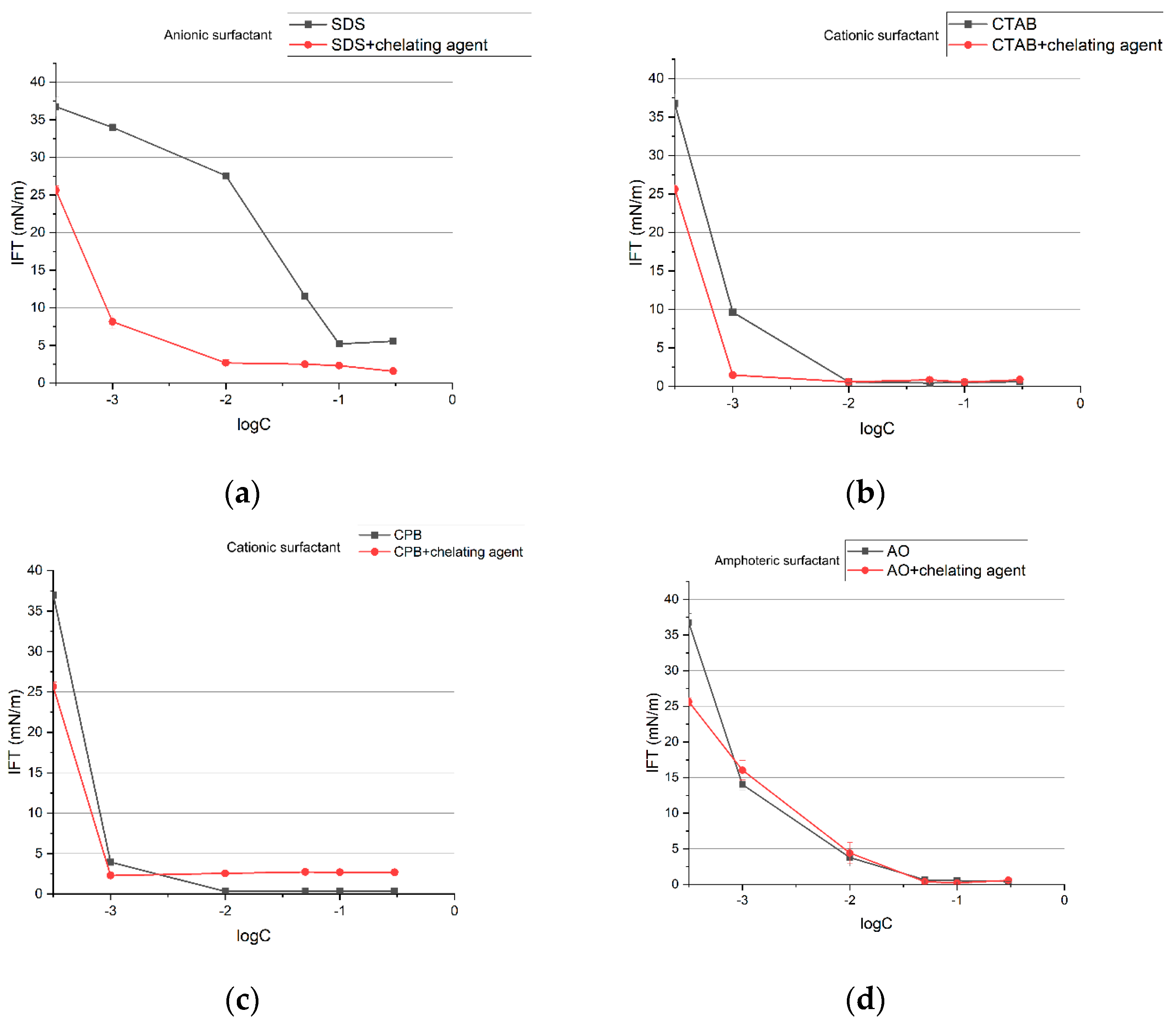
3.1. IFT Measurement
3.2. Wettability Alteration of Model Rock
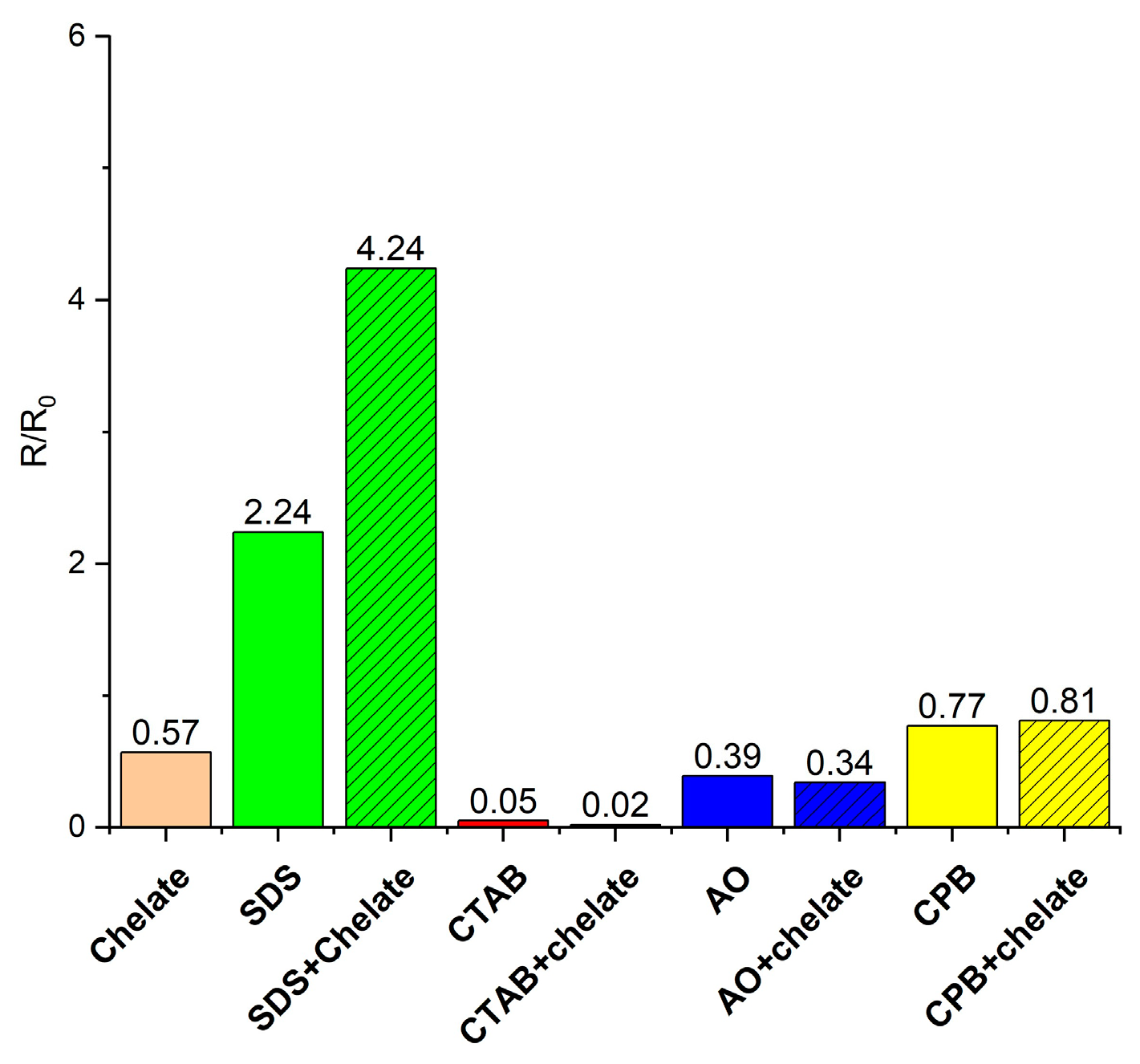
3.3. Effect of the Wettability Alteration on the Dissolution Capacity of CA
3.4. Wettability Alteration of Reservoir Rock
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Xu, Z.X.; Li, S.Y.; Li, B.F.; Chen, D.Q.; Liu, Z.Y.; Li, Z.M. A Review of Development Methods and EOR Technologies for Carbonate Reservoirs. Pet. Sci. 2020, 17, 990–1013. [Google Scholar] [CrossRef]
- Pashapouryeganeh, F.; Zargar, G.; Kadkhodaie, A.; Rabiee, A.; Misaghi, A.; Zakariaei, S.J.S. Experimental Evaluation of Designed and Synthesized Alkaline-Surfactant-Polymer (ASP) for Chemical Flooding in Carbonate Reservoirs. Fuel 2022, 321, 124090. [Google Scholar] [CrossRef]
- Galkin, S.V.; Martyushev, D.A.; Osovetsky, B.M.; Kazymov, K.P.; Song, H. Evaluation of Void Space of Complicated Potentially Oil-Bearing Carbonate Formation Using X-ray Tomography and Electron Microscopy Methods. Energy Rep. 2022, 8, 6245–6257. [Google Scholar] [CrossRef]
- Lian, P.Q.; Cheng, L.S.; Ma, C.Y. The Characteristics of Relative Permeability Curves In Naturally Fractured Carbonate Reservoirs. J. Can. Pet. Technol. 2012, 51, 137–142. [Google Scholar] [CrossRef]
- Manrique, E.J.; Muci, V.E.; Gurfinkel, M.E. EOR Field Experiences in Carbonate Reservoirs in the United States. SPE Reserv. Eval. Eng. 2007, 10, 667–686. [Google Scholar] [CrossRef]
- Sun, S.Q.; Sloan, R. Quantification of Uncertainty in Recovery Efficiency Predictions: Lessons Learned from 250 Mature Carbonate Fields. In Proceedings of the SPE Annual Technical Conference and Exhibition, Denver, CO, USA, 5 October 2003. [Google Scholar] [CrossRef]
- Lin, R.; Yu, Z.; Zhao, J.; Dai, C.; Sun, Y.; Ren, L.; Xie, M. Experimental Evaluation of Tight Sandstones Reservoir Flow Characteristics under CO2–Brine–Rock Multiphase Interactions: A Case Study in the Chang 6 Layer, Ordos Basin, China. Fuel 2022, 309, 122167. [Google Scholar] [CrossRef]
- Ibrahem, Y.; Morozov, V.P.; Sudakov, V.; Idrisov, I.; Kolchugin, A.N. Sedimentary Diagenesis and Pore Characteristics for the Reservoir Evaluation of Domanik Formations (Semiluksk and Mendymsk) in the Central Part of Volga-Ural Petroleum Province. Pet. Res. 2022, 7, 32–46. [Google Scholar] [CrossRef]
- Buckley, J.S.; Liu, Y.; Monsterleet, S. Mechanisms of Wetting Alteration by Crude Oils. SPE J. 1998, 3, 54–61. [Google Scholar] [CrossRef]
- Saraji, S.; Goual, L.; Piri, M. Dynamic Adsorption of Asphaltenes on Quartz and Calcite Packs in the Presence of Brine Films. Colloids Surf. A Physicochem. Eng. Asp. 2013, 434, 260–267. [Google Scholar] [CrossRef]
- Liu, Y.; Buckley, J.S. Evolution of Wetting Alteration by Adsorption from Crude Oil. SPE Form. Eval. 1997, 12, 5–11. [Google Scholar] [CrossRef]
- Gonzalez, G.; Travalloni-Louvisse, A.M. Adsorption of Asphaltenes and Its Effect on Oil Production. SPE Prod. Facil. 1993, 8, 91–96. [Google Scholar] [CrossRef]
- Saraji, S.; Goual, L.; Piri, M. Adsorption of Asphaltenes in Porous Media under Flow Conditions. Energy Fuels 2010, 24, 6009–6017. [Google Scholar] [CrossRef]
- Adams, J.J. Asphaltene Adsorption, a Literature Review. Energy Fuels 2014, 28, 2831–2856. [Google Scholar] [CrossRef]
- Alipour Tabrizy, V.; Denoyel, R.; Hamouda, A.A. Characterization of Wettability Alteration of Calcite, Quartz and Kaolinite: Surface Energy Analysis. Colloids Surf. A Physicochem. Eng. Asp. 2011, 384, 98–108. [Google Scholar] [CrossRef]
- Alvim, R.S.; Lima, F.C.D.A.; Sánchez, V.M.; Headen, T.F.; Boek, E.S.; Miranda, C.R. Adsorption of Asphaltenes on the Calcite (10.4) Surface by First-Principles Calculations. RSC Adv. 2016, 6, 95328–95336. [Google Scholar] [CrossRef]
- Mohammed, S.; Gadikota, G. Dynamic Wettability Alteration of Calcite, Silica and Illite Surfaces in Subsurface Environments: A Case Study of Asphaltene Self-Assembly at Solid Interfaces. Appl. Surf. Sci. 2020, 505, 144516. [Google Scholar] [CrossRef]
- Bagrintseva, K.I. Carbonate Reservoir Rocks, 1st ed.; Scrinever Publishing: Beverly, MA, USA, 2015; p. 355. [Google Scholar]
- Zhang, Y.; Zhao, P.; Cai, M.; Lu, F.; Wu, X.; Guo, Z. Occurrence State and Forming Mechanism of Microscopic Remaining Oil Controlled by Dynamic and Static Factors. J. Pet. Sci. Eng. 2020, 193, 107330. [Google Scholar] [CrossRef]
- Iglauer, S.; Fernø, M.A.; Shearing, P.; Blunt, M.J. Comparison of Residual Oil Cluster Size Distribution, Morphology and Saturation in Oil-Wet and Water-Wet Sandstone. J. Colloid Interface Sci. 2012, 375, 187–192. [Google Scholar] [CrossRef]
- Owens, W.W.; Archer, D.L. Effect of Rock Wettability on Oil- Water Relative Permeability Relationships. J. Pet. Technol. 1971, 23, 873–878. [Google Scholar] [CrossRef]
- Anderson, W.G. Wettability Literature Survey—Part 5: The Effects of Wettability on Relative Permeability. J. Pet. Technol. 1987, 39, 1453–1468. [Google Scholar] [CrossRef]
- Ahmadi, R.; Farmani, Z.; Osfouri, S.; Azin, R. Condensate Blockage Remediation in a Gas Reservoir through Wettability Alteration Using Natural CaCO3 Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2019, 579, 123702. [Google Scholar] [CrossRef]
- Jin, J.; Sun, J.; Rong, K.; Lv, K.; Nguyen, T.A.H.; Wang, R.; Huang, X.; Bai, Y.; Liu, J.; Wang, J. Gas-Wetting Alteration by Fluorochemicals and Its Application for Enhancing Gas Recovery in Gas-Condensate Reservoirs: A Review. Energies 2020, 13, 4591. [Google Scholar] [CrossRef]
- Isah, A.; Arif, M.; Hassan, A.; Mahmoud, M.; Iglauer, S. Fluid-Rock Interactions and Its Implications on EOR: Critical Analysis, Experimental Techniques and Knowledge Gaps. Energy Rep. 2022, 8, 6355–6395. [Google Scholar] [CrossRef]
- Pal, S.; Mushtaq, M.; Banat, F.; Al Sumaiti, A.M. Review of Surfactant-Assisted Chemical Enhanced Oil Recovery for Carbonate Reservoirs: Challenges and Future Perspectives. Pet. Sci. 2018, 15, 77–102. [Google Scholar] [CrossRef]
- Hou, B.F.; Wang, Y.F.; Huang, Y. Mechanistic Study of Wettability Alteration of Oil-Wet Sandstone Surface Using Different Surfactants. Appl. Surf. Sci. 2015, 330, 56–64. [Google Scholar] [CrossRef]
- Nieto-Alvarez, D.A.; Zamudio-Rivera, L.S.; Luna-Rojero, E.E.; Rodríguez-Otamendi, D.I.; Marín-León, A.; Hernández-Altamirano, R.; Mena-Cervantes, V.Y.; Chávez-Miyauchi, T.E. Adsorption of Zwitterionic Surfactant on Limestone Measured with High-Performance Liquid Chromatography: Micelle-Vesicle Influence. Langmuir 2014, 30, 12243–12249. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, D.; Olatunji, K. Surfactant Adsorption Investigation in Ultra-Lower Permeable Rocks. In Proceedings of the SPE Low Perm Symposium, Denver, CO, USA, 5 May 2016. [Google Scholar] [CrossRef]
- Chen, P.; Mohanty, K.K. Wettability Alteration in High Temperature Carbonate Reservoirs. In Proceedings of the 18th SPE Improved Oil Recovery Symposium, Tulsa, OK, USA, 12 April 2014. [Google Scholar] [CrossRef]
- Shafiq, M.U.; Mahmud, H.K.B.; Zahoor, M.K.; Shahid, A.S.A.; Rezaee, R.; Arif, M. Investigation of Change in Different Properties of Sandstone and Dolomite Samples during Matrix Acidizing Using Chelating Agents. J. Pet. Explor. Prod. Technol. 2019, 9, 2793–2809. [Google Scholar] [CrossRef]
- Hassan, A.M.; Al-Hashim, H.S. Cost Effective Chelating Agent Eor Fluid System for Carbonate Reservoirs. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 25 April 2016. [Google Scholar] [CrossRef]
- Nasr El-Din, H.A.; Mahmoud, M.A.; Abdelgawad, K.Z. Chelating-Agent Enhanced Oil Recovery for Sandstone and Carbonate Reservoirs. SPE J. 2015, 20, 483–495. [Google Scholar] [CrossRef]
- Hassan, A.M.; Al-Hashim, H.S. Surface Charge Study of EDTA Interaction with Carbonate Rock during Chelating Agent Flooding. J. Pet. Sci. Eng. 2020, 191, 107163. [Google Scholar] [CrossRef]
- Deng, X.; Patil, S.; Kamal, M.S.; Mahmoud, M.; Sultan, A.; Saikia, T. Wettability Alteration of Carbonate Rock by Chelating Agents and Viscoelastic Surfactants: Synergetic Impact. Energy Fuels 2022, 36, 7391–7401. [Google Scholar] [CrossRef]
- Janjua, A.N.; Sultan, A.S.; Saikia, T.; Kamal, M.S.; Mahmoud, M. Experimental Investigation of Noble Viscoelastic Surfactant and Chelating Agent for Heavy Oil Enhanced Oil Recovery in High-Pressure–High-Temperature Carbonate Reservoirs. J. Surfactants Deterg. 2021, 24, 289–300. [Google Scholar] [CrossRef]
- Deng, X.; Kamal, M.S.; Patil, S.; Hussain, S.M.S.; Mahmoud, M.; Al Shehri, D.; Abu-Khamsin, S.; Mohanty, K. Wettability Alteration on Carbonate Rock by the Mixture of the Gemini Surfactant and Chelating Agent. Energy Fuels 2022, 36, 13652–13664. [Google Scholar] [CrossRef]
- Deng, X.; Patil, S.; Kamal, M.S.; Shakil, S.M.; Mahmoud, M.; Zhou, X.; Shalabi, E.A.; Hassan, A. Wettability Alteration of Carbonate Rock by Chelating Agents. In Proceedings of the Gas & Oil Technology Showcase and Conference, Dubai, United Arab Emirates, 13 March 2023. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. The Influence of Chelating Agents on the Kinetics of Calcite Dissolution. J. Colloid Interface Sci. 1998, 204, 187–197. [Google Scholar] [CrossRef]
- Fredd, C.N.; Fogler, H.S. Alternative Stimulation Fluids and Their Impact on Carbonate Acidizing. SPE J. 1998, 3, 34–40. [Google Scholar] [CrossRef]
- Kumar, H.T.; Muhemmed, S.; Nasr-El-Din, H.A. Impact of Oil Saturation, CO2 Evolution, and Rock Wettability on Acid Efficiencies during Carbonate Acidizing: A Three-Phase Perspective. In Proceedings of the SPE International Conference and Exhibition on Formation Damage Control, Lafayette, LA, USA, 12 February 2020. [Google Scholar] [CrossRef]
- Abdollahi, R.; Shadizadeh, S.R. The Effect of Spent Acid on Carbonate Rock Wettability during a Matrix Acidizing Treatment. Pet. Sci. Technol. 2014, 32, 450–454. [Google Scholar] [CrossRef]
- Novikov, V.A.; Martyushev, D.A.; Li, Y.; Yang, Y. A New Approach for the Demonstration of Acidizing Parameters of Carbonates: Experimental and Field Studies. J. Pet. Sci. Eng. 2022, 213, 110363. [Google Scholar] [CrossRef]
- Roustaei, A.; Bagherzadeh, H. Experimental Investigation of SiO2 Nanoparticles on Enhanced Oil Recovery of Carbonate Reservoirs. J. Pet. Explor. Prod. Technol. 2015, 5, 27–33. [Google Scholar] [CrossRef]
- Rezaei Gomari, K.A.; Hamouda, A.A. Effect of Fatty Acids, Water Composition and PH on the Wettability Alteration of Calcite Surface. J. Pet. Sci. Eng. 2006, 50, 140–150. [Google Scholar] [CrossRef]
- Kasha, A.; Al-Hashim, H.; Abdallah, W.; Taherian, R.; Sauerer, B. Effect of Ca2+, Mg2+ and SO42− Ions on the Zeta Potential of Calcite and Dolomite Particles Aged with Stearic Acid. Colloids Surf. A Physicochem. Eng. Asp. 2015, 482, 290–299. [Google Scholar] [CrossRef]
- Yunusov, T.I.; Davletshina, L.F.; Magadova, L.A.; Silin, M.A. Study of Chelating Agent—Surfactant Interactions on the Interphase as Possibly Useful for the Well Stimulation. Energies 2023, 16, 1679. [Google Scholar] [CrossRef]
- Kumar, B. Effect of Salinity on the Interfacial Tension of Model and Crude Oil Systems. Master’s Thesis, University of Calgary, Calgary, AB, Canada, 2012. [Google Scholar]
- Klimenko, A.; Molinier, V.; Bourrel, M. Mechanisms Underlying the Adhesion of Crude Oil to Mineral Surfaces: Relevance of Oil-Brine Interactions. J. Pet. Sci. Eng. 2020, 190, 107036. [Google Scholar] [CrossRef]
- Madaan, P.; Tiyagi, V.K. Quaternary Pyridinium Salts: A Review. J. Oleo Sci. 2008, 57, 197–215. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena? 4th ed; John Wiley and Sons: Hoboker, NJ, USA, 2012. [Google Scholar]
- Xu, L.; Fu, Q. Proper Selection of Surfactant Additives Ensures Better Well Stimulation in the Unconventional Oil and Gas Formations. In Proceedings of the SPE Middle East Unconventional Gas Conference and Exhibition, Abu Dhabi, United Arab Emirates, 23 January 2012. [Google Scholar] [CrossRef]
- Somasundaran, P.; Agar, G.E. The Zero Point of Charge of Calcite. J. Colloid Interface Sci. 1967, 24, 433–440. [Google Scholar] [CrossRef]
- Shadizadeh, S.R.; Amirpour, M. Reservoir Rock Wettability Alteration Using Different Types of Surfactants: Experimental Assessment. Energy Sources Part A Recovery Util. Environ. Eff. 2023, 45, 383–389. [Google Scholar] [CrossRef]
- Mohammadi, M.S.; Moghadasi, J.; Kordestany, A. A Laboratory Investigation into Wettability Alteration of Carbonate Rock by Surfactants: The Effect of Salinity, PH, and Surfactant Concentration. Iran. J. Oil Gas Sci. Technol. 2014, 3, 1–10. [Google Scholar]
- Strand, S.; Høgnesen, E.J.; Austad, T. Wettability Alteration of Carbonates—Effects of Potential Determining Ions (Ca2+ and SO42−) and Temperature. Colloids Surf. A Physicochem. Eng. Asp. 2006, 275, 1–10. [Google Scholar] [CrossRef]
- Mannhardt, K.; Novosad, J.J.; Jha, K.N.N. Adsorption of Foam-Forming Surfactants In Berea Sandstone. J. Can. Pet. Technol. 1994, 33, PETSOC-94-02-04. [Google Scholar] [CrossRef]
- Zhao, J.; Dai, C.; Fang, J.; Feng, X.; Yan, L.; Zhao, M. Surface Properties and Adsorption Behavior of Cocamidopropyl Dimethyl Amine Oxide under High Temperature and High Salinity Conditions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 93–98. [Google Scholar] [CrossRef]
- Souraki, Y.; Hosseini, E.; Yaghodous, A. Wettability Alteration of Carbonate Reservoir Rock Using Amphoteric and Cationic Surfactants: Experimental Investigation. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 41, 349–359. [Google Scholar] [CrossRef]
- Standnes, D.C.; Austad, T. Wettability Alteration in Carbonates: Interaction between Cationic Surfactant and Carboxylates as a Key Factor in Wettability Alteration from Oil-Wet to Water-Wet Conditions. Colloids Surf. A Physicochem. Eng. Asp. 2003, 216, 243–259. [Google Scholar] [CrossRef]
- Tetko, I.V.; Gasteiger, J.; Todeschini, R.; Mauri, A.; Livingstone, D.; Ertl, P.; Palyulin, V.A.; Radchenko, E.V.; Zefirov, N.S.; Makarenko, A.S.; et al. Virtual Computational Chemistry Laboratory—Design and Description. J. Comput. Aided. Mol. Des. 2005, 19, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Fredd, C.N.; Scott Fogler, H. Influence of Transport and Reaction on Wormhole Formation in Porous Media. AIChE J. 1998, 44, 1933–1949. [Google Scholar] [CrossRef]
- Silin, M.A.; Magadova, L.A.; Davletshina, L.F.; Yunusov, T.I.; Mikulov, V.A. Experimental and Theoretical Study of Carbonate Rocks Dissolution in Chelating Reagents. Proc. Gubkin Univ. 2022, 307, 20–36. [Google Scholar] [CrossRef]



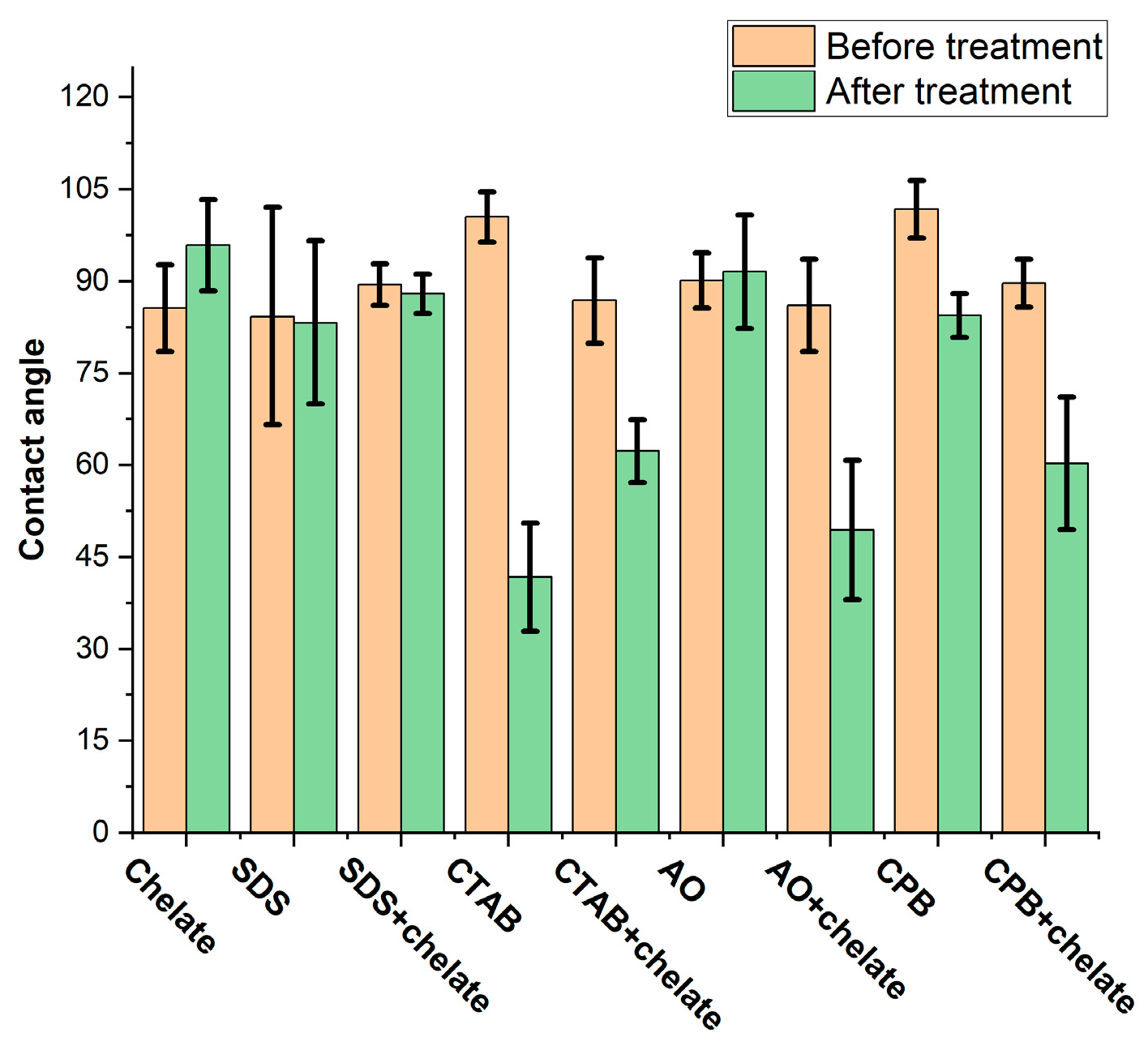
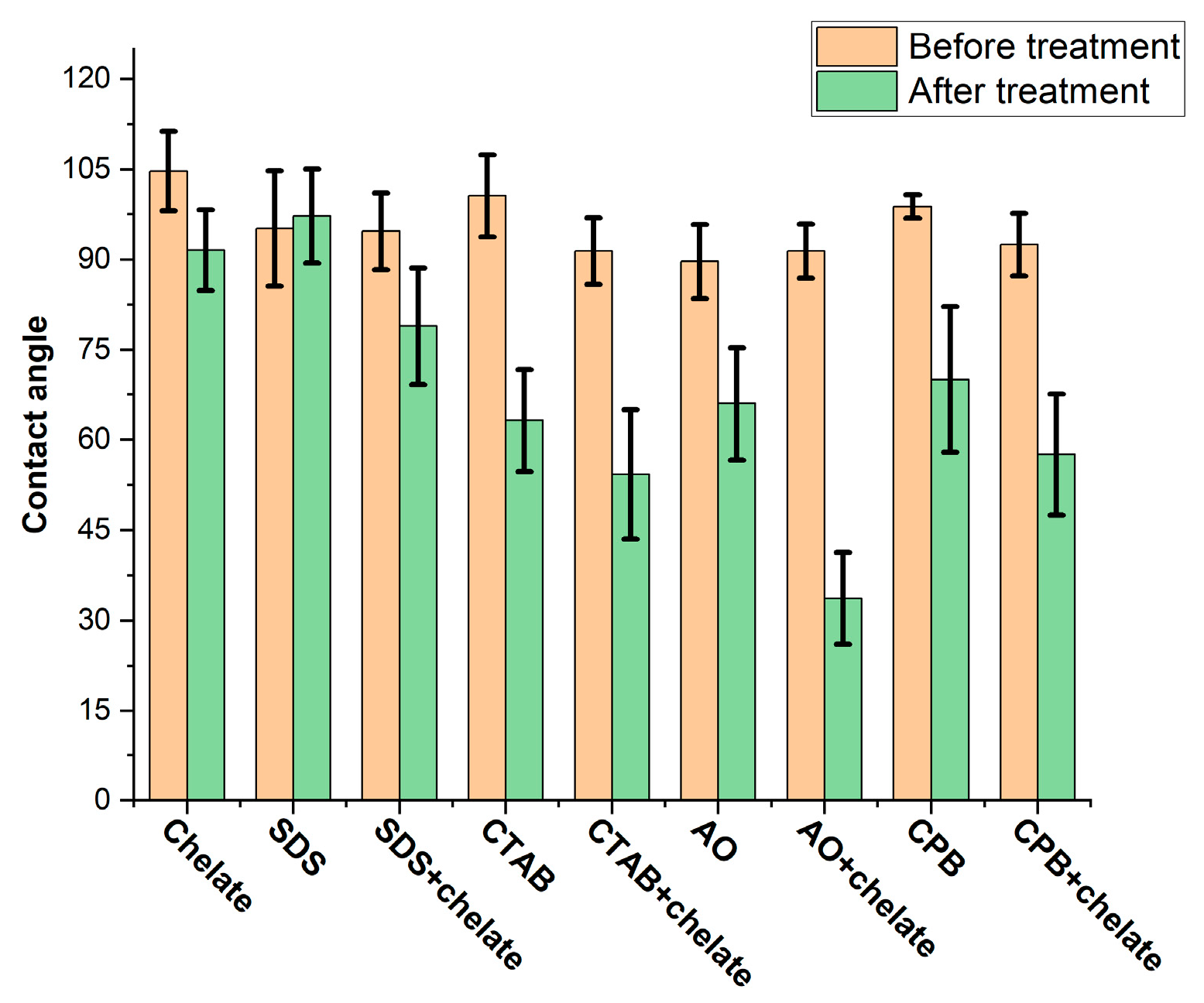
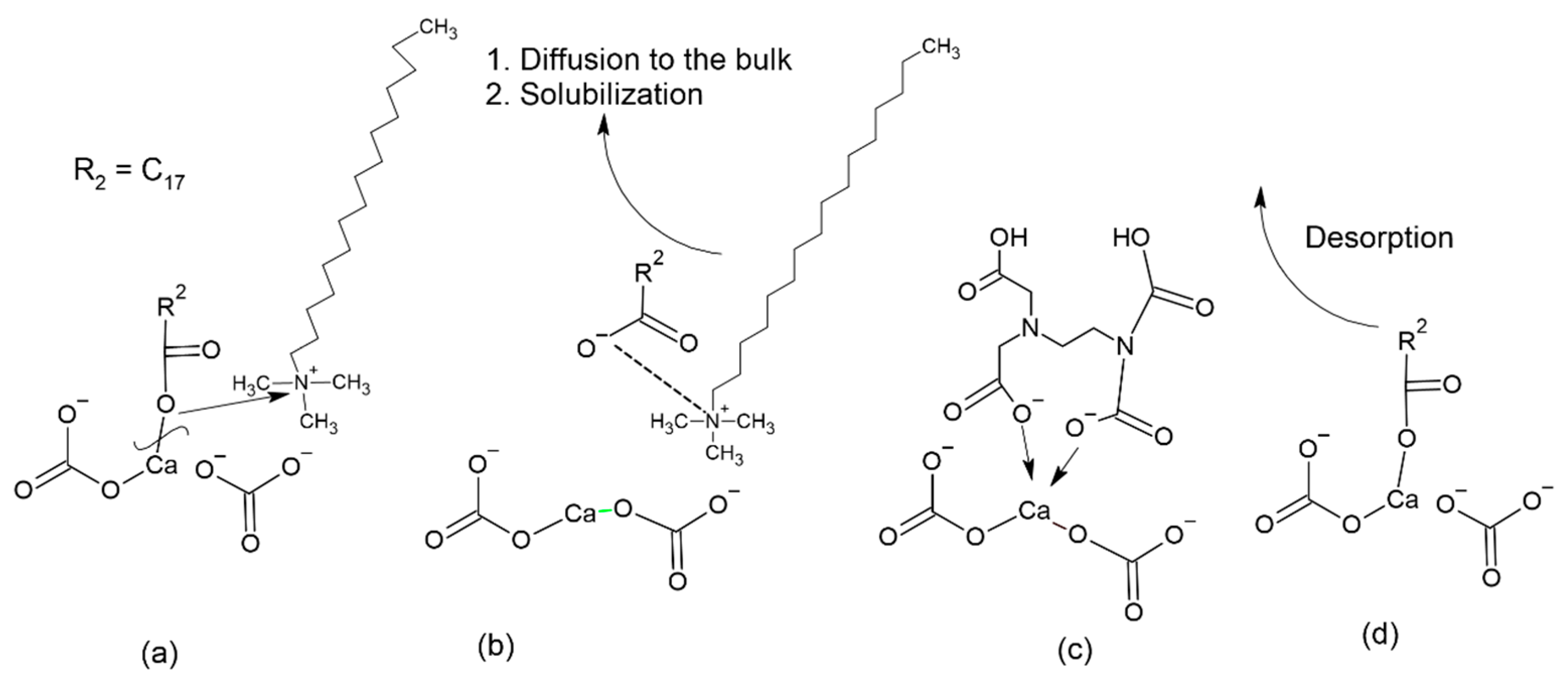

| Density, kg/m3 | Viscosity, cP | Asphaltenes, % wt. | Resins, % wt. |
|---|---|---|---|
| 0.850 | 27 | 0.37 | 15.9 |
| Sample | DC, g/g | |
|---|---|---|
| 25 °C | 120 °C | |
| Hydrophilic sample, CA | 0.057 ± 0.003 | 0.084 ± 0.002 |
| Hydrophobic sample, CA | 0.029 ± 0.002 | 0.018 ± 0.005 |
| Hydrophobic sample, CA-surfactant mixture | 0.050 ± 0.011 | 0.086 ± 0.002 |
| Sample | Contact Angle, ° | |
|---|---|---|
| 25 °C | 120 °C | |
| Control sample, hydrophilic | 57.11 ± 7.19 | |
| Control sample, hydrophobic | 82.17 ± 5.80 | |
| Hydrophobic sample, treated with plain CA | 77.52 ± 4.10 | 98.34 ± 4.73 |
| Hydrophobic sample, treated with CA-surfactant mixture | 35.48 ± 5.05 | 27.83 ± 4.14 |
| R Value | Temperature of the Treatment, °C | |
|---|---|---|
| 25 °C | 120 °C | |
| Before ageing | 0.73 | |
| After ageing | 1.62 | |
| CA | 0.47 | 0.39 |
| CA-surfactant mixture | 0.89 | 0.51 |
| Surfactant | 2.45 | 1.13 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yunusov, T.I.; Davletshina, L.F.; Klimov, D.N.; Magadova, L.A.; Silin, M.A. Study of Wettability Alteration of Hydrophobic Carbonate Rock by Surfactant-Containing Chelating Agent Solutions. Appl. Sci. 2023, 13, 9664. https://doi.org/10.3390/app13179664
Yunusov TI, Davletshina LF, Klimov DN, Magadova LA, Silin MA. Study of Wettability Alteration of Hydrophobic Carbonate Rock by Surfactant-Containing Chelating Agent Solutions. Applied Sciences. 2023; 13(17):9664. https://doi.org/10.3390/app13179664
Chicago/Turabian StyleYunusov, Timur Ildarovich, Lyutsia Faritovna Davletshina, Dmitriy Nikolaevich Klimov, Lyubov Abdulaevna Magadova, and Mikhail Alexandrovich Silin. 2023. "Study of Wettability Alteration of Hydrophobic Carbonate Rock by Surfactant-Containing Chelating Agent Solutions" Applied Sciences 13, no. 17: 9664. https://doi.org/10.3390/app13179664
APA StyleYunusov, T. I., Davletshina, L. F., Klimov, D. N., Magadova, L. A., & Silin, M. A. (2023). Study of Wettability Alteration of Hydrophobic Carbonate Rock by Surfactant-Containing Chelating Agent Solutions. Applied Sciences, 13(17), 9664. https://doi.org/10.3390/app13179664







