Clinical Approaches to the Three-Dimensional Endodontic Obturation Protocol for Teeth with Periapical Bone Lesions
Abstract
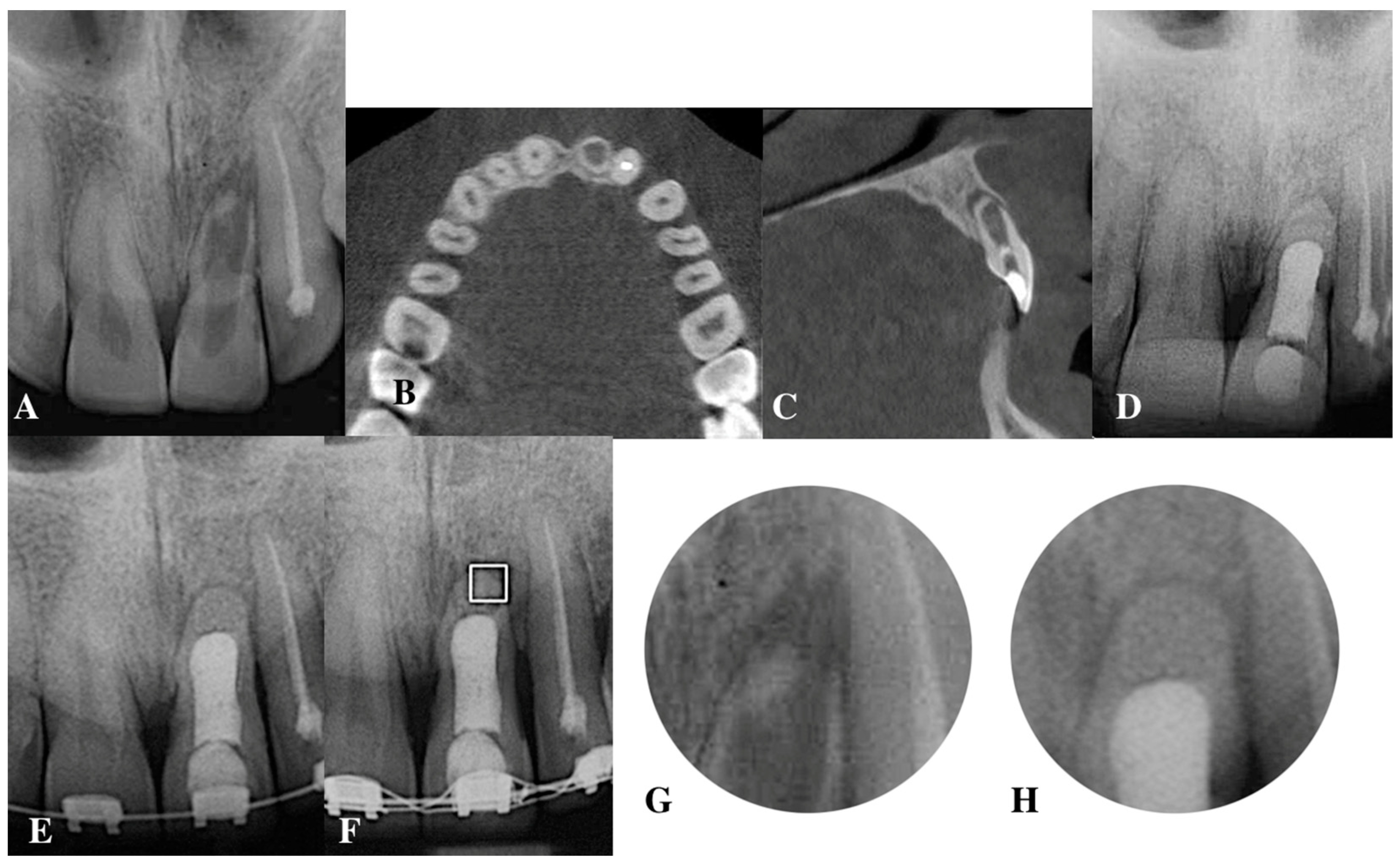
:1. Introduction
2. Requirement Properties for Root Canal Sealer in the Treatment of Teeth with Periapical Lesions
3. Factors Affecting Obturation Protocol in Teeth with Periapical Bone Lesions
4. Contemporary Sealers with Indications for the Treatment of Teeth with Periapical Lesions
4.1. Epoxy Resin-Based Sealers: AH 26, AH Plus
4.2. Calcium Hydroxide-Based Canal Sealers
4.3. Bioceramic Sealers
4.3.1. Calcium Silicate-Based Sealers
- iRootSP sealer
- Total Fill BC sealer
- MTA Fillapex
4.3.2. Calcium Phosphate-Based Sealer
4.4. Nanomaterials of Hydroxyapatite as Root Canal Filling Materials
5. Apical Plug in Teeth with Periapical Lesions
5.1. Apical Plug in Teeth with Apical Resorption and “Open Apex”
5.2. Apical Barrier
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tomson, R.M.E.; Polycarpou, N.; Tomson, P.L. Contemporary obturation of the root canal system. Br. Dent. J. 2014, 216, 315–322. [Google Scholar] [CrossRef]
- Ozok, A.; van der Sluis, L.; Wu, M.; Wesselink, P. Sealing ability of a new polydimethylsiloxane based root canal filling material. J. Endod. 2008, 34, 204–207. [Google Scholar] [CrossRef]
- Haapasalo, M.; Shen, Y.; Qian, W.; Gao, Y. Irrigation in Endodontics. Dent. Clin. N. Am. 2010, 54, 291–312. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, F.; Massone, E.J.; Artaza, L.P. Comparison of the sealing capacity of three endodontic filling technique. J. Endod. 1995, 21, 1–3. [Google Scholar] [CrossRef]
- Kikly, A.; Jaâfoura, S.; Kammoun, D.; Sahtout, S. Sealing Ability of Endodontic Cements: An In Vitro Study. Int. J. Dent. 2020, 2020, 5862598. [Google Scholar] [CrossRef] [PubMed]
- Basmadjian-Charles, C.; Farge, P.; Bourgeois, D.; Lebrun, T. Factors influencing the long-term results of endodontic treatment: A review of the literature. Int. Dent. J. 2002, 52, 81–86. [Google Scholar] [CrossRef]
- Lin, Z.-M.; Jhugroo, A.; Ling, J.-Q. An evaluation of the sealing ability of a polycaprolactone-based root canal filling material (Resilon) after retreatment. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2007, 104, 846–851. [Google Scholar] [CrossRef] [PubMed]
- Van der Sluis, L.; Wu, M.; Wesselink, P. An evaluation of the quality of root fillings in mandibular incisors and maxillary and mandibular canines using different methodologies. J. Dent. 2005, 33, 683–688. [Google Scholar] [CrossRef]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. Int. Endod. J. 2011, 44, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Karabucak, B.; Kim, A.; Chen, V.; Iqbal, M.K. The comparison of Gutta-Percha and Resilon penetration into lateral canals with different thermoplastic delivery systems. J. Endod. 2008, 34, 847–849. [Google Scholar] [CrossRef]
- Schilder, H. Filling root canals in three dimensions. J. Endod. 2006, 32, 281–290. [Google Scholar] [CrossRef]
- Šimundić Munitić, M.; Poklepović Peričić, T.; Utrobičić, A.; Bago, I.; Puljak, L. Antimicrobial efficacy of commercially available endodontic bioceramic root canal sealers: A systematic review. PLoS ONE 2019, 14, e0223575. [Google Scholar] [CrossRef]
- Dagna, A.; Colombo, M.; Poggio, C.; Russo, G.; Pellegrini, M.; Pietrocola, G.; Beltrami, R. In Vitro Antibacterial Activity of Different Bioceramic Root Canal Sealers. Ceramics 2022, 5, 901–907. [Google Scholar] [CrossRef]
- Mangat, P.; Dhingra, A.; Muni, S.; Bhullar, H.K. To compare and evaluate the antimicrobial activity of three different root canal sealers: An In Vitro Study. J. Conserv. Dent. 2020, 23, 571–576. [Google Scholar] [CrossRef]
- Ørstavik, D. Materials used for root canal obturation: Technical, biological and clinical testing. Endod. Top. 2005, 12, 25–38. [Google Scholar] [CrossRef]
- Williams, D. Definitions in Biomaterials. In Progress in Biomedical Engineering, Proceedings of the Consensus Conference of the European Society for Biomaterials, Chester, England, 3–5 March 1986; Elsevier: Amsterdam, The Netherlands, 1987; Volume 4. [Google Scholar]
- Bryan, T.E.; Khechen, K.; Brackett, M.; Messer, R.L.; El-Awady, A.; Primus, C.M.; Gutmann, J.L.; Tay, F.R. In vitro osteogenic potential of an experimental calcium silicate-based root canal sealer. J. Endod. 2010, 36, 1163–1169. [Google Scholar] [CrossRef]
- Yang, Q.; Lu, D. Premixed Biological Hydraulic Cement Paste Composition and Using the Same. Google Patents EP2142225A1, 13 January 2010. [Google Scholar]
- De Miranda Candeiro, G.T.; Correia, F.C.; Duarte, M.A.H.; Ribeiro-Siqueira, D.C.; Gavini, G. Evaluation of radiopacity, pH, release of calcium ions, and flow of a bioceramic root canal sealer. J. Endod. 2012, 38, 842–845. [Google Scholar] [CrossRef]
- ISO 6876/2001; Dental Root Canal Sealing Materials. International Organization for Standardization: Geneva, Switzerland, 2001.
- Tanomaru, J.M.G.; Duarte, M.A.H.; Gonçalves, M.; Tanomaru-Filho, M. Radiopacity evaluation of root canal sealers containing calcium hydroxide and MTA. Braz. Oral. Res. 2009, 23, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Okabe, T.; Sakamoto, M.; Takeuchi, H.; Matsushima, K. Effects of pH on mineralization ability of human dental pulp cells. J. Endod. 2006, 32, 198–201. [Google Scholar] [CrossRef]
- Zhang, H.; Shen, Y.; Ruse, N.D.; Haapasalo, M. Antibacterial activity of endodontic sealers by modified direct contact test against Enterococcus faecalis. J. Endod. 2009, 35, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, E.T.; Gomes, B.P.F.A.; Ferraz, C.C.R.; Sousa, E.L.R.; Teixeira, F.B.; Souza-Filho, F.J. Microorganisms from canals of root-filled teeth with periapical lesions. Int. Endod. J. 2003, 36, 1–11. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.; Chandler, N. Calcium hydroxide-based root canal sealers: A review. J. Endod. 2009, 35, 475–480. [Google Scholar] [CrossRef]
- Sousa-Neto, M.D.; Coelho, F.I.S.; Marchesan, M.A.; Alfredo, E.; Silva-Sousa, Y.T.C. Ex vivo study of the adhesion of an epoxy-based sealer to human dentine submitted to irradiation with Er: YAG and Nd: YAG lasers. Int. Endod. J. 2005, 38, 866–870. [Google Scholar] [CrossRef]
- Schwartz, R. Adhesive dentistry and endodontics. Part 2: Bonding in the root canal system—The promise and the problems: A review. J. Endod. 2006, 32, 1125–1134. [Google Scholar] [CrossRef]
- Kumar, S.A.; Shivanna, V.; Naian, M.T.; Shivamurthy, G. Comparative evaluation of the apical sealing ability and adaptation to dentine of three resin-based sealers: An in vitro study. J. Conserv. Dent. 2011, 14, 16–20. [Google Scholar] [CrossRef]
- Friedman, S. Considerations and concepts of case selection in the management of post-treatment endodontic disease (treatment failures). Endod. Top. 2002, 1, 54–78. [Google Scholar] [CrossRef]
- Gulabivala, K.; Ng, Y.L. Factors that affect the outcomes of root canal treatment and retreatment—A reframing of the principles. Int. Endod. J. 2023, 56, 82–115. [Google Scholar] [CrossRef]
- Gusiyska, A. Factors affecting the prognosis of endodontic retreatment of teeth with bone lesions. Int. J. Sci. Res. 2022, 11, 386–392. [Google Scholar]
- Basrani, B.; Malkhassian, G. Update on Irrigation Disinfection. Available online: https://www.aae.org/specialty/update-on-irrigation-disinfection/ (accessed on 1 May 2023).
- Gyulbenkiyan, E.; Gusiyska, A.; Vassileva, R.; Dyulgerova, E. Scanning electron microscopic evaluation of the sealer/dentine interface of two sealers using two protocols of irrigation. J. IMAB 2020, 26, 2887–2891. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q.; Zou, X.; Yue, L. Evaluation of four final irrigation protocols for cleaning root canal walls. Int. J. Oral. Sci. 2020, 12, 29. [Google Scholar] [CrossRef]
- Zancan, R.F.; Hadis, M.; Burgess, D.; Zhang, Z.J.; Di Maio, A.; Tomson, P.; Duarte, M.A.H.; Camilleri, J. A matched irrigation and obturation strategy for root canal therapy. Sci. Rep. 2021, 11, 4666. [Google Scholar] [CrossRef]
- Lim, M.; Jung, C.; Shin, D.-H.; Cho, Y.-B.; Song, M. Calcium silicate-based root canal sealers: A literature review. Restor. Dent. Endod. 2020, 45, e35. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Soltani, M.K.; Shalavi, S.; Yazdizadeh, M.; Jafarzadeh, M. Calcium hydroxide-based root canal sealers: An updated literature review. Compend. Contin. Educ. Dent. 2014, 35, 334–340. [Google Scholar] [PubMed]
- Shah, N.; Logani, A.; Mishra, N.; Kaur, A. Biotoxicity of commonly used root canal sealers: A meta-analysis. J. Conserv. Dent. 2015, 18, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Best, S.; Porter, A.; Thian, E.; Huang, J. Bioceramics: Past, present and for the future. J. Eur. Ceram. Soc. 2008, 28, 1319–1327. [Google Scholar] [CrossRef]
- Kock, K.; Brave, D. Bioceramics, part I: The clinician’s viewpoint. Dent. Today 2012, 31, 130–135. [Google Scholar]
- Damas, B.; Wheater, M.; Bringas, J.; Hoen, M. Cytotoxicity comparison of mineral trioxide aggregates and EndoSequence bioceramic root repair materials. J. Endod. 2011, 37, 372–375. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Z.; Peng, B. Assessment of a new root canal sealer’s apical sealing ability. Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. Endod. 2009, 107, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Loushine, B.A.; Bryan, T.E.; Looney, S.W.; Gillen, B.M.; Loushine, R.J.; Weller, R.N.; Pashley, D.H.; Tay, F.R. Setting properties and cytotoxicity evaluation of a premixed bioceramic root canal sealer. J. Endod. 2011, 37, 673–677. [Google Scholar] [CrossRef]
- Borisova-Papancheva, T.; Svetlozazrova, S.; Vasileva, V. Comparative characteristics of some sealer types used in root canal obturation. Var. Med. For. 2022, 11, 179–185. [Google Scholar]
- Balgerie, E.; van der Sluis, L.; Vallaeys, K.; Gurgel-gergelin, M.; Dimer, F. Sealer penetration and adaptation in the dentinal tubules: A scanning electron microscopic study. J. Endod. 2013, 37, 1567–1569. [Google Scholar] [CrossRef] [PubMed]
- Camilleri, J.; Gandolfi, M.G.; Siboni, F.; Prati, C. Dynamic sealing ability of MTA root canal sealer. Int. Endod. J. 2011, 44, 9–20. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Zeng, C.; Liu, Y.; Gong, Q.; Jiang, H. Clinical outcome of bioceramic sealer iRoot SP extrusion in root canal treatment: A retrospective analysis. Head. Face Med. 2022, 18, 28. [Google Scholar] [CrossRef]
- Wu, L.; Xue, K.; Hu, G.; Du, H.; Gan, K.; Zhu, J.; Du, T. Effects of iRoot SP on osteogenic differentiation of human stem cells from apical papilla. BMC Oral. Health 2021, 21, 407. [Google Scholar] [CrossRef] [PubMed]
- Koch, K.; Brave, D. Bioceramic technology: The game changer in endodontics. Endod. Pract. 2009, 2, 17–21. [Google Scholar]
- Zhang, W.; Li, Z.; Peng, B. Ex vivo cytotoxicity of a new calcium silicate-based canal filling material. Int. Endod. J. 2010, 43, 769–774. [Google Scholar] [CrossRef]
- Zhou, H.-M.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.F.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Premixed Bioceramic Endodontic Materials, TotalFill. Available online: https://www.fkg.ch/products/endodontics/obturation/totalfill (accessed on 1 May 2023).
- Available online: https://angelus.ind.br/produto/mta-fillapex (accessed on 1 May 2023).
- Wang, Z.; Shen, Y.; Haapasalo, M. Antimicrobial and Antibiofilm Properties of Bioceramic Materials in Endodontics. Materials 2021, 14, 7594. [Google Scholar] [CrossRef]
- Silva, E.J.; Accorsi-Mendonça, T.; Pedrosa, A.C.; Granjeiro, J.M.; Zaia, A.A. Long-Term Cytotoxicity, pH and Dissolution Rate of AH Plus and MTA Fillapex. Braz. Dent. J. 2016, 27, 419–423. [Google Scholar] [CrossRef]
- Andreasen, J.O.; Munksgaard, E.C.; Bakland, L.K. Comparison of fracture resistance in root canals of immature sheep teeth after filling with calcium hydroxide or MTA. Dent. Traumatol. 2006, 22, 154–156. [Google Scholar] [CrossRef]
- Massi, S.; Tanomaru-Filho, M.; Silva, G.F.; Duarte, M.A.H.; Grizzo, L.T.; Buzalaf, M.A.R.; Guerreiro-Tanomaru, J.M. pH, calcium ion release, and setting time of an experimental mineral trioxide aggregate-based root canal sealer. J. Endod. 2011, 37, 844–846. [Google Scholar] [CrossRef]
- LeGeros, R.; Chohayeb, A.; Shulman, A. Apatitic calcium phosphates: Possible dental restorative materials. J. Dent. Res. 1982, 61, 343. [Google Scholar]
- Krell, K.; Wefel, J. A calcium phosphate cement root canal sealer—Scanning electron microscopic analysis. J. Endod. 1984, 10, 571–576. [Google Scholar] [CrossRef]
- Seo, D.-G.; Lee, D.; Kim, Y.-M.; Song, D.; Kim, S.-Y. Biocompatibility and Mineralization Activity of Three Calcium Silicate-Based Root Canal Sealers Compared to Conventional Resin-Based Sealer in Human Dental Pulp Stem Cells. Materials 2019, 12, 2482. [Google Scholar] [CrossRef] [PubMed]
- Donnermeyer, D.; Bürklein, S.; Dammaschke, T.; Schäfer, E. Endodontic sealers based on calcium silicates: A systematic review. Odontology 2019, 107, 421–436. [Google Scholar] [CrossRef] [PubMed]
- Dolci, G.; Mongiorgi, R.; Prati, C.; Valdre, G. Novel apatites designed for dentinal hypersensitivity therapy. J. Dent. Univ. Ital. Dent. Industr. Assoc. 2001, 2, 9–21. [Google Scholar]
- Gusiyska, A. Orthograde Treatment of Chronic Apical Periodontitis—Biological Approaches. Ph.D. Thesis, Medical University of Sofia, Sofia, Bulgaria, 2012. [Google Scholar]
- Farea, M.; Masudi, S.M.; Wan Bakar, W.Z.; Pameijer, C.H. Apical sealing of a nano-hydroxyapatite sealer using cold lateral and continuous wave condensation techniques: An in vitro study. Endod. Pract. 2009, 11, 18–22. [Google Scholar]
- Ilieva, R.; Dyulgerova, E.; Petrov, O.; Aleksandrova, R.; Titorenkova, R. Effects of high energy dry milling on biphase calcium phosphates. Adv. Appl. Ceram. 2013, 112, 219–226. [Google Scholar] [CrossRef]
- Ilieva, R.; Dyulgerova, E.; Petrov, O.; Tarassov, M.; Gusiyska, A.; Vasileva, R. Preparation of hydroxyapatite/hyaluronan biomimetic nano-hybrid material for reconstruction of critical size bone defects. Bulg. Chem. Commun. 2018, 50, 93–101. [Google Scholar]
- Van Pham, K.; Tran, T.A. Effectiveness of MTA apical plug in dens evaginatus with open apices. BMC Oral. Health 2021, 21, 566. [Google Scholar] [CrossRef]
- Abu Hasna, A.; de Paula Ramos, L.; Campos, T.M.B.; de Castro Lopes, S.L.P.; Rachi, M.A.; de Oliveira, L.D.; Carvalho, C.A.T. Biological and chemical properties of five mineral oxides and of mineral trioxide aggregate repair high plasticity: An in vitro study. Sci. Rep. 2022, 12, 14123. [Google Scholar] [CrossRef] [PubMed]
- Abu Hasna, A.; Pereira Santos, D.; de Oliveira, T.R.G.; Pinto, A.B.A.; Pucci, C.R.; Lage-Marques, J.L. Apicoectomy of Perforated Root Canal Using Bioceramic Cement and Photodynamic Therapy. Int. J. Dent. 2020, 9, 6677588. [Google Scholar] [CrossRef] [PubMed]
- Pace, R.; Giuliani, V.; Prato, L.P.; Baccetti, T.; Pagavino, G. Apical plug technique using mineral trioxide aggregate: Results from a case series. Int. Endod. J. 2007, 40, 478–484. [Google Scholar] [CrossRef]
- Gusiyska, A.; Ilieva, R. Nanosize Biphasic Calcium Phosphate used for Treatment of Periapical Lesions. Int. J. Curr. Res. 2015, 7, 11564–11567. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gusiyska, A.; Dyulgerova, E. Clinical Approaches to the Three-Dimensional Endodontic Obturation Protocol for Teeth with Periapical Bone Lesions. Appl. Sci. 2023, 13, 9755. https://doi.org/10.3390/app13179755
Gusiyska A, Dyulgerova E. Clinical Approaches to the Three-Dimensional Endodontic Obturation Protocol for Teeth with Periapical Bone Lesions. Applied Sciences. 2023; 13(17):9755. https://doi.org/10.3390/app13179755
Chicago/Turabian StyleGusiyska, Angela, and Elena Dyulgerova. 2023. "Clinical Approaches to the Three-Dimensional Endodontic Obturation Protocol for Teeth with Periapical Bone Lesions" Applied Sciences 13, no. 17: 9755. https://doi.org/10.3390/app13179755





