Acceleration Techniques for Teeth Movements in Extractive Orthodontic Therapy
Abstract
:1. Introduction
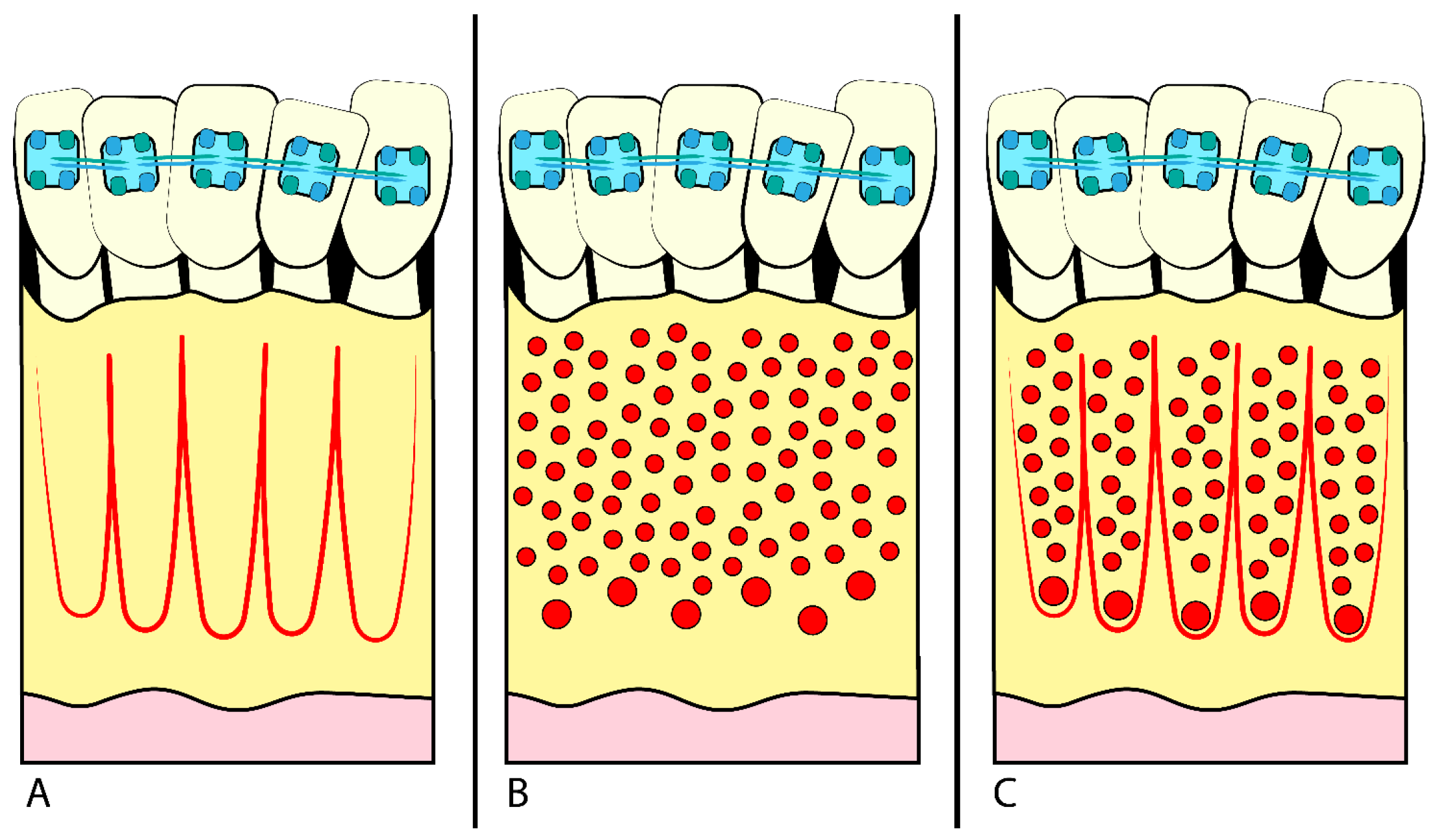
- Corticotomy is the execution of small incisions in the alveolar bone surrounding the teeth to facilitate their movement (Figure 1) [1], using several different techniques (chisel and hammer, piezosurgery, etc.) [4]. The goal is to stimulate local biological response and bone remodelling without damaging the surrounding tissues.
- MOPs are small perforations in the alveolar bone around the teeth, obtained using miniscrews or fine needles [8]. The drilling process stimulates the local inflammatory response and accelerates bone remodelling, allowing for faster and more efficient OTM (Figure 1B) [5]. MOPs can be performed safely and with minor patient morbidity, reducing orthodontic TT [8,10].
- In some cases, nonsteroidal anti-inflammatory drugs (NSAIDs) or drugs-modulating calcium and phosphorus metabolism can also facilitate OTM and reduce pain [17].
- DAD involves a device constantly forcing the teeth to stimulate bone remodelling [18]. Osteogenic distraction is often used to treat severe skeletal discrepancies and requires close collaboration between the orthodontist and the oral-maxillofacial surgeon [19,20]. The process creates a controlled fracture in the bone, followed by applying a distraction device to lengthen the bone over time, forming new bone. This allows significant corrections of skeletal deformities and functional and aesthetic improvements [18]. Although this technique can be highly effective, it is associated with an increased risk of complications and requires careful patient management throughout the treatment process [21].
2. Materials and Methods
2.1. Protocol
2.2. Information Sources and Search Strategy
2.3. Eligibility Criteria
2.4. Synthesis Methods
3. Results
- Corticotomy, 15 articles
- PRF/PRP, 4 articles
- LLLT, 13 articles
- MOPs, 4 articles
- Vibration, 5 articles
- DAD, 1 article
- LLLT + corticotomy, 1 article
- Drugs, 1 article
4. Discussion
4.1. Corticotomies
4.2. Micro-Osteo-Perforations (MOPs)
4.3. Vibration Therapy
4.4. Low-Level Laser Therapy (LLLT)
4.5. PRP and PRF
4.6. Drugs Therapy
4.7. Dentoalveolar Osteodistraction
5. Limitation
6. Conclusions
- Techniques like corticotomies and micro-osteo perforations (MOPs) exhibit 1.5 to 2 times faster acceleration than traditional methods.
- Piezoincisions are effective with variable success rates, offering time benefits but potential costs and discomfort.
- Vibrational therapy’s impact on tooth movement is debated.
- Pulsed electromagnetic field significantly shortens treatment times.
- Low-level laser therapy speeds up tooth movement and offers analgesic benefits.
- PRF, PRP, and Vitamin D treatments increase movement speed.
- Dentoalveolar distraction aids shorter treatment, particularly in patients with vertical skeletal dimensions, minimizing anchoring loss.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CGr | Control Group |
| C Side | Control Side |
| CT | Clinical trial |
| DAD | Dentoalveolar Distraction |
| DG | Distalisation group |
| DNA | Deoxyribonucleic acid |
| ExGr | Experimental Group |
| GaAlAs | Gallium Aluminum Arsenide |
| GCF | Gingival Crevicular Fluid |
| IL-1β: | L’interleuchina-1 beta |
| LAFC | Laser-assisted flapless corticotomy |
| LLLI | Low-level Laser Irradiation |
| LLLT | Low-level Laser Therapy |
| L-PRF: | Leukocyte and Platelet-Rich Fibrin |
| MOPs | Micro-osteoperforations |
| NiTi | Nickel-Titanium |
| NSAIDs | Nonsteroidal anti-inflammatory drugs |
| OPG | Osteoprotegerin |
| OTM | Orthodontic tooth movement |
| P | Prospective study |
| PBM | Photobiomodulation |
| PEMF | Pulsed Electromagnetic Field |
| PRF | Platelet-rich fibrinogen |
| PRP | Platelet-rich plasma |
| RAP | Regional Accleratory Phenomena |
| RCT | Randomised clinical trial |
| RNA | Ribonucleic acid |
| sRANKL | Soluble Receptor Activator of Nuclear factor κb |
| SW | Straightwire |
| TNF-α: | Tumor Necrosis Factor Alfa |
| TT | Treatment Time |
| VT | Vibration therapy |
References
- Wilcko, W.M.; Wilcko, T.; Bouquot, J.E.; Ferguson, D.J. Rapid Orthodontics with Alveolar Reshaping: Two Case Reports of Decrowding. Int. J. Periodontics Restor. Dent. 2001, 21, 9–19. [Google Scholar]
- Coloccia, G.; Inchingolo, A.D.; Inchingolo, A.M.; Malcangi, G.; Montenegro, V.; Patano, A.; Marinelli, G.; Laudadio, C.; Limongelli, L.; Di Venere, D.; et al. Effectiveness of Dental and Maxillary Transverse Changes in Tooth-Borne, Bone-Borne, and Hybrid Palatal Expansion through Cone-Beam Tomography: A Systematic Review of the Literature. Medicina 2021, 57, 288. [Google Scholar] [CrossRef] [PubMed]
- Mummolo, S.; Marchetti, E.; Albani, F.; Campanella, V.; Pugliese, F.; Di Martino, S.; Tecco, S.; Marzo, G. Comparison between Rapid and Slow Palatal Expansion: Evaluation of Selected Periodontal Indices. Head. Face Med. 2014, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.J. Orthodontic Treatment Acceleration with Corticotomy-Assisted Exposure of Palatally Impacted Canines. Angle Orthod. 2007, 77, 417–420. [Google Scholar] [CrossRef]
- Teixeira, C.C.; Khoo, E.; Tran, J.; Chartres, I.; Liu, Y.; Thant, L.M.; Khabensky, I.; Gart, L.P.; Cisneros, G.; Alikhani, M. Cytokine Expression and Accelerated Tooth Movement. J. Dent. Res. 2010, 89, 1135–1141. [Google Scholar] [CrossRef] [PubMed]
- Miles, P.; Fisher, E. Assessment of the Changes in Arch Perimeter and Irregularity in the Mandibular Arch during Initial Alignment with the AcceleDent Aura Appliance vs No Appliance in Adolescents: A Single-Blind Randomized Clinical Trial. Am. J. Orthod. Dentofac. Orthop. 2016, 150, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Aksakalli, S.; Calik, B.; Kara, B.; Ezirganli, S. Accelerated Tooth Movement with Piezocision and Its Periodontal-Transversal Effects in Patients with Class II Malocclusion. Angle Orthod. 2015, 86, 59–65. [Google Scholar] [CrossRef]
- Alikhani, M.; Raptis, M.; Zoldan, B.; Sangsuwon, C.; Lee, Y.B.; Alyami, B.; Corpodian, C.; Barrera, L.M.; Alansari, S.; Khoo, E.; et al. Effect of Micro-Osteoperforations on the Rate of Tooth Movement. Am. J. Orthod. Dentofac. Orthop. 2013, 144, 639–648. [Google Scholar] [CrossRef]
- Quinzi, V.; Tecco, S.; Nota, A.; Caggiati, E.; Mummolo, S.; Marzo, G. Mesial Rotation of the Upper First Molar: Association with Anterior Dental Crowding in Mixed and Permanent Dentition. Appl. Sci. 2020, 10, 5301. [Google Scholar] [CrossRef]
- Quinzi, V.; Ferro, R.; Rizzo, F.A.; Marranzini, E.M.; Federici Canova, F.; Mummolo, S.; Mattei, A.; Marzo, G. The Two by Four Appliance: A Nationwide Cross-Sectional Survey. Eur. J. Paediatr. Dent. 2018, 19, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Davidovitch, Z. Tooth Movement. Crit. Rev. Oral Biol. Med. 1991, 2, 411–450. [Google Scholar] [CrossRef] [PubMed]
- Kau, C.; Nguyen, J.T.; English, J. The Clinical Evaluation of a Novel Cyclical Force Generating Device in Orthodontics. Orthod. Pract. US 2010, 1.1, 1–4. [Google Scholar]
- Scarano, A.; Lorusso, F.; Inchingolo, F.; Postiglione, F.; Petrini, M. The Effects of Erbium-Doped Yttrium Aluminum Garnet Laser (Er: YAG) Irradiation on Sandblasted and Acid-Etched (SLA) Titanium, an In Vitro Study. Materials 2020, 13, 4174. [Google Scholar] [CrossRef]
- Cruz, D.R.; Kohara, E.K.; Ribeiro, M.S.; Wetter, N.U. Effects of Low-Intensity Laser Therapy on the Orthodontic Movement Velocity of Human Teeth: A Preliminary Study. Lasers Surg. Med. 2004, 35, 117–120. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef]
- Alissa, R.; Esposito, M.; Horner, K.; Oliver, R. The Influence of Platelet-Rich Plasma on the Healing of Extraction Sockets: An Explorative Randomised Clinical Trial. Eur. J. Oral. Implantol. 2010, 3, 121–134. [Google Scholar] [PubMed]
- Kim, S.-J.; Park, Y.-G.; Kang, S.-G. Effects of Corticision on Paradental Remodeling in Orthodontic Tooth Movement. Angle Orthod. 2009, 79, 284–291. [Google Scholar] [CrossRef]
- Wang, X.; Mei, M.; Han, G.; Luan, Q.; Zhou, Y. Effectiveness of Modified Periodontally Accelerated Osteogenic Orthodontics in Skeletal Class II Malocclusion Treated by a Camouflage Approach. Am. J. Transl. Res. 2022, 14, 979–989. [Google Scholar] [PubMed]
- Boyne, P.J. Experimental Evaluation of the Osteogenic Potential of Bone Graft Materials. Annu. Meet. Am. Inst. Oral. Biol. 1969, 13–21. [Google Scholar]
- Quinzi, V.; Panetta, G.; Filippi, P.; Rizzo, F.A.; Mancini, L.; Mummolo, S. Autotransplatation of Immature Third Molars as Substitutes for Congenitally Missing Second Premolars: An Alternative Solution in a Young Patient with Oligodontia. J. Biol. Regul. Homeost. Agents 2020, 34, 155–163. [Google Scholar] [PubMed]
- Liou, E.J.-W.; Figueroa, A.A.; Polley, J.W. Rapid Orthodontic Tooth Movement into Newly Distracted Bone after Mandibular Distraction Osteogenesis in a Canine Model. Am. J. Orthod. Dentofac. Orthop. 2000, 117, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Healthcare Interventions: Explanation and Elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef] [PubMed]
- Abbas, N.H.; Sabet, N.E.; Hassan, I.T. Evaluation of Corticotomy-Facilitated Orthodontics and Piezocision in Rapid Canine Retraction. Am. J. Orthod. Dentofac. Orthop. 2016, 149, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Addanki, P.; Gooty, J.R.; Palaparthy, R. Clinical and Radiographic Comparative Evaluation of Buccal and Palatal Corticotomy with Buccal Corticotomy in Periodontally Accelerated Osteogenic Orthodontics with Surgical Bur. Contemp. Clin. Dent. 2017, 8, 321–326. [Google Scholar] [CrossRef]
- Al-Imam, G.M.F.; Ajaj, M.A.; Hajeer, M.Y.; Al-Mdalal, Y.; Almashaal, E. Evaluation of the Effectiveness of Piezocision-Assisted Flapless Corticotomy in the Retraction of Four Upper Incisors: A Randomized Controlled Clinical Trial. Dent. Med. Probl. 2019, 56, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Alfawal, A.M.H.; Hajeer, M.Y.; Ajaj, M.A.; Hamadah, O.; Brad, B. Evaluation of Piezocision and Laser-Assisted Flapless Corticotomy in the Acceleration of Canine Retraction: A Randomized Controlled Trial. Head. Face Med. 2018, 14, 4. [Google Scholar] [CrossRef]
- Angel, S.L.; Samrit, V.D.; Kharbanda, O.P.; Duggal, R.; Kumar, V.; Chauhan, S.S.; Coshic, P. Effects of Submucosally Administered Platelet-Rich Plasma on the Rate of Tooth Movement. Angle Orthod. 2022, 92, 73–79. [Google Scholar] [CrossRef]
- Arumughan, S.; Somaiah, S.; Muddaiah, S.; Shetty, B.; Reddy, G.; Roopa, S. A Comparison of the Rate of Retraction with Low-Level Laser Therapy and Conventional Retraction Technique. Contemp. Clin. Dent. 2018, 9, 260–266. [Google Scholar] [CrossRef]
- Attri, S.; Mittal, R.; Batra, P.; Sonar, S.; Sharma, K.; Raghavan, S.; Rai, K.S. Comparison of Rate of Tooth Movement and Pain Perception during Accelerated Tooth Movement Associated with Conventional Fixed Appliances with Micro-Osteoperforations—A Randomised Controlled Trial. J. Orthod. 2018, 45, 225–233. [Google Scholar] [CrossRef]
- Baeshen, H.A. The Effect of Partial Corticotomy on the Rate of Maxillary Canine Retraction: Clinical and Radiographic Study. Molecules 2020, 25, 4837. [Google Scholar] [CrossRef]
- Bajaj, I.; Garg, A.; Gupta, D. Comparative Effect of Micro-Osteoperforation and Photo-Bio-Modulation on the Rate of Maxillary Canine Retraction: A Split Mouth Randomized Clinical Trial. La Clin. Ter. 2022, 173, 39–45. [Google Scholar] [CrossRef]
- Bhad (Patil), W.A.; Karemore, A.A. Efficacy of Pulsed Electromagnetic Field in Reducing Treatment Time: A Clinical Investigation. Am. J. Orthod. Dentofac. Orthop. 2022, 161, 652–658. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, P.; Bhattacharya, H.; Anjum, A.; Bhandari, R.; Agarwal, D.K.; Gupta, A.; Ansar, J. Assessment of Corticotomy Facilitated Tooth Movement and Changes in Alveolar Bone Thickness—A CT Scan Study. J. Clin. Diagn. Res. 2014, 8, ZC26–ZC30. [Google Scholar] [CrossRef]
- Chandran, M.; Muddaiah, S.; Nair, S.; Shetty, B.; Somaiah, S.; Reddy, G.; Abraham, B. Clinical and Molecular-Level Comparison between Conventional and Corticotomy-Assisted Canine Retraction Techniques. J. World Fed. Orthod. 2018, 7, 128–133. [Google Scholar] [CrossRef]
- Farhadian, N.; Miresmaeili, A.; Borjali, M.; Salehisaheb, H.; Farhadian, M.; Rezaei-Soufi, L.; Alijani, S.; Soheilifar, S.; Farhadifard, H. The Effect of Intra-Oral LED Device and Low-Level Laser Therapy on Orthodontic Tooth Movement in Young Adults: A Randomized Controlled Trial. Int. Orthod. 2021, 19, 612–621. [Google Scholar] [CrossRef]
- Farid, K.A.; Eid, A.A.; Kaddah, M.A.; Elsharaby, F.A. The Effect of Combined Corticotomy and Low Level Laser Therapy on the Rate of Orthodontic Tooth Movement: Split Mouth Randomized Clinical Trial. Laser Ther. 2019, 28, 275–283. [Google Scholar] [CrossRef]
- Feizbakhsh, M.; Zandian, D.; Heidarpour, M.; Farhad, S.Z.; Fallahi, H.R. The Use of Micro-Osteoperforation Concept for Accelerating Differential Tooth Movement. J. World Fed. Orthod. 2018, 7, 56–60. [Google Scholar] [CrossRef]
- Gibreal, O.; Hajeer, M.Y.; Brad, B. Efficacy of Piezocision-Based Flapless Corticotomy in the Orthodontic Correction of Severely Crowded Lower Anterior Teeth: A Randomized Controlled Trial. Eur. J. Orthod. 2019, 41, 188–195. [Google Scholar] [CrossRef]
- Gibreal, O.; Al-modallal, Y.; Al-assaf, M. Evaluation of the Efficacy of 3D-Guided Piezosurgery in Accelerating Mandibular Orthodontic Teeth Alignment: A Randomized Controlled Trial in Adults. Dentistry 3000 2022, 10, 281–288. [Google Scholar] [CrossRef]
- AlSayed Hasan, M.M.A.; Sultan, K.; Hamadah, O. Low-Level Laser Therapy Effectiveness in Accelerating Orthodontic Tooth Movement: A Randomized Controlled Clinical Trial. Angle Orthod. 2017, 87, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, A.; Horodynski, M.; Fusco, R.; Palaia, G.; Polimeni, A.; Romeo, U.; Barbato, E.; Galluccio, G. Photobiomodulation Therapy on Orthodontic Movement: Analysis of Preliminary Studies with a New Protocol. Int. J. Environ. Res. Public. Health 2020, 17, 3547. [Google Scholar] [CrossRef] [PubMed]
- Isola, G.; Matarese, M.; Briguglio, F.; Grassia, V.; Picciolo, G.; Fiorillo, L.; Matarese, G. Effectiveness of Low-Level Laser Therapy during Tooth Movement: A Randomized Clinical Trial. Materials 2019, 12, 2187. [Google Scholar] [CrossRef] [PubMed]
- Khera, A.; Raghav, P.; Mehra, V.; Wadhawan, A.; Gupta, N.; Phull, T. Effect of Customized Vibratory Device on Orthodontic Tooth Movement: A Prospective Randomized Control Trial. J. Orthod. Sci. 2022, 11, 18. [Google Scholar] [CrossRef]
- Kumar, V.; Batra, P.; Sharma, K.; Raghavan, S.; Srivastava, A. Comparative Assessment of the Rate of Orthodontic Tooth Movement in Adolescent Patients Undergoing Treatment by First Bicuspid Extraction and En Mass Retraction, Associated with Low-Frequency Mechanical Vibrations in Passive Self-Ligating and Conventional Brackets: A Randomized Controlled Trial. Int. Orthod. 2020, 18, 696–705. [Google Scholar] [CrossRef] [PubMed]
- Kundi, I.; Alam, M.K.; Shaheed, S. Micro-Osteo Perforation Effects as an Intervention on Canine Retraction. Saudi Dent. J. 2020, 32, 15–20. [Google Scholar] [CrossRef]
- Kurt, G.; İşeri, H.; Kişnişçi, R.; Özkaynak, Ö. Rate of Tooth Movement and Dentoskeletal Effects of Rapid Canine Retraction by Dentoalveolar Distraction Osteogenesis: A Prospective Study. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 204–213. [Google Scholar] [CrossRef]
- Le, L.; Le, K.; Do, T. Influence of Low-Level Laser Treatment on Tooth Movement in Orthodontic Treatment. J. Int. Dent. Med. Res. 2023, 15, 1614–1619. [Google Scholar]
- Liao, Z.; Elekdag-Turk, S.; Turk, T.; Grove, J.; Dalci, O.; Chen, J.; Zheng, K.; Ali Darendeliler, M.; Swain, M.; Li, Q. Computational and Clinical Investigation on the Role of Mechanical Vibration on Orthodontic Tooth Movement. J. Biomech. 2017, 60, 57–64. [Google Scholar] [CrossRef]
- Mahmoudzadeh, M.; Poormoradi, B.; Alijani, S.; Farhadian, M.; Kazemisaleh, A. Efficacy of Er,Cr Laser Incision Corticotomy in Rapid Maxillary Canine Retraction: A Split-Mouth Randomized Clinical Trial. J. Lasers Med. Sci. 2020, 11, 442–449. [Google Scholar] [CrossRef]
- Moradinejad, M.; Chaharmahali, R.; Shamohammadi, M.; Mir, M.; Rakhshan, V. Low-Level Laser Therapy, Piezocision, or Their Combination vs. Conventional Treatment for Orthodontic Tooth Movement: A Hierarchical 6-Arm Split-Mouth Randomized Clinical Trial. J. Orofac. Orthop. 2022. [Google Scholar] [CrossRef]
- Qamruddin, I.; Alam, M.K.; Mahroof, V.; Fida, M.; Khamis, M.F.; Husein, A. Photobiostimulatory Effect of a Single Dose of Low-Level Laser on Orthodontic Tooth Movement and Pain. Pain. Res. Manag. 2021, 2021, 6690542. [Google Scholar] [CrossRef] [PubMed]
- Qamruddin, I.; Alam, M.K.; Mahroof, V.; Fida, M.; Khamis, M.F.; Husein, A. Effects of Low-Level Laser Irradiation on the Rate of Orthodontic Tooth Movement and Associated Pain with Self-Ligating Brackets. Am. J. Orthod. Dentofac. Orthop. 2017, 152, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Naji, R.; Zeitounlouian, T.; Alomari, E.; Youssef, M. Evaluation of the Efficacy of Platelet-Rich Plasma (PRP) and Injectable Platelet-Rich Fibrin (i-PRF) in the Acceleration of Canine Retraction: A Randomized Controlled Trial. J. Int. Oral. Health 2022, 14, 243. [Google Scholar] [CrossRef]
- Inchingolo, F.; Ballini, A.; Cagiano, R.; Inchingolo, A.D.; Serafini, M.; De Benedittis, M.; Cortelazzi, R.; Tatullo, M.; Marrelli, M.; Inchingolo, A.M.; et al. Immediately Loaded Dental Implants Bioactivated with Platelet-Rich Plasma (PRP) Placed in Maxillary and Mandibular Region. Clin Ter 2015, 166, e146-52. [Google Scholar]
- Simre, S.S.; Rajanikanth, K.; Bhola, N.; Jadhav, A.; Patil, C.; Mishra, A. Comparative Assessment of Corticotomy Facilitated Rapid Canine Retraction Using Piezo versus Bur: A Randomized Clinical Study. J. Oral. Biol. Craniofac Res. 2022, 12, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Storniolo-Souza, J.; Lima, L.M.; Pinzan, A.; Alvarez, F.; Pereira, S.C.d.C.; Janson, G. Influence of Low-Level Laser Irradiation on Orthodontic Movement and Pain Level—A Randomized Clinical Trial. Orthod. Waves 2020, 79, 105–112. [Google Scholar] [CrossRef]
- Inchingolo, F.; Tatullo, M.; Abenavoli, F.M.; Marrelli, M.; Inchingolo, A.D.; Gentile, M.; Inchingolo, A.M.; Dipalma, G. Non-Syndromic Multiple Supernumerary Teeth in a Family Unit with a Normal Karyotype: Case Report. Int. J. Med. Sci. 2010, 7, 378–384. [Google Scholar] [CrossRef]
- Sultana, S.; Ab Rahman, N.; Zainuddin, S.L.A.; Ahmad, B. Effect of Piezocision Procedure in Levelling and Alignment Stage of Fixed Orthodontic Treatment: A Randomized Clinical Trial. Sci. Rep. 2022, 12, 6230. [Google Scholar] [CrossRef]
- Taha, K.; Conley, R.S.; Arany, P.; Warunek, S.; Al-Jewair, T. Effects of Mechanical Vibrations on Maxillary Canine Retraction and Perceived Pain: A Pilot, Single-Center, Randomized-Controlled Clinical Trial. Odontology 2020, 108, 321–330. [Google Scholar] [CrossRef]
- Barhate, U.H.; Duggal, I.; Mangaraj, M.; Sharan, J.; Duggal, R.; Jena, A.K. Effects of Autologous Leukocyte-Platelet Rich Fibrin (L-PRF) on the Rate of Maxillary Canine Retraction and Various Biomarkers in Gingival Crevicular Fluid (GCF): A Split Mouth Randomized Controlled Trial. Int. Orthod. 2022, 20, 100681. [Google Scholar] [CrossRef]
- Varella, A.M.; Revankar, A.V.; Patil, A.K. Low-Level Laser Therapy Increases Interleukin-1β in Gingival Crevicular Fluid and Enhances the Rate of Orthodontic Tooth Movement. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 535–544.e5. [Google Scholar] [CrossRef]
- Varughese, S.T.; Shamanna, P.U.; Goyal, N.; Thomas, B.S.; Lakshmanan, L.; Pulikkottil, V.J.; Ahmed, M.G. Effect of Vitamin D on Canine Distalization and Alveolar Bone Density Using Multi-Slice Spiral CT: A Randomized Controlled Trial. J. Contemp. Dent. Pract. 2019, 20, 1430–1435. [Google Scholar] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Fiorillo, L.; Cervino, G.; Cicciù, M. Economic Inequalities and Temporomandibular Disorders: A Systematic Review with Meta-analysis. J. Oral. Rehabil. 2023, 50, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Crimi, S.; Badnjević, A.; Cervino, G.; Bianchi, A.; Cicciù, M. Correlation between Temporomandibular Disorders (TMD) and Posture Evaluated Trough the Diagnostic Criteria for Temporomandibular Disorders (DC/TMD): A Systematic Review with Meta-Analysis. J. Clin. Med. 2023, 12, 2652. [Google Scholar] [CrossRef] [PubMed]
- Quinzi, V.; Saccomanno, S.; Manenti, R.J.; Giancaspro, S.; Coceani Paskay, L.; Marzo, G. Efficacy of Rapid Maxillary Expansion with or without Previous Adenotonsillectomy for Pediatric Obstructive Sleep Apnea Syndrome Based on Polysomnographic Data: A Systematic Review and Meta-Analysis. Appl. Sci. 2020, 10, 6485. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Ferrara, I.; Viapiano, F.; Netti, A.; Campanelli, M.; Buongiorno, S.; Latini, G.; Carpentiere, V.; Ciocia, A.M.; Ceci, S.; et al. Rapid Maxillary Expansion on the Adolescent Patient: Systematic Review and Case Report. Children 2022, 9, 1046. [Google Scholar] [CrossRef]
- Dinoi, M.; Marchetti, E.; Garagiola, U.; Caruso, S.; Mummolo, S.; Marzo, G. Orthodontic Treatment of an Unerupted Mandibular Canine Tooth in a Patient with Mixed Dentition: A Case Report. J. Med. Case Rep. 2016, 10, 170. [Google Scholar] [CrossRef]
- Bakr, A.R.; Nadim, M.A.; Sedky, Y.W.; El Kady, A.A. Effects of Flapless Laser Corticotomy in Upper and Lower Canine Retraction: A Split-Mouth, Randomized Controlled Trial. Cureus 2023, 15, e37191. [Google Scholar] [CrossRef] [PubMed]
- Alkebsi, A.; Al-Maaitah, E.; Al-Shorman, H.; Abu Alhaija, E. Three-Dimensional Assessment of the Effect of Micro-Osteoperforations on the Rate of Tooth Movement during Canine Retraction in Adults with Class II Malocclusion: A Randomized Controlled Clinical Trial. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Papadopoulou, A.K.; Gandedkar, N.; Dalci, K.; Darendeliler, M.A.; Dalci, O. The Effect of Micro-Osteoperforations on Orthodontic Space Closure Investigated over 12 Weeks: A Split-Mouth, Randomized Controlled Clinical Trial. Eur. J. Orthod. 2022, 44, 427–435. [Google Scholar] [CrossRef]
- Pasini, M.; Giuca, M.R.; Ligori, S.; Mummolo, S.; Fiasca, F.; Marzo, G.; Quinzi, V. Association between Anatomical Variations and Maxillary Canine Impaction: A Retrospective Study in Orthodontics. Appl. Sci. 2020, 10, 5638. [Google Scholar] [CrossRef]
- Campanella, V.; Gallusi, G.; Nardi, R.; Mea, A.; Di Taranto, V.; Montemurro, E.; Marzo, G.; Libonati, A. Dentinal Substrate Variability and Bonding Effectiveness: SEM Investigation. J. Biol. Regul. Homeost. Agents 2020, 34, 49–54. [Google Scholar] [PubMed]
- Mummolo, S.; Nota, A.; Marchetti, E.; Padricelli, G.; Marzo, G. The 3D Tele Motion Tracking for the Orthodontic Facial Analysis. Biomed. Res. Int. 2016, 2016, 4932136. [Google Scholar] [CrossRef] [PubMed]
- El-Angbawi, A.; McIntyre, G.T.; Fleming, P.S.; Bearn, D.R. Non-Surgical Adjunctive Interventions for Accelerating Tooth Movement in Patients Undergoing Fixed Orthodontic Treatment. Cochrane Database Syst. Rev. 2015, 2016, CD010887. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Xiao, J.; Li, X.; Li, Y.; Zhao, Z. The Effectiveness of Vibrational Stimulus to Accelerate Orthodontic Tooth Movement: A Systematic Review. BMC Oral. Health 2017, 17, 143. [Google Scholar] [CrossRef]
- Aljabaa, A.; Almoammar, K.; Aldrees, A.; Huang, G. Effects of Vibrational Devices on Orthodontic Tooth Movement: A Systematic Review. Am. J. Orthod. Dentofac. Orthop. 2018, 154, 768–779. [Google Scholar] [CrossRef]
- Qamar, Z.; Alghamdi, A.M.S.; Bin Haydarah, N.K.; Balateef, A.A.; Alamoudi, A.A.; Abumismar, M.A.; Shivakumar, S.; Cicciù, M.; Minervini, G. Impact of Temporomandibular Disorders on Oral Health-related Quality of Life: A Systematic Review and Meta-analysis. J. Oral. Rehabil. 2023, 50, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Cirulli, N.; Inchingolo, A.D.; Patano, A.; Ceci, S.; Marinelli, G.; Malcangi, G.; Coloccia, G.; Montenegro, V.; Di Pede, C.; Ciocia, A.M.; et al. Innovative Application of Diathermy in Orthodontics: A Case Report. Int. J. Environ. Res. Public. Health 2022, 19, 7448. [Google Scholar] [CrossRef]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef]
- Shaadouh, R.I.; Hajeer, M.Y.; Mahmoud, G.; Murad, R.M.T. Systematic Review: Is High-Energy Laser Therapy (HELT) With Flapless Corticotomy Effective in Accelerating Orthodontic Tooth Movement? Cureus 2022, 14, e22337. [Google Scholar] [CrossRef]
- Erdur, E.A.; Karakaslı, K.; Oncu, E.; Ozturk, B.; Hakkı, S. Effect of Injectable Platelet-Rich Fibrin (i-PRF) on the Rate of Tooth Movement. Angle Orthod. 2021, 91, 285–292. [Google Scholar] [CrossRef]
- Minervini, G.; Franco, R.; Marrapodi, M.M.; Ronsivalle, V.; Shapira, I.; Cicciù, M. Prevalence of Temporomandibular Disorders in Subjects Affected by Parkinson Disease: A Systematic Review and Metanalysis. J. Oral. Rehabil. 2023. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Inchingolo, F.; Rapone, B.; Lucchina, A.G.; Qorri, E.; Lorusso, F. Role of Autologous Platelet Gel (APG) in Bone Healing: A Rabbit Study. Appl. Sci. 2021, 11, 395. [Google Scholar] [CrossRef]
- Krishna, V.B.; Duggal, I.; Sharan, J.; Mangaraj, M.; Duggal, R.; Jena, A.K. Effect of Leukocyte-Platelet-Rich Fibrin (L-PRF) on the Rate of Orthodontic Tooth Movement and Expression of Various Biomarkers in Gingival Crevicular Fluid. Clin. Oral. Invest. 2023, 27, 2311–2319. [Google Scholar] [CrossRef]
- Chackartchi, T.; Iezzi, G.; Goldstein, M.; Klinger, A.; Soskolne, A.; Piattelli, A.; Shapira, L. Sinus Floor Augmentation Using Large (1–2 Mm) or Small (0.25–1 Mm) Bovine Bone Mineral Particles: A Prospective, Intra-Individual Controlled Clinical, Micro-Computerized Tomography and Histomorphometric Study. Clin. Oral. Implant. Res. 2011, 22, 473–480. [Google Scholar] [CrossRef]
- Maspero, C.; Abate, A.; Inchingolo, F.; Dolci, C.; Cagetti, M.G.; Tartaglia, G.M. Incidental Finding in Pre-Orthodontic Treatment Radiographs of an Aural Foreign Body: A Case Report. Children 2022, 9, 421. [Google Scholar] [CrossRef]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Xhajanka, E.; Romeo, D.M.; Romeo, M.; Zappone, C.M.F.; Malcangi, G.; Scarano, A.; Lorusso, F.; et al. The Effectiveness of Osseodensification Drilling Protocol for Implant Site Osteotomy: A Systematic Review of the Literature and Meta-Analysis. Materials 2021, 14, 1147. [Google Scholar] [CrossRef]
- İşeri, H.; Kişnişci, R.; Bzizi, N.; Tüz, H. Rapid Canine Retraction and Orthodontic Treatment with Dentoalveolar Distraction Osteogenesis. Am. J. Orthod. Dentofac. Orthop. 2005, 127, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Liou, E.J.W.; Huang, C.S. Rapid Canine Retraction through Distraction of the Periodontal Ligament. Am. J. Orthod. Dentofac. Orthop. 1998, 114, 372–382. [Google Scholar] [CrossRef] [PubMed]



| Articles screening strategy | (Keywords: acceleration) AND (dental movement) AND (orthodontic) |
| Boolean Indicators: (“A” AND “B”) | |
| Timespan: from 1 April 2003 to 1 April 2023 | |
| Electronic Database: Pubmed, Science Direct, Scopus, and Web of Science |
| Author (Year) | Study Design | Number of Patients | Average Age (Years) | Dental Movement Acceleration Techniques | Orthodontic Treatment | Outcomes |
|---|---|---|---|---|---|---|
| Abbas (2016) [23] | RCT | 20 | 15–25 | Piezocision/control group (CGr); corticotomy/CGr | Roth prescription brackets; closed coils NiTi springs 150 g force | Orthodontics supported via corticotomies and piezocision is 1.5–2 times faster than traditional orthodontics. |
| Addanki (2017) [24] | RCT | 16 | 20–40 | Buccal and palatal bur corticotomy/buccal bur corticotomy (control) | SW brackets | No difference between the two groups. |
| Aksakalli (2015) [7] | RCT | 10 | 16.3 ± 2.4 | Piezocision/corticocision (blade 15) | Roth prescription brackets; elastomeric chain 150 g force and medium anchorage (transpalatal arch) | Movement in the side undergoing piezocision is twice as fast as in the CGr. |
| Al Imam (2019) [25] | RCT | 42 | 19.15 | Piezocision | MBT prescription brackets; NiTi coil springs 150 g; medium anchorage (transpalatal arch) | The incisor retraction time in the experimental group has decreased by 27%. |
| Alfawal (2018) [26] | RCT | 36 | 15–27 | Piezocision/CGr; laser-assisted flapless corticotomy (LAFC) ER: YAG laser | MBT prescription brackets; NiTi closed coil spring 150 g force | The experimental side had a higher rate of OTM in the first and second months and a 25% reduction in overall canine retraction duration. |
| Angel et al. (2022) [27] | RCT | 10 | 16–24 | Injection of PRP | Roth prescription brackets; medium anchorage (Nance palatal button) | Movement occurred 35% more increased on the i-PRP side than on the CGr. |
| Arumughan et Al. (2018) [28] | RCT | 12 | LLLT: 810 nm wavelength laser (100 mW power, continuous wave). | MBT prescription brackets; NiTi closed-coil spring 150 g force | LLLT speeds OTM by 12.555% compared to the conventional retraction approach. | |
| Attri et al. (2018) [29] | 2-arm parallel RCT | 60 | 13–20 | MOPs | MBT prescription brackets | Increased OTM with MOPs. |
| Baeshen (2020) [30] | CT with the split-mouth | 20 | 16 ± 2.8 | Partial corticotomy | SW brackets; elastomeric chain 150 g force; medium anchorage (transpalatal arch) | The rate of canine retraction was significantly higher on the corticotomy side than on the CGr (p < 0.05). |
| Bajaj et al. (2022) [31] | split mouth RCT | 30 | 18–25 | MOPs and PBM | MBT prescription brackets | The retraction rate is 1.1 times higher with MOPs than with PBM. |
| Bhad (Patil) e Karemore (2022) [32] | A clinical study with a split-mouth design | 19 | 18–24 | PEMF therapy | SW; NiTi closed-coil springs. | The rate of OTM in the experimental group was significantly higher than the CGr, with a mean increase in M1 of 41% and a mean increase in M2 of 31%. |
| Bhattacharya (2014) [33] | RCT | 20 | 18.8 ± 3.48 | Corticotomy | MBT prescription brackets; NiTi closed coil spring 250 g force; medium anchorage (transpalatal arch) | The corticotomy group’s meantime for en masse retraction was 131 ± 7.5 d, compared to 234 ± 9 d for the traditional approach. |
| Chandran (2018) [34] | RCT | 20 | 14.5 | Bur corticotomy | MBT prescription brackets; active tie-back 100 g force | Alveolar corticotomy enhanced the rate of canine retraction by about 40%. |
| Cruz (2004) [14] | RCT | 11 | 15 | LLLT | Roth prescription brackets from right to left canines;12 mm NiTi closed coil spring | Laser Group is faster than CGr with a ratio of 1.34. |
| Farhadian et Al. (2021) [35] | RCT | 60 | LLLT group (20.9 ± 5.5); LED group (21.7 ± 4.2); CGr (22.7 ± 5.3). | LLLT Group: GaAlAs (810 nm; 100 mW) performed on days 0, 3, 30, and 60. LED Group: intraoral LED device (wavelength: 640 nm; 10 j/cm2; 40 mW/cm2), 5 min/day | MBT and Roth prescription brackets. Medium anchorage (trans-palatal arch, on second molars) 6-mm NiTi closed-coil spring 150 g force | The laser group had a considerably higher rate of canine retraction than the CGr (p = 0.004). This variable is also 26% higher in the LED group than in the CGr; the difference is not statistically significant (p = 0.17). |
| Farid et Al. (2019) [36] | RCT Split mouth | 16 | 21.5 ± 3.2 | LLLT: In-Ga-As diode laser (940 nm; 0.5 W/cm2 power density, 5 J/cm2 Fluence, CW, 240 s time irradiations), weekly for the first month and twice monthly for the next three months | Roth prescription brackets; Medium anchorage (trans-palatal arch). 6-mm NiTi closed-coil spring | LLLT paired with corticotomy did not achieve a higher rate of canine retraction than the gold standard corticotomy approach alone. |
| Feizbakhsh et al. (2018) [37] | RCT | 20 | 28 | MOPs | Roth prescription brackets | The retraction rate was twice as high in the MOPs group than in the control group. |
| Gibreal (2019) [38] | RCT | 34 | 16–27 | Piezocision | MBT prescription brackets; power chain | 59% less TT in piezocision group. |
| Gibreal (2022) [39] | Parallel-group RCT | 34 | 20.86 | 3D-guided piezo-assisted orthodontic treatment/conventional orthodontic | MBT prescription brackets; 5 incisions in the labial cortical plate between the six anterior teeth. | OTM time was decreased by 48% in the experimental group. This could be explained via the regional acceleratory phenomenon (RAP) following the intentional bone injury. |
| Hasan et A. (2017) [40] | RCT | 26 | 20.07 ± 3.13 | LLLT: 830 nm; 2.25-J/cm2 | MBT prescription brackets | LLLT is an efficient way to accelerate OTM. |
| Impellizzeri et al. (2020) [41] | RCT | 3 | 16 | LLLT | SW brackets; lace-back | After 1 month of follow-up, the laser side was 32% faster than the placebo. |
| Isola et Al. (2019) [42] | RCT | 41 | 13.4 ± 2.1 | LLLT: 810 nm laser applied on 3 points (1 W, continuous wave 66.7 J/cm2; 8 J) at 3, 7, and 14 days and every 15 days until the space closed. | Self-ligating brackets system; Closed NiTi coil spring (9 mm long, 50 N). | LLLT therapy is effective in accelerating OTM. |
| Khera et al. (2022) [43] | RCT | 25 | 18–25 | A customised vibratory device is similar to AcceleDent Aura, with a frequency of 30 Hz and force of 0.25 N (25 g). | 0.018” MBT prescription brackets | There is no statistically significant difference between the experimental and CGrs. |
| Kumar et al. (2020) [44] | RCT | 65 | Group 1 (17 ± 0.80), Group 2 (17.40 ± 0.7), Group 3 (16.90 ± 1.1) | Low-frequency vibrations (30 Hz) using a custom-made vibratory device | Group 1: Passive self-ligating brackets (MBT prescription) with low-frequency vibrations Group 2: Conventional MBT brackets with low-frequency vibrations Group 3: Conventional MBT brackets without low-frequency vibrations | There are no significant differences in the rate of space closure between the three groups (p > 0.05). |
| Kundi et al. (2020) [45] | Parallel group RCT | 30 | 27.5 ± 4.4 | MOPs | MBT prescription brackets | Acceleration of OTM by 2–3 times. |
| Kurt et al. (2017) [46] | P | 33 | DAD group: 15.8 ± 1.96; DG group: 16.02 ± 2.8 | The distraction of the Alveolar Bone (DAD) and Distalisation group (DG) | SW Brackets | DAD Group: Canines retracted 7.9 ± 1.49 mm in 11.8 ± 1.3 days; DG group: Canine distalisation achieved 5.29 ± 2.01 mm in 200 ± 57 days; significant distal displacement of maxillary incisors (1.96 ± 2.79 mm) and canines (5.29 ± 2.01 mm). |
| Le et Al. (2023) [47] | CS | 16 | 22.53 ± 3.54 | LLLT: GaAlAs laser (810 nm; 100 mW continuous mode, twice-a-month irradiation; 5.1 J/cm2 | MBT prescription bracket | In orthodontic therapy, LLLt had a positive influence on OTM speed. |
| Liao et al. (2017) [48] | CS | 13 | 13.6 | Vibration using an Oral B (USA) Hamming Bird Vibrating Unit | Coil springs attached to maxillary first molar and canine brackets | OTM was higher with vibration compared to non-vibration. |
| Mahmoudzadeh et al. (2020) [49] | RCT | 12 | 18.91 ± 3.87 | Laser corticotomy | MBT prescription brackets; 9-mm-long nickel-titanium closed coil springs | At one month, OTM under laser was 2.5 times higher than the control. |
| Moradinejad et Al. (2022) [50] | RCT | 32 | 19.13 ± 2.27 | LLLT + Piezocision | MBT prescription bracket; short-size elastic chain | Piezocision is superior in accelerating movement compared to LLLT. Speed is higher with the combination of piezocision and LLLT. |
| Qamruddin et Al. (2021) [51] | CS | 20 | 20.25 ± 3.88 | LLLT: GaAlAs (940 nm; 100 mW for 3 s) | MBT prescription brackets; NiTi closed-coil spring 150 g force | The use of LLLT at regular orthodontic sessions (3 weeks apart) speeds up OTM. |
| Qamruddin et Al. (2017) [52] | RCT | 22 | 19.8 ± 3.1 | LLLT: GaAlAs laser (940 nm) applied at baseline and then repeated after three weeks for two more consecutive follow-up visits | Self-ligating brackets; 6 mm NiTi closed coil springs 150 g force | LLLT applied at 3-week intervals can accelerate OTM. |
| Naji et al. (2022) [53] | RCT | 40 | 21.3 ± 1.8 | Injection of PRF and PRP | Roth 0.018-inch brackets; Ricketts Retraction Spring (Blue-Elgiloy, 0.016 * × 0.022 inches) | PRP determined a more pronounced acceleration of canine retraction than i-PRP. |
| Sakthi et al. (2014) [54] | RCT | 40 | n.d. | Bur decortication | Roth prescription; NiTi closed coil spring 250 g force; no anchorage | The average space closure velocity in the maxilla was 1.8 mm/month, and the mandible was 1.57 mm/month, compared to 1.02 mm/month in the maxilla and 0.87 mm/month in the CGr. |
| Simre (2022) [55] | RCT | 24 | 20.50 ± 2.58 | Piezocision-conventional bur corticotomy | SW brackets; NiTi closed coil springs | Corticotomy with bur was 1.5–2 times more rapid, whereas piezocision was 1.5 times faster. |
| Storniolo-Souza (2020) [56] | RCT | 11 | 14.04 | LLLT | SW brackets; NiTi closed coil springs (12 mm length) | High retraction speed of the mandibular canine laser side. |
| Subrahmanya (2020) [57] | P | 15 | 18–26 | Piezoincision | SW brackets; elastomeric chain 150 g force; medium anchorage (BTP) | 1.5 times acceleration of movement. |
| Sultana (2022) [58] | RCT | 13 | 20.83 ± 2.32 | Piezoincision | MBT prescription Brackets; NiTi closed coil spring 250 g force; medium anchorage (transpalatal arch) | The piezocision group completed the levelling and alignment phase faster than the CGr. |
| Taha et al. (2020) [59] | Single-center pilot RCT | 21 | 15.09 ± 1.7 CGr and 15.9 ± 1.29 in the ExGr | AcceleDent Aura (OrthoAccel Technologies Inc., Bellaire, USA) is used in the ExGr for 20 min daily. | MBT prescription brackets | There were no statistically significant differences in OTM between the control and ExGrs: 1.21 ± 0.32 mm/month in the CGr and 1.12 ± 0.20 mm/month in the ExGr. |
| Uday H Barhate et al. (2022) [60] | RCT | 15 | 18–25 | Injection of L-PRF | Standard Edgewise appliance of 0.018“slot dimension | A slight acceleration was found in the first four weeks. |
| Varella (2018) [61] | P | 10 | 17.7 years | LLLT | MBT prescription Brackets; 9-mm-long NiTi closed coil spring | The laser side is two times faster than the control side (C Side) with high production of IL-1b. |
| Varughese et al. (2019) [62] | RCT | 15 | 22.5 | Periodontal injection of calcitriol (1.25 DHC) on the experimental side and injection of placebo gel on the C Side. | SW brackets; closed NiTi coil springs 150 g force | Significantly greater canine distalisation on the experimental side compared to the C Side. |
| Yassaei (2016) [63] | RCT | 11 | 19 ± 4.21 | LLLT | edgewise appliance; NiTi closed coil springs | LLT did not lead to statistically significant differences. |
| Zeitunlouian et al. (2021) [64] | RCT | 21 | 20.85 ± 3.85 | injection of PRF | MBT prescription Brackets | Statistically significant orthodontic movement acceleration at T2. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dipalma, G.; Patano, A.; Ferrara, I.; Viapiano, F.; Netti, A.; Ceci, S.; Azzollini, D.; Ciocia, A.M.; Malcangi, G.; Inchingolo, A.D.; et al. Acceleration Techniques for Teeth Movements in Extractive Orthodontic Therapy. Appl. Sci. 2023, 13, 9759. https://doi.org/10.3390/app13179759
Dipalma G, Patano A, Ferrara I, Viapiano F, Netti A, Ceci S, Azzollini D, Ciocia AM, Malcangi G, Inchingolo AD, et al. Acceleration Techniques for Teeth Movements in Extractive Orthodontic Therapy. Applied Sciences. 2023; 13(17):9759. https://doi.org/10.3390/app13179759
Chicago/Turabian StyleDipalma, Gianna, Assunta Patano, Irene Ferrara, Fabio Viapiano, Anna Netti, Sabino Ceci, Daniela Azzollini, Anna Maria Ciocia, Giuseppina Malcangi, Alessio Danilo Inchingolo, and et al. 2023. "Acceleration Techniques for Teeth Movements in Extractive Orthodontic Therapy" Applied Sciences 13, no. 17: 9759. https://doi.org/10.3390/app13179759
APA StyleDipalma, G., Patano, A., Ferrara, I., Viapiano, F., Netti, A., Ceci, S., Azzollini, D., Ciocia, A. M., Malcangi, G., Inchingolo, A. D., Inchingolo, F., Palermo, A., & Inchingolo, A. M. (2023). Acceleration Techniques for Teeth Movements in Extractive Orthodontic Therapy. Applied Sciences, 13(17), 9759. https://doi.org/10.3390/app13179759














