Abstract
Milk consumption has increased constantly, with milk being part of the diet of a large proportion of the global population. As a result of this growing demand, the increased competition in the dairy market, and the increasing complexity of the supply chain, the producers in the sector of milk and dairy products resort to technological fraud, which is considered to be a predominant problem in countries without specific legislation. Therefore, further research is required to educate the public about fraud or carelessness in milk production. Over time, as counterfeiting methods have become more complex, detection techniques have had to be developed in the same sequence. This paper aims to review the main adulterants, the counterfeiting techniques, and various methods of detecting counterfeiting.
1. Introduction
Milk is undoubtedly a ubiquitous food in the human diet. This is the first food of mammals and, as such, provides all the energy and nutrients needed for proper growth and development. The nutritional richness of milk is indisputable; it is a good source of protein with high biological value and polyvalent roles in immune function as well as nutrient transport and absorption, and of course, it contains essential vitamins and minerals [1].
According to annex VII, part III of the REGULATION (EU) No 1308/2013, milk and milk products are defined as the normal mammary secretion of milking animals, without any addition or extraction thereof, intended for consumption as liquid milk or for further processing [2,3].
At the same time, fraud from a legal point of view is defined by Directive (EU) No 2017/1371 from Regulation (EC) No 178/2002 of the EU food law, as the general framework from the point of view of the protection of consumers’ interests with regards to the prevention of fraudulent or deceptive practices, the adulteration of food, and any other practices that may mislead the consumer [4,5].
Adulteration of milk and dairy products has become a worldwide concern, however the history of milk counterfeiting is very old. In the old German Empire, milk was diluted, and then its consistency was restored by adding sugar, flour, or calcium carbonate. In addition, this process dates back to 1850, when 8000 babies died in New York from milk produced by the Swill factories because the milk came from animals fed on by-products from distilleries and was then adulterated by dilution with water, bleached by adding plaster, and thickened with starch. Until the early 1900s, the milk was often adulterated with foreign substances obtained from sick cows or mishandled during milking and storage. As a result, milk was often the host of tuberculosis, cholera, typhoid fever, and other life-threatening diseases. It was not until the end of the 19th century, when scientists began to fully understand theories about microorganisms, that they realized that diseases are transferred through milk and that they could intervene to eliminate this risk. Finally, the last resounding case of milk adulteration worldwide is represented by the melamine contamination of infant products in China in 2008 [6,7,8,9,10,11,12,13].
Unfortunately, milk and dairy products are among the most counterfeited foods worldwide. The reasons behind this fraud are mainly the perishable nature of milk, the shortage of supply and demand to meet urban demand, and the lack of adequate detection methods [14]. According to studies, milk is the second most susceptible to counterfeiting, after olive oil. Thus, it is adulterated with harmful substances that increase its quantity but considerably reduce its quality [11,12,15].
In addition to its microbiological quality and safety, the quality of milk is usually defined on the basis of its nutrient levels (mainly protein and fat). These parameters were used to calculate the payment to the supplier. In general, the parameters usually used to assess milk quality are fat, protein, solids (non-fat), and freezing point. The adulterants added to milk improve the value of these parameters, thus increasing milk quality in a dishonest manner [11,16]. As it is equally important to inform consumers about common adulterants and their possible effects on health, appropriate consumer awareness can be considered a solution to warn against dishonest practices and counterfeiting in the food industry [11,12,13,15].
Although consumers have a right to believe that the milk they purchase will be pure and unadulterated, this is regrettably not always the case. Adulteration is normally carried out either for financial gain or because of a lack of proper hygiene during processing, storage, transport, and marketing. Eventually, it reaches the stage where the consumer is either deceived or becomes a victim of disease. Milk is produced throughout the year. However, heat stress during the summer months significantly lowers milk production. Milk is transported from the point of production to the largest cities mainly through intermediaries. Such milk is often subject to skimming or dilution to increase profit. Adulterants such as starch, wheat flour, urea, cane sugar, and vegetable oil are added to preserve its composition. Milk is a perishable product, so in the summer months it is likely to spoil during transport. Therefore, intermediaries add chemical preservatives, including sodium bicarbonate, formaldehyde, hydrogen peroxide, penicillin, and streptopenicillin. People consume liquid milk that has been altered and diluted to such an extent that its nutritional properties are reduced, leading largely to general public health problems and malnutrition [17].
The negligence that occurs in developing countries seems to be largely encouraged by the lack of strict control by food safety authorities. Additional suggestions for fraud prevention in milk and other dairy products and strategies for improvement include: better tailored education for farmers and creating networks to prevent malpractice that could present itself as fraud; improved implementation of dairy fraud detection methods; and implementation of additional anti-fraud measures during supplier audits. For example, the supplier should verify that a warning policy has been implemented, and auditors should collect and analyze milk samples to validate supplier claims [9].
2. Materials and Methods
The current review was restricted to articles with English full-text availability. MDPI, Google Scholar, MedCrave, Springer Link, Springer Nature, Elsevier, and Juniper Publishers were among the databases used. The most common search terms were: milk quality, milk adulteration, potential milk adulterants, and detection methods. In addition, searches were conducted using each adulterant in turn. We also looked for additional references in the bibliographies of the included papers. In our review of the literature, we discovered a sizable number of studies that mostly discussed the chemical makeup of milk and how the most crucial elements of milk change when adulterants are added. The results of the thorough search are sorted into categories and listed according to the best techniques discovered. We only included the more than 120 research articles and review papers that were discovered after 2009 because that is when the majority of adulterant detection techniques were created, which was necessary following the significant finding of the falsification of powdered milk from China in 2008.
3. Results and Discussion
3.1. The Most Common Milk Adulterations
The different types of adulterants found in milk can be categorized into intentionally or accidentally added adulterants. However, only adulterants that have been added intentionally will be presented below. In addition, water, vegetable proteins, whey, and milk of different species (cow, buffalo, goat, sheep, camel, etc.) form the main constituents of economically motivated adulterants and do not pose a serious health risk. Also, adulterants such as urea (to increase the non-protein nitrogen content and make milk white), formalin and boric/benzoic acid (to increase the shelf life of milk), detergents (to emulsify the oil in diluted milk), chlorine (to compensate for the density of diluted milk after adulteration), and ammonium sulphate (to maintain milk density) pose serious health risks [11,12,13,18,19,20]. Figure 1 shows the most common types of falsification of raw milk.
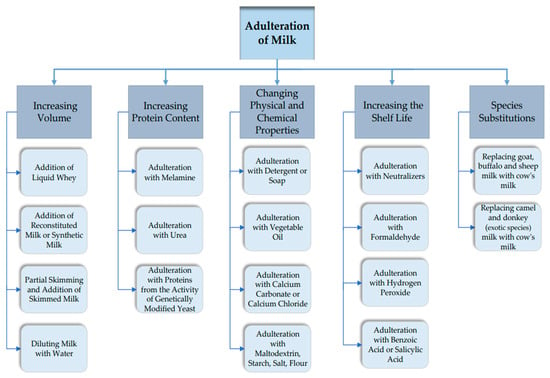
Figure 1.
The most common forms of adulteration of raw milk.
3.1.1. Adulteration That Generally Aimed at Increasing the Volume of Milk
This type of adulteration can be performed by adding water or skimmed milk to whole milk, as well as by double forging by both adding water and reducing the fat content of the milk [7,16].
Addition of Liquid Whey (a By-Product of Cheese Making)
This is a well-known practice to increase the volume of milk, and it is noticed especially in cases where a large amount of cottage cheese is produced, but it has serious economic effects, by obtaining products derived from milk with low nutritional value [21]. The advantage of whey addition in moderate proportion is that it does not significantly change the milk’s lactose and protein content without producing a series of sensory changes noticeable to consumers. [22]. In addition, the whey added to the milk does not change the overall milk density but reduces the solids and fat content, which decrease in proportion to the whey added. In this particular case, it is the fat that reflects the adulteration. Moreover, the addition of whey cheese to milk is very difficult to detect by formal analytical procedures, making it necessary to implement new experimental procedures/tests [11,12,20,23,24]. Casein macropeptide (CMP) content analysis is a standard method for detecting and quantifying whey that has been purposefully added to milk. This K-casein fragment is hydrophilic and should not be present in milk, but it is still soluble in whey [22].
Addition of Reconstituted Milk or Synthetic Milk
Synthetic milk is a remarkable imitation of milk, which contains vegetable oil, urea, and emulsifiers. It is well-known for its fat, nitrogen, glucose, and foam content, as well as a specific gravity similar to that of natural milk. When the synthetic milk is mixed with normal milk in different proportions, it becomes identical to it, including flavor. It is reported that synthetic milk is used to alter milk by 5–10% [3,13]. In addition, Table 1 lists the physical and chemical characteristics of natural and synthetic milk that differ most frequently. Unlike synthetic milk, natural milk does not produce foam when rubbed on the palm. Both milks’ pHs differ significantly from one another. Natural milk has a pH range of 6.6 to 6.7, but synthetic milk has a pH range of 10 to 11. Synthetic milk has a very alkaline character. Natural milk doesn’t change color when heated, while synthetic milk turns yellow when it’s boiled. While artificial milk gradually turns pale yellow over time, natural milk stays white when stored. Yet, the percentages of non-fat solids and fats are the same in each [25].

Table 1.
Notable differences between the physical and chemical characteristics of natural and synthetic milk–data from Mudgil, D. et al., 2013 [25].
Partial Skimming and Addition of Skimmed Milk
This practice is most often used when milk with a high fat content, milked in the evening, is skimmed and added on top of whole milk. Milk fat is one of the most valuable components and therefore can be subject to fraud; hence, removing a quantity of fat leads to changes in the characteristics of the milk: the density increases and the fat content decreases in proportion to the degree of skimming. The dry matter in the milk may increase, or its value may remain unchanged [11,26].
Diluting Milk with Water
This is the most common, simplest, and oldest method of adulteration, based on the percentage reduction of all milk components and the addition of chemicals to bring the density and color to normal parameters. The milk will increase considerably in volume; hence, the producers will earn a significant profit, but the nutritional value and other quality control parameters of the milk (such as density, fat, solid-non-fat, protein, and freezing point) will decrease. Due to the fluctuations of these characteristics over a wide range during the lactation period, when a small amount of water is added, they are not below normal limits. The addition of an increased concentration of hard water to milk showed a negative effect on milk quality by increasing acidity, thus reducing the shelf life of milk. Raw milk diluted by farmers with water, regardless of its type, could be a major source of contamination. This water can be contaminated with feces, manure, industrial materials, and household effluents, and milk can in turn harbor a variety of microorganisms and harmful chemicals, thus constituting a considerable source of pathogens. Adulteration of milk with water can be detected by using a lactodensimeter or by checking the freezing point of the milk. Therefore, dilution of milk with water can lead to changes in nutritional, hygienic, and technological quality in addition to changes in chemical composition, as seen in Table 2 [16,27,28].

Table 2.
Variations of milk quality control parameters according to the degree of dilution with water.
3.1.2. Adulterations Involving the Increased Protein Content
Adulterants rich in nitrogen have become a well-known topic in recent years, mainly because of food safety incidents. This adulteration is common and difficult to detect because the Kjeldahl and Dumas methods that are used to quantify the total protein content of milk cannot distinguish the nature of the non-protein nitrogen. The most commonly used adulterants in this category are whey, melamine, and urea, and this is because of their high nitrogen content and low cost [3,11,13].
Adulteration of Milk with Melamine
According to the World Health Organization, melamine is a nitrogen-rich substance that is used to increase the apparent protein content and therefore the economic value of milk. Until melamine contamination was reported in China in 2008, the limit of melamine in EU food legislation was not established. Both the European Commission and the United States Food and Drug Administration imposed the maximum allowable limit, which was later followed by the Codex Alimentarius Commission in a new ruling in 2010. According to Regulation 1881/2006, the maximum level of melamine in food, except infant formula, is 2.5 mg/kg, and in powdered infant formula and follow-on formula, the maximum level allowed is 1 mg/kg. Although harmful and causing kidney and urinary problems, including infant death when it reacts with cyanuric acid, melamine is not a carcinogenic compound and has low oral toxicity. The toxic dose of melamine is on par with tht of common table salt, with an average lethal dose (LD50) greater than 3 g per kilogram of body weight [8,12,29]. The following are some of the ways that melamine can get into milk and milk-related products: adulteration of milk by direct addition to increase protein content; the application of the insecticide cyromazine on crops (cyromazine is converted to melamine in the animal’s body and may therefore be present as a contaminant in the animal’s milk and tissues); utilization of fertilizers with nitrogen when the nitrogen source is melamine; transfer of melamine from dairy industry plastics and packaging materials; permeation in cows’ milk and tissue when animals graze on fertilized pasture (melamine is fast transferred from grass to milk in 8 h, with a 3% efficiency for low concentrations and a 2.1% efficiency for pastures with high melamine concentrations) [29].
Adulteration of Milk with Urea
Urea is used as an adulterating agent in milk when water is added to it in order to reduce the milk’s density and whiteness. Urea acts as a preservative, increases solid-non-fat and non-protein nitrogen, decreases titratable acidity, and suppresses milk fermentation. At the same time, it makes the milk viscous, giving the impression of thicker milk. Urea, being a natural component of milk, accounts for most of the non-protein nitrogen in milk, i.e., 55%. The amount of protein in the feed ration and the wellbeing of the animals have a direct impact on the concentration of urea in milk, which varies from farm to farm. It has been demonstrated that the content of urea in milk increases with the amount of protein in the feed taken over the previous 24 h. According to the Food Safety and Standards Authority of India (FSSAI), the maximum permissible limit for urea in milk is 70 mg/100 mL. The adulteration of milk with urea could be performed in two ways: by intentionally adding urea or by adding synthetic milk to natural milk. Contrary to the addition of water, which decreases the density of the adulterated milk, the addition of urea enhances it. Because of this, it’s common practice to preserve the density of tainted milk by mixing water and urea. For instance, diluted milk (20%) has a density of 1022 kg/m3, and adding 3% urea to diluted milk (20%) makes it nearly as dense (1030 kg/m3) as unadulterated milk. The milk, water, and urea combination becomes denser as the urea concentration rises for a certain level of dilution [10,11,13,18,30].
Adulteration of Milk with Proteins Resulting from the Activity of Genetically Modified Yeasts
The technology for obtaining these types of proteins allows them to be applied anywhere, even in urbanized areas. This technology is still being studied and the aim is to make these caseins allergen-free. Since these proteins are based on GMO technology, no milk protein produced in this way has so far received regulatory approval in Europe. In the US, products containing proteins identical to those of natural milk, produced by a controlled fermentation process, are already on the market. In the future, it is likely that these proteins will also be the main ingredient in synthetic milk as a response to the huge demand for the product, the drawbacks of factory farming, and concerns about lactose allergies, hormones, and antibiotics [10,31].
3.1.3. Counterfeiting Involving the Addition of Unusual Substances
This practice is applied in order to hide another type of falsification and involves the addition of the following compounds:
Detergents or Soap
This type of compound is added to achieve the natural characteristics of milk, especially to make the milk thicker and to emulsify and dissolve the vegetable oil previously added to replace the extracted fat, forming a solution that resembles the froth of freshly milked milk. Detergent is added mainly to synthetic milk, which is similar to natural milk in being white in color and is produced by mixing urea, detergent, vegetable oil, neutralizers, sugar, and water. Detergents have been shown to be the essential components of such a milk-like preparation. Anionic detergents are widely used in such practices precisely because of their low cost and easy availability [11,13,19,32].
Vegetable Oil
Milk fat is an important component of food and plays a significant role in the economics, nutrition, and physical and chemical properties of milk. About 70% of the milk fat is made up of saturated fatty acids, whereas 30% is unsaturated fatty acids. Diet, lactation, individual characteristics, breed, state of health, and environmental conditions all have an impact on an animal’s fatty acid composition. The most significant factor is the nutrition of dairy cows. However, the incorporation of vegetable oils alters the content, type, and distribution of fat droplets in the protein network, causing changes in the microstructure and textural behavior of dairy products. Olive oil in particular is used to replace milk fat, and it is the added element after the fat content is removed. As a result of having a fatty acid profile that is nearly equal to milk fat, palm oil is yet another frequently utilized oil. In order to identify blends of vegetable oils in animal fats, cholesterol has also frequently been utilized. Typically, milk fat contains 204.3 to 382.4 mg/100 g of cholesterol. Due to the high cholesterol content of milk fat, adulteration with foreign oils will be obvious due to the change in cholesterol levels. Since milk fatty acids are short-chain fatty acids (caprylic, capric, and butyric) and vegetable fats are long-chain fatty acids, only a simple analysis of the fatty acid profile by chromatographic method can show that milk has been adulterated with vegetable oils [11,12,33,34].
Calcium Carbonate or Calcium Chloride
It is added to correct the density and mask the dilution of the milk with water. When the calcium value rises above the normal range, the milk can be said to be falsified [11,16].
Maltodextrin
Maltodextrins are polysaccharides containing dextrose and are obtained either chemically or by enzymatic hydrolysis of starch. Maltodextrins are classified on the basis of the amount of reducing sugar in relation to total carbohydrates, ranging from 3 to 20%. Maltodextrin, which is used in foods and beverages as a sweetener, thickener, and/or stabilizer, has a solubility of about 1.2 kg/L. Maltodextrin imparts important functional properties such as bulking, gelling, binding, prevention of crystallization, promotion of dispersibility, and freezing control. It has been reported that maltodextrin is added as an adulterant to milk with the main aim of increasing the density and yield of the food product with maltodextrin as an ingredient [12,20,35].
Starch, Wheat Flour or Rice Flour
These are added precisely to increase the density of the milk but also to increase the milk solids content. Functional maize starch is also specially designed for introduction into the milk industry. It successfully replaces modified starches with much better stability and is suitable for use in food processes that undergo heating or shearing. It has no impact on product, color, or taste [7,11,12,16]. In addition, rice proteins are used as milk supplements in various food products for consumers with lactose intolerance [13].
Salt
It is added mainly to get a correct lactometer reading by increasing the milk density. It is the most commonly used because its properties lend themselves very well to such adulteration. It is relatively difficult to detect, as there is quite a large amount of chlorides in milk, and in some particular situations (milk from cows with mastitis), the chlorides exceed the normal maximum limit. However, the natural content of chlorides in mixed milk (expressed as sodium chloride) varies between 120 and 170 mg/100 mL of milk, with an average of 140 mg/100 mL and up to 200 mg/100 mL in colostral milk or milk from cows with mastitis [11,35].
3.1.4. Adulterations Associated with Increasing the Shelf Life of Milk
Neutralizers
These are added to cover the acidity and sour taste of the milk. The most popular neutralizers are sodium bicarbonate, hydrated lime, sodium hydroxide, and sodium carbonate. Although the addition of such alkaline substances is not allowed by law, producers tend to neutralize milk to avoid rejection in milk collection centers and factories [10,11,16].
Formaldehyde
This is an antiseptic, a disinfectant, and a good preservative. It is used to preserve milk for a long period of time (1 mL per 10 L of milk preserves it for about 10 days), especially during transport and storage to avoid refrigeration costs. It is a toxic and very dangerous substance, considered carcinogenic, and a high dose of formaldehyde can affect the liver and cause kidney damage [10,12,13,14,36].
Hydrogen Peroxide
It has a similar role to formaldehyde and helps increase shelf life by acting as a preservative. It decreases the souring of milk when hygiene and low-temperature storage rules are not followed by stopping bacterial growth. Hydrogen peroxide (H2O2) is largely used as a preservative in milk and dairy products around the world to activate the inherent lactoperoxidase enzyme system in areas where refrigeration is not widely available. Although, due to chemical processes within raw milk, it may contain small amounts of hydrogen peroxide (1–2 mg/L), the concentration necessary to inactivate pathogens is 10 times higher. In addition, the presence of H2O2 in high concentrations can lead to modifications in the milk’s chemical composition. At the same time, the addition of hydrogen peroxide for the purpose of preserving milk is prohibited for the following reasons: it masks to some extent negligence in the observance of hygienic conditions; it has a germicidal action that is not selective, acting more strongly on lactic bacteria than on spoilage microflora; used in larger quantities to ensure preservation for 1–2 days, it can impart undesirable sensory properties to milk (bitter, irritating taste); added even in small quantities, hydrogen peroxide, through the active oxygen released, causes incipient oxidation of milk fat. The US Food and Drug Administration (FDA) only allows hydrogen peroxide in milk that is used for cheese production [11,13,37].
Acids
These are added to milk as preservatives. The most common are salicylic acid and benzoic acid, which are responsible for increasing shelf life. Salicylic acid added to milk at a rate of 0.04–0.05% preserves the product for several days. Salicylic acid and its salts are prohibited for use in the preservation of milk because of their harmful effects on the body and other negative implications. Benzoic acid is widely used in foodstuffs as a preservative. Although benzoic acid is generally recognized as safe (GRAS) under the food regulations, benzoic acid is not a permitted preservative in milk and milk beverages in the EU and China. WHO has assessed and established an acceptable daily intake (ADI) for benzoic acid of 0–5 mg/kg body weight [10,13,15,38,39,40].
3.1.5. Adulterations Involving Species Substitutions
Adulteration caused by the replacement of milk with higher nutritional value (e.g., goat, sheep, buffalo), including exotic species such as donkey and camel, with cheaper cow milk is explained by seasonal fluctuations and lower yields of more expensive milk. This process has an overall negative economic impact and could represent an issue from an ethical, religious, or cultural perspective.
In certain EU countries, especially those in the Mediterranean area, Eastern Europe, and the Middle East, a variety of cheeses are prized as traditional products made from goat’s milk, sheep’s milk, their milk in a mixture, or buffalo milk. These sorts of cheeses generally have higher market prices and are therefore more prone to adulteration of the raw material from which they are made. There is now a growing market for non-cow’s milk in some countries, particularly goat’s milk, but also for other aspects such as its superior digestibility. In addition, according to studies, goat’s milk may be a possible alternative to cow’s milk as it is considered to be hypoallergenic in comparison with cow’s milk. However, because the proteins are very similar, people allergic to cow’s milk proteins can be affected by milk from any species, demonstrating the importance of correct labeling. Therefore, milk from any species should be listed on food labels, according to the legal requirement, since it could pose a health risk to consumers who are allergic. [3,41,42].
The composition of milk from different types of farm animals differs significantly in physico-chemical indicators such as the mass fraction of proteins and fats, minerals, vitamins, enzymes, etc. An important identification criterion for the type of milk is also the polymorphism of caseins, the constituent technological components of raw milk that determine its possibility of industrial processing. In order to avoid possible fraudulent substitution of goat and sheep milk by cow’s milk, it is necessary to develop analytical procedures capable of detecting such frauds and protecting consumers against misleading mislabeling [13,41,42,43].
3.2. Methods for Detecting Adulterants in Milk
The control of milk quality is very important for safety reasons. Therefore, the adulteration of milk decreases its quality and can even affect its safety. The methods for detecting adulteration in milk are generally classified into qualitative and quantitative detection methods. Although the quantitative detection methods include complex biotechnological and electrical methods as well as sophisticated molecular tools for food authentication and traceability, the qualitative detection methods based on color chemical reactions (biochemical and physical-chemical) are advantageous because they are simple, fast, and very easy to perform, even if they are not very accurate [16,44]. Over time, as counterfeiting methods have become more complex, detection techniques have had to be developed in the same sequence. While Regulation (EU) 2018/150 of 30 January 2018 provides the general methods for the analysis and quality evaluation of milk and milk products, the detection of various adulterants as well as food adulteration practices are performed through more sophisticated and complex analytical techniques [45].
3.2.1. Physical Methods (Quantitative)
The most commonly applied methods based on the physical properties of milk are, among others, the density, the freezing point, and the refractive index. These techniques are easy to perform and relatively cheap and convenient; however, due to natural variations in milk composition, they may not be highly accurate. In addition, the physical methods have limitations (i.e., seasonal changes, composition of milk, regional factors) due to natural variations, such as lower sensitivity in comparison to chemical and instrumental ones [12,35,46].
3.2.2. Chemical Methods (Qualitative)
Chemical methods for detecting milk adulteration are based on observable physico-chemical changes and these can be observed in Figure 2. They can be performed in any biosafety level 1 laboratory with the availability of chemical reagents and the necessary precautions. Chemical changes may occur as a result of a chemical reaction between the adulterant in the milk and a specific chemical reagent, resulting in the appropriate colorimetric detection [47,48].
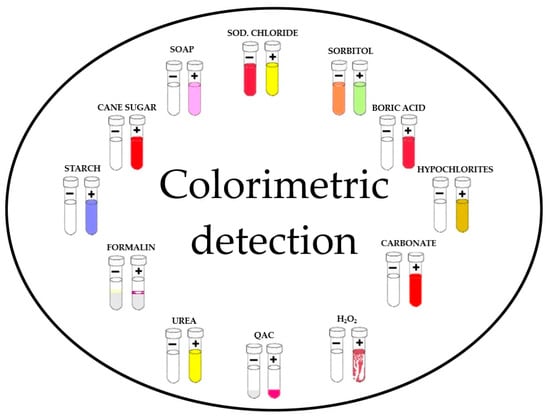
Figure 2.
Color-based reactions for the determination of adulterants in milk (adapted from Chaudhry, H.R. et al., 2015) QAC = Quaternary Ammonium Compounds [47].
Even if they are easy to perform and rapid, these methods lack precision because they rely on a sufficient concentration of milk adulterant. In addition, such methods require a multi-step approach, including separate tests for each adulterant to be identified. In addition, it was discovered through the specialized literature review that the systematic improvement effort pertaining to the execution of qualitative tests to detect adulterants in milk received insufficient attention. Recently, efforts have been made to improve the majority of the qualitative tests for the identification of typical milk adulterants. The testing techniques for the qualitative tests have been improved in two separate ways: by standardizing the chemical reagents used and by improving the environment in which the tests are conducted. When reviewing and evaluating available qualitative tests for the detection of common adulterants reported in milk, it was observed that there is a wide variation related to the performance of several tests in terms of aspects such as sensitivity, convenience, and cost. Given the demands for improved performance, the existing qualitative tests for the determination of common adulterants have undergone numerous changes, and the most relevant tests are listed in Table 3 [12,20,35,47].

Table 3.
List of qualitative tests for finding common milk adulterants that have been reported (adapted from Aparnathi et al. [35]).
3.2.3. Instrumental Methods (Quantitative)
To effectively protect consumers and promote trade, an acceptable level of food quality and safety must be guaranteed. Modern biotechnology offers a range of rapid, sensitive, and accurate methods for the detection and analysis of adulterants in food products. However, recent incidents of adulterated milk contamination have shown that standards in milk quality control are insufficient in identifying poor quality milk. Therefore, different methods have been studied and applied precisely to combat these problems. In addition, such detection methods require large investments. Another important aspect is that the type of quantitative detection technique depends on the nature of the adulterants in milk. Although there are fairly well-known techniques based on portable equipment, those designed and developed recently require experimental set-up and much more expensive equipment, as well as a number of operational procedures [16]. About ten years ago, studies of milk and milk products could only be conducted in laboratories using chemical or physico-chemical techniques. Since they are direct procedures, they are seen to be the most trustworthy; nonetheless, such an analysis is time-consuming (the analysis might take up to 15 h), a process that calls for expertise and specialized knowledge on the part of the personnel, as well as high-purity chemicals. This causes a rise in the cost of the assessment of raw materials, as well as a reduction in the accuracy of the results due to imperfect reagents, the presence of human factor in the laboratory, and the subjective evaluation of the results.
Although in principle they are simple methods, systematic errors in the analysis, such as a significant difference of up to 0.3% fat, often arise when the indicators of the used device, even if these are calibrated according to the manufacturer’s data, differ from the indicators obtained by reference methods, such as the Gerber method for fat determination. This leads to discrepancies between the milk analyzer readings and the results obtained from the chemical analysis data precisely because the Gerber method was not correctly carried out. In addition, thanks to new technologies, quality analysis is carried out much faster and depends less on the qualifications of employees. Therefore, priority is currently given to indirect methods of data collection. The analytical methods by which milk authenticity can be demonstrated are presented in Figure 3 [12,13,48,49].
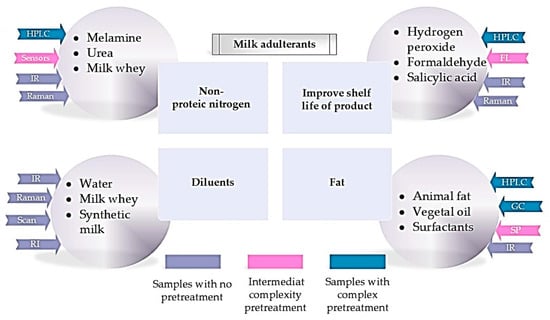
Figure 3.
A description of the main milk adulterants, along with information on sample pretreatment complexity and the analytical techniques used for determining the most commonly used (adapted from Nascimento et al., 2017). FL: molecular fluorescence; GC: gas chromatography; HPLC: high performance liquid chromatography; IR: infrared spectrometry; RI: measurement of refractive index; Scan: scanometry; SP: UV-vis spectrophotometry [48].
Techniques for Identifying Adulteration in Nitrogen Content
As it is already mentioned, this common adulteration is difficult to detect because the Kjeldahl and Dumas methods used to quantify the total protein content of milk cannot distinguish with accuracy the nature of the non-protein nitrogen. For the detection of these nitrogenous chemicals in milk, many analytical techniques have been created since 2010. The most popular techniques include gas chromatography, high-performance liquid chromatography (GC and HPLC), NIR, MID, Raman infrared spectroscopy, enzyme-linked immune sorbent assay (ELISA), capillary electrophoresis (CE), and nuclear magnetic resonance (NMR) [48,49]. There are several analytical techniques for detecting melamine in liquid and powdered milk, but regulatory agencies prefer and frequently use HPLC to identify this adulterant because of its great sensitivity and molecular specificity. There are many effective detectors that can be used, including mass spectrometry (MS), fluorescence, and UV-vis spectrophotometry. For the detection of melamine in liquid milk and powdered infant formula, HPLC with UV or fluorescence detection has also been suggested. This was taken into account precisely because, in comparison to HPLC-MS/MS, the equipment needed for these approaches is much simpler and less expensive. Due to the fact that other organic substances absorb in the same spectral region as melamine (about 240 nm), they can, however, be less selective, particularly in the HPLC-UV approach. In addition, infrared spectroscopy and chemometric techniques make for a perfect methodology for evaluating the authenticity and quality of milk sold on the market [48,50]. Due to their high nitrogen content and low price, melamine, urea, and whey are the most common adulterants used for this purpose. The analytical procedures for their identification are described in Table 4 [46].

Table 4.
Different techniques to identify adulterants added to increase protein content.
These methods have a number of drawbacks, including expensive instrumentation, the need for qualified analysts, the length of time, immobility, human interaction with dangerous reactants, chemical waste during testing, and sensitivity to stray gases in the sample [55].
Studies based on biosensors have shown that they can be used to detect compounds added to milk to boost the protein content in a quick, easy, high-throughput, multi-target, and low-cost manner. These technologies are thought to be new and appealing to get over the drawbacks of standard approaches and instrumental analysis [42,56,57]. To determine the presence of melamine in infant formula, Fodey et al. developed an optical biosensor-based immunoassay. This quick and accurate technique relied on a substance with a chemical structure similar to melamine, which was utilized as a hapten to develop a polyclonal antibody and as an immobilized antigen on the surface of a biosensor chip. The only disadvantage of the procedure would be the antibody’s considerable cross-reactivity with the insecticide cyromazine, whose metabolite is melamin [58].
Another study to identify melamine is based on a portable surface plasmon resonance (SPR) biosensor, which is a non-invasive, label-free sensor that uses polarized electromagnetic waves to investigate real-time interactions between an analyte in solution and immobilized biomolecular recognition elements. The three types of immunoassays used in the study, namely the direct test, displacement test, and competitive test, demonstrated great selectivity for the chemical compound. The competitive test, which was completed in 15 min and had a detection limit of 0.02 g/mL, was the most efficient [59].
Kaneko et al. created an aptamer-based biosensor for detecting melamine in raw milk. The procedure involved microarray-based screening and chemiluminescence detection to optimize the screened biosensor. At 2 mM melamine, the biosensor demonstrated considerably increased signal intensity [60].
Hao et al. made possible the simple and rapid detection of melamine in milk by using a reusable fading-wave fiber optic biosensor. The complete test process, including regeneration, has thus been shown to take less than 15 min. Because of the surface chemistry of the melamine-bovine serum albumin conjugates on the probe surface, not only are immobilized melamine activities towards the antibody maintained, but non-specific adsorption is reduced by immersing the fiber surface in bovine serum albumin. In this study, cross-reactivity against organic molecules structurally related to melamine was low [61].
Several studies have been published on the detection of urea as an adulterant in milk utilizing biosensors. Ezhilan et al. have created a promising technique for determining melamine and urea in cow’s milk samples. It was represented by an acetylcholinesterase cyclic voltammetric biosensor based on a Pt electrode modified with zinc oxide nanospheres. To examine the electrochemical changes on the biosensor electrode, cyclic voltammetry was used. The biosensor was subjected to testing with urea and melamine at levels ranging from 1 to 20 nM and was able to identify the adulterants down to 3 and 1 pM, respectively. The bioelectrode demonstrated 100% permeability to the binary combination of melamine and urea, which improved selectivity [55].
Entrapping the urease enzyme in a polymer matrix of poly (carbamoylsulphonate) (PCS) and polyethyleneimine (PEI) resulted in the development of a potentiometric biosensor for the detection of urea. The stand-alone urea biosensor device detects urea in milk samples using a screen-printed Ag/AgCl reference electrode. This biosensor was thus built to respond quickly and consistently to changes in test urea concentrations. Was utilized as well on unprocessed milk samples, with a limit of detection of 2.5 × 10−5 M/L [62].
Naik et al. developed an Android-integrated biosensor system to identify fraudulently added urea in milk, improve health and consumer comprehension, and facilitate communication between the food testing laboratories and concerned authorities. As a result, data from a flow injection urea biosensor served as a point of contact for the analyst, the customer, and the appropriate higher authorities. Initially, adulterated milk samples were evaluated using the biosensor, and the results were saved on a computer. Once the data had been evaluated, it was transmitted to the analyst’s Android phone in graphic form, with the response signal flow visible in real time. This tool can be successfully used in milk collection centers, individually by the consumer, or by authorities for data collection and long-term milk quality monitoring [63].
The use of a simple portable optical screening sensor provides a semi-quantitative method for detecting urea in milk. This sensor is made of air-stabilized lipid layers with urease inserted within them. Without this enzyme, lipid films became fluorescent when exposed to a UV lamp. A small amount of aqueous urea solution activated the fluorescence. This allowed for the quick detection of urea at concentrations as low as 10−8 M [64].
The use of thermal biosensors in milk testing is increasing, as are the demands for such sensors that are simple to operate and have long-term stability. This class of biosensors is based on the heat record generated during a biological reaction. The method of determining urea offers the advantages of enzymatic selectivity, rapid analysis time, and low reagent use. A flow injection analysis-enzyme thermistor (FIA-ET) biosensor was created by covalently immobilizing the enzyme urease on glass with regulated pores and packing it in a column into the thermistor, which selectively degraded the urea in the test sample. In addition, the specific heat produced by the urea hydrolysis process was shown to be related to the content of urea in the milk sample [65].
Fat Content Adulteration Identification Techniques
Liquid chromatography (LC) and gas chromatography (GC), particularly when combined with mass spectrometry (MS), have both recently been proposed as analytical methods for quick screening or selective confirmation of the quality and authenticity of milk fat. Due to the ability to observe and compare fatty acids and triacylglycerols to reference standards, the gas chromatographic method is most often used to analyze the origin of fat. According to the number of carbon atoms, the milk triacylglycerol classes are separated into fatty acids using the gas-chromatographic method. It also allows the simultaneous determination of 60 fatty acids in each sample, and the spectrum of fatty acids can be observed with the help of chromatograms. There are a number of factors that intervene in the separation of fatty acids such as the temperature of the column, the type of the carrier phase, the speed with which it circulates, the type and thickness of the film of the stationary phase, the inner diameter of the column, the pressure of the vapors in the column, the length of the column, and the volume of the sample that was injected [66,67,68]. Precisely because determining the purity of milk fat is as difficult as identifying seawater in ocean water, differences in fatty acid composition are most useful for this purpose. The gas-chromatographic method proved its effectiveness in a study conducted by Kumar et al., in which they investigated the identification of adulteration of milk fat with soybean oil and buffalo depot fat. They were based on the fact that soybean oil has a high amount of linoleic acid and buffalo depot fat has a high content of oleic acid, both of which are devoid of short-chain fatty acids, characteristic of fats of dairy origin. These markers led to the identification of 5% v/v added of both adulterants, and the method proved to be a specific quantitative method [69]. Therefore, the gas-chromatographic method is a laborious method in terms of sample processing, requiring reagents, expensive equipment, and qualified personnel, but it is a precise method for determining the spectrum of fatty acids contained in milk samples [66,67,68].
Regarding the existence of another method to identify the adulteration of milk fat, Ntakatsane et al. studied the possible use of fluorescence spectroscopy in the identification of samples according to their fat source and the detection of milk fat adulterated with vegetable oil. On the other hand, spectroscopic techniques provide information on a mixture’s constituents in a single spectrum, typically without the need for derivatization. For the classification of various types of milk or the detection of adulteration, statistical analysis is necessary when the spectrum differences are not always clearly visible [33,70].
In addition, Garcia et al. evaluated the potential application of matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-QTOF MS) as an effective technique for the analysis of oils and fats by providing characteristic profiles of triacylglycerides. The approach may also be able to define the type of fat or oil supplied while giving an estimate of the adulteration level because comprehensive TAG profiles are available [71].
Techniques for Identifying Additional Adulterants Added to Increase the Shelf Life of Milk
It is absolutely necessary to identify the adulterants added in order to extend the shelf life of milk, precisely because they have harmful effects on human health. Many chemicals, including formaldehyde, salicylic acid, hydrogen peroxide, hypochlorite, and potassium dichromate, are included in this group of adulterants and are difficult to identify due to their ability to decompose. To identify these adulterants, analytical approaches including fluorimetry, portable spectroscopy using chemometric instruments, spectrophotometry, amperometry, and HPLC have been reported in the literature, but these require expensive equipment, and the development of cheap and rapid detection instruments is needed in the future. In recent years, a series of techniques have been proposed that perform for this purpose, and the most relevant examples are presented in Table 5 and Table 6 [48,72].

Table 5.
Biosensors for the detection of adulterants added to milk to increase the protein content.

Table 6.
Different techniques to identify adulterants added to increase the shelf life of milk.
Techniques for Identifying Adulteration with Water and Other Substances
According to data from the literature, the detection of added water in milk is not an easy task at all. Utilizing a lactometer or another traditional technique, the specific gravity is measured. These strategies fall short, though, if the weight after the adulteration is maintained at the same level [81,82,83]. Over time, many methods have been used to determine this type of adulteration, such as high performance liquid chromatography (HPLC), capillary electrophoresis, Fourier transform infrared spectroscopy (FTIR), and methods for determining the cryoscopic point. Even if these techniques work, they are expensive, time-consuming, and require high-purity chemicals and expensive equipment, which leads to higher prices and slower outcomes. [81]. Other methods studied were spectroscopic methods based on electrical impedance, which, in addition to adding water, are able to detect even fomaldehyde, hydrogen peroxide, and sodium hydroxide. The electrical analysis of adulterated milk samples in the study of Durante and his collaborators helped to determine the quantity of adulterants, allowing the development of real-time monitoring systems for the detection of these frauds [82]. In a different investigation by Ashoorirad et al., the electrical impedance of milk was measured using a VDP bioimpedance sensor to ascertain its electrical characteristics. The study of milk samples contaminated with deionized water revealed a decrease in conductivity using a measurement device made up of a bioimpedance sensor, an impedance analyzer, and a computer or laptop for data gathering and processing. The study’s findings demonstrated that milk impedance spectra can be utilized as a low-cost, reliable, and accessible method to regulate milk quality in the dairy industry. This method is suited for identifying changes in milk [81]. Dave et al. investigated a non-contact technique for using the apparatus with the milk sample, retaining its consistency and quality, and making it reusable for another test. However, there are some drawbacks to this approach as well. The milk’s fat content, which alters its density and, as a result, directly influences the refractive index and, thus, the optical sensor’s reading, is the primary factor that has a negative impact. It has been shown that a sample of buffalo milk altered in proportion by approximately 70% will provide readings identical to those of a pure sample of cow’s milk, hence, it is also necessary to specify the origin of the species from which the milk is derived during the analysis. This system’s ability to detect adulteration with water is really impressive, but it needs to be improved in a number of ways, including by describing the milk’s fat content and species as well as bringing it to room temperature to get more accurate results [83].
Different Methods of Species Identification
Currently, the analytical methods based on DNA analysis have progressed rapidly and have been successfully applied to testing the authenticity of milk [3]. Most DNA tests allow the identification of the species of origin in processed foods such as dairy products and are based on the polymerase chain reaction (PCR) technique. DNA-based methods have clear advantages, such as simplicity, high specificity, and sensitivity. Moreover, the ubiquity of nucleic acids in each cell type and their superior stability compared to proteins are two of their most important advantages. PCR is a detection technique that can be used to identify adulterants in milk, including milk from various sources, in both qualitative and quantitative ways. The several PCR versions, such as multiplex PCR, real-time PCR, restriction fragment length polymorphism (RFLP), etc., are used to identify microbiological and exogenous milk from diverse sources in raw and processed milk. Due to a few limitations, PCR is not currently used as a standard procedure for detecting adulterants in milk. The potential use of PCR as a detection tool is restricted by its incapacity to detect adulterants in milk without DNA and its inhibition by high concentrations of compounds like fat and protein [3,11,12].
According to EU legislation, isoelectric focusing of γ-cazeins after plasmolysis should be used as a reference method to ensure that the products obtained are exactly sheep’s milk, goat’s milk, or buffalo milk, or a mixture of sheep’s, goat’s, and buffalo milk. The method may be used to detect either raw or heat-treated and caseinized cow’s milk in fresh or ripened sheep’s, goat’s, and buffalo’s milk cheeses or mixtures thereof [3].
Since the late 1980s, ELISA has been utilized for species identification in milk and dairy products. Recently, a commercially available kit has been developed that is based on a competitive ELISA using a mouse monoclonal antibody raised against bovine ĸ-casein and allows screening of both raw milk and heat-treated cow and buffalo milk in milk and cheese from other species and sources. The enzyme-linked immunosorbent assay, or ELISA, is a widely used laboratory procedure. The possibility of using ELISA to identify adulteration using relatively thermostable proteins s caseins has been raised. In a study by Ren et al., a quick ELISA technique for the identification of cow milk in yak milk that has been tampered with was created utilizing a high-affinity anti-bovine-casein monoclonal antibody. As a result of the test, the minimal detection limit was 1% (v/v) yak milk containing cow milk, and the linear range for adulteration detection was 1 to 80%, which is a wide enough range for regular adulteration testing. This test was not influenced by a series of treatments applied to the milk, such as acidification, thermal treatment, or the addition of rennet [3,11,12,42,84].
In addition, biosensors may give quick, quantitative, and direct determinations and are thus frequently used in the field of food testing. In their study, Kourti et al. developed a label-free optical immunosensor that utilized Mach-Zehnder interferometry to identify milk adulteration, employing a competitive immunoassay to quantify bovine k-casein in sheep and goat milk. With a total testing period of 12 min, a limit of detection of 0.04 g/mL concerning bovine k-casein was achieved, which corresponds to roughly 0.06% cow milk in undiluted ewe/goat milk. Several factors were tuned in order to produce this immunoassay, including the exact amount of immobilized bovine k-casein, the amount of anti-k-casein antibodies, the prior incubation period, and the effect of the matrix. As a result of the outstanding analytical efficiency of the proposed assay as well as the portability of the sensor system, this device is appropriate for on-site detection of milk adulteration [85].
In another study, Sakti et al. evaluated the performance of the quartz crystal microbalance (QCM) covered with polystyrene to detect the adulteration of milk of different species with cow’s milk. The technique was created based on the difference in protein profiles between cow’s milk and goat’s milk. The recently introduced QCM immunosensor to recognize cow milk presence has a limit of detection of 1 ppm. These detection limits were satisfactory for employing the biosensor for the detection of adulteration of goat’s milk with cow’s milk [86].
4. Conclusions
As milk is consumed everywhere in the world, its adulteration represents a global concern. People are concerned about the quality and purity of milk due to the increasing fraudulent practice of adulterating milk. As a consequence, the consumers’ health may be harmed by milk adulterants such as water, vegetable and animal fat, extraneous proteins, and chemical additives such as melamine, urea, ammonium sulphate, formalin, acids (e.g., boric acid, benzoic acid, salicylic acid), caustic soda, hydrogen peroxide, detergents, and sugars that are knowingly added. Various techniques have been developed over time to identify adulterants in milk, but the most accurate are instrumental. Therefore, there is an increasing need for the development of reliable, affordable, and non-expensive methods and technologies that could detect and stop the practices of adulteration. This review provides an understanding of the many types of additional adulterants as well as the most effective qualitative and quantitative approaches for their identification in order to preserve the harmless and nutritional character of milk.
Author Contributions
Conceptualization: A.-D.I., A.I.C. and M.B.; Methodology: A.-D.I. and A.I.C.; Writing—original draft preparation: A.-D.I.; Writing—review and editing: A.-D.I. and M.B.; Visualization: M.B.; Supervision: A.I.C. and M.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2013/1308 of 17 December 2013 amending establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Off. J. Eur. Union 2013, L347, 671–854. [Google Scholar]
- Morin, J.L.; Lees, M. Food Integrity Handbook: A Guide to Food Authenticity Issues and Analytical Solutions; Eurofins Analytics France: Nantes, France, 2018. [Google Scholar] [CrossRef]
- European Commission. Directive (EU) 2017/1371 of the European Parliament and of the Council of 5 July 2017 on the fight against fraud to the Union’s financial interests by means of criminal law. Off. J. Eur. Union 2017, L198, 29–41. [Google Scholar]
- European Commission. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Union 2002, L31, 1–24. [Google Scholar]
- Hau, A.K.-C.; Kwan, T.; Li, P.-T. Melamine toxicity and the kidney. J. Am. Soc. Nephrol. 2009, 20, 245–250. [Google Scholar]
- El-Loly, M.M.; Mansour, A.; Ahmed, R. Evaluation of raw milk for common commercial additives and heat treatments. Internet J. Food Saf. 2013, 15, 7–10. [Google Scholar]
- Domingo, E.; Tirelli, A.A.; Nunes, C.A.; Guerreiro, M.C.; Pinto, S.M. Melamine detection in milk using vibrational spectroscopy and chemometrics analysis: A review. Food Res. Int. 2014, 60, 131–139. [Google Scholar]
- Handford, C.E.; Campbell, K.; Elliott, C.T. Impacts of Milk Fraud on Food Safety and Nutrition with Special Emphasis on Developing Countries. Compr. Rev. Food Sci. Food Saf. 2015, 15, 130–142. [Google Scholar] [CrossRef]
- Singh, P.; Gandhi, N. Milk Preservatives and Adulterants: Processing, Regulatory and Safety Issues. Food Rev. Int. 2015, 31, 236–261. [Google Scholar] [CrossRef]
- Poonia, A.; Jha, A.; Sharma, R.; Singh, H.B.; Rai, A.K.; Sharma, N. Detection of adulteration in milk: A review. Int. J. Dairy Technol. 2017, 70, 23–42. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M. Food frauds: Global incidents and misleading situations. Trends Food Sci. Technol. 2021, 114, 424–442. [Google Scholar]
- Momtaz, M.; Bubli, S.Y.; Khan, M.S. Mechanisms and Health Aspects of Food Adulteration: A Comprehensive Review. Foods 2023, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Tanzina, A.; Shoeb, A. Common milk adulteration and their detection techniques. Int. J. Food Contam. 2016, 24, 127–139. [Google Scholar] [CrossRef]
- Gawali, S.P. Common milk adulteration and their detection techniques: A review. Int. J. Multidiscip. Educ. Res. 2021, 2, 30–35. [Google Scholar]
- Kedjia, H.M. Milk Adulteration: Option to Maintain a Quality Product; Addis Ababa University: Addis Ababa, Ethiopia, 2018. [Google Scholar]
- Draaiyer, J. Milk Testing and Payment Systems: Resource Book: A Practical Guide to Assist Milk Producer Groups; FAO: Rome, Italy, 2009. [Google Scholar]
- Kumar, V.; Dash, S. Evaporation-Based Low-Cost Method for the Detection of Adulterant in Milk. ACS Omega 2021, 6, 27200–27207. [Google Scholar] [CrossRef]
- Avula, V.; Sundar, S.P.; Sree Rekha, B.; Kalpana, M. Analytical methods to detect the Adulterants in Milk—An Overview. Asian J. Res. Pharm. Sci. 2022, 12, 272–276. [Google Scholar] [CrossRef]
- Tian, H.; Chen, S.; Li, D.; Lou, X.; Chen, C.; Yu, H. Simultaneous detection for adulterations of maltodextrin, sodium carbonate, and whey in raw milk using Raman spectroscopy and chemometrics. J. Dairy Sci. 2022, 105, 7242–7252. [Google Scholar] [CrossRef]
- Lima, J.S.; Ribeiro, D.C.; Neto, H.A.; Campos, S.V.; Leite, M.O.; Fortini, M.E.D.R.; de Carvalho, B.P.M.; Almeida, M.V.O.; Fonseca, L.M. A machine learning proposal method to detect milk tainted with cheese whey. J. Dairy Sci. 2022, 105, 9496–9508. [Google Scholar] [CrossRef]
- Condé, V.A.; Valente, G.F.S.; Minighi, E.C. Milk fraud by the addition of whey using an artificial neural network. Cienc. Rural. 2020, 50, e20190312. [Google Scholar] [CrossRef]
- Aquino, L.; Silva, A.; Freitas, M.; Felicio, T.; Cruz, A.; Conte-Junior, C. Identifying cheese whey an adulterant in milk: Limited contribution of a sensometric approach. Food Res. Int. 2014, 62, 233–237. [Google Scholar] [CrossRef]
- Vera-Bravo, R.; Hernández, A.V.; Peña, S.; Alarcón, C.; Loaiza, A.E.; Celis, C.A. Cheese Whey Milk Adulteration Determination Using Casein Glycomacropeptide as an Indicator by HPLC. Foods 2022, 11, 3201. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S. Synthetic milk: A threat to Indian dairy industry. Carpathian J. Food Sci. Technol. 2013, 5, 64–68. [Google Scholar]
- Hanganu, A.; Chira, N.-A. When detection of dairy food fraud fails: An alternative approach through proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 8454–8466. [Google Scholar] [CrossRef]
- Tesfay, T.; Kebede, A.; Seifu, E. Physico chemical properties of cow milk produced and marketed in dire dawa town, eastern ethiopia. Food Sci. Qual. Manag. 2015, 42, 56–61. [Google Scholar]
- Vujadinovic, D.; Beribaka, M.; Vukic, M.; Marjanoviić-Balaban, Ž. Comparison of methods for determining the falsification milk. J. Hyg. Eng. Des. 2017, 18, 19–24. [Google Scholar]
- Jalili, M. A Review Paper on Melamine in Milk and Dairy Products. J. Dairy Veter. Sci. 2017, 1, 555566. [Google Scholar] [CrossRef]
- Minetto, T.A.; França, B.D.; da Silva Dariz, G.; Veiga, E.A.; Galvão, A.C.; da Silva Robazza, W. Identifying adulteration of raw bovine milk with urea through electrochemical impedance spectroscopy coupled with chemometric techniques. Food Chem. 2022, 385, 132678. [Google Scholar]
- Slane, C. Fake Milk Is Real News, as Synthetic Alternatives Threaten Traditional Dairy Farms; The University of the West of England: Bristol, UK, 2019. [Google Scholar]
- Barui, A.K.; Sharma, R.; Rajput, Y.S.; Singh, S. A rapid paper chromatographic method for detection of anionic detergent in milk. J. Food Sci. Technol. 2013, 50, 826–829. [Google Scholar]
- Ntakatsane, M.; Liu, X.; Zhou, P. Short communication: Rapid detection of milk fat adulteration with vegetable oil by fluorescence spectroscopy. J. Dairy Sci. 2013, 96, 2130–2136. [Google Scholar] [CrossRef]
- El-Nabawy, M.; Awad, S.; Ibrahim, A. Validation of the Methods for the Non-Milk Fat Detection in Artificially Adulterated Milk with Palm Oil. Food Anal. Methods 2023, 16, 798–807. [Google Scholar]
- Aparnathi, K.D.; Shaikh, A.I.; Patel, S.I. Qualitative Tests for Detection of Common Adulteration in Milk; Directorate of Research Anand Agricultural University: Anand, India, 2020. [Google Scholar]
- Mabood, F.; Hussain, J.; Moo, A.N.; Gilani, S.A.; Farooq, S.; Naureen, Z.; Jabeen, F.; Ahmed, M.; Al-Harrasi, A. Detection and Quantification of Formalin Adulteration in Cow Milk Using Near Infrared Spectroscopy Combined with Multivariate Analysis. Adv. Dairy Res. 2017, 5, 167. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Merkuleva, A.D.; Andreev, S.V.; Sakharov, K.A. Method for determination of hydrogen peroxide in adulterated milk using high performance liquid chromatography. Food Chem. 2019, 283, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Hong, H.; Liang, X.; Liu, D. Assessment of benzoic acid levels in milk in China. Food Control 2009, 20, 414–418. [Google Scholar]
- Yang, Z.; Ma, C.; Gu, J.; Wu, Y.; Zhu, C.; Li, L.; Chen, G.; Gao, H.; Yin, W.; Wang, Z.; et al. SERS detection of benzoic acid in milk by using Ag-COF SERS substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120534. [Google Scholar] [CrossRef]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef] [PubMed]
- Zachar, P.; Šoltés, M.; Kasarda, R.; Novotný, J.; Novikmecová, M.; Marcincáková, D. Analytical methods for the species identification of milk and milk products. Mljekarstvo Cas. Za Unaprjedenje Proizv. i Prerade Mlijeka 2011, 61, 199–207. [Google Scholar]
- Mafra, I.; Honrado, M.; Amaral, J.S. Animal Species Authentication in Dairy Products. Foods 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Gilmanov, K.; Vafin, R.; Bliadze, V.G.; Michailova, I. The Problem of Falsification of Milk Species Appliance; All-Russian Dairy Research Institute: Moscow, Russia, 2020. [Google Scholar]
- Fanelli, V.; Mascio, I.; Miazzi, M.M.; Savoia, M.A.; De Giovanni, C.; Montemurro, C. Molecular Approaches to Agri-Food Traceability and Authentication: An Updated Review. Foods 2021, 10, 1644. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/150 of 30 January 2018 amending Implementing Regulation (EU) 2016/1240 as regards methods for the analysis and quality evaluation of milk and milk products eligible for public inter-vention and aid for private storage. Off. J. Eur. Union 2018, L26, 14–47. [Google Scholar]
- Nagraik, R.; Sharma, A.; Kumar, D.; Chawla, P.; Kumar, A.P. Milk adulterant detection: Conventional and biosensor based approaches: A review. Sens. Bio-Sens. Res. 2021, 33, 100433. [Google Scholar] [CrossRef]
- Chaudhry, H.R.; Khushi, M.Z.; Rabbani, M. Laboratory Manual Quality Control of Milk: Quality Control of Milk; University of Vetrinary and Animal Science: Lahore, Pakistan, 2015. [Google Scholar]
- Nascimento, C.F.; Santos, P.M.; Pereira-Filho, E.R.; Rocha, F.R. Recent advances on determination of milk adulterants. Food Chem. 2017, 221, 1232–1244. [Google Scholar]
- Smirnova, A.; Konoplev, G.; Mukhin, N.; Stepanova, O.; Steinmann, U. Milk as a Complex Multiphase Polydisperse System: Approaches for the Quantitative and Qualitative Analysis. J. Compos. Sci. 2020, 4, 151. [Google Scholar]
- Chen, H.; Tan, C.; Lin, Z.; Wu, T. Detection of melamine adulteration in milk by near-infrared spectroscopy and one-class partial least squares. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Manzi, P. Melamine detection in milk and dairy products: Traditional analytical methods and recent developments. Food Anal. Methods 2018, 11, 128–147. [Google Scholar] [CrossRef]
- Lutter, P.; Savoy-Perroud, M.C.; Campos-Gimenez, E.; Meyer, L.; Goldmann, T.; Bertholet, M.C.; Delatour, T. Screening and confirmatory methods for the determination of melamine in cow’s milk and milk-based powdered infant formula: Validation and proficiency-tests of ELISA, HPLC-UV, GC-MS and LC-MS/MS. Food Control 2011, 22, 903–913. [Google Scholar] [CrossRef]
- Liang, W.; Wei, Y.; Gao, M.; Yan, X.; Zhu, X.; Guo, W. Detection of melamine adulteration in milk powder by using optical spectroscopy technologies in the last decade—A review. Food Anal. Methods 2020, 13, 2059–2069. [Google Scholar]
- Dai, X.; Fang, X.; Su, F.; Yang, M.; Li, H.; Zhou, J.; Xu, R. Accurate analysis of urea in milk and milk powder by isotope dilution gas chromatography–mass spectrometry. J. Chromatogr. B 2010, 878, 1634–1638. [Google Scholar] [CrossRef]
- Ezhilan, M.; Gumpu, M.B.; Ramachandra, B.L.; Nesakumar, N.; Babu, K.J.; Krishnan, U.M.; Rayappan, J.B.B. Design and development of electrochemical biosensor for the simultaneous detection of melamine and urea in adulterated milk samples. Sens. Actuators B Chem. 2017, 238, 1283–1292. [Google Scholar] [CrossRef]
- Singh, M. Nanosensor Platforms for Detection of Milk Adulterants. Sens. Actuators Rep. 2023, 5, 100159. [Google Scholar]
- Shalileh, F.; Sabahi, H.; Dadmehr, M.; Hosseini, M. Sensing approaches toward detection of urea adulteration in milk. Microchem. J. 2023, 193, 108990. [Google Scholar]
- Fodey, T.L.; Thompson, C.S.; Traynor, I.M.; Haughey, S.A.; Kennedy, D.G.; Crooks, S.R. Development of an optical biosensor based immunoassay to screen infant formula milk samples for adulteration with melamine. Anal. Chem. 2011, 83, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, H.; Chua, F.Z.; Li, S.F.Y. Rapid detection of melamine based on immunoassay using portable surface plasmon resonance biosensor. Sens. Actuators B Chem. 2013, 178, 541–546. [Google Scholar] [CrossRef]
- Kaneko, N.; Horii, K.; Akitomi, J.; Kato, S.; Shiratori, I.; Waga, I. An aptamer-based biosensor for direct, label-free detection of melamine in raw milk. Sensors 2018, 18, 3227. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.J.; Zhou, X.H.; Zhang, Y.; Liu, L.H.; Long, F.; Song, L.; Shi, H.C. Melamine detection in dairy products by using a reusable evanescent wave fiber-optic biosensor. Sens. Actuators B Chem. 2014, 204, 682–687. [Google Scholar] [CrossRef]
- Trivedi, U.B.; Lakshminarayana, D.; Kothari, I.L.; Patel, N.G.; Kapse, H.N.; Makhija, K.K.; Panchal, C.J.; Patel, P.B. Potentiometric biosensor for urea determination in milk. Sens. Actuators B Chem. 2009, 140, 260–266. [Google Scholar] [CrossRef]
- Naik, P.P.; Mishra, G.K.; Danielsson, B.; Bhand, S. Android integrated urea biosensor for public health awareness. Sens. Bio-Sens. Res. 2015, 3, 12–17. [Google Scholar] [CrossRef][Green Version]
- Nikoleli, G.P.; Nikolelis, D.P.; Methenitis, C. Construction of a simple optical sensor based on air stable lipid film with incorporated urease for the rapid detection of urea in milk. Anal. Chim. Acta 2010, 675, 58–63. [Google Scholar] [CrossRef]
- Mishra, G.K.; Mishra, R.K.; Bhand, S. Flow injection analysis biosensor for urea analysis in adulterated milk using enzyme thermistor. Biosens. Bioelectron. 2010, 26, 1560–1564. [Google Scholar] [CrossRef]
- Trbović, D.; Petronijević, R.; Đorđević, V. Chromatography methods and chemometrics for determination of milk fat adulterants. IOP Conf. Ser. Earth Environ. Sci. 2017, 85, 012025. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Vega, S.; Díaz, G.; Sánchez, J.; Coronado, M.; Ramírez, A.; Schettino, B. Detection of non-milk fat in milk fat by gas chromatography and linear discriminant analysis. J. Dairy Sci. 2009, 92, 1846–1855. [Google Scholar] [CrossRef]
- Simionato, J.I.; Garcia, J.C.; Santos, G.T.D.; Oliveira, C.C.; Visentainer, J.V.; Souza, N.E.D. Validation of the determination of fatty acids in milk by gas chromatography. J. Braz. Chem. Soc. 2010, 21, 520–524. [Google Scholar] [CrossRef]
- Kumar, A.; Upadhyay, N.; Padghan, P.V.; Gandhi, K.; Lal, D.; Sharma, V. Detection of vegetable oil and animal depot fat adulteration in anhydrous milk fat (Ghee) using fatty acid composition. MOJ Food Process. Technol. 2015, 1, 00013. [Google Scholar] [CrossRef]
- Saleem, M. Fluorescence spectroscopy based detection of adulteration in desi ghee. J. Fluoresc. 2020, 30, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.S.; Sanvido, G.B.; Saraiva, S.A.; Zacca, J.J.; Cosso, R.G.; Eberlin, M.N. Bovine milk powder adulteration with vegetable oils or fats revealed by MALDI-QTOF MS. Food Chem. 2012, 131, 722–726. [Google Scholar] [CrossRef]
- Lima, L.S.; Rossini, E.L.; Pezza, L.; Pezza, H.R. Bioactive paper platform for detection of hydrogen peroxide in milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117774. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Matias, A.; Jorge, P.; Saraiva, C.; Mendes, J.; Araújo, J.; Coelho, L.C. Optical Biosensor for the Detection of Hydrogen Peroxide in Milk. Chem. Proc. 2021, 5, 55. [Google Scholar]
- Song, J.; Wan, Y.; Yang, C.; Deng, Q.; Cui, Y.; Yan, Z.; Liu, Y. Synthesis of CuxO/Ag nanoparticles on exfoliated graphene: Application for enhanced electrochemical detection of H2O2 in milk. Sci. Rep. 2023, 13, 6640. [Google Scholar] [CrossRef]
- Veríssimo, M.I.; Gamelas, J.A.; Fernandes, A.J.; Evtuguin, D.V.; Gomes, M.T.S. A new formaldehyde optical sensor: Detecting milk adulteration. Food Chem. 2020, 318, 126461. [Google Scholar] [CrossRef]
- De Freitas Rezende, F.B.; Cheibub, A.M.D.S.S.; Netto, A.D.P.; de Carvalho Marques, F.F. Determination of formaldehyde in bovine milk using a high sensitivity HPLC-UV method. Microchem. J. 2017, 134, 383–389. [Google Scholar] [CrossRef]
- Silva, A.F.S.; Goncalves, I.C.; Rocha, F.R. Smartphone-based digital images as a novel approach to determine formaldehyde as a milk adulterant. Food Control 2021, 125, 107956. [Google Scholar] [CrossRef]
- Coimbra, P.T.; Bathazar, C.F.; Guimarães, J.T.; Coutinho, N.M.; Pimentel, T.C.; Neto, R.P.; Cruz, A.G. Detection of formaldehyde in raw milk by time domain nuclear magnetic resonance and chemometrics. Food Control 2020, 110, 107006. [Google Scholar] [CrossRef]
- Fu, X.; Wu, J.; Xu, H.; Wan, P.; Fu, H.; Mei, Q. Luminescence nanoprobe in the near-infrared-II window for ultrasensitive detection of hypochlorite. Anal. Chem. 2021, 93, 15696–15702. [Google Scholar] [CrossRef] [PubMed]
- Bastan, N.; Ahmadi, M.; Madrakian, T.; Afkhami, A.; Khalili, S.; Majidi, M.; Moradi, M. A paired emitter–detector diode-based photometer for the determination of sodium hypochlorite adulteration in milk. Sci. Rep. 2023, 13, 6217. [Google Scholar] [CrossRef] [PubMed]
- Ashoorirad, M.; Baghbani, R.; Ghalamboran, M.R. Bioimpedance sensor to detect water content in milk based on van Der Pauw method. IET Nanobiotechnol. 2021, 15, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Durante, G.; Becari, W.; Lima, F.A.; Peres, H.E. Electrical impedance sensor for real-time detection of bovine milk adulteration. IEEE Sens. J. 2015, 16, 861–865. [Google Scholar] [CrossRef]
- Dave, A.; Banwari, D.; Srivastava, S.; Sadistap, S. Optical sensing system for detecting water adulteration in milk. In Proceedings of the 2016 IEEE Global Humanitarian Technology Conference (GHTC), Seattle, WA, USA, 13–16 October 2016; pp. 634–639. [Google Scholar]
- Ren, Q.R.; Zhang, H.; Guo, H.Y.; Jiang, L.; Tian, M.; Ren, F.Z. Detection of cow milk adulteration in yak milk by ELISA. J. Dairy Sci. 2014, 97, 6000–6006. [Google Scholar] [CrossRef] [PubMed]
- Kourti, D.; Angelopoulou, M.; Misiakos, K.; Makarona, E.; Economou, A.; Petrou, P.; Kakabakos, S. Detection of Adulteration of Milk from Other Species with Cow Milk through an Immersible Photonic Immunosensor. Eng. Proc. 2023, 35, 582. [Google Scholar]
- Sakti, S.P.; Chabibah, N.; Ayu, S.P.; Padaga, M.C.; Aulanni’am, A.A. Development of QCM biosensor with specific cow milk protein antibody for candidate milk adulteration detection. J. Sens. 2016, 2016, 1807647. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).