A Review of Milk Frauds and Adulterations from a Technological Perspective
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
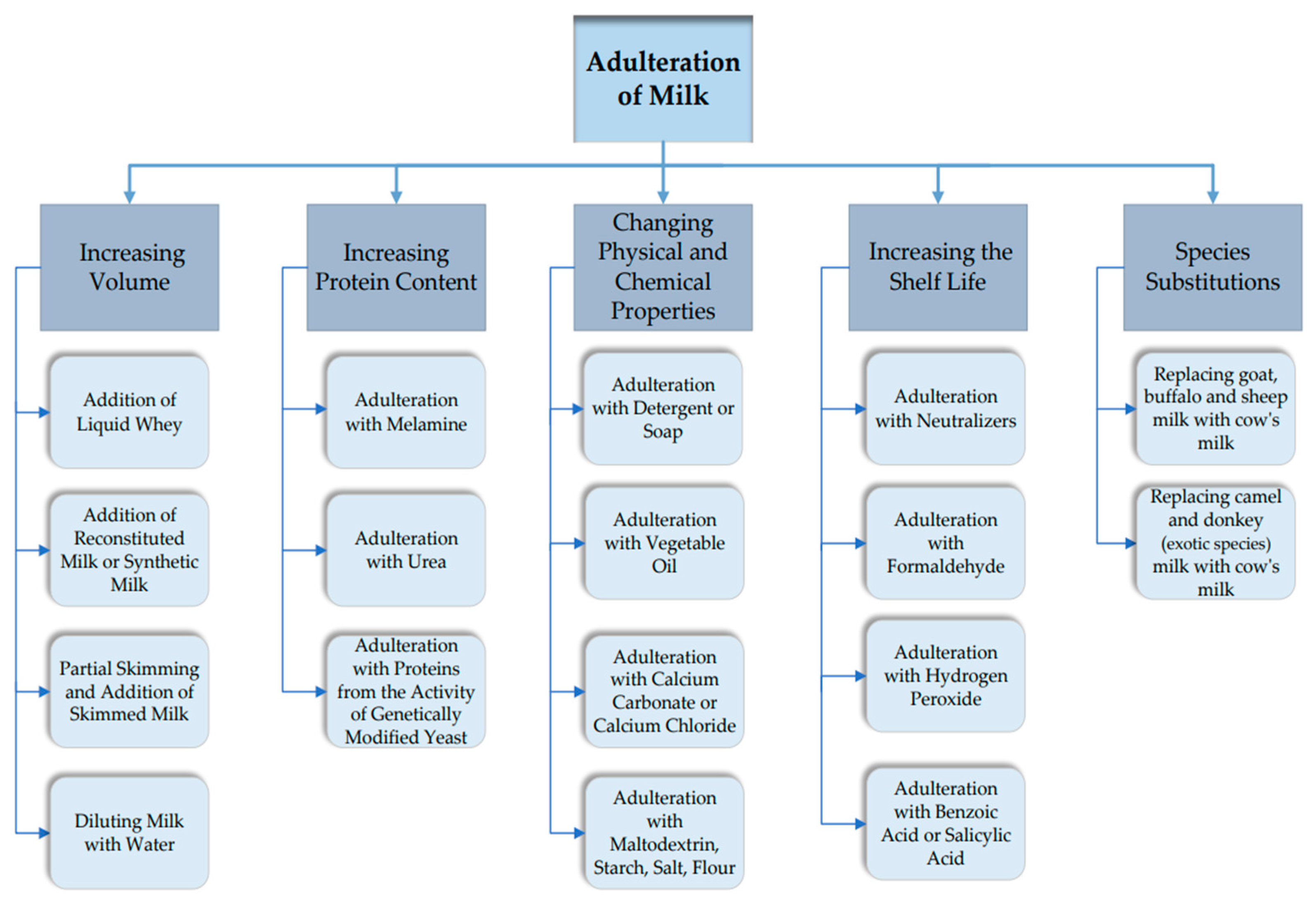
3.1. The Most Common Milk Adulterations
3.1.1. Adulteration That Generally Aimed at Increasing the Volume of Milk
Addition of Liquid Whey (a By-Product of Cheese Making)
Addition of Reconstituted Milk or Synthetic Milk
Partial Skimming and Addition of Skimmed Milk
Diluting Milk with Water
3.1.2. Adulterations Involving the Increased Protein Content
Adulteration of Milk with Melamine
Adulteration of Milk with Urea
Adulteration of Milk with Proteins Resulting from the Activity of Genetically Modified Yeasts
3.1.3. Counterfeiting Involving the Addition of Unusual Substances
Detergents or Soap
Vegetable Oil
Calcium Carbonate or Calcium Chloride
Maltodextrin
Starch, Wheat Flour or Rice Flour
Salt
3.1.4. Adulterations Associated with Increasing the Shelf Life of Milk
Neutralizers
Formaldehyde
Hydrogen Peroxide
Acids
3.1.5. Adulterations Involving Species Substitutions
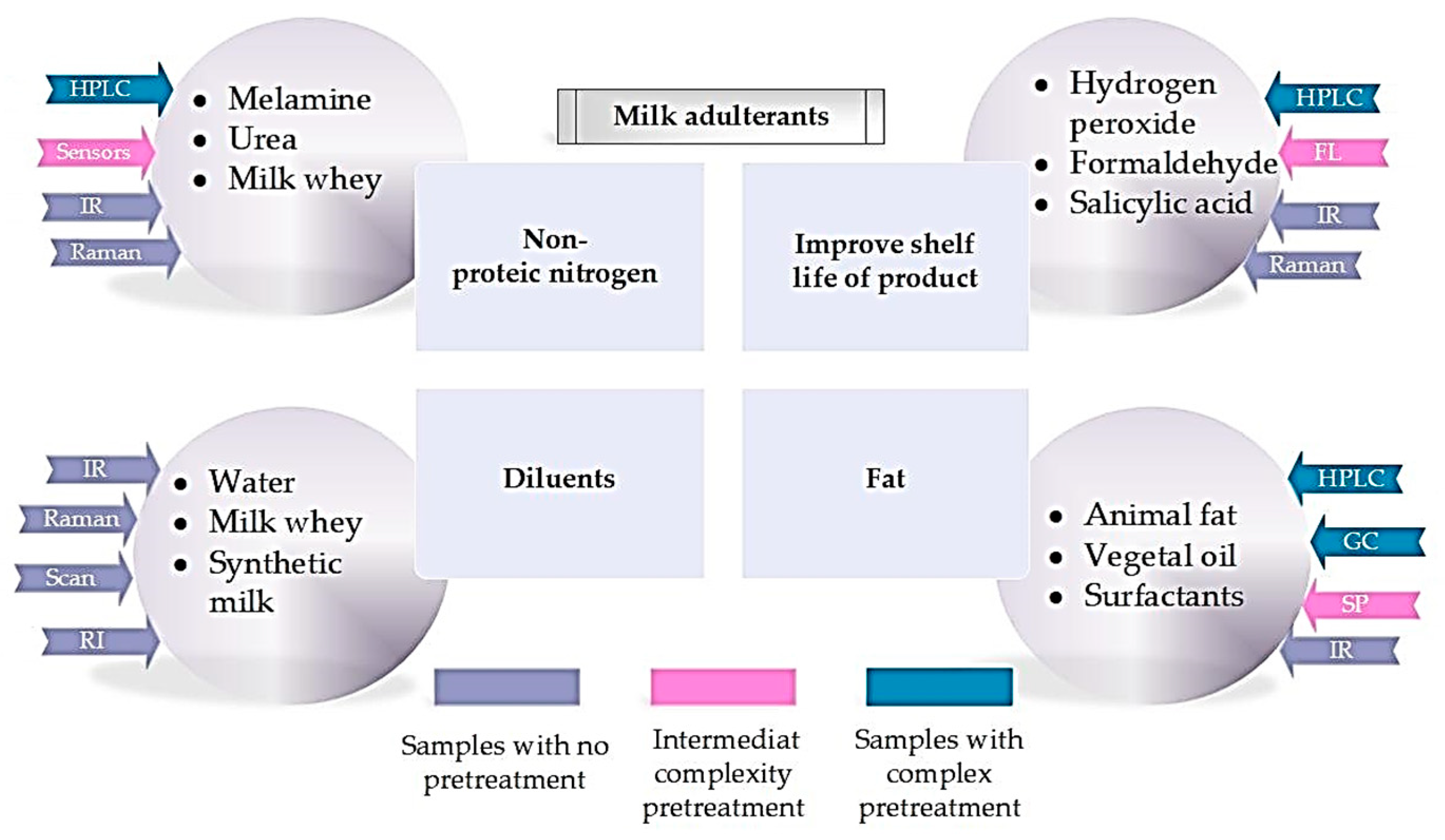
3.2. Methods for Detecting Adulterants in Milk
3.2.1. Physical Methods (Quantitative)
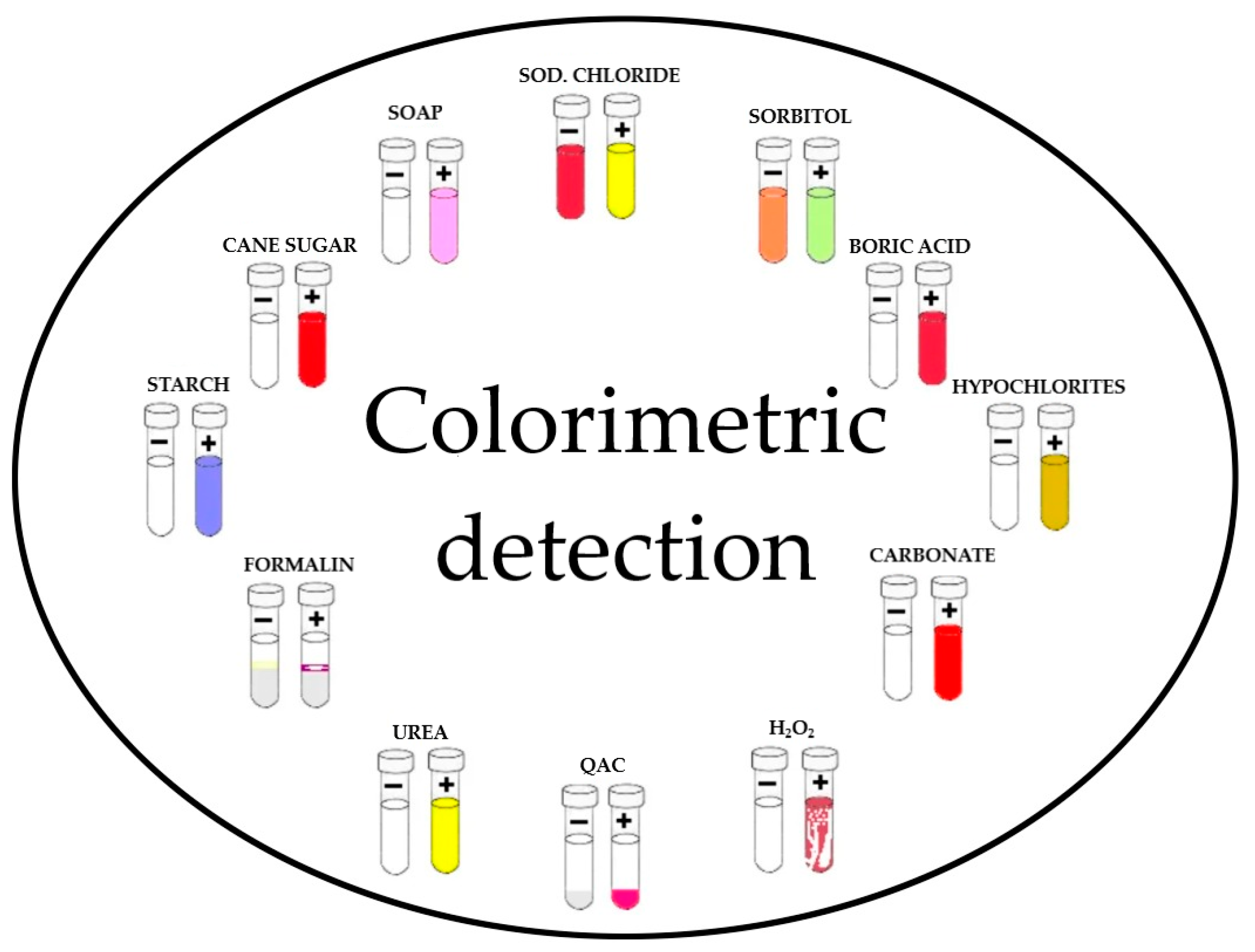
3.2.2. Chemical Methods (Qualitative)
3.2.3. Instrumental Methods (Quantitative)
Techniques for Identifying Adulteration in Nitrogen Content
Fat Content Adulteration Identification Techniques
Techniques for Identifying Additional Adulterants Added to Increase the Shelf Life of Milk
Techniques for Identifying Adulteration with Water and Other Substances
Different Methods of Species Identification
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pereira, P.C. Milk nutritional composition and its role in human health. Nutrition 2014, 30, 619–627. [Google Scholar] [PubMed]
- European Commission. Commission Implementing Regulation (EU) 2013/1308 of 17 December 2013 amending establishing a common organisation of the markets in agricultural products and repealing Council Regulations (EEC) No 922/72, (EEC) No 234/79, (EC) No 1037/2001 and (EC) No 1234/2007. Off. J. Eur. Union 2013, L347, 671–854. [Google Scholar]
- Morin, J.L.; Lees, M. Food Integrity Handbook: A Guide to Food Authenticity Issues and Analytical Solutions; Eurofins Analytics France: Nantes, France, 2018. [Google Scholar] [CrossRef]
- European Commission. Directive (EU) 2017/1371 of the European Parliament and of the Council of 5 July 2017 on the fight against fraud to the Union’s financial interests by means of criminal law. Off. J. Eur. Union 2017, L198, 29–41. [Google Scholar]
- European Commission. Regulation (EC) No 178/2002 of the European Parliament and of the Council of 28 January 2002 laying down the general principles and requirements of food law, establishing the European Food Safety Authority and laying down procedures in matters of food safety. Off. J. Eur. Union 2002, L31, 1–24. [Google Scholar]
- Hau, A.K.-C.; Kwan, T.; Li, P.-T. Melamine toxicity and the kidney. J. Am. Soc. Nephrol. 2009, 20, 245–250. [Google Scholar]
- El-Loly, M.M.; Mansour, A.; Ahmed, R. Evaluation of raw milk for common commercial additives and heat treatments. Internet J. Food Saf. 2013, 15, 7–10. [Google Scholar]
- Domingo, E.; Tirelli, A.A.; Nunes, C.A.; Guerreiro, M.C.; Pinto, S.M. Melamine detection in milk using vibrational spectroscopy and chemometrics analysis: A review. Food Res. Int. 2014, 60, 131–139. [Google Scholar]
- Handford, C.E.; Campbell, K.; Elliott, C.T. Impacts of Milk Fraud on Food Safety and Nutrition with Special Emphasis on Developing Countries. Compr. Rev. Food Sci. Food Saf. 2015, 15, 130–142. [Google Scholar] [CrossRef]
- Singh, P.; Gandhi, N. Milk Preservatives and Adulterants: Processing, Regulatory and Safety Issues. Food Rev. Int. 2015, 31, 236–261. [Google Scholar] [CrossRef]
- Poonia, A.; Jha, A.; Sharma, R.; Singh, H.B.; Rai, A.K.; Sharma, N. Detection of adulteration in milk: A review. Int. J. Dairy Technol. 2017, 70, 23–42. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M. Food frauds: Global incidents and misleading situations. Trends Food Sci. Technol. 2021, 114, 424–442. [Google Scholar]
- Momtaz, M.; Bubli, S.Y.; Khan, M.S. Mechanisms and Health Aspects of Food Adulteration: A Comprehensive Review. Foods 2023, 12, 199. [Google Scholar] [CrossRef] [PubMed]
- Tanzina, A.; Shoeb, A. Common milk adulteration and their detection techniques. Int. J. Food Contam. 2016, 24, 127–139. [Google Scholar] [CrossRef]
- Gawali, S.P. Common milk adulteration and their detection techniques: A review. Int. J. Multidiscip. Educ. Res. 2021, 2, 30–35. [Google Scholar]
- Kedjia, H.M. Milk Adulteration: Option to Maintain a Quality Product; Addis Ababa University: Addis Ababa, Ethiopia, 2018. [Google Scholar]
- Draaiyer, J. Milk Testing and Payment Systems: Resource Book: A Practical Guide to Assist Milk Producer Groups; FAO: Rome, Italy, 2009. [Google Scholar]
- Kumar, V.; Dash, S. Evaporation-Based Low-Cost Method for the Detection of Adulterant in Milk. ACS Omega 2021, 6, 27200–27207. [Google Scholar] [CrossRef]
- Avula, V.; Sundar, S.P.; Sree Rekha, B.; Kalpana, M. Analytical methods to detect the Adulterants in Milk—An Overview. Asian J. Res. Pharm. Sci. 2022, 12, 272–276. [Google Scholar] [CrossRef]
- Tian, H.; Chen, S.; Li, D.; Lou, X.; Chen, C.; Yu, H. Simultaneous detection for adulterations of maltodextrin, sodium carbonate, and whey in raw milk using Raman spectroscopy and chemometrics. J. Dairy Sci. 2022, 105, 7242–7252. [Google Scholar] [CrossRef]
- Lima, J.S.; Ribeiro, D.C.; Neto, H.A.; Campos, S.V.; Leite, M.O.; Fortini, M.E.D.R.; de Carvalho, B.P.M.; Almeida, M.V.O.; Fonseca, L.M. A machine learning proposal method to detect milk tainted with cheese whey. J. Dairy Sci. 2022, 105, 9496–9508. [Google Scholar] [CrossRef]
- Condé, V.A.; Valente, G.F.S.; Minighi, E.C. Milk fraud by the addition of whey using an artificial neural network. Cienc. Rural. 2020, 50, e20190312. [Google Scholar] [CrossRef]
- Aquino, L.; Silva, A.; Freitas, M.; Felicio, T.; Cruz, A.; Conte-Junior, C. Identifying cheese whey an adulterant in milk: Limited contribution of a sensometric approach. Food Res. Int. 2014, 62, 233–237. [Google Scholar] [CrossRef]
- Vera-Bravo, R.; Hernández, A.V.; Peña, S.; Alarcón, C.; Loaiza, A.E.; Celis, C.A. Cheese Whey Milk Adulteration Determination Using Casein Glycomacropeptide as an Indicator by HPLC. Foods 2022, 11, 3201. [Google Scholar] [CrossRef] [PubMed]
- Mudgil, D.; Barak, S. Synthetic milk: A threat to Indian dairy industry. Carpathian J. Food Sci. Technol. 2013, 5, 64–68. [Google Scholar]
- Hanganu, A.; Chira, N.-A. When detection of dairy food fraud fails: An alternative approach through proton nuclear magnetic resonance spectroscopy. J. Dairy Sci. 2021, 104, 8454–8466. [Google Scholar] [CrossRef]
- Tesfay, T.; Kebede, A.; Seifu, E. Physico chemical properties of cow milk produced and marketed in dire dawa town, eastern ethiopia. Food Sci. Qual. Manag. 2015, 42, 56–61. [Google Scholar]
- Vujadinovic, D.; Beribaka, M.; Vukic, M.; Marjanoviić-Balaban, Ž. Comparison of methods for determining the falsification milk. J. Hyg. Eng. Des. 2017, 18, 19–24. [Google Scholar]
- Jalili, M. A Review Paper on Melamine in Milk and Dairy Products. J. Dairy Veter. Sci. 2017, 1, 555566. [Google Scholar] [CrossRef]
- Minetto, T.A.; França, B.D.; da Silva Dariz, G.; Veiga, E.A.; Galvão, A.C.; da Silva Robazza, W. Identifying adulteration of raw bovine milk with urea through electrochemical impedance spectroscopy coupled with chemometric techniques. Food Chem. 2022, 385, 132678. [Google Scholar]
- Slane, C. Fake Milk Is Real News, as Synthetic Alternatives Threaten Traditional Dairy Farms; The University of the West of England: Bristol, UK, 2019. [Google Scholar]
- Barui, A.K.; Sharma, R.; Rajput, Y.S.; Singh, S. A rapid paper chromatographic method for detection of anionic detergent in milk. J. Food Sci. Technol. 2013, 50, 826–829. [Google Scholar]
- Ntakatsane, M.; Liu, X.; Zhou, P. Short communication: Rapid detection of milk fat adulteration with vegetable oil by fluorescence spectroscopy. J. Dairy Sci. 2013, 96, 2130–2136. [Google Scholar] [CrossRef]
- El-Nabawy, M.; Awad, S.; Ibrahim, A. Validation of the Methods for the Non-Milk Fat Detection in Artificially Adulterated Milk with Palm Oil. Food Anal. Methods 2023, 16, 798–807. [Google Scholar]
- Aparnathi, K.D.; Shaikh, A.I.; Patel, S.I. Qualitative Tests for Detection of Common Adulteration in Milk; Directorate of Research Anand Agricultural University: Anand, India, 2020. [Google Scholar]
- Mabood, F.; Hussain, J.; Moo, A.N.; Gilani, S.A.; Farooq, S.; Naureen, Z.; Jabeen, F.; Ahmed, M.; Al-Harrasi, A. Detection and Quantification of Formalin Adulteration in Cow Milk Using Near Infrared Spectroscopy Combined with Multivariate Analysis. Adv. Dairy Res. 2017, 5, 167. [Google Scholar] [CrossRef]
- Ivanova, A.S.; Merkuleva, A.D.; Andreev, S.V.; Sakharov, K.A. Method for determination of hydrogen peroxide in adulterated milk using high performance liquid chromatography. Food Chem. 2019, 283, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Qi, P.; Hong, H.; Liang, X.; Liu, D. Assessment of benzoic acid levels in milk in China. Food Control 2009, 20, 414–418. [Google Scholar]
- Yang, Z.; Ma, C.; Gu, J.; Wu, Y.; Zhu, C.; Li, L.; Chen, G.; Gao, H.; Yin, W.; Wang, Z.; et al. SERS detection of benzoic acid in milk by using Ag-COF SERS substrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 267, 120534. [Google Scholar] [CrossRef]
- Del Olmo, A.; Calzada, J.; Nuñez, M. Benzoic acid and its derivatives as naturally occurring compounds in foods and as additives: Uses, exposure, and controversy. Crit. Rev. Food Sci. Nutr. 2017, 57, 3084–3103. [Google Scholar] [CrossRef] [PubMed]
- Zachar, P.; Šoltés, M.; Kasarda, R.; Novotný, J.; Novikmecová, M.; Marcincáková, D. Analytical methods for the species identification of milk and milk products. Mljekarstvo Cas. Za Unaprjedenje Proizv. i Prerade Mlijeka 2011, 61, 199–207. [Google Scholar]
- Mafra, I.; Honrado, M.; Amaral, J.S. Animal Species Authentication in Dairy Products. Foods 2022, 11, 1124. [Google Scholar] [CrossRef] [PubMed]
- Gilmanov, K.; Vafin, R.; Bliadze, V.G.; Michailova, I. The Problem of Falsification of Milk Species Appliance; All-Russian Dairy Research Institute: Moscow, Russia, 2020. [Google Scholar]
- Fanelli, V.; Mascio, I.; Miazzi, M.M.; Savoia, M.A.; De Giovanni, C.; Montemurro, C. Molecular Approaches to Agri-Food Traceability and Authentication: An Updated Review. Foods 2021, 10, 1644. [Google Scholar] [CrossRef]
- European Commission. Commission Implementing Regulation (EU) 2018/150 of 30 January 2018 amending Implementing Regulation (EU) 2016/1240 as regards methods for the analysis and quality evaluation of milk and milk products eligible for public inter-vention and aid for private storage. Off. J. Eur. Union 2018, L26, 14–47. [Google Scholar]
- Nagraik, R.; Sharma, A.; Kumar, D.; Chawla, P.; Kumar, A.P. Milk adulterant detection: Conventional and biosensor based approaches: A review. Sens. Bio-Sens. Res. 2021, 33, 100433. [Google Scholar] [CrossRef]
- Chaudhry, H.R.; Khushi, M.Z.; Rabbani, M. Laboratory Manual Quality Control of Milk: Quality Control of Milk; University of Vetrinary and Animal Science: Lahore, Pakistan, 2015. [Google Scholar]
- Nascimento, C.F.; Santos, P.M.; Pereira-Filho, E.R.; Rocha, F.R. Recent advances on determination of milk adulterants. Food Chem. 2017, 221, 1232–1244. [Google Scholar]
- Smirnova, A.; Konoplev, G.; Mukhin, N.; Stepanova, O.; Steinmann, U. Milk as a Complex Multiphase Polydisperse System: Approaches for the Quantitative and Qualitative Analysis. J. Compos. Sci. 2020, 4, 151. [Google Scholar]
- Chen, H.; Tan, C.; Lin, Z.; Wu, T. Detection of melamine adulteration in milk by near-infrared spectroscopy and one-class partial least squares. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Ritota, M.; Manzi, P. Melamine detection in milk and dairy products: Traditional analytical methods and recent developments. Food Anal. Methods 2018, 11, 128–147. [Google Scholar] [CrossRef]
- Lutter, P.; Savoy-Perroud, M.C.; Campos-Gimenez, E.; Meyer, L.; Goldmann, T.; Bertholet, M.C.; Delatour, T. Screening and confirmatory methods for the determination of melamine in cow’s milk and milk-based powdered infant formula: Validation and proficiency-tests of ELISA, HPLC-UV, GC-MS and LC-MS/MS. Food Control 2011, 22, 903–913. [Google Scholar] [CrossRef]
- Liang, W.; Wei, Y.; Gao, M.; Yan, X.; Zhu, X.; Guo, W. Detection of melamine adulteration in milk powder by using optical spectroscopy technologies in the last decade—A review. Food Anal. Methods 2020, 13, 2059–2069. [Google Scholar]
- Dai, X.; Fang, X.; Su, F.; Yang, M.; Li, H.; Zhou, J.; Xu, R. Accurate analysis of urea in milk and milk powder by isotope dilution gas chromatography–mass spectrometry. J. Chromatogr. B 2010, 878, 1634–1638. [Google Scholar] [CrossRef]
- Ezhilan, M.; Gumpu, M.B.; Ramachandra, B.L.; Nesakumar, N.; Babu, K.J.; Krishnan, U.M.; Rayappan, J.B.B. Design and development of electrochemical biosensor for the simultaneous detection of melamine and urea in adulterated milk samples. Sens. Actuators B Chem. 2017, 238, 1283–1292. [Google Scholar] [CrossRef]
- Singh, M. Nanosensor Platforms for Detection of Milk Adulterants. Sens. Actuators Rep. 2023, 5, 100159. [Google Scholar]
- Shalileh, F.; Sabahi, H.; Dadmehr, M.; Hosseini, M. Sensing approaches toward detection of urea adulteration in milk. Microchem. J. 2023, 193, 108990. [Google Scholar]
- Fodey, T.L.; Thompson, C.S.; Traynor, I.M.; Haughey, S.A.; Kennedy, D.G.; Crooks, S.R. Development of an optical biosensor based immunoassay to screen infant formula milk samples for adulteration with melamine. Anal. Chem. 2011, 83, 5012–5016. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, H.; Chua, F.Z.; Li, S.F.Y. Rapid detection of melamine based on immunoassay using portable surface plasmon resonance biosensor. Sens. Actuators B Chem. 2013, 178, 541–546. [Google Scholar] [CrossRef]
- Kaneko, N.; Horii, K.; Akitomi, J.; Kato, S.; Shiratori, I.; Waga, I. An aptamer-based biosensor for direct, label-free detection of melamine in raw milk. Sensors 2018, 18, 3227. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.J.; Zhou, X.H.; Zhang, Y.; Liu, L.H.; Long, F.; Song, L.; Shi, H.C. Melamine detection in dairy products by using a reusable evanescent wave fiber-optic biosensor. Sens. Actuators B Chem. 2014, 204, 682–687. [Google Scholar] [CrossRef]
- Trivedi, U.B.; Lakshminarayana, D.; Kothari, I.L.; Patel, N.G.; Kapse, H.N.; Makhija, K.K.; Panchal, C.J.; Patel, P.B. Potentiometric biosensor for urea determination in milk. Sens. Actuators B Chem. 2009, 140, 260–266. [Google Scholar] [CrossRef]
- Naik, P.P.; Mishra, G.K.; Danielsson, B.; Bhand, S. Android integrated urea biosensor for public health awareness. Sens. Bio-Sens. Res. 2015, 3, 12–17. [Google Scholar] [CrossRef]
- Nikoleli, G.P.; Nikolelis, D.P.; Methenitis, C. Construction of a simple optical sensor based on air stable lipid film with incorporated urease for the rapid detection of urea in milk. Anal. Chim. Acta 2010, 675, 58–63. [Google Scholar] [CrossRef]
- Mishra, G.K.; Mishra, R.K.; Bhand, S. Flow injection analysis biosensor for urea analysis in adulterated milk using enzyme thermistor. Biosens. Bioelectron. 2010, 26, 1560–1564. [Google Scholar] [CrossRef]
- Trbović, D.; Petronijević, R.; Đorđević, V. Chromatography methods and chemometrics for determination of milk fat adulterants. IOP Conf. Ser. Earth Environ. Sci. 2017, 85, 012025. [Google Scholar] [CrossRef]
- Gutiérrez, R.; Vega, S.; Díaz, G.; Sánchez, J.; Coronado, M.; Ramírez, A.; Schettino, B. Detection of non-milk fat in milk fat by gas chromatography and linear discriminant analysis. J. Dairy Sci. 2009, 92, 1846–1855. [Google Scholar] [CrossRef]
- Simionato, J.I.; Garcia, J.C.; Santos, G.T.D.; Oliveira, C.C.; Visentainer, J.V.; Souza, N.E.D. Validation of the determination of fatty acids in milk by gas chromatography. J. Braz. Chem. Soc. 2010, 21, 520–524. [Google Scholar] [CrossRef]
- Kumar, A.; Upadhyay, N.; Padghan, P.V.; Gandhi, K.; Lal, D.; Sharma, V. Detection of vegetable oil and animal depot fat adulteration in anhydrous milk fat (Ghee) using fatty acid composition. MOJ Food Process. Technol. 2015, 1, 00013. [Google Scholar] [CrossRef]
- Saleem, M. Fluorescence spectroscopy based detection of adulteration in desi ghee. J. Fluoresc. 2020, 30, 181–191. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.S.; Sanvido, G.B.; Saraiva, S.A.; Zacca, J.J.; Cosso, R.G.; Eberlin, M.N. Bovine milk powder adulteration with vegetable oils or fats revealed by MALDI-QTOF MS. Food Chem. 2012, 131, 722–726. [Google Scholar] [CrossRef]
- Lima, L.S.; Rossini, E.L.; Pezza, L.; Pezza, H.R. Bioactive paper platform for detection of hydrogen peroxide in milk. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 227, 117774. [Google Scholar] [CrossRef]
- Vasconcelos, H.; Matias, A.; Jorge, P.; Saraiva, C.; Mendes, J.; Araújo, J.; Coelho, L.C. Optical Biosensor for the Detection of Hydrogen Peroxide in Milk. Chem. Proc. 2021, 5, 55. [Google Scholar]
- Song, J.; Wan, Y.; Yang, C.; Deng, Q.; Cui, Y.; Yan, Z.; Liu, Y. Synthesis of CuxO/Ag nanoparticles on exfoliated graphene: Application for enhanced electrochemical detection of H2O2 in milk. Sci. Rep. 2023, 13, 6640. [Google Scholar] [CrossRef]
- Veríssimo, M.I.; Gamelas, J.A.; Fernandes, A.J.; Evtuguin, D.V.; Gomes, M.T.S. A new formaldehyde optical sensor: Detecting milk adulteration. Food Chem. 2020, 318, 126461. [Google Scholar] [CrossRef]
- De Freitas Rezende, F.B.; Cheibub, A.M.D.S.S.; Netto, A.D.P.; de Carvalho Marques, F.F. Determination of formaldehyde in bovine milk using a high sensitivity HPLC-UV method. Microchem. J. 2017, 134, 383–389. [Google Scholar] [CrossRef]
- Silva, A.F.S.; Goncalves, I.C.; Rocha, F.R. Smartphone-based digital images as a novel approach to determine formaldehyde as a milk adulterant. Food Control 2021, 125, 107956. [Google Scholar] [CrossRef]
- Coimbra, P.T.; Bathazar, C.F.; Guimarães, J.T.; Coutinho, N.M.; Pimentel, T.C.; Neto, R.P.; Cruz, A.G. Detection of formaldehyde in raw milk by time domain nuclear magnetic resonance and chemometrics. Food Control 2020, 110, 107006. [Google Scholar] [CrossRef]
- Fu, X.; Wu, J.; Xu, H.; Wan, P.; Fu, H.; Mei, Q. Luminescence nanoprobe in the near-infrared-II window for ultrasensitive detection of hypochlorite. Anal. Chem. 2021, 93, 15696–15702. [Google Scholar] [CrossRef] [PubMed]
- Bastan, N.; Ahmadi, M.; Madrakian, T.; Afkhami, A.; Khalili, S.; Majidi, M.; Moradi, M. A paired emitter–detector diode-based photometer for the determination of sodium hypochlorite adulteration in milk. Sci. Rep. 2023, 13, 6217. [Google Scholar] [CrossRef] [PubMed]
- Ashoorirad, M.; Baghbani, R.; Ghalamboran, M.R. Bioimpedance sensor to detect water content in milk based on van Der Pauw method. IET Nanobiotechnol. 2021, 15, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Durante, G.; Becari, W.; Lima, F.A.; Peres, H.E. Electrical impedance sensor for real-time detection of bovine milk adulteration. IEEE Sens. J. 2015, 16, 861–865. [Google Scholar] [CrossRef]
- Dave, A.; Banwari, D.; Srivastava, S.; Sadistap, S. Optical sensing system for detecting water adulteration in milk. In Proceedings of the 2016 IEEE Global Humanitarian Technology Conference (GHTC), Seattle, WA, USA, 13–16 October 2016; pp. 634–639. [Google Scholar]
- Ren, Q.R.; Zhang, H.; Guo, H.Y.; Jiang, L.; Tian, M.; Ren, F.Z. Detection of cow milk adulteration in yak milk by ELISA. J. Dairy Sci. 2014, 97, 6000–6006. [Google Scholar] [CrossRef] [PubMed]
- Kourti, D.; Angelopoulou, M.; Misiakos, K.; Makarona, E.; Economou, A.; Petrou, P.; Kakabakos, S. Detection of Adulteration of Milk from Other Species with Cow Milk through an Immersible Photonic Immunosensor. Eng. Proc. 2023, 35, 582. [Google Scholar]
- Sakti, S.P.; Chabibah, N.; Ayu, S.P.; Padaga, M.C.; Aulanni’am, A.A. Development of QCM biosensor with specific cow milk protein antibody for candidate milk adulteration detection. J. Sens. 2016, 2016, 1807647. [Google Scholar] [CrossRef]
| Type of Milk | Main Chemical Characteristics | Main Physical Characteristics |
|---|---|---|
| pH: Slightly acidic, almost neutral, 6.4–6.8 | Taste: Palatable | |
| Urea test: Pale yellow color | Texture: No soapiness | |
| Natural Milk | Urea Concentration: 0.2–0.7 mg/mL | Color: White |
| Sugar test (Resorcinol): Negative | Odor: Not distinctive | |
| Neutralizers test (Rosalic acid): Negative | Storage: Curdling but no color change | |
| pH: Extremely alkaline, 10.5 | Taste: Bitter | |
| Urea test: Bright yellow color | Texture: When rubbed, it gives off a soapy feel. | |
| Urea concentration: 14 mg/mL | Color: White | |
| Synthetic Milk | Sugar test (Resorcinol): Positive | Odor: When produced, the combination has a soapy scent that goes away after being stored at 4 °C overnight. |
| Neutralizers test (Rosalic acid): Positive | Storage: After some time, it becomes yellowish |
| Models | Density [g/cm3] | Viscosity [mPas] | Freezing Point [°C] | pH | Fat of Milk [%] |
|---|---|---|---|---|---|
| Undiluted milk | 1.0571 | 33.83 | −0.501 | 6.70 | 3.20 |
| 5% H2O | 1.0531 | 32.83 | −0.484 | 6.72 | 3.00 |
| 10% H2O | 1.0517 | 32.63 | −0.457 | 6.73 | 2.90 |
| 15% H2O | 1.0505 | 31.63 | −0.433 | 6.75 | 2.80 |
| 20% H2O | 1.0478 | 31.33 | −0.405 | 6.76 | 2.55 |
| 25% H2O | 1.0469 | 30.90 | −0.378 | 6.77 | 2.45 |
| 30% H2O | 1.0447 | 29.27 | −0.352 | 6.78 | 2.25 |
| 35% H2O | 1.0436 | 28.90 | −0.327 | 6.78 | 2.00 |
| 40% H2O | 1.0430 | 28.73 | −0.301 | 6.80 | 1.80 |
| 45% H2O | 1.0412 | 27.90 | −0.275 | 6.80 | 1.73 |
| 50% H2O | 1.0397 | 27.13 | −0.250 | 6.81 | 1.48 |
| Adulterants | Details of Qualitative Tests |
|---|---|
| Test | |
| Detergent | Methylene blue |
| Urea | p-dimethylaminobenzaldehyde test (DMAB) |
| Ammonium salts | Nessler |
| Sucrose | Seliwanoff |
| Glucose | Barfoed |
| Maltodextrin | Iodine |
| Starch | Iodine Silver nitrate test |
| Salt | |
| Nitrate | Diphenylamine |
| Sulphate | Barium chloride |
| Hydrogen peroxide | Iodometric test |
| Formaldehyde | Hehner Rosolic acid |
| Neutralizers |
| Adulterant | Detection Technique | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Melamine | Chromatography coupled with mass spectrometry | Quantitative, sensitive, and specific detection | The need for derivatization of analytes, reagents, and expensive equipment | [51] |
| Melamine | Liquid chromatography coupled with mass spectrometry | High specificity and sensitivity with simple sample pretreatment | Expensive equipment, limited sample flow, and absence of portability | [51,52] |
| Melamine | Raman spectroscopy | Fast, non-destructive method allows direct analysis | High-cost instrumentation | [52] |
| Melamine | NIR spectroscopy | Useful for the examination of heterogeneous samples with minimal preparation | Data collection and analysis require sensitive detectors, and data processing is complex | [52] |
| Melamine | MALDI-TOF-MS | The use of heterogeneous samples with less sample volume | Sample pretreatment requires time, expensive equipment, and qualified personnel | [53] |
| Melamine | HPLC-MS | Molecular specificity and high sensitivity | Sample pretreatment has limited application in routine analysis | [49,50] |
| Melamine | HPLC-UV | Simple equipment compared to HPLC-MS; high selectivity in combination with fluorimetric detectors | Less selective method, laborious treatment of the sample, inability to confirm the target analyte | [51,52] |
| Melamine | ELISA | Selectivity, sensitivity, simple sample preparation, simultaneous analysis of 40 samples for one hour | Semi-quantitative determination due to the relatively large standard deviation, and cross-reactivity with cyromazine | [51] |
| Urea | GC-IDMS | Used to compare the outcomes of conventional procedures. Excellent repeatability and reproducibility | Laborious and expensive sample preparation. Optimization of derivatization conditions | [54] |
| Whey | HPLC (cGMP) | Utilized in both qualitative and quantitative tests, the procedure is precise, quick, and easy to implement in the laboratory. It can identify urea in even traces or extremely small levels | Expensive, complex technique, requires specialized knowledge, sample preparation, the columns used can clog, and the sample volume introduced is limited | [54] |
| Name of the Adulterant | Biosensor Class | Detection Limit | Reference |
|---|---|---|---|
| Melamine | Optical biosensor | 0.5 μg/mL | [58] |
| Melamine | SPR | 0.02 μg/mL | [59] |
| Melamine | Aptamer based | 2 mM | [60] |
| Melamine | EWFI | 5.14 μg/L | [61] |
| Melamine and urea | Electrochemical | 3 pM, 1 pM | [55] |
| Urea | Potentiometric | 2.5 × 10−5 M/L | [62] |
| Urea | Android integrated | 10 mM | [63] |
| Urea | Optical sensor | 10−8 M | [64] |
| Urea | FIA-ET | 0.1–250 mM | [65] |
| Adulterant | Detection Technique | Advantage | Disadvantage | Reference |
|---|---|---|---|---|
| Hydrogen peroxide | Optical Biosensor | Quick, economical, and environmentally friendly methods with good repeatability, concentrations as low as 0.001% v/v can be measured. | The systematic optimization of the membrane used. The need for reagents. | [73] |
| Hydrogen peroxide | Bioactive paper platform | Simple, cheap, disposable, accessible, and flexible. Minimal amounts of reagent and sample are needed. | All reagents used must be of analytical quality. The need for the subsequent use of chemometric methods. | [72] |
| Hydrogen peroxide | HPLC with a diode-array based on the oxidation of triphenylphosphine into triphenylphosphine oxide | Optimized method validated with acceptable linearity. Suitable for use in most laboratories (routine technique). | A small possibility exists for milk components to interact with triphenylphosphine. | [37] |
| Hydrogen peroxide | Non-enzymatic sensor of Cux O/Ag nanoparticles on exfoliated graphene | High sensitivity over an extremely wide range of concentrations. Exceptional selectivity and long-term stability. | A lot of materials and chemicals are needed for the careful construction of this sensor. | [74] |
| Formaldehyde | Optical sensor | It could be used in contact with aqueous solutions because it is insoluble in water. Low cost, easy to use. | The need for reagents, sample processing, and sensor configuration. | [75] |
| Formaldehyde | HPLC-UV | Simple, accurate, interference-free method | The need for the derivatization reaction. | [76] |
| Formaldehyde | Smartphone-based digital images | An innovative, quick, and affordable method. No toxic reagents are used, and a small sample volume is required. | The need for chemical derivatization. | [77] |
| Formaldehyde | Time-Domain magnetic resonance (TD-NMR) | Excellent repeatability and reproducibility. It does not require the preparation of samples. | Must be used together with chemometric methods. | [78] |
| Hypochlorite | Luminescence Nanoprobe in the Near-Infrared-II Window | Sensitive and selective detection. Low-cost, non-invasive detection. | The difficult design of nanocrystals. | [79] |
| Hypochlorite | A paired emitter–detector diode-based photometer | The photometer has the potential to be portable and can be used by consumers. | Supplementing with a potentiometer and a phone app. | [80] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, A.-D.; Cîrîc, A.I.; Begea, M. A Review of Milk Frauds and Adulterations from a Technological Perspective. Appl. Sci. 2023, 13, 9821. https://doi.org/10.3390/app13179821
Ionescu A-D, Cîrîc AI, Begea M. A Review of Milk Frauds and Adulterations from a Technological Perspective. Applied Sciences. 2023; 13(17):9821. https://doi.org/10.3390/app13179821
Chicago/Turabian StyleIonescu, Alina-Daiana, Alexandru Ionuț Cîrîc, and Mihaela Begea. 2023. "A Review of Milk Frauds and Adulterations from a Technological Perspective" Applied Sciences 13, no. 17: 9821. https://doi.org/10.3390/app13179821





