An Experimental Study on the Combustion Characteristics of a Methane Diffusion Flame within a Confined Space under Sub-Atmospheric Pressure
Abstract
1. Introduction
2. Experimental Methodology
3. Results and Discussion
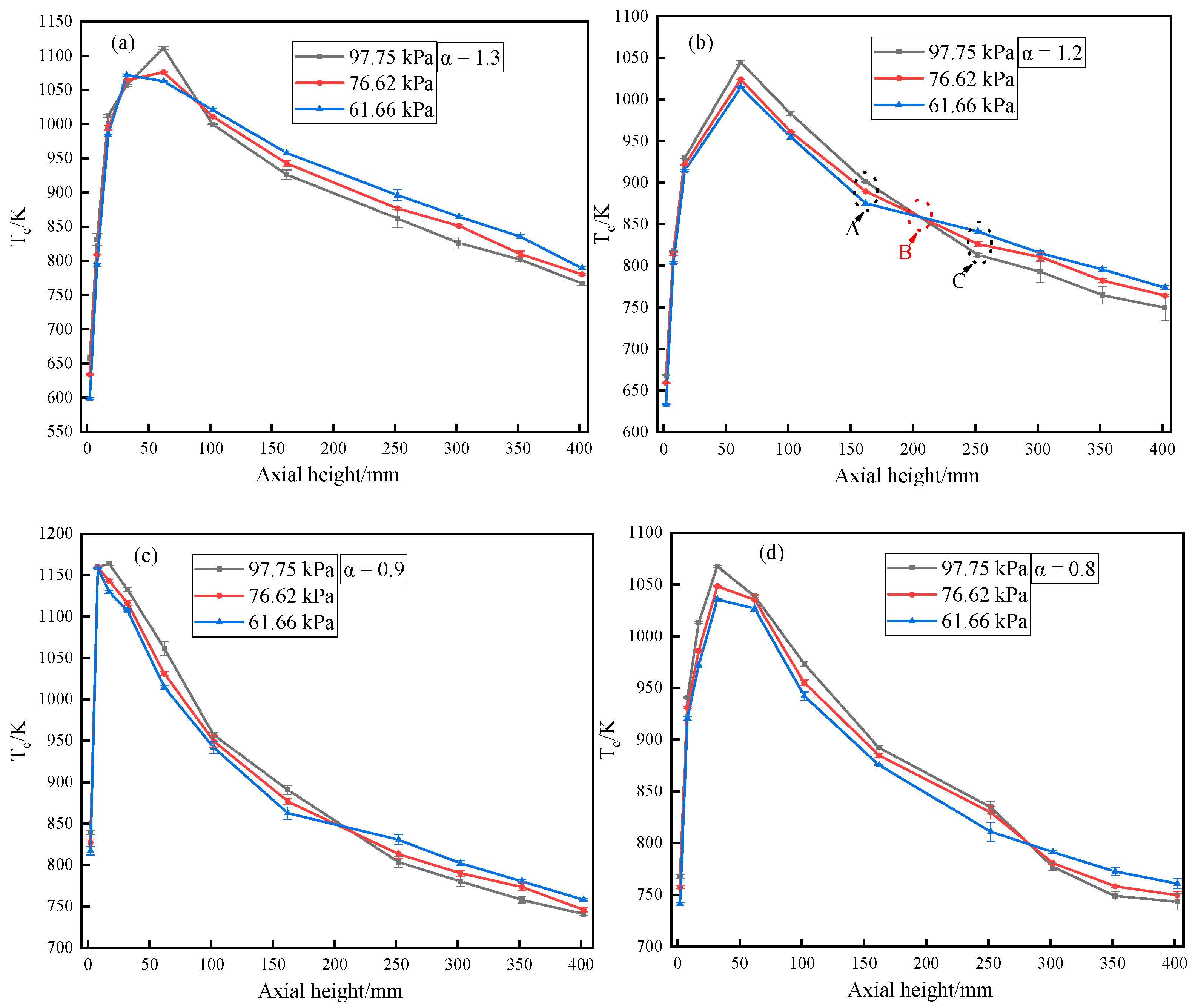
3.1. Temperature Distribution
3.2. Flame Appearance
3.3. Smoke Point
3.4. CO and NOx Emission
4. Discussion
- (1)
- The at the front of the furnace decreases with decreasing pressure, whereas at the rear of the furnace increases with decreasing pressure.
- (2)
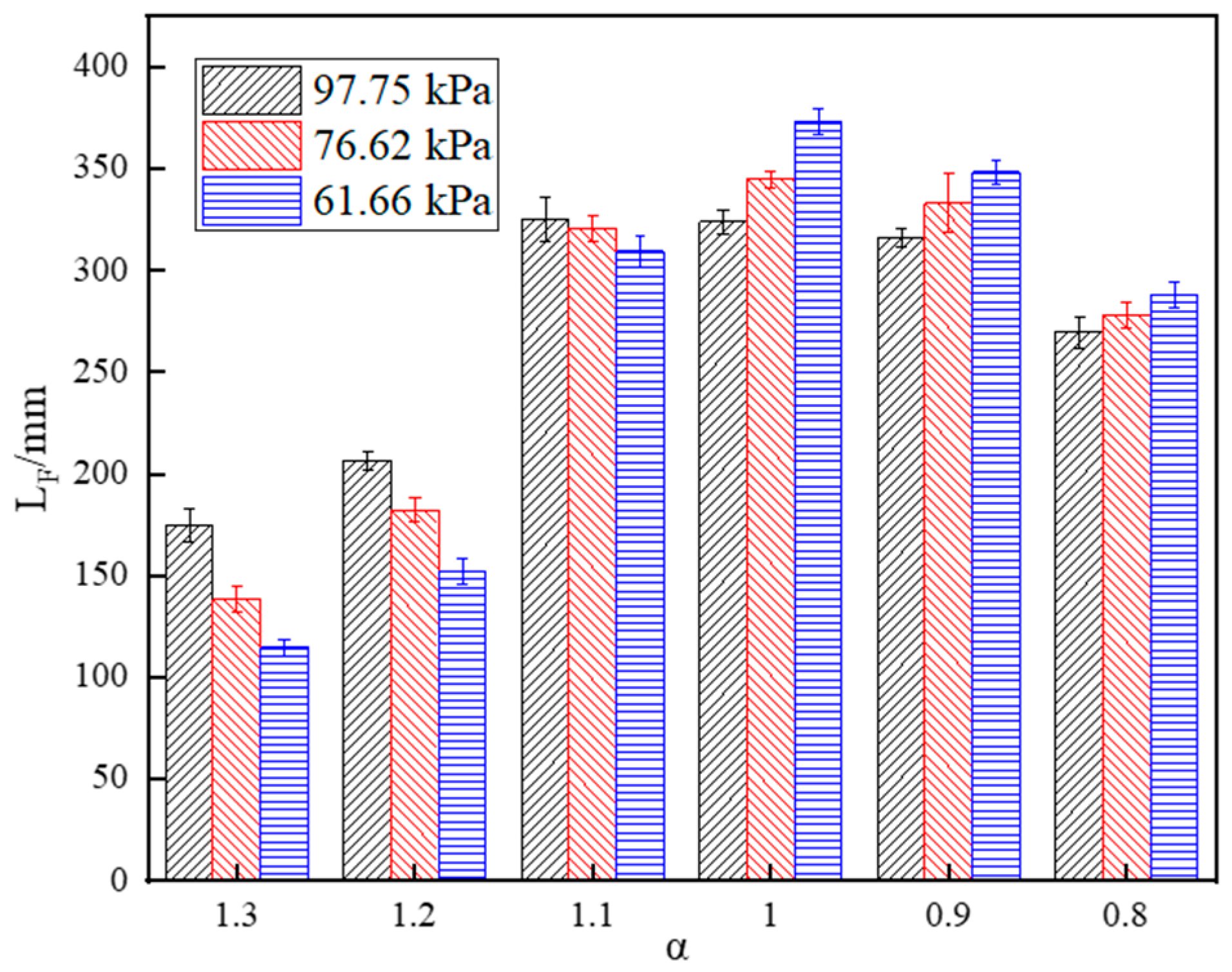
- Under fuel-lean combustion, the flame length decreases with decreasing pressure. However, the flame length appears as a reverse trend under fuel-rich combustion. In addition, the rise jet velocity and reduced burning rate result in an increase in the blue region height of the flame under sub-atmospheric pressure.
- (3)
- The smoke point fuel flow rate, flame length and residence time increase with decreasing pressure, following the law of the negative exponent.
- (4)
- The CO emission decreases with decreasing pressure, which indicates that the reduced pressure makes methane combustion more complete. For NO emission, the reduced pressure results in an opposite tendency under fuel-lean and -rich combustion. With decreased pressure, the NO emission decreases under fuel-lean combustion but increases under fuel-rich combustion.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shan, Y.; Zheng, H.; Guan, D.; Li, C.; Mi, Z.; Meng, J.; Schroeder, H.; Ma, J.; Ma, Z. Energy consumption and CO2 emissions in Tibet and its cities in 2014. Earths Future 2017, 5, 854–864. [Google Scholar] [CrossRef]
- Zhou, Y.; Huang, X.; Peng, S.; Li, L. Comparative study on the combustion characteristics of an atmospheric induction stove in the plateau and plain regions of China. Appl. Therm. Eng. 2017, 111, 301–307. [Google Scholar] [CrossRef]
- Amell, A.A. Influence of altitude on the height of blue cone in a premixed flame. Appl. Therm. Eng. 2007, 27, 408–412. [Google Scholar] [CrossRef]
- Andres, A.; Layrisser, I. Performance study of an induced air porous radiant burner for household applications at high altitude. Appl. Therm. Eng. 2015, 83, 31–39. [Google Scholar]
- Zhang, J.; Du, Y.; Yu, J.; Wang, Y.; Wang, C.; Che, D.; Da, Y. The influence of altitude on combustion characteristics within gas-fired boiler by numerical simulation. Asia-Pac. J. Chem. Eng. 2023, 18, e2880. [Google Scholar] [CrossRef]
- Yang, S.S.; Gülder, M. Pressure dependence of sooting characteristics of m-xylene and n-octane doped laminar methane diffusion flames from 2 to 10 bar. Combust. Flame 2020, 220, 203–209. [Google Scholar] [CrossRef]
- Jia, L.U.; Liao, G.X.; Tao, C.F. Experimental study of flame shape of methane jet diffusion flame. J. Saf. Environ. 2010, 10, 164–168. [Google Scholar]
- Zhang, X.; Duan, Y.; Zhang, R.; Wei, H.; Chen, L. Optical study of oxygen enrichment on methane combustion characteristics under high compression-ratio conditions. Fuel 2022, 328, 125251. [Google Scholar] [CrossRef]
- Yang, M.; He, Y.; Li, H.; Zhang, X.; Li, Z.; Wang, J. Combustion of laminar non-premixed acetylene jet at two different altitudes. Combust. Sci. Technol. 2012, 184, 1950–1969. [Google Scholar] [CrossRef]
- Hu, L.H.; Qiang, W.; Fei, T.; Delichatsios, M.; Zhang, X.C. Axial temperature profile in vertical buoyant turbulent jet fire in a reduced pressure atmosphere. Fuel 2013, 106, 779–786. [Google Scholar] [CrossRef]
- Zeng, Y.; Fang, J.; Wang, J.; Li, J.; Tu, R.; Zhang, Y. Momentum-dominated methane jet flame at sub-atmospheric pressure. Procedia Eng. 2013, 62, 924–931. [Google Scholar] [CrossRef][Green Version]
- Kim, C.H.; El-Leathy, A.M.; Xu, F.; Faeth, G.M. Soot surface growth and oxidation in laminar diffusion flames at pressures of 0.1–1.0 atm. Combust. Flame 2004, 136, 191–207. [Google Scholar] [CrossRef]
- Li, H. Study on Combustion Characteristics and Flame Morphologies of Gas Jets at Subatmospheric Pressures. Ph.D. Thesis, University of Science and technology of China, Hefei, China, 2014. [Google Scholar]
- Gu, M.; Liu, F.; Consalvi, J.L.; Gülder, M. Effects of pressure on soot formation in laminar coflow methane/air diffusion flames doped with n-heptane and toluene between 2 and 8 atm. Proc. Combust. Inst. 2020, 38, 1403–1412. [Google Scholar] [CrossRef]
- Yang, M. Experimental and Computational Study on the Effects of Low Atmospheric Pressure on the Gas Fuel Combustion Characteristics and Smoke Properties under High Altitudes. Ph.D. Thesis, University of Science and technology of China, Hefei, China, 2011. [Google Scholar]
- Berry, T.L.; Roberts, W.L. Measurement of smoke point in velocity-matched coflow laminar diffusion flames with pure fuels at elevated pressures. Combust. Flame 2006, 145, 571–578. [Google Scholar] [CrossRef]
- Dai, Z.; Faeth, G.M. Hydrodynamic suppression of soot formation in laminar coflowing jet diffusion flames. Proc. Combust. Inst. 2000, 28, 2085–2092. [Google Scholar] [CrossRef]
- Urban, D.L.; Yuan, Z.G.; Sunderland, R.B.; Lin, K.C.; Dai, Z.; Faeth, G.M. Smoke-point properties of non-buoyant round laminar jet diffusion flames. Proc. Combust. Inst. 2000, 28, 1965–1972. [Google Scholar] [CrossRef]
- Li, H.; He, Y.; Wang, J. Determination of smoke point of laminar acetylene diffusion flames under subatmospheric pressures. Combust. Sci. Technol. 2014, 186, 1237–1248. [Google Scholar] [CrossRef]
- Xie, K.; Li, Y.Z.; Zhang, J.; Wang, C.L.; Liu, Z.G.; Wang, G.Q.; Tan, X.F.; Zheng, C.S.; Xiong, Y.X. Study on statistical invariance of probability processing and fluctuation characteristics of consecutive images of horizontal spray flame under low-pressure. Case Stud. Therm. Eng. 2022, 38, 102329. [Google Scholar] [CrossRef]
- Yang, X.; Ma, S.J.; Gao, J.M.; Du, Q.; Zhang, Y.; Dong, H.M.; Wu, S.H.; Qin, Y.K. Mechanism and effect assessment of sub-atmospheric pressure and co-flow air to suppress the flicker of buoyancy-driven methane laminar diffusion flame br. Fuel Process. Technol. 2023, 242, 107649. [Google Scholar] [CrossRef]
- Derk, G.; Boyer, E.; Risha, G.A.; Yetter, R.A.; Smooke, M.D. Experimental and numerical investigation of high-pressure nitromethane combustion. Proc. Combust. Inst. 2021, 38, 3325–3332. [Google Scholar] [CrossRef]
- Li, H.; Zhou, Z.; Niu, Y.; Yao, J.; Zhou, D.; Wang, J. Effect of pressure and type of fuel on laminar diffusion flame height at subatmospheric pressures. Chem. Technol. Fuels Oils 2015, 51, 389–396. [Google Scholar] [CrossRef]
- Chu, H.; Gu, M.; Zhou, H.; Liu, F. Calculations of narrow-band transimissities and the Planck mean absorption coefficients of real gases using line-by-line and statistical narrow-band models. Front. Energy 2014, 8, 41–48. [Google Scholar] [CrossRef]
- Yao, W.; Hu, X.; Rong, J.; Wang, J.; Zhang, H. Experimental study of large-scale fire behavior under low pressure at high altitude. J. Fire Sci. 2013, 31, 481–494. [Google Scholar] [CrossRef]
- Mandatori, P.M.; Gülder, Ö.L. Soot formation in laminar ethane diffusion flames at pressures from 0.2 to 3.3 MPa. Proc. Combust. Inst. 2011, 33, 577–584. [Google Scholar] [CrossRef]
- Miller, I.M.; Maahs, H.G. High Pressure Flame System for Pollution Studies with Results for Methane-Air Diffusion Flames; Technical Report NASA TN D-8407; NASA: Washington, DC, USA, 1977.
- Li, Z.H.; He, Y.; Zhang, H.; Wang, J. Combustion characteristics of n-heptane and wood crib fires at different altitudes. Proc. Combust. Inst. 2009, 32, 2481–2488. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, G.M.; Kim, D.J. Effects of operating pressure on flame oscillation and emission characteristics in a partially premixed swirl combustor. Exp. Therm. Fluid Sci. 2011, 35, 165–171. [Google Scholar] [CrossRef]
- Kim, J.R.; Akamatsu, F.; Choi, G.M.; Kim, D.J. Observation of local heat release rate with changing combustor pressure in the CH4/air flame (wrinkled laminar regime). Thermochim. Acta 2009, 491, 109–115. [Google Scholar] [CrossRef]

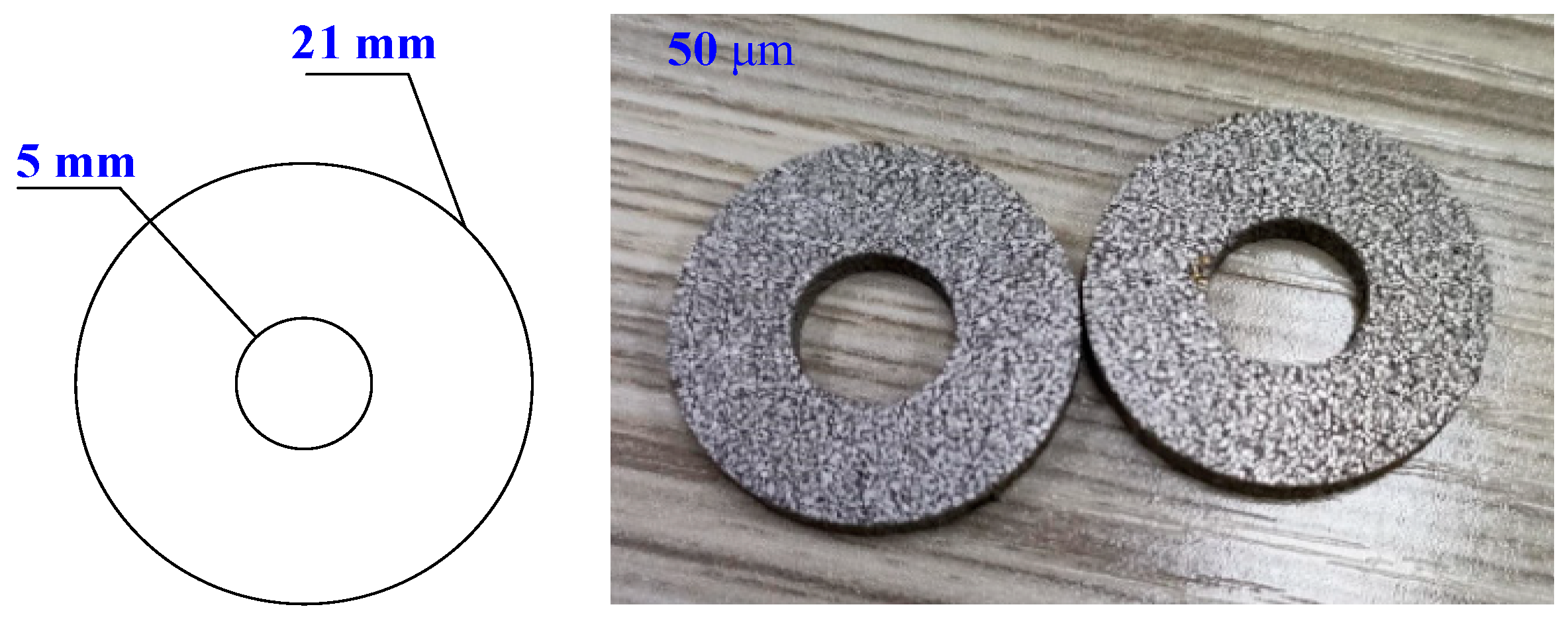



| Equipment Type | Application | Measurement Span | Uncertainty |
|---|---|---|---|
| Mass flowmeter | Air/methane | Air: 10 L·min−1 Methane: 1 L·min−1 | ±2% |
| K-type thermocouple | Centerline temperature | 0~1523 K | ±0.5% |
| Pressure gauge | Reactor pressure | 0~110 kPa | ±0.1% |
| Testo 335 | O2 | 0~25% | ±0.1% under 0~4.99%; ±0.5%, 5~25% |
| CO | 0~10,000 ppm | ±10%, 0~200 ppm; ±5%, 201~2000 ppm | |
| NO | 0~2000 ppm | ±10%, 0~99 ppm; ±5%, 100~1999 ppm |
| Case | P/kPa | /m | /m·s−1 | α | /mg·s−1 |
|---|---|---|---|---|---|
| 1 | 97.75 | 300 | 0.074 | 0.8 | 61.63 |
| 2 | 0.9 | 69.33 | |||
| 3 | 1.0 | 77.03 | |||
| 4 | 1.1 | 84.74 | |||
| 5 | 1.2 | 92.44 | |||
| 6 | 1.3 | 100.14 | |||
| 8 | 76.62 | 2295 | 0.094 | 0.8 | 61.63 |
| 9 | 0.9 | 69.33 | |||
| 10 | 1.0 | 77.03 | |||
| 11 | 1.1 | 84.74 | |||
| 12 | 1.2 | 92.44 | |||
| 13 | 1.3 | 100.14 | |||
| 15 | 61.66 | 4000 | 0.012 | 0.8 | 61.63 |
| 16 | 0.9 | 69.33 | |||
| 17 | 1.0 | 77.03 | |||
| 18 | 1.1 | 84.74 | |||
| 19 | 1.2 | 92.44 | |||
| 20 | 1.3 | 100.14 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Du, Y.; Zong, S.; Zhao, N.; Da, Y.; Deng, L.; Che, D. An Experimental Study on the Combustion Characteristics of a Methane Diffusion Flame within a Confined Space under Sub-Atmospheric Pressure. Appl. Sci. 2023, 13, 9848. https://doi.org/10.3390/app13179848
Zhang J, Du Y, Zong S, Zhao N, Da Y, Deng L, Che D. An Experimental Study on the Combustion Characteristics of a Methane Diffusion Flame within a Confined Space under Sub-Atmospheric Pressure. Applied Sciences. 2023; 13(17):9848. https://doi.org/10.3390/app13179848
Chicago/Turabian StyleZhang, Jingkun, Yongbo Du, Siyu Zong, Nan Zhao, Yaodong Da, Lei Deng, and Defu Che. 2023. "An Experimental Study on the Combustion Characteristics of a Methane Diffusion Flame within a Confined Space under Sub-Atmospheric Pressure" Applied Sciences 13, no. 17: 9848. https://doi.org/10.3390/app13179848
APA StyleZhang, J., Du, Y., Zong, S., Zhao, N., Da, Y., Deng, L., & Che, D. (2023). An Experimental Study on the Combustion Characteristics of a Methane Diffusion Flame within a Confined Space under Sub-Atmospheric Pressure. Applied Sciences, 13(17), 9848. https://doi.org/10.3390/app13179848







