Disease Severity Index in Parkinson’s Disease Based on Self-Organizing Maps
Abstract
:1. Introduction
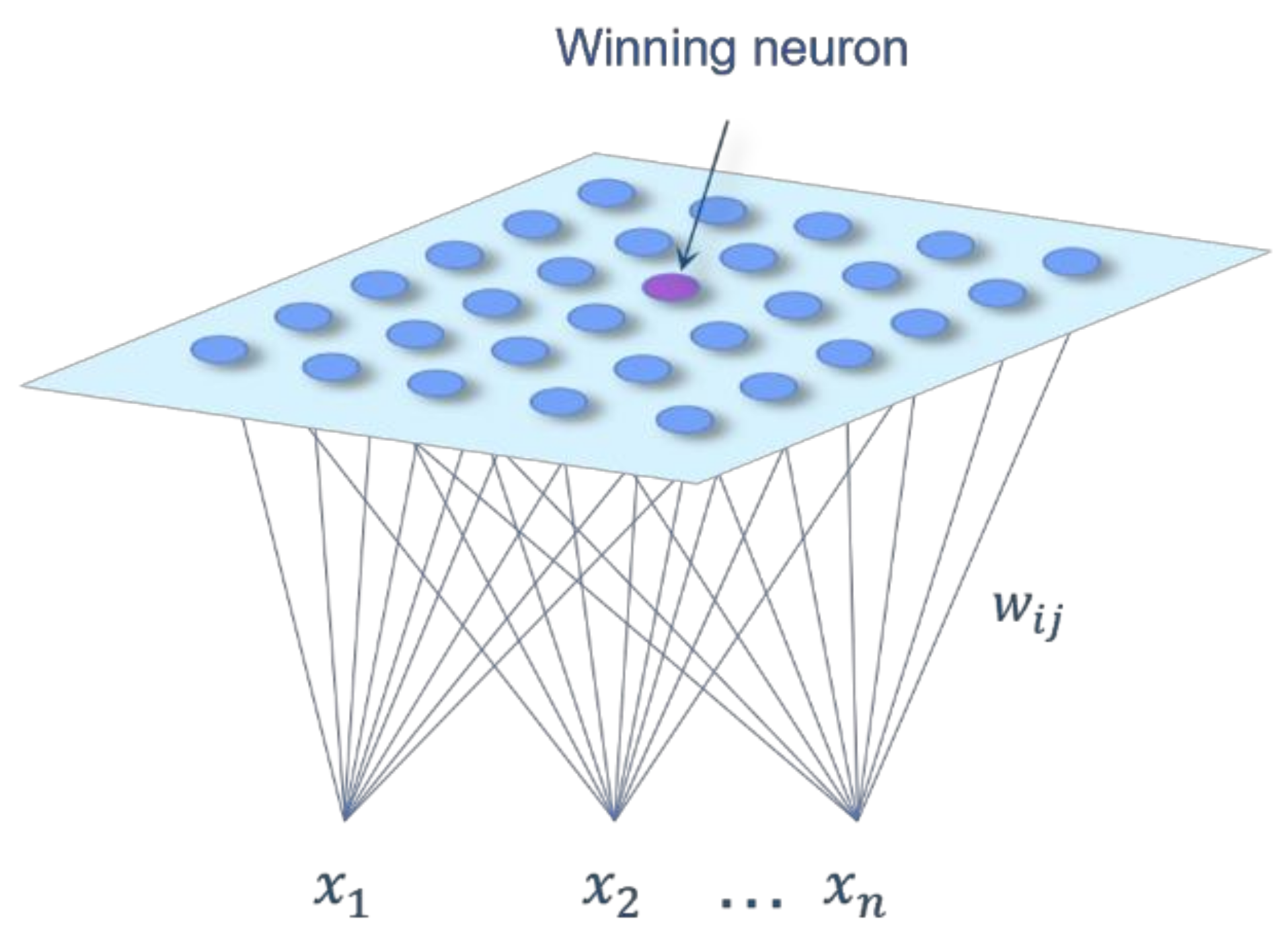
2. Materials and Methods
2.1. Coding of the Data
2.2. Included and Excluded Data
2.3. Selection of the Data Processing Methodology
3. Results
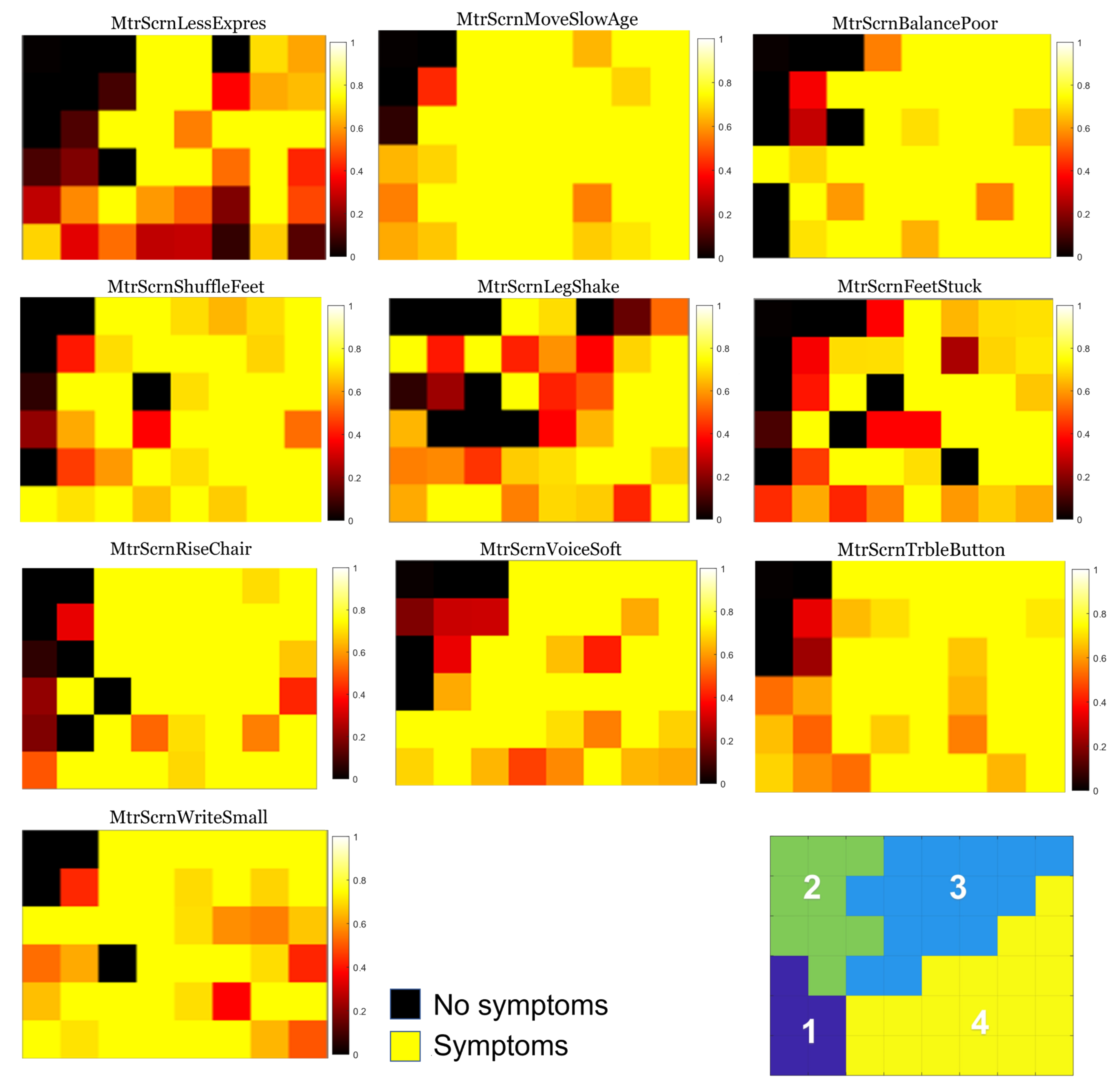
3.1. Individual Symptom Analysis
3.2. Validation of Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization (WHO). Parkinson Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease (accessed on 1 July 2023).
- Seppi, K.; Ray Chaudhuri, K.; Coelho, M.; Fox, S.H.; Katzenschlager, R.; Perez Lloret, S.; Weintraub, D.; Sampaio, C.; Chahine, L.; Hametner, E.; et al. Update on treatments for nonmotor symptoms of Parkinson’s disease—An evidence-based medicine review. Mov. Disord. 2019, 34, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Sivanandy, P.; Leey, T.C.; Xiang, T.C.; Ling, T.C.; Wey Han, S.A.; Semilan, S.L.A.; Hong, P.K. Systematic Review on Parkinson’s Disease Medications, Emphasizing on Three Recently Approved Drugs to Control Parkinson’s Symptoms. Int. J. Environ. Res. Public Health 2021, 19, 364. [Google Scholar] [CrossRef] [PubMed]
- The Lancet. Parkinson’s disease: A complex disease revisited. Lancet 2017, 390, 430. [Google Scholar] [CrossRef]
- Abbas, S.Q.; Chi, L.; Chen, Y.-P.P. Transformed Domain Convolutional Neural Network for Alzheimer’s Disease Diagnosis using Structural MRI. Pattern Recognit. 2022, 133, 109031. [Google Scholar] [CrossRef]
- Alenezi, F.; Armghan, A.; Polat, K. A multi-stage melanoma recognition framework with deep residual neural network and hyperparameter optimization-based decision support in dermoscopy images. Expert Syst. Appl. 2023, 215, 119352. [Google Scholar] [CrossRef]
- Singh, V.; Asari, V.K.; Rajasekaran, R. A Deep Neural Network for Early Detection and Prediction of Chronic Kidney Disease. Diagnostics 2022, 12, 116. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Tang, G.; Yuan, X.; Lin, Q.; Mao, L.; Song, H.; Xue, Y.; Wu, S.; Ouyang, R.; Hou, H.; et al. Combination of Blood Routine Examination and T-SPOT.TB Assay for Distinguishing Between Active Tuberculosis and Latent Tuberculosis Infection. Front. Cell. Infect. Microbiol. 2021, 11, 575650. [Google Scholar] [CrossRef] [PubMed]
- Mian, T.S. An Unsupervised Neural Network Feature Selection and 1D Convolution Neural Network Classification for Screening of Parkinsonism. Diagnostics 2022, 12, 1796. [Google Scholar] [CrossRef] [PubMed]
- Bhavekar, G.S.; Goswami, A.D. A hybrid model for heart disease prediction using recurrent neural network and long short term memory. Int. J. Inf. Technol. 2022, 14, 1781–1789. [Google Scholar] [CrossRef]
- Trabassi, D.; Serrao, M.; Varrecchia, T.; Ranavolo, A.; Coppola, G.; De Icco, R.; Tassorelli, C.; Castiglia, S.F. Machine Learning Approach to Support the Detection of Parkinson’s Disease in IMU-Based Gait Analysis. Gait Posture 2022, 97, 15–16. [Google Scholar] [CrossRef]
- Ai, L.; Wang, J.; Yao, R. Classification of parkinsonian and essential tremor using empirical mode decomposition and support vector machine. Digit. Signal Process. 2011, 21, 543–550. [Google Scholar] [CrossRef]
- Loconsole, C.; Cascarano, G.D.; Brunetti, A.; Trotta, G.F.; Losavio, G.; Bevilacqua, V.; Di Sciascio, E. A model-free technique based on computer vision and sEMG for classification in Parkinson’s disease by using computer-assisted handwriting analysis. Pattern Recognit. Lett. 2019, 121, 28–36. [Google Scholar] [CrossRef]
- Kohonen, T. Self-Organizing Maps; Springer: Berlin/Heidelberg, Germany, 2001. [Google Scholar] [CrossRef]
- Novis, S.; Machado, F.; Costa, V.B.; Foguel, D.; Cruz, M.W.; de Seixas, J.M. Applying an artificial neural network model for developing a severity score for patients with hereditary amyloid polyneuropathy. Amyloid 2017, 24, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cheng, Z. Cross-Sectional Studies. Chest 2020, 158, S65–S71. [Google Scholar] [CrossRef] [PubMed]
- Bock, M.A.; Brown, E.G.; Zhang, L.; Tanner, C. Association of Motor and Nonmotor Symptoms With Health-Related Quality of Life in a Large Online Cohort of People With Parkinson Disease. Neurology 2022, 98, e2194–e2203. [Google Scholar] [CrossRef] [PubMed]
- White, D.R.; Speller, R.; Morgan, T.J.; Showalter, C.K.; Shope, T.B.; Rothenberg, L.N.; Pentlow, K.S. A SURVEY OF 20 CT SYSTEMS. J. Comput. Assist. Tomogr. 1979, 3, 573. [Google Scholar] [CrossRef]
- Łuczak, M. Hierarchical clustering of time series data with parametric derivative dynamic time warping. Expert Syst. Appl. 2016, 62, 116–130. [Google Scholar] [CrossRef]
- Haykin, S. Redes Neurais—Principios E Prática; Bookman: 2003. Available online: https://books.google.co.jp/books?hl=zh-CN&lr=&id=bhMwDwAAQBAJ&oi=fnd&pg=PP1&dq=Redes+Neurais%E2%80%94PriPrincip+E+Pr%C3%A1tica&ots=08zoPPPQNs&sig=aqvY-GdlV85uZSru8I4V9LRL5ec&redir_esc=y#v=onepage&q=Redes%20NNeurai%E2%80%94Principios%20E%20Pr%C3%A1tica&f=false (accessed on 3 July 2023).
- Victor Coelho Cascão, L. Modelos De Inteligencia Computacional Para Apoio À Triagem De Pacientes E Diagnostico Clinico De Tuberculose Pulmonar. Master’s Thesis, Universidade Federal do Rio de Janeiro, Rio de Janeiro, Brazil, 2011. Available online: http://pee.ufrj.br/teses/textocompleto/2011101801.pdf (accessed on 16 May 2023).
- Jayanth Krishnan, K.; Mitra, K. A Modified Kohonen Map Algorithm for Clustering Time Series Data. Expert Syst. Appl. 2022, 201, 117249. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Weintraub, D.; Aarsland, D.; Chaudhuri, K.R.; Dobkin, R.D.; Leentjens, A.F.; Rodriguez-Violante, M.; Schrag, A. The neuropsychiatry of Parkinson’s disease: Advances and challenges. Lancet Neurol. 2022, 21, 89–102. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.; Zhu, C.; Zhang, Z.; Liu, Y. Automated Detection of Parkinson’s Disease Based on Multiple Types of Sustained Phonations Using Linear Discriminant Analysis and Genetically Optimized Neural Network. IEEE J. Transl. Eng. Health Med. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Berus, L.; Klancnik, S.; Brezocnik, M.; Ficko, M. Classifying Parkinson’s Disease Based on Acoustic Measures Using Artificial Neural Networks. Sensors 2018, 19, 16. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.; Schumacher, N.U.; Pürner, D.; Machetanz, G.; Demleitner, A.F.; Feneberg, E.; Hagemeier, M.; Lingor, P. Parkinson’s disease therapy: What lies ahead? J. Neural Transm. 2023, 130, 793–820. [Google Scholar] [CrossRef] [PubMed]








| Groups | Designation | Description of Questions |
|---|---|---|
| Group 1 | MtrScrnRiseChair | Having trouble getting up from a chair? |
| MtrScrnWriteSmall | Is your handwriting smaller than before? | |
| MtrScrnVoiceSoft | Do people say that your voice is softer than before? | |
| MtrScrnBalancePoor | Is your balance poor? | |
| MtrScrnFeetStuck | Do your feet ever seem to stick to the ground? | |
| MtrScrnLessExpres | Does your face seem less expressive than before? | |
| MtrScrnLegShake | Do your arms or legs shake? | |
| MtrScrnTrbleButton | Do you have trouble buttoning buttons? | |
| MtrScrnShuffleFeet | Do you drag your feet and/or take small steps when walking? | |
| MtrScrnMoveSlowAge | Do you move slower than other people your age? | |
| Group 2 | MoveSpeech | In the last week, have you had problems with your speech? |
| MoveSaliva | In the past week, have you usually had a lot of saliva when you were awake or asleep? | |
| MoveChew | In the past week, have you generally had trouble swallowing pills or eating meals? Do you need your pills chopped or crushed or your meals softened, chopped, or mixed to prevent gagging? | |
| MoveEat | In the past week, have you generally had trouble handling your food and using cutlery? For example, do you have trouble handling snacks or using forks, knives, spoons, or chopsticks? | |
| MoveDress | In the past week, have you generally had trouble getting dressed? For example, are you slow or do you need help buttoning, zipping, putting on, or taking off clothes or jewelry? | |
| MoveHygiene | In the past week, have you been generally slow or need help washing, showering, shaving, brushing your teeth, combing your hair, or doing other personal hygiene? | |
| MoveWrite | In the past week, have people generally had trouble reading your handwriting? | |
| MoveHobby | In the past week, have you generally had trouble doing your hobbies or other things you enjoy doing? | |
| MoveSleep | In the past week, have you usually had trouble turning over in bed? | |
| MoveTremor | During the past week, have you usually had tremors? | |
| MoveUp | During the last week, have you usually had difficulty getting out of a bed, a car seat, or a deep chair? | |
| MoveWalk | During the last week, have you usually had problems with balance and walking? | |
| MoveFreeze | During the last week, on your usual day of walking, do you suddenly stop or freeze as if your feet were glued to the ground? | |
| Group 3 | Mobility | Having trouble getting around? |
| Self-care | Do you have difficulty washing or dressing yourself? | |
| Activities | Do you have difficulties carrying out your usual activities? | |
| Pains | Do you have pain or discomfort? | |
| Anxiety | Are you anxious or depressed? |
| Groups | Answers | Codification |
|---|---|---|
| Group 1 | 0—No | 0 |
| 1—Yes | 1 | |
| Group 2 | 0—No | 0 |
| 1—Slight | ||
| 2—Smooth | ||
| 3—Moderate | 1 | |
| 4—Severe | ||
| Group 3 | 0—No | 0 |
| 1—Slight | ||
| 2—Moderate | 1 | |
| 3—Serious 4—Extremely serious |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, S.M.; Nery, S.B.M.; Magalhães, B.G.; Almeida, K.J.; Gaspar, P.D. Disease Severity Index in Parkinson’s Disease Based on Self-Organizing Maps. Appl. Sci. 2023, 13, 10019. https://doi.org/10.3390/app131810019
Araújo SM, Nery SBM, Magalhães BG, Almeida KJ, Gaspar PD. Disease Severity Index in Parkinson’s Disease Based on Self-Organizing Maps. Applied Sciences. 2023; 13(18):10019. https://doi.org/10.3390/app131810019
Chicago/Turabian StyleAraújo, Suellen M., Sabrina B. M. Nery, Bianca G. Magalhães, Kelson James Almeida, and Pedro D. Gaspar. 2023. "Disease Severity Index in Parkinson’s Disease Based on Self-Organizing Maps" Applied Sciences 13, no. 18: 10019. https://doi.org/10.3390/app131810019
APA StyleAraújo, S. M., Nery, S. B. M., Magalhães, B. G., Almeida, K. J., & Gaspar, P. D. (2023). Disease Severity Index in Parkinson’s Disease Based on Self-Organizing Maps. Applied Sciences, 13(18), 10019. https://doi.org/10.3390/app131810019








