Laboratory-Scale Optimization of Celestine Concentration Using a Hydrocyclone System
Abstract
1. Introduction
2. Materials and Methods
2.1. Montevive Celestine Mineral
2.2. Sample Preparation
2.3. Dense Media
2.3.1. Viscosity Determination
2.3.2. Stability Study
- In a 250 mL measuring cylinder, 250 mL of medium was prepared (water and heavy mineral) at 3.00 kg/L (Figure S2A).
- Then, the medium was well-mixed, preventing it from adhering to the cylinder walls.
- Time and height data were taken from the dense medium column using a ruler and stopwatch (Figure S2B).
- Due to the fast-decanting speed, a video of the process was recorded (Figure S2C).
2.4. Dense Medium Separation Experiments
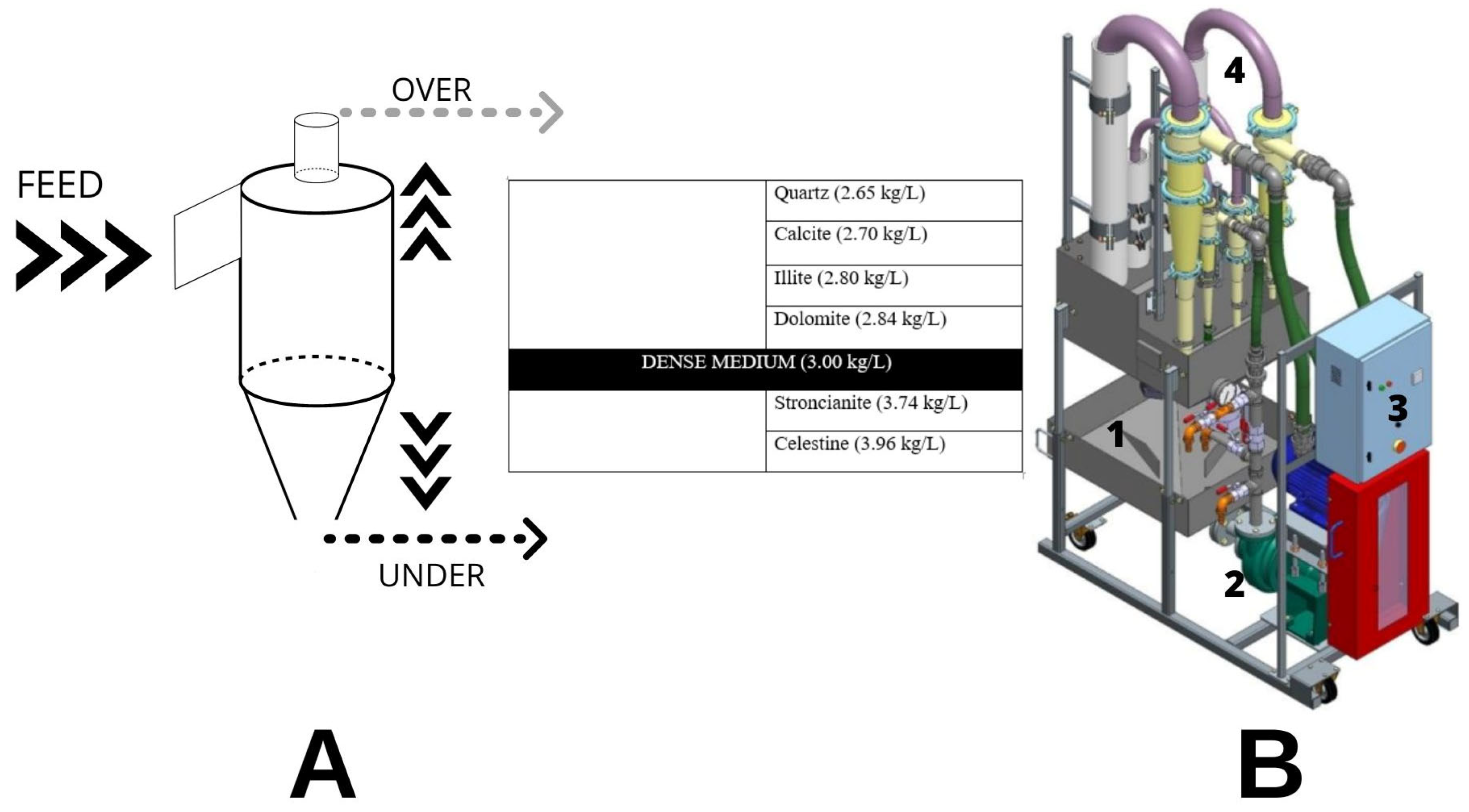
2.4.1. Laboratory Scale Separation System
- 160 L sump (Figure S3);
- AMP 3/2 CMAR 7.5 kW pump;
- Frequency converter to run the pump and control speed (Figure S4B);
- Wika Manometer 0–4 bar, 0.1 bar precision;
- 75 mm hydrocyclone (Figure S3 and Figure S4C).
2.4.2. Mineral Separation Experiments
2.5. XRD Analysis
3. Results and Discussion
3.1. Characterization of Dense Media
3.1.1. Stability of Dense Media
3.1.2. Viscosity of Dense Media
3.2. Celestine Mineral Concentration by DMS
3.2.1. Theoretical and Empirical Mass of Heavy Mineral in the Suspension
3.2.2. Mineral Mass Recovered in the Output Streams
3.3. Characterization of the Mineral by XRD
3.3.1. Characterization of Run-of-Mine Mineral Fed into the Input Stream
3.3.2. Characterization of the Mineral Composition in Output Streams
- Celestine concentration in the output streams
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jimeno, C.L.; González, C.M. Las materias primas minerales y la transición energética. Cuad. De Estrateg. 2022, 209, 61–174. [Google Scholar]
- Ariza-Rodríguez, N.; Rodríguez-Navarro, A.B.; Calero de Hoces, M.; Martin, J.M.; Muñoz-Batista, M.J. Chemical and Mineralogical Characterization of Montevive Celestine Mineral. Minerals 2022, 12, 1261. [Google Scholar] [CrossRef]
- García-Veigas, J.; Rosell, L.; Cendón, D.I.; Gibert, L.; Martín, J.M.; Torres-Ruiz, J.; Ortí, F. Large celestine orebodies formed by early-diagenetic replacement of gypsified stromatolites (Upper Miocene, Montevive-Escúzar deposit, Granada Basin, Spain). Ore Geol. Rev. 2015, 64, 187–199. [Google Scholar] [CrossRef]
- Martin, J.M.; Ortega Huertas, M.; Torres Ruiz, J. Genesis and Evolution of Strontium Deposits of the Granada Basin (Southeastern Spain)—Evidence of Diagenetic Replacement of a Stromatolite Belt. Sediment. Geol. 1984, 39, 281–298. [Google Scholar] [CrossRef]
- Aslan, N.; Canbazoglu, M. Processing of Thinkener Underflow from Celestite Concentrator by Multi Gravity Separator. Ph.D. Thesis, Cumhuriyet University, Department of Mining Engineering, Sivas, Turkey, 1996. [Google Scholar]
- Bosman, J. The art and science of dense medium selection. J. South. Afr. Inst. Min. Metall. 2014, 114, 529–536. [Google Scholar]
- Caner Orhan, E.; Can, M.; Olgun, Z.; Özer, A. Performance Evaluation Practices at Dense Medium Separation Circuits.; Litvinenko, V., Ed.; Springer International Publishing: Cham, Switzerland, 2016; pp. 839–847. [Google Scholar]
- De Korte, G.J.; Engelbrecht, J. Dense Medium Cyclones. Int. J. Coal Prep. Util. 2014, 34, 49–58. [Google Scholar] [CrossRef]
- Gupta, A.; Yan, D. Mineral Processing Design and Operations, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; Available online: http://www.sciencedirect.com/science/article/pii/B9780444635891000277 (accessed on 19 April 2023).
- Ma, G.; Bu, X.; Xie, G.; Peng, Y.; Sha, J.; Xia, W.; Wu, E. Comparative Study of Separation Performance of a Spiral and Dense-medium Cyclone on Cleaning Coal. Int. J. Coal Prep. Util. 2021, 41, 108–116. [Google Scholar] [CrossRef]
- Napier-Munn, T. The dense medium cyclone—Past, present and future. Miner. Eng. 2018, 116, 107–113. [Google Scholar] [CrossRef]
- Silva, A.C.; Schons Silva, E.M.; Vieira Matos, J.D. Hydrocyclones simulation using a new modification in Plitt’s equation. IFAC Proc. Vol. 2013, 46, 12–17. [Google Scholar] [CrossRef]
- Umucu, Y. Investigation of separation performance of dense medium cyclone using computer simulation. Physicochem. Probl. Miner. Process. 2015, 51, 303–314. [Google Scholar] [CrossRef]
- Vakamalla, T.; Narasimha, M. Rheology-based CFD modeling of magnetite medium segregation in a dense medium cyclone. Powder Technol. 2015, 277, 275–286. [Google Scholar] [CrossRef]
- Wills, B.A.; Finch, J.A. Dense Medium Separation (DMS). In Wills’ Mineral Processing Technology, 8th ed.; Butterworth-Heinemann: Boston, MA, USA, 2016; 20p. [Google Scholar]
- Hernáinz, F.; Calero, M. The effect of the degree of grinding on the flotation of celestite ore. Adv. Powder Technol. 2001, 12, 481–491. [Google Scholar] [CrossRef]
- Ozkan, A.; Uebeyiay, H. Selective flocculation of celestite from celestite-calcite fines. Indian J. Chem. Technol. 2008, 15, 383–387. [Google Scholar]
- Cebeci, Y.; Ulusoy, U.; Erişen., M. Concentration of Celestite by Oil Agglomeration. In Proceedings of the 8th International Mineral Processing Symposium, Antalya, Turkey, 16–18 October 2000. [Google Scholar]
- Cebeci, Y.; Sönmez, İ. Investigation of spherical oil agglomeration properties of celestite. J. Colloid Interface Sci. 2004, 273, 198–204. Available online: https://www.sciencedirect.com/science/article/pii/S0021979703011391 (accessed on 1 May 2023). [CrossRef] [PubMed]
- Izerdem, D.; Orhan, E.C.; Ozcan, O.; Alpay, E. Application of density tracers in a dense medium circuit: A case study. Miner. Eng. 2018, 121, 39–46. [Google Scholar] [CrossRef]
- Magwai, M.K.; Bosman, J. The effect of cyclone geometry and operating conditions on spigot capacity of dense medium cyclones. Int. J. Miner. Process. 2008, 86, 94–103. [Google Scholar] [CrossRef]
- Marion, C.; Williams, H.; Langlois, R.; Kokkilic, O.; Coelho, F.; Awais, M.; Rowson, N.A.; Waters, K.E. The potential for dense medium separation of mineral fines using a laboratory Falcon Concentrator. Miner. Eng. 2017, 105, 7–9. [Google Scholar] [CrossRef][Green Version]
- Aktaş, Z.; Karacan, F.; Olcay, A. Centrifugal float–sink separation of fine Turkish coals in dense media. Fuel Process. Technol. 1998, 55, 235–250. [Google Scholar] [CrossRef]
- Klima, M.; Xu, D.; Cho, H. A Preliminary Investigation of Dense-Medium Centrifugation for Fine Coal Separations. In Metallurgy, and Exploration; Society for Mining: San Francisco, CA, USA, 1995; pp. 138–142. [Google Scholar]
- Rao, T.C.; Barnwal, J.P.; Govindarajan, B. Studies on a Vorsyl separator as an alternate for a dense medium cyclone. Int. J. Miner. Process. 1998, 53, 49–57. [Google Scholar] [CrossRef]
- Dou, D.; Zhou, D.; Yang, J. A new partition curve model of dense-medium cyclone based on process parameters. Int. J. Coal Prep. Util. 2020, 40, 459–472. [Google Scholar] [CrossRef]
- Shi, F. Determination of ferrosilicon medium rheology and stability. Miner. Eng. 2016, 98, 60–70. [Google Scholar] [CrossRef]
- Shi, F.N.; Napier-Munn, T. A model for slurry rheology. Int. J. Miner. Process. 1996, 47, 103–123. [Google Scholar] [CrossRef]
- Eriez. Available online: https://www.eriez.com/ (accessed on 22 November 2022).
- Geer, M.R.; Sokaski, M.; West, J.M.; Yancey, H.F. The Role of Viscosity in Dense-Medium Coal Cleaning; United States Department of the Interior, Bureau of Mines: Washington, DA, USA, 1957; Volume 5354. [Google Scholar]
- Klein, B. Rheology and Stability of Magnetite Dense Media. 1992. Available online: https://open.library.ubc.ca/collections/831/items/1.0081158 (accessed on 1 May 2023).
- Barnes, H.A.; Hutton, J.F.; Walters, K. (Eds.) Chapter 7—Rheology of Suspensions. In Rheology Series; Elsevier: Amsterdam, The Netherlands, 1989; Volume 3, pp. 115–139. Available online: https://www.sciencedirect.com/science/article/pii/B9780444874696500111 (accessed on 1 May 2023).
- Mabuza, N.T.; Pocock, J.; Loveday, B.K. The use of surface active chemicals in heavy medium viscosity reduction. Miner. Eng. 2005, 18, 25–31. Available online: https://www.sciencedirect.com/science/article/pii/S0892687504002183 (accessed on 1 May 2023). [CrossRef]
- Kirchberg, S.; Abdin, Y.; Ziegmann, G. Influence of particle shape and size on the wetting behavior of soft magnetic micropowders. Powder Technol. 2011, 207, 311–317. [Google Scholar] [CrossRef]
- Collins, B.; Napier-Munn, T.J.; Sciarone, M. The production, properties, and selection of ferrosilicon powders for heavy-medium separation. J. South. Afr. Inst. Min. Metall. 1974, 75, 103–115. [Google Scholar]
- Appel, I.; Behrens, S. Influence of the particle parameters on the stability of magnetic dopants in a ferrolyotropic suspension. J. Magn. Magn. Mater. 2017, 431, 49–53. [Google Scholar] [CrossRef]
- López-López, M.; Iglesias, G.; Durán, J.; González-Caballero, F. On the Stability of Magnetic Colloids. 2008, 63, 31–46. Available online: http://annales.umcs.lublin.pl/tt_p.php?rok=2008&tom=63&;sectio=AA&;numer_artykulu=04&;zeszyt=0 (accessed on 1 May 2023).
- Norton, I.T.; Spyropoulos, F.; Cox, P. (Eds.) Viscosity and Oscillatory Rheology; Wiley: Hoboken, NJ, USA, 2010; pp. 7–28. [Google Scholar] [CrossRef]
- Napier-Munn, T.; Scott, I.A. The effect of demagnetisation and ore contamination on the viscosity of the medium in a dense medium cyclone plant. Miner. Eng. 1990, 3, 607–613. [Google Scholar] [CrossRef]
- Menéndez, M.; Gent, M.; Toraño, J.; Diego, I. Optimization of multigravity separation for recovery of ultrafine coal. Min. Metall. Explor. 2007, 24, 253–263. [Google Scholar] [CrossRef]
- Yang, X.; Yang, G.; Liu, P.; Li, X.; Jiang, L.; Zhang, J. Study on the Desliming Performance of a Novel Hydrocyclone Sand Washer. Separations 2022, 9, 74. [Google Scholar] [CrossRef]
- Oats, W.J.; Ozdemir, O.; Nguyen, A.V. Effect of mechanical and chemical clay removals by hydrocyclone and dispersants on coal flotation. Miner. Eng. 2010, 23, 413–419. [Google Scholar] [CrossRef]
- Tao, Y.J.; Zhao, Y.N.; Xian, Y.S.; Song, A.; Wang, Y.P.; Shi, Z.X. Coal Desliming Using Annular Rinse Water Hydrocyclone; Trans Tech Publ.: Stafa-Zurich, Switzerland, 2020; pp. 28–40. [Google Scholar]
- Gajda, D.; Lutyński, M.; Kujawska, M. Substitution of Magnetite in Dense Medium Separation by Zinc-Lead Waste; IOP Publishing: London, UK, 2018; p. 012036. [Google Scholar]
- He, Y.B.; Laskowski, J.S. Effect of dense medium properties on the separation performance of a dense medium cyclone. Miner. Eng. 1994, 7, 209–221. [Google Scholar] [CrossRef]








| Sample | Celestine (%) | Stroncianite (%) | Quartz (%) | Dolomite (%) | Calcite (%) |
|---|---|---|---|---|---|
| 70 | 67.45 | 2.03 | 6.79 | 8.08 | 15.01 |
| 70D | 69.28 | 1.81 | 3.54 | 9.04 | 15.55 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariza-Rodríguez, N.; Rodríguez-Navarro, A.B.; de Hoces, M.C.; Muñoz-Batista, M.J. Laboratory-Scale Optimization of Celestine Concentration Using a Hydrocyclone System. Appl. Sci. 2023, 13, 10206. https://doi.org/10.3390/app131810206
Ariza-Rodríguez N, Rodríguez-Navarro AB, de Hoces MC, Muñoz-Batista MJ. Laboratory-Scale Optimization of Celestine Concentration Using a Hydrocyclone System. Applied Sciences. 2023; 13(18):10206. https://doi.org/10.3390/app131810206
Chicago/Turabian StyleAriza-Rodríguez, Noemi, Alejandro B. Rodríguez-Navarro, Mónica Calero de Hoces, and Mario J. Muñoz-Batista. 2023. "Laboratory-Scale Optimization of Celestine Concentration Using a Hydrocyclone System" Applied Sciences 13, no. 18: 10206. https://doi.org/10.3390/app131810206
APA StyleAriza-Rodríguez, N., Rodríguez-Navarro, A. B., de Hoces, M. C., & Muñoz-Batista, M. J. (2023). Laboratory-Scale Optimization of Celestine Concentration Using a Hydrocyclone System. Applied Sciences, 13(18), 10206. https://doi.org/10.3390/app131810206









