Extended High Frequency Thresholds and Their Relationship to Distortion Product Otoacoustic Emissions, Hearing Acuity, Age, Gender, Presence of Spontaneous Otoacoustic Emissions, and Side of Measurement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.3. Statistical Analysis
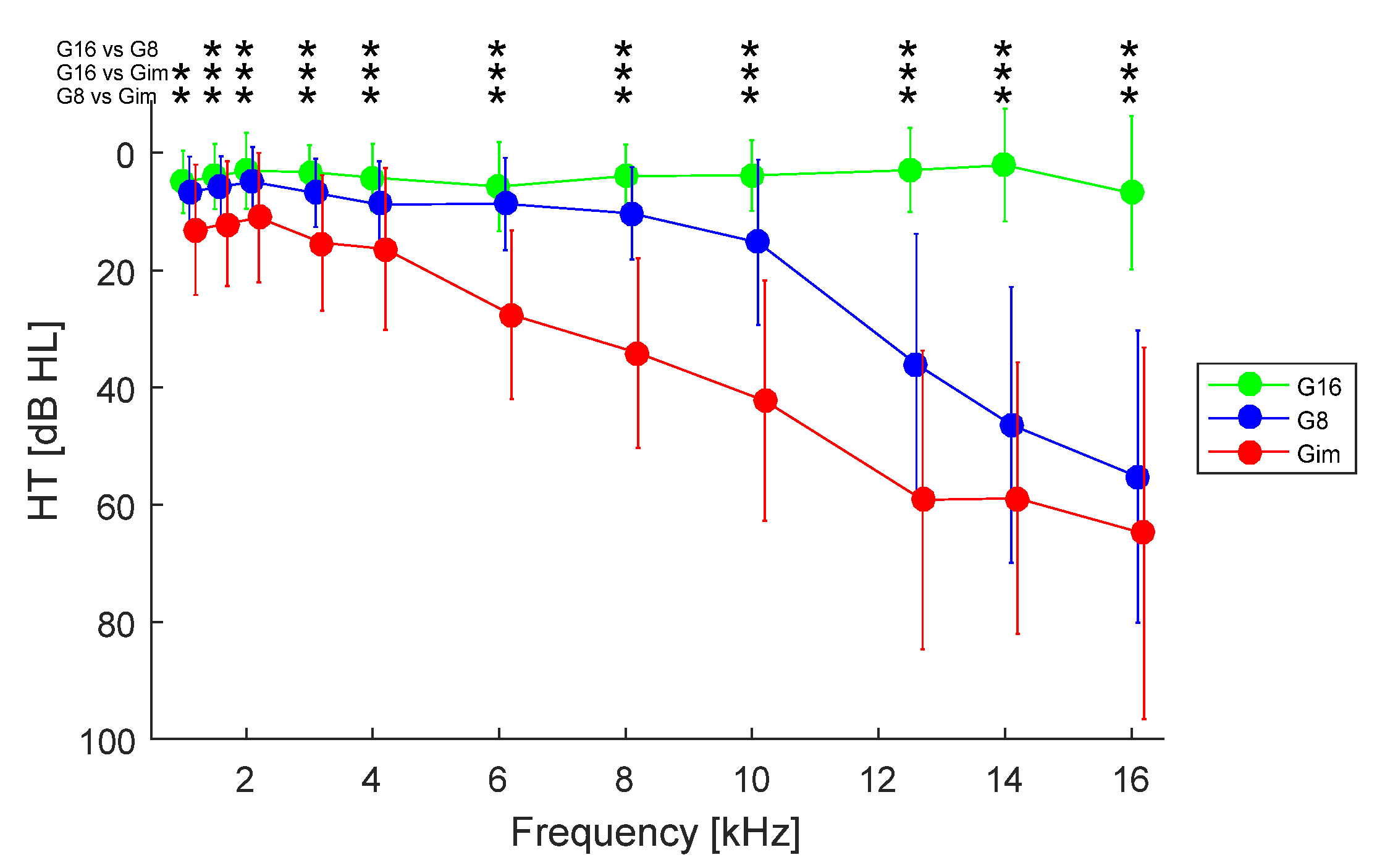
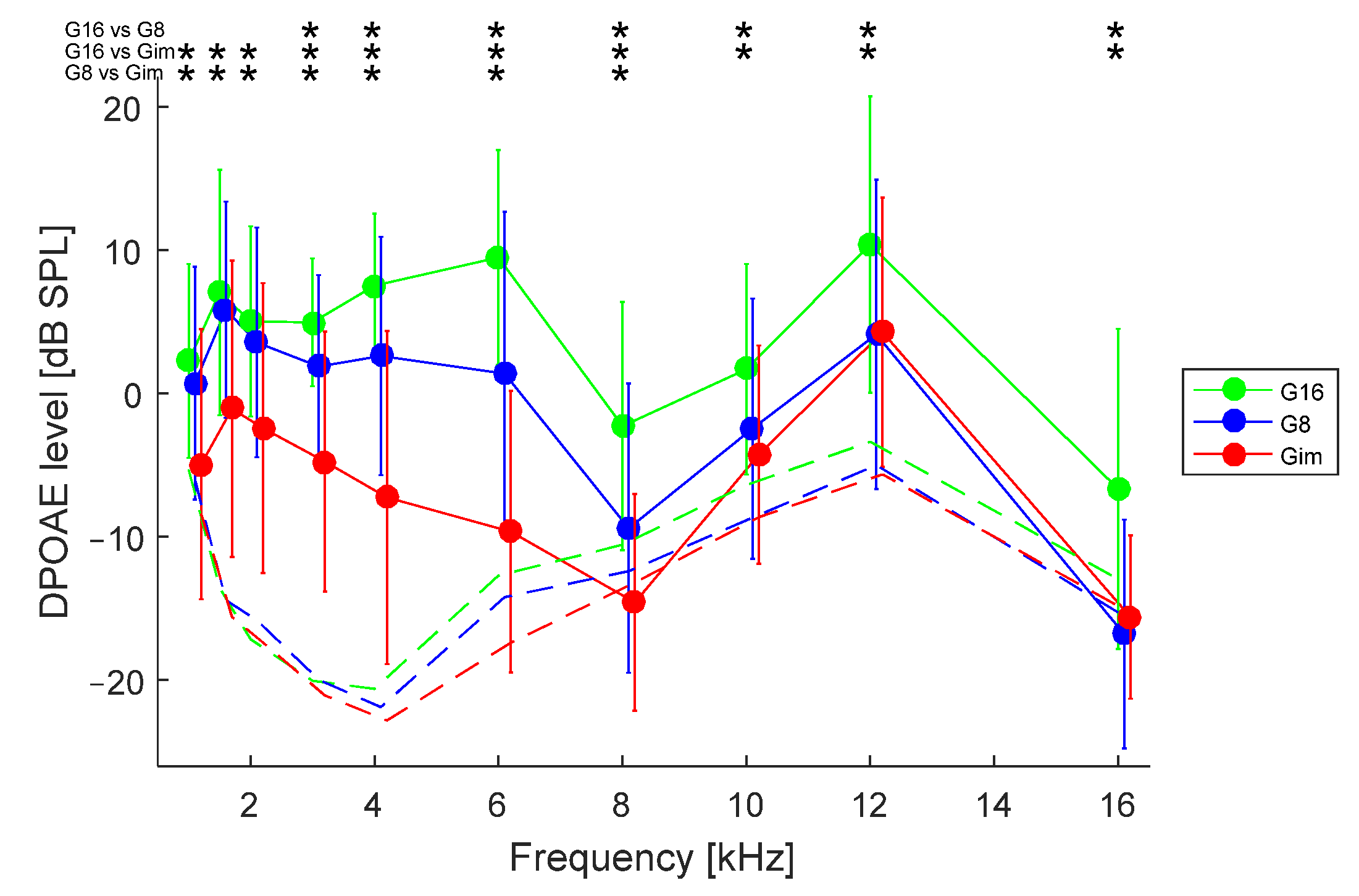
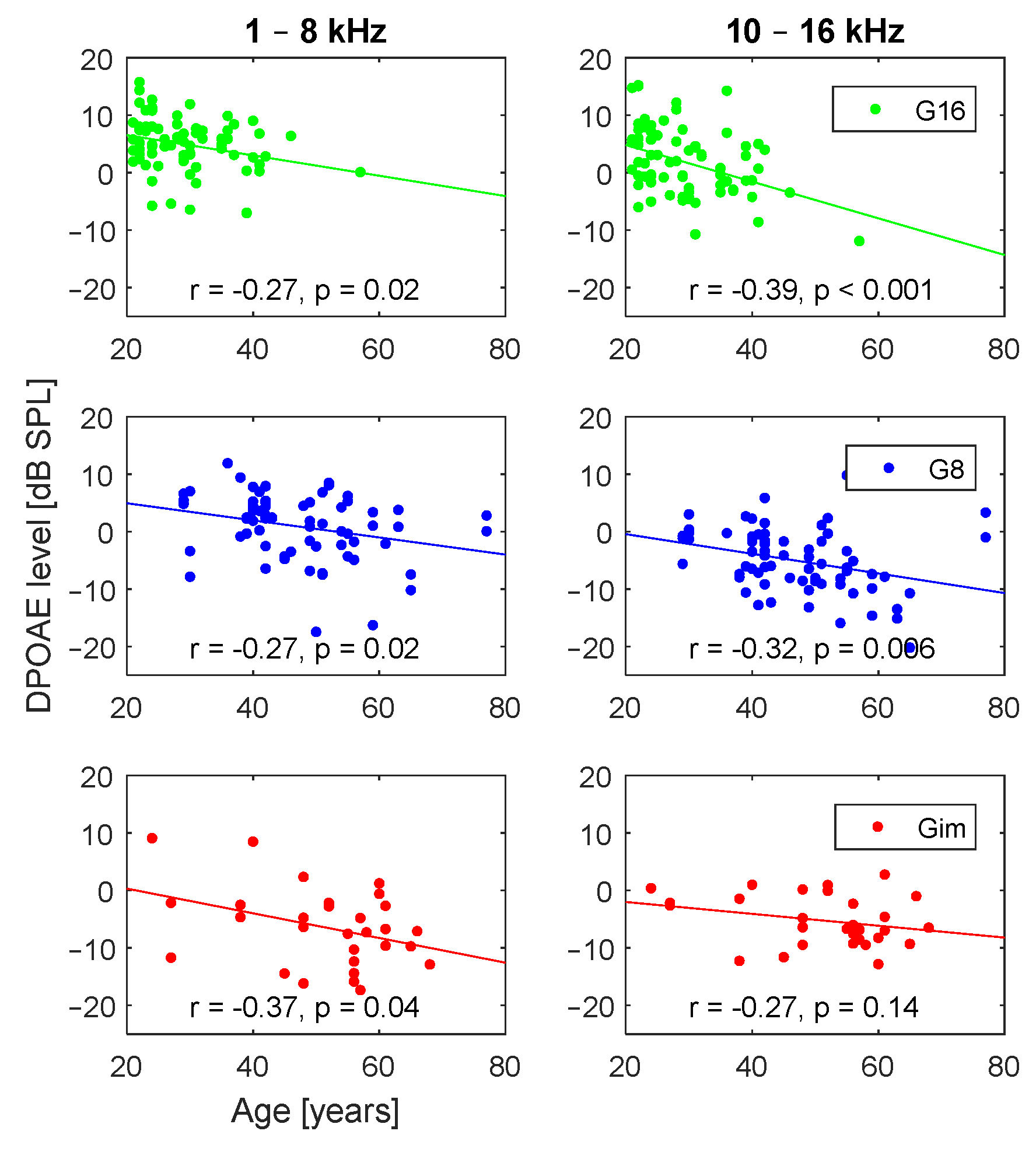
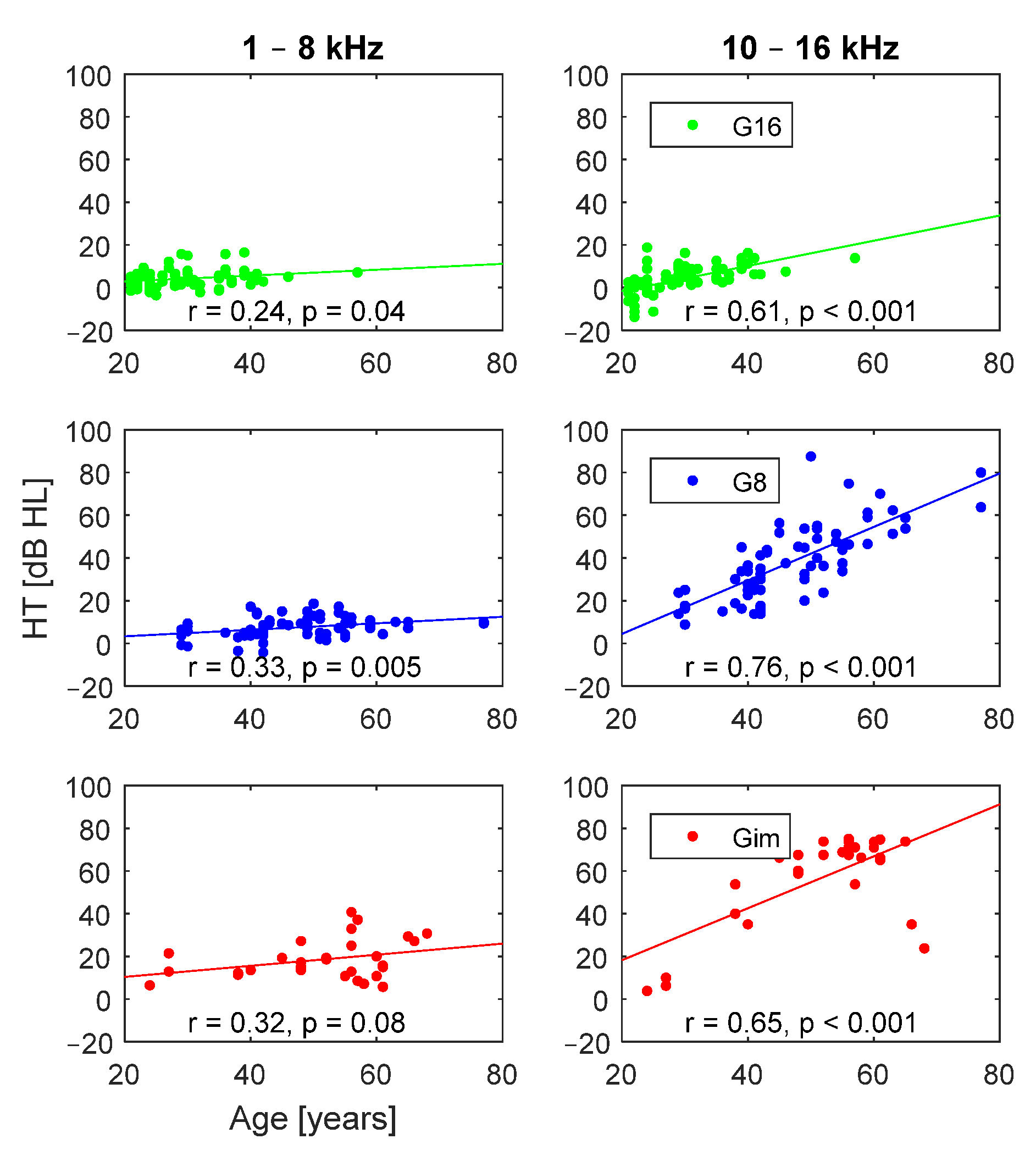
3. Results
3.1. Effect of Hearing Loss
3.2. Effect of Age
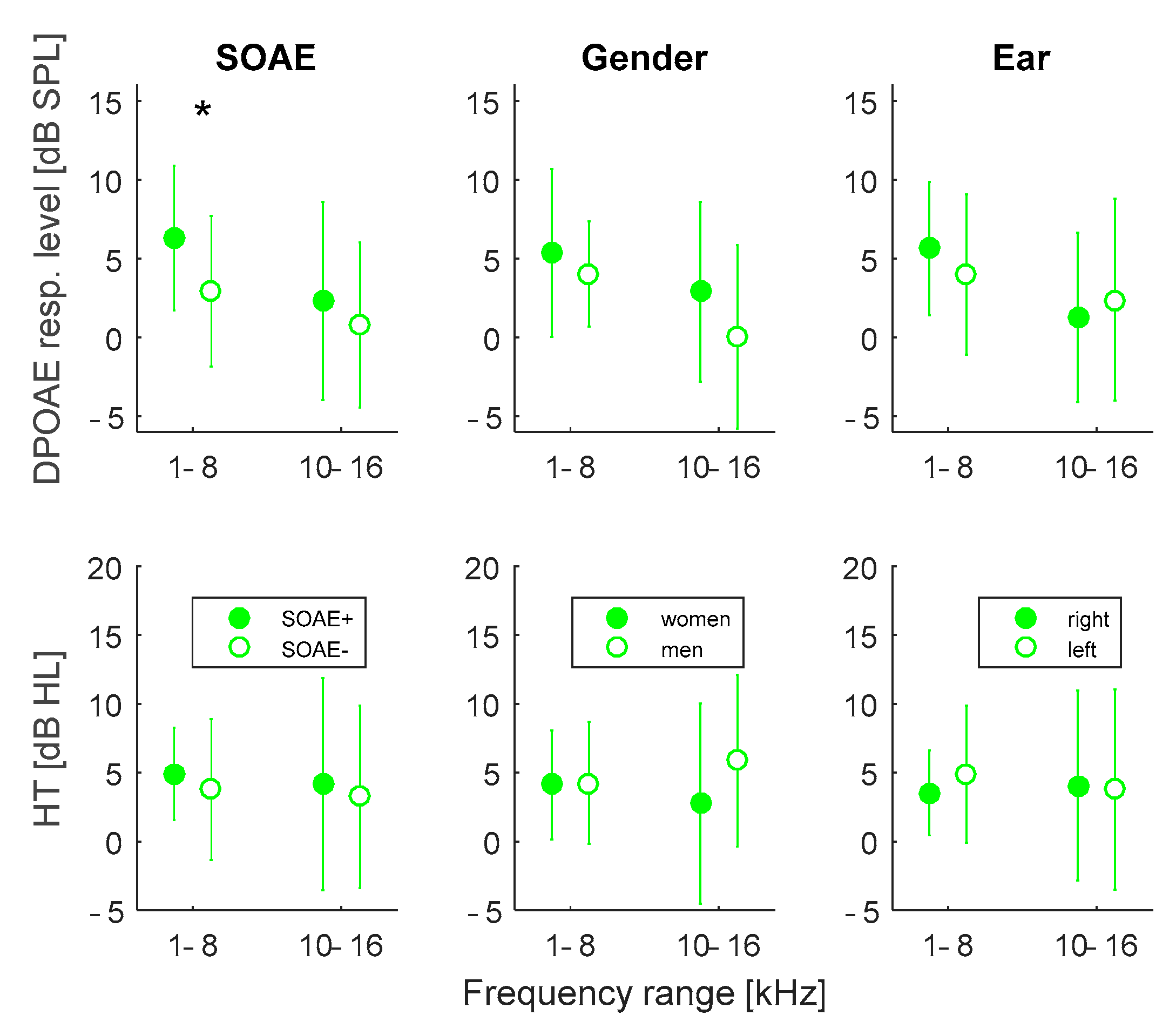
3.3. Effects of SOAE Presence, Gender, and Ear Side
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hunter, L.L.; Monson, B.B.; Moore, D.R.; Dhar, S.; Wright, B.A.; Munro, K.J.; Zadeh, L.M.; Blankenship, C.M.; Stiepan, S.M.; Siegel, J.H. Extended high frequency hearing and speech perception implications in adults and children. Hear. Res. 2020, 397, 107922. [Google Scholar] [CrossRef]
- Kemp, D.T. Stimulated acoustic emissions from within the human auditory system. J. Acoust. Soc. Am. 1978, 64, 1386–1391. [Google Scholar] [CrossRef]
- Kemp, D.T. Otoacoustic emissions, their origin in cochlear function, and use. Br. Med. Bull. 2002, 63, 223–241. [Google Scholar] [CrossRef]
- Job, A.; Raynal, M.; Kossowski, M.; Studler, M.; Ghernaouti, C.; Baffioni-venturi, A.; Roux, A.; Darolles, C.; Guelorget, A. Otoacoustic detection of risk of early hearing loss in ears with normal audiograms: A 3-year follow-up study. Hear. Res. 2009, 251, 10–16. [Google Scholar] [CrossRef]
- Hao, J.; Fu, X.; Zhang, C.; Zhang, X.; Zhao, S.; Li, Y. Early detection of hearing impairment in patients with diabetes mellitus with otoacoustic emission. A systematic review and meta-analysis. Acta Oto-Laryngol. 2016, 137, 179–185. [Google Scholar] [CrossRef]
- Glavin, C.C.; Siegel, J.; Dhar, S. Distortion Product Otoacoustic Emission (DPOAE) Growth in Aging Ears with Clinically Normal Behavioral Thresholds. J. Assoc. Res. Otolaryngol. 2021, 22, 659–680. [Google Scholar] [CrossRef]
- Paping, D.E.; van der Schroef, M.; Helleman, H.W.; Goedegebure, A.; Baatenburg de Jong, R.J.; Vroegop, J.L. Distortion Product Otoacoustic Emissions in Screening for Early Stages of High-frequency Hearing Loss in Adolescents. Noise Health 2022, 24, 20–26. [Google Scholar]
- Gorga, M.P.; Neely, S.T.; Bergman, B.M.; Beauchaine, K.L.; Kaminski, J.R.; Peters, J.; Schulte, L.; Jesteadt, W. A comparison of transient-evoked and distortion product otoacoustic emissions in normal-hearing and hearing-impaired subjects. J. Acoust. Soc. Am. 1993, 94, 2639–2648. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Kochanek, K.; Trzaskowski, B.; Pilka, E.; Skarzynski, P.H.; Skarzynski, H. Tone-Burst and Click-Evoked Otoacoustic Emissions in Subjects with Hearing Loss Above 0.25, 0.5, and 1 kHz. Ear Hear. 2012, 33, 757–767. [Google Scholar] [CrossRef]
- Zhang, V.W.; McPherson, B.; Shi, B.X.; Tang, J.L.; Wong, B.Y. Neonatal hearing screening: A combined click evoked and tone burst otoacoustic emission approach. Int. J. Pediatr. Otorhinolaryngol. 2008, 72, 351–360. [Google Scholar] [CrossRef]
- Arnold, D.J.; Lonsbury-Martin, B.L.; Martin, G.K. High-Frequency Hearing Influences Lower-Frequency Distortion-Product Otoacoustic Emissions. Arch. Otolaryngol. Neck Surg. 1999, 125, 215. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.; Lapsley Miller, J.A.; Heller, L.M. Distortion-Product Otoacoustic Emissions as a Screening Tool for Noise-Induced Hearing Loss. Noise Health 2001, 3, 43–60. [Google Scholar] [PubMed]
- Kapoor, N.; Mani, K.V.; Shukla, M. Distortion product oto-acoustic emission: A superior tool for hearing assessment than pure tone audiometry. Noise Health 2019, 21, 164–168. [Google Scholar]
- Mishra, S.K.; Saxena, U.; Rodrigo, H. Extended High-frequency Hearing Impairment Despite a Normal Audiogram: Relation to Early Aging, Speech-in-noise Perception, Cochlear Function, and Routine Earphone Use. Ear Hear. 2022, 43, 822–835. [Google Scholar] [CrossRef]
- Mishra, S.K.; Saxena, U.; Rodrigo, H. Hearing Impairment in the Extended High Frequencies in Children Despite Clinically Normal Hearing. Ear Hear. 2022, 43, 1653–1660. [Google Scholar] [CrossRef]
- Schmuziger, N.; Probst, R.; Smurzynski, J. Otoacoustic emissions and extended high-frequency hearing sensitivity in young adults Emisiones otoacústicas y sensibilidad extendida a frecuencias altas en adultos jóvenes. Int. J. Audiol. 2005, 44, 24–30. [Google Scholar] [CrossRef]
- Al-Malky, G.; Suri, R.; Dawson, S.J.; Sirimanna, T.; Kemp, D. Aminoglycoside antibiotics cochleotoxicity in paediatric cystic fibrosis (CF) patients: A study using extended high-frequency audiometry and distortion product otoacoustic emissions. Int. J. Audiol. 2011, 50, 112–122. [Google Scholar] [CrossRef]
- Mepani, A.M.; Kirk, S.A.; Hancock, K.E.; Bennett, K.; de Gruttola, V.; Liberman, M.C.; Maison, S.F. Middle Ear Muscle Reflex and Word Recognition in “Normal-Hearing” Adults. Ear Hear. 2020, 41, 25–38. [Google Scholar] [CrossRef]
- Gravel, J.S.; Roberts, J.E.; Roush, J.; Grose, J.; Besing, J.; Burchinal, M.; Neebe, E.; Wallace, I.F.; Zeisel, S. Early Otitis Media with Effusion, Hearing Loss, and Auditory Processes at School Age. Ear Hear. 2006, 27, 353–368. [Google Scholar] [CrossRef]
- Probst, R.; Lonsbury-Martin, B.L.; Martin, G.K. A review of otoacoustic emissions. J. Acoust. Soc. Am. 1991, 89, 2027–2067. [Google Scholar] [CrossRef]
- Kuroda, T. Clinical investigation on spontaneous otoacoustic emission (SOAE) in 447 ears. Auris Nasus Larynx 2007, 34, 29–38. [Google Scholar] [CrossRef]
- Penner, M.J.; Zhang, T. Prevalence of spontaneous otoacoustic emissions in adults revisited. Hear. Res. 1997, 103, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, W.W.; Blinowska, K.J.; Kochanek, K.; Skarzynski, H. Synchronized spontaneous otoacoustic emissions analyzed in a time-frequency domain. J. Acoust. Soc. Am. 2008, 124, 3720–3729. [Google Scholar] [CrossRef]
- Kulawiec, J.T.; Orlando, M.S. The Contribution of Spontaneous Otoacoustic Emissions to the Click Evoked Otoacoustic Emissions. Ear Hear. 1995, 16, 515–520. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Koziel, M.; Kochanek, K.; Skarzynski, H. Otoacoustic Emissions in Smoking and Nonsmoking Young Adults. Clin. Exp. Otorhinolaryngol. 2015, 8, 303. [Google Scholar] [CrossRef]
- Kuroda, T.; Fukuda, S.; Chida, E.; Kashiwamura, M.; Matsumura, M.; Ohwatari, R.; Inuyama, Y. Effects of spontaneous otoacoustic emis-sions on distortion product otoacoustic emission. Auris Nasus Larynx. 2001, 28, 33–38. [Google Scholar] [CrossRef]
- McFadden, D.; Mishra, R. On the relation between hearing sensitivity and otoacoustic emissions. Hear. Res. 1993, 71, 208–213. [Google Scholar] [CrossRef]
- Baiduc, R.R.; Lee, J.; Dhar, S. Spontaneous otoacoustic emissions, threshold microstructure, and psychophysical tuning over a wide frequency range in humans. J. Acoust. Soc. Am. 2014, 135, 300–314. [Google Scholar] [CrossRef]
- Dreisbach, L.E.; Siegel, J.H. Distortion-product otoacoustic emissions measured at high frequencies in humans. J. Acoust. Soc. Am. 2001, 110, 2456–2469. [Google Scholar] [CrossRef]
- Hecker, D.J.; Lohscheller, J.; Bader, C.A.; Delb, W.; Schick, B.; Dlugaiczyk, J. A new method to analyze distortion product otoacoustic emissions (DPOAEs) in the high-frequency range up to 18 kHz using windowed periodograms. IEEE Trans. Biomed. Eng. 2011, 58, 2369–2377. [Google Scholar] [CrossRef]
- Keefe, D.H.; Goodman, S.S.; Ellison, J.C.; Fitzpatrick, D.F.; Gorga, M.P. Detecting high-frequency hearing loss with click-evoked otoacoustic emissions. J. Acoust. Soc. Am. 2011, 129, 245–261. [Google Scholar] [CrossRef]
- Poling, G.L.; Siegel, J.H.; Lee, J.; Lee, J.; Dhar, S. Characteristics of the 2f1-f2 distortion product otoacoustic emission in a normal hearing population. J. Acoust. Soc. Am. 2014, 135, 287–299. [Google Scholar] [CrossRef]
- Poling, G.L.; Vlosich, B.; Dreisbach, L.E. Emerging Distortion Product Otoacoustic Emission Techniques to Identify Preclinical Warning Signs of Basal Cochlear Dysfunction Due to Ototoxicity. Appl. Sci. 2019, 9, 3132. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Pilka, E.; Ganc, M.; Kochanek, K.; Skarzynski, H. Ultra-High Frequency Distortion Product Otoacoustic Emissions for Detection of Hearing Loss and Tinnitus. Int. J. Environ. Res. Public Health 2022, 19, 2123. [Google Scholar] [CrossRef]
- Ratner, B. The correlation coefficient: Its values range between +1/−1, or do they? J. Target. Meas. Anal. Mark. 2009, 17, 139–142. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Bramhall, N.F.; McMillan, G.P.; Mashburn, A.N. Subclinical Auditory Dysfunction: Relationship Between Distortion Product Otoacoustic Emissions and the Audiogram. Am. J. Audiol. 2021, 30, 854–869. [Google Scholar] [CrossRef]
- Dreisbach, L.E.; Torre III, P.; Kramer, S.J.; Kopke, R.; Jackson, R.; Balough, B. Influence of Ultrahigh-Frequency Hearing Thresholds on Distortion-Product Otoacoustic Emission Levels at Conventional Frequencies. J. Am. Acad. Audiol. 2008, 19, 325–336. [Google Scholar] [CrossRef]
- Yeend, I.; Beach, E.F.; Sharma, M.; Dillon, H. The effects of noise exposure and musical training on suprathreshold auditory processing and speech perception in noise. Hear. Res. 2017, 353, 224–236. [Google Scholar] [CrossRef]
- Aziz, A.; Md Daud, M.K.; Nik Othman, N.A.; Abd Rahman, N. Early Detection of High-frequency Presbycusis Among Normal Hearing Individuals. Otol. Neurotol. 2020, 41, e989–e992. [Google Scholar] [CrossRef]
- Engdahl, B. Otoacoustic Emissions in the General Adult Population of Nord-Trondelag, Norway: I. Distributions by Age, Gender, and Ear Side. Int. J. Audiol. 2002, 41, 64–77. [Google Scholar] [CrossRef]
- Robinette, M.S. Clinical Observations with Evoked Otoacoustic Emissions at Mayo Clinic. J. Am. Acad. Audiol. 2003, 14, 213–224. [Google Scholar] [CrossRef]
- Cacace, A.T.; McClelland, W.A.; Weiner, J.; McFarland, D.J. Individual differences and the reliability of 2F1-F2 distortion-product otoacoustic emissions: Effects of time-of-day, stimulus variables, and gender. J. Speech Hear. Res. 1996, 39, 1138–1148. [Google Scholar] [CrossRef]
- Dunckley, K.T.; Dreisbach, L.E. Gender effects on high frequency distortion product otoacoustic emissions in humans. Ear Hear. 2004, 25, 554–564. [Google Scholar] [CrossRef]
- Avan, P.; Elbez, M.; Bonfils, P. Click-evoked otoacoustic emissions and the influence of high-frequency hearing losses in humans. J. Acoust. Soc. Am. 1997, 101, 2771–2777. [Google Scholar] [CrossRef]
- Souza, N.N.; Dhar, S.; Neely, S.T.; Siegel, J.H. Comparison of nine methods to estimate ear-canal stimulus levels. J. Acoust. Soc. Am. 2014, 136, 1768–1787. [Google Scholar] [CrossRef]
- Jedrzejczak, W.W.; Gos, E.; Pilka, E.; Skarzynski, P.H.; Skarzynski, H.; Hatzopoulos, S. Pitfalls in the Detection of Hearing Loss via Otoacoustic Emissions. Appl. Sci. 2021, 11, 2184. [Google Scholar] [CrossRef]
- Trzaskowski, B.; Jędrzejczak, W.W.; Piłka, E.; Cieślicka, M.; Skarżyński, H. Otoacoustic Emissions before and after Listening to Music on a Personal Player. Med. Sci. Monit. 2014, 20, 1426–1431. [Google Scholar] [CrossRef]
- Dudarewicz, A.; Zamojska-Daniszewska, M.; Zaborowski, K.; Pawlaczyk-Łuszczyńska, M. Hearing status of people occupationally exposed to ultrasonic noise. Int. J. Occup. Med. Environ. Health 2022, 35, 309–325. [Google Scholar] [CrossRef]

| DPOAE 1–8 kHz | DPOAE 10–16 kHz | Age | |
|---|---|---|---|
| HT 1–8 kHz | −0.31 *** | −0.05 | 0.24 ** |
| HT 10–16 kHz | −0.22 ** | −0.17 * | 0.78 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jedrzejczak, W.W.; Pilka, E.; Pastucha, M.; Kochanek, K.; Skarzynski, H. Extended High Frequency Thresholds and Their Relationship to Distortion Product Otoacoustic Emissions, Hearing Acuity, Age, Gender, Presence of Spontaneous Otoacoustic Emissions, and Side of Measurement. Appl. Sci. 2023, 13, 10311. https://doi.org/10.3390/app131810311
Jedrzejczak WW, Pilka E, Pastucha M, Kochanek K, Skarzynski H. Extended High Frequency Thresholds and Their Relationship to Distortion Product Otoacoustic Emissions, Hearing Acuity, Age, Gender, Presence of Spontaneous Otoacoustic Emissions, and Side of Measurement. Applied Sciences. 2023; 13(18):10311. https://doi.org/10.3390/app131810311
Chicago/Turabian StyleJedrzejczak, W. Wiktor, Edyta Pilka, Malgorzata Pastucha, Krzysztof Kochanek, and Henryk Skarzynski. 2023. "Extended High Frequency Thresholds and Their Relationship to Distortion Product Otoacoustic Emissions, Hearing Acuity, Age, Gender, Presence of Spontaneous Otoacoustic Emissions, and Side of Measurement" Applied Sciences 13, no. 18: 10311. https://doi.org/10.3390/app131810311








