Applications of Tumor Cells in an In Vitro 3D Environment
Abstract
1. Introduction
2. 2D vs. 3D Cell Cultures
3. Scaffolds and Matrices
4. Organoids
5. Co-Culture Systems
6. Microfluidic Organ-on-a-Chip-Based Cell Culture Devices
7. Applications of 3D Models of Cancer Cells
7.1. Colorectal Cancer
7.2. Prostate Cancer
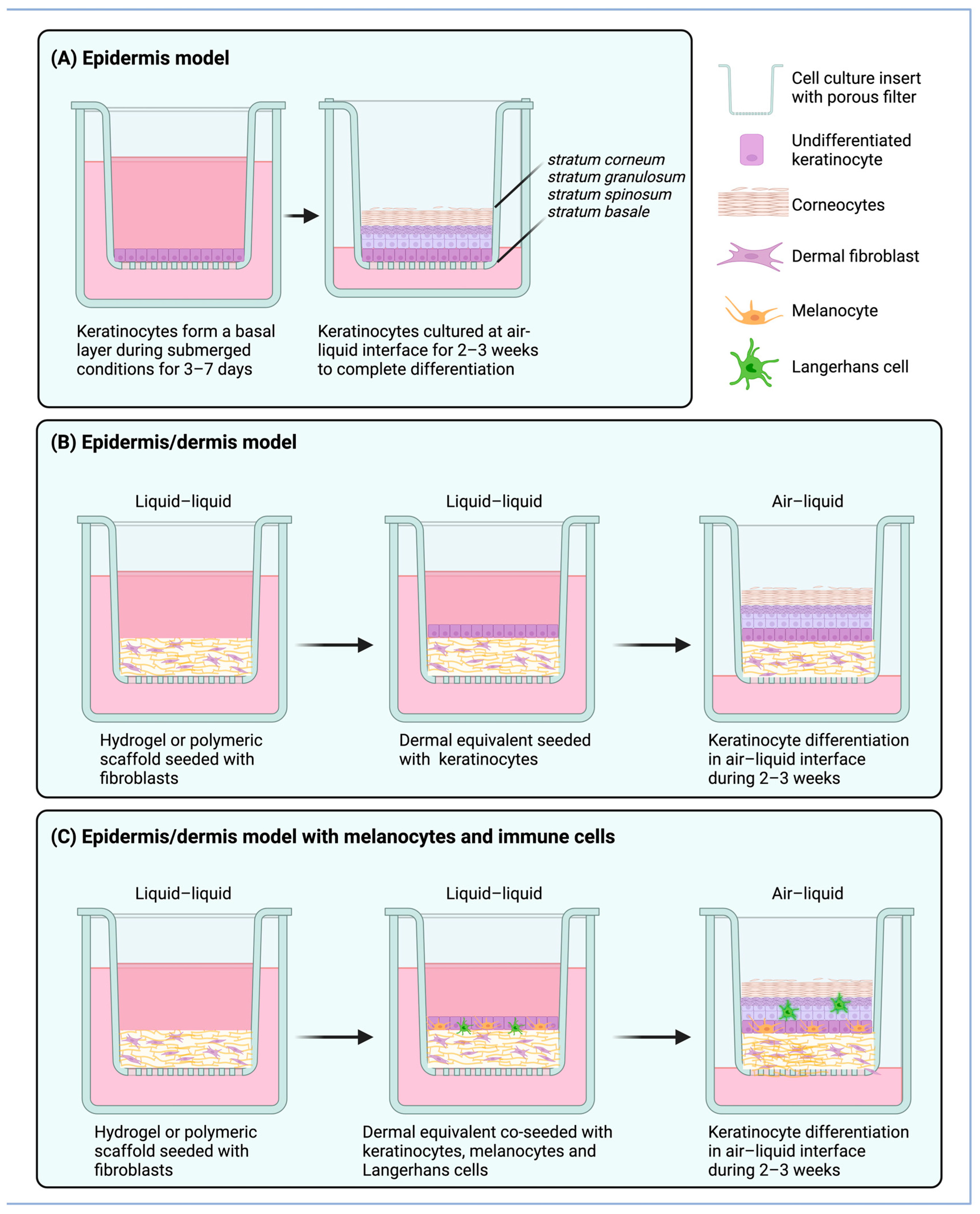
7.3. ALI Systems of the Skin
7.4. Melanocytes
7.5. Immune Cells
7.6. The Vascular System
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Badr-Eldin, S.M.; Aldawsari, H.M.; Kotta, S.; Deb, P.K.; Venugopala, K.N. Three-Dimensional In Vitro Cell Culture Models for Efficient Drug Discovery: Progress So Far and Future Prospects. Pharmaceuticals 2022, 15, 926. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef]
- Thoma, C.R.; Zimmermann, M.; Agarkova, I.; Kelm, J.M.; Krek, W. 3D cell culture systems modeling tumor growth determinants in cancer target discovery. Adv. Drug Deliv. Rev. 2014, 69, 29–41. [Google Scholar] [CrossRef]
- Zhang, L.; Liao, W.; Chen, S.; Chen, Y.; Cheng, P.; Lu, X.; Ma, Y. Towards a New 3Rs Era in the construction of 3D cell culture models simulating tumor microenvironment. Front. Oncol. 2023, 13, 1146477. [Google Scholar] [CrossRef]
- Yoon, P.S.; Del Piccolo, N.; Shirure, V.S.; Peng, Y.; Kirane, A.; Canter, R.J.; Fields, R.C.; George, S.C.; Gholami, S. Advances in modeling the immune microenvironment of colorectal cancer. Front. Immunol. 2021, 11, 614300. [Google Scholar] [CrossRef]
- Naba, A.; Clauser, K.R.; Whittaker, C.A.; Carr, S.A.; Tanabe, K.K.; Hynes, R.O. Extracellular matrix signatures of human primary metastatic colon cancers and their metastases to liver. BMC Cancer 2014, 14, 518. [Google Scholar] [CrossRef]
- Poltavets, V.; Kochetkova, M.; Pitson, S.M.; Samuel, M.S. The role of the extracellular matrix and its molecular and cellular regulators in cancer cell plasticity. Front. Oncol. 2018, 8, 431. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D cell culture systems: Tumor application, advantages, and disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Li, Z.-L.; Wang, Z.-J.; Wei, G.-H.; Yang, Y.; Wang, X.-W. Changes in extracellular matrix in different stages of colorectal cancer and their effects on proliferation of cancer cells. World J. Gastrointest. Oncol. 2020, 12, 267. [Google Scholar] [CrossRef]
- Chandra, R.; Karalis, J.D.; Liu, C.; Murimwa, G.Z.; Voth Park, J.; Heid, C.A.; Reznik, S.I.; Huang, E.; Minna, J.D.; Brekken, R.A. The colorectal cancer tumor microenvironment and its impact on liver and lung metastasis. Cancers 2021, 13, 6206. [Google Scholar] [CrossRef]
- Kapałczyńska, M.; Kolenda, T.; Przybyła, W.; Zajączkowska, M.; Teresiak, A.; Filas, V.; Ibbs, M.; Bliźniak, R.; Łuczewski, Ł.; Lamperska, K. 2D and 3D cell cultures–a comparison of different types of cancer cell cultures. Arch. Med. Sci. 2018, 14, 910–919. [Google Scholar] [CrossRef]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-dimensional in vitro culture models in oncology research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling physiological events in 2D vs. 3D cell culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef]
- Reidy, E.; Leonard, N.A.; Treacy, O.; Ryan, A.E. A 3D view of colorectal cancer models in predicting therapeutic responses and resistance. Cancers 2021, 13, 227. [Google Scholar] [CrossRef]
- Fontana, F.; Raimondi, M.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. Three-dimensional cell cultures as an in vitro tool for prostate cancer modeling and drug discovery. Int. J. Mol. Sci. 2020, 21, 6806. [Google Scholar] [CrossRef]
- Anthon, S.G.; Valente, K.P. Vascularization Strategies in 3D Cell Culture Models: From Scaffold-Free Models to 3D Bioprinting. Int. J. Mol. Sci. 2022, 23, 14582. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef]
- Bray, L.J.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C. Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 2015, 53, 609–620. [Google Scholar] [CrossRef]
- Worthington, P.; Pochan, D.J.; Langhans, S.A. Peptide hydrogels–versatile matrices for 3D cell culture in cancer medicine. Front. Oncol. 2015, 5, 92. [Google Scholar] [CrossRef]
- Benton, G.; Kleinman, H.K.; George, J.; Arnaoutova, I. Multiple uses of basement membrane-like matrix (BME/Matrigel) in vitro and in vivo with cancer cells. Int. J. Cancer 2011, 128, 1751–1757. [Google Scholar] [CrossRef]
- Marchini, A.; Gelain, F. Synthetic scaffolds for 3D cell cultures and organoids: Applications in regenerative medicine. Crit. Rev. Biotechnol. 2022, 42, 468–486. [Google Scholar] [CrossRef] [PubMed]
- Johansson, U.; Widhe, M.; Shalaly, N.D.; Arregui, I.L.; Nilebäck, L.; Tasiopoulos, C.P.; Åstrand, C.; Berggren, P.-O.; Gasser, C.; Hedhammar, M. Assembly of functionalized silk together with cells to obtain proliferative 3D cultures integrated in a network of ECM-like microfibers. Sci. Rep. 2019, 9, 6291. [Google Scholar] [CrossRef] [PubMed]
- Widhe, M.; Shalaly, N.D.; Hedhammar, M. A fibronectin mimetic motif improves integrin mediated cell biding to recombinant spider silk matrices. Biomaterials 2016, 74, 256–266. [Google Scholar] [CrossRef] [PubMed]
- Tasiopoulos, C.P.; Widhe, M.; Hedhammar, M. Recombinant spider silk functionalized with a motif from fibronectin mediates cell adhesion and growth on polymeric substrates by entrapping cells during self-assembly. ACS Appl. Mater. Interfaces 2018, 10, 14531–14539. [Google Scholar] [CrossRef]
- Åstrand, C.; Chotteau, V.; Falk, A.; Hedhammar, M. Assembly of FN-silk with laminin-521 to integrate hPSCs into a three-dimensional culture for neural differentiation. Biomater. Sci. 2020, 8, 2514–2525. [Google Scholar] [CrossRef]
- Gustafsson, L.; Tasiopoulos, C.P.; Jansson, R.; Kvick, M.; Duursma, T.; Gasser, T.C.; van der Wijngaart, W.; Hedhammar, M. Recombinant spider silk forms tough and elastic nanomembranes that are protein-permeable and support cell attachment and growth. Adv. Funct. Mater. 2020, 30, 2002982. [Google Scholar] [CrossRef]
- Tasiopoulos, C.P.; Gustafsson, L.; van der Wijngaart, W.; Hedhammar, M. Fibrillar nanomembranes of recombinant spider silk protein support cell Co-culture in an in vitro blood vessel wall model. ACS Biomater. Sci. Eng. 2021, 7, 3332–3339. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Simian, M.; Bissell, M.J. Organoids: A historical perspective of thinking in three dimensions. J. Cell Biol. 2017, 216, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Koo, B.-K.; Knoblich, J.A. Human organoids: Model systems for human biology and medicine. Nat. Rev. Mol. Cell Biol. 2020, 21, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Mitrakas, A.G.; Tsolou, A.; Didaskalou, S.; Karkaletsou, L.; Efstathiou, C.; Eftalitsidis, E.; Marmanis, K.; Koffa, M. Applications and Advances of Multicellular Tumor Spheroids: Challenges in Their Development and Analysis. Int. J. Mol. Sci. 2023, 24, 6949. [Google Scholar] [CrossRef]
- El-Schich, Z. Digital holographic microscopy: A noninvasive method to analyze the formation of spheroids. BioTechniques 2021, 71, 598–603. [Google Scholar] [CrossRef]
- Cacace, T.; Bianco, V.; Ferraro, P. Quantitative phase imaging trends in biomedical applications. Opt. Lasers Eng. 2020, 135, 106188. [Google Scholar] [CrossRef]
- Balasubramani, V.; Kujawińska, M.; Allier, C.; Anand, V.; Cheng, C.-J.; Depeursinge, C.; Hai, N.; Juodkazis, S.; Kalkman, J.; Kuś, A. Roadmap on digital holography-based quantitative phase imaging. J. Imaging 2021, 7, 252. [Google Scholar] [CrossRef]
- Nguyen, T.; Pradeep, S.; Judson-Torres, R.; Reed, J.; Teitell, M.; Zangle, T. Quantitative phase imaging: Recent advances and expanding potential in biomedicine. ACS Nano 2022, 16, 11516. [Google Scholar] [CrossRef] [PubMed]
- Hellesvik, M.; Øye, H.; Aksnes, H. Exploiting the potential of commercial digital holographic microscopy by combining it with 3D matrix cell culture assays. Sci. Rep. 2020, 10, 14680. [Google Scholar] [CrossRef] [PubMed]
- Shannon, A.E.; Boos, C.E.; Hummon, A.B. Co-culturing multicellular tumor models: Modeling the tumor microenvironment and analysis techniques. Proteomics 2021, 21, 2000103. [Google Scholar] [CrossRef]
- Marconi, A.; Quadri, M.; Saltari, A.; Pincelli, C. Progress in melanoma modelling in vitro. Exp. Dermatol. 2018, 27, 578–586. [Google Scholar] [CrossRef]
- Sahai, E.; Astsaturov, I.; Cukierman, E.; DeNardo, D.G.; Egeblad, M.; Evans, R.M.; Fearon, D.; Greten, F.R.; Hingorani, S.R.; Hunter, T. A framework for advancing our understanding of cancer-associated fibroblasts. Nat. Rev. Cancer 2020, 20, 174–186. [Google Scholar] [CrossRef]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [PubMed]
- Shiga, K.; Hara, M.; Nagasaki, T.; Sato, T.; Takahashi, H.; Takeyama, H. Cancer-associated fibroblasts: Their characteristics and their roles in tumor growth. Cancers 2015, 7, 2443–2458. [Google Scholar] [CrossRef] [PubMed]
- Bauleth-Ramos, T.; Feijão, T.; Gonçalves, A.; Shahbazi, M.-A.; Liu, Z.; Barrias, C.; Oliveira, M.J.; Granja, P.; Santos, H.A.; Sarmento, B. Colorectal cancer triple co-culture spheroid model to assess the biocompatibility and anticancer properties of polymeric nanoparticles. J. Control. Release 2020, 323, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Anseth, K.S. Recent advances in 3D models of tumor invasion. Curr. Opin. Biomed. Eng. 2021, 19, 100310. [Google Scholar] [CrossRef]
- Hakim, M.; Kermanshah, L.; Abouali, H.; Hashemi, H.M.; Yari, A.; Khorasheh, F.; Alemzadeh, I.; Vossoughi, M. Unraveling Cancer Metastatic Cascade Using Microfluidics-based Technologies. Biophys. Rev. 2022, 14, 517–543. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Ciardiello, D.; Vitiello, P.P.; Cardone, C.; Martini, G.; Troiani, T.; Martinelli, E.; Ciardiello, F. Immunotherapy of colorectal cancer: Challenges for therapeutic efficacy. Cancer Treat. Rev. 2019, 76, 22–32. [Google Scholar] [CrossRef]
- Biller, L.H.; Schrag, D. Diagnosis and treatment of metastatic colorectal cancer: A review. Jama 2021, 325, 669–685. [Google Scholar]
- Johnson, H.; El-Schich, Z.; Ali, A.; Zhang, X.; Simoulis, A.; Wingren, A.G.; Persson, J.L. Gene-Mutation-Based Algorithm for Prediction of Treatment Response in Colorectal Cancer Patients. Cancers 2022, 14, 2045. [Google Scholar] [CrossRef]
- Guinney, J.; Dienstmann, R.; Wang, X.; De Reynies, A.; Schlicker, A.; Soneson, C.; Marisa, L.; Roepman, P.; Nyamundanda, G.; Angelino, P. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015, 21, 1350–1356. [Google Scholar] [CrossRef] [PubMed]
- Janes, M.R.; Zhang, J.; Li, L.-S.; Hansen, R.; Peters, U.; Guo, X.; Chen, Y.; Babbar, A.; Firdaus, S.J.; Darjania, L. Targeting KRAS mutant cancers with a covalent G12C-specific inhibitor. Cell 2018, 172, 578–589.e517. [Google Scholar] [CrossRef]
- Feist, P.E.; Sun, L.; Liu, X.; Dovichi, N.J.; Hummon, A.B. Bottom-up proteomic analysis of single HCT 116 colon carcinoma multicellular spheroids. Rapid Commun. Mass Spectrom. 2015, 29, 654–658. [Google Scholar] [CrossRef] [PubMed]
- McMahon, K.M.; Volpato, M.; Chi, H.; Musiwaro, P.; Poterlowicz, K.; Peng, Y.; Scally, A.J.; Patterson, L.H.; Phillips, R.M.; Sutton, C.W. Characterization of changes in the proteome in different regions of 3D multicell tumor spheroids. J. Proteome Res. 2012, 11, 2863–2875. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak, A.; Szaryńska, M.; Kmieć, Z. In vitro characterization of spheres derived from colorectal cancer cell lines. Int. J. Oncol. 2018, 52, 599–612. [Google Scholar] [CrossRef] [PubMed]
- Olejniczak-Kęder, A.; Szaryńska, M.; Wrońska, A.; Siedlecka-Kroplewska, K.; Kmieć, Z. Effects of 5-FU and anti-EGFR antibody in combination with ASA on the spherical culture system of HCT116 and HT29 colorectal cancer cell lines. Int. J. Oncol. 2019, 55, 223–242. [Google Scholar] [CrossRef]
- Ivanov, D.P.; Grabowska, A.M. Spheroid arrays for high-throughput single-cell analysis of spatial patterns and biomarker expression in 3D. Sci. Rep. 2017, 7, 41160. [Google Scholar] [CrossRef]
- Rajcevic, U.; Knol, J.C.; Piersma, S.; Bougnaud, S.; Fack, F.; Sundlisaeter, E.; Søndenaa, K.; Myklebust, R.; Pham, T.V.; Niclou, S.P. Colorectal cancer derived organotypic spheroids maintain essential tissue characteristics but adapt their metabolism in culture. Proteome Sci. 2014, 12, 39. [Google Scholar] [CrossRef]
- Barbáchano, A.; Fernández-Barral, A.; Bustamante-Madrid, P.; Prieto, I.; Rodríguez-Salas, N.; Larriba, M.J.; Muñoz, A. Organoids and colorectal cancer. Cancers 2021, 13, 2657. [Google Scholar] [CrossRef]
- Ponsioen, B.; Post, J.B.; Buissant des Amorie, J.R.; Laskaris, D.; van Ineveld, R.L.; Kersten, S.; Bertotti, A.; Sassi, F.; Sipieter, F.; Cappe, B. Quantifying single-cell ERK dynamics in colorectal cancer organoids reveals EGFR as an amplifier of oncogenic MAPK pathway signalling. Nat. Cell Biol. 2021, 23, 377–390. [Google Scholar] [CrossRef]
- Zoetemelk, M.; Rausch, M.; Colin, D.J.; Dormond, O.; Nowak-Sliwinska, P. Short-term 3D culture systems of various complexity for treatment optimization of colorectal carcinoma. Sci. Rep. 2019, 9, 7103. [Google Scholar] [CrossRef] [PubMed]
- Zoetemelk, M.; Ramzy, G.M.; Rausch, M.; Nowak-Sliwinska, P. Drug-drug interactions of irinotecan, 5-fluorouracil, folinic acid and oxaliplatin and its activity in colorectal carcinoma treatment. Molecules 2020, 25, 2614. [Google Scholar] [CrossRef] [PubMed]
- Ramzy, G.M.; Boschung, L.; Koessler, T.; Delucinge-Vivier, C.; Docquier, M.; McKee, T.A.; Rubbia-Brandt, L.; Nowak-Sliwinska, P. FOLFOXIRI Resistance Induction and Characterization in Human Colorectal Cancer Cells. Cancers 2022, 14, 4812. [Google Scholar] [CrossRef] [PubMed]
- Gandaglia, G.; Leni, R.; Bray, F.; Fleshner, N.; Freedland, S.J.; Kibel, A.; Stattin, P.; Van Poppel, H.; La Vecchia, C. Epidemiology and prevention of prostate cancer. Eur. Urol. Oncol. 2021, 4, 877–892. [Google Scholar] [CrossRef] [PubMed]
- Marima, R.; Hull, R.; Mathabe, K.; Setlai, B.; Batra, J.; Sartor, O.; Mehrotra, R.; Dlamini, Z. Prostate cancer racial, socioeconomic, geographic disparities: Targeting the genomic landscape and splicing events in search for diagnostic, prognostic and therapeutic targets. Am. J. Cancer Res. 2021, 11, 1012. [Google Scholar]
- Saranyutanon, S.; Deshmukh, S.K.; Dasgupta, S.; Pai, S.; Singh, S.; Singh, A.P. Cellular and molecular progression of prostate cancer: Models for basic and preclinical research. Cancers 2020, 12, 2651. [Google Scholar] [CrossRef]
- Shen, M.M.; Abate-Shen, C. Molecular genetics of prostate cancer: New prospects for old challenges. Genes Dev. 2010, 24, 1967–2000. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, C.; Zhang, Y.; Shi, C. Application of organoid models in prostate cancer research. Front. Oncol. 2021, 11, 736431. [Google Scholar] [CrossRef]
- Catalona, W.J. Prostate cancer screening. Med. Clin. 2018, 102, 199–214. [Google Scholar] [CrossRef]
- Tan, G.H.; Nason, G.; Ajib, K.; Woon, D.T.S.; Herrera-Caceres, J.; Alhunaidi, O.; Perlis, N. Smarter screening for prostate cancer. World J. Urol. 2019, 37, 991–999. [Google Scholar] [CrossRef]
- Donovan, K.A.; Walker, L.M.; Wassersug, R.J.; Thompson, L.M.; Robinson, J.W. Psychological effects of androgen-deprivation therapy on men with prostate cancer and their partners. Cancer 2015, 121, 4286–4299. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, Q.; Liu, X.; Liu, C.; Liu, R.; Rycaj, K.; Zhang, D.; Liu, B.; Jeter, C.; Calhoun-Davis, T. Defining a Population of Stem-like Human Prostate Cancer Cells That Can Generate and Propagate Castration-Resistant Prostate CancerDefining Castration-Resistant Prostate Cancer Stem Cells. Clin. Cancer Res. 2016, 22, 4505–4516. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, N.; Yang, J.; Shepherd, P.D.; Li-Ning-Tapia, E.M.; Labanca, E.; Manyam, G.C.; Ravoori, M.K.; Kundra, V.; Araujo, J.C.; Efstathiou, E. The MD Anderson Prostate Cancer Patient-derived Xenograft Series (MDA PCa PDX) Captures the Molecular Landscape of Prostate Cancer and Facilitates Marker-driven Therapy DevelopmentMDA PCa PDXs, A Prostate Cancer PDX Resource. Clin. Cancer Res. 2020, 26, 4933–4946. [Google Scholar] [CrossRef] [PubMed]
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Application of prostate cancer models for preclinical study: Advantages and limitations of cell lines, patient-derived xenografts, and three-dimensional culture of patient-derived cells. Cells 2019, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Petrić, T.; Sabol, M. Let’s Go 3D! New Generation of Models for Evaluating Drug Response and Resistance in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 5293. [Google Scholar] [CrossRef]
- Ben-David, U.; Beroukhim, R.; Golub, T.R. Genomic evolution of cancer models: Perils and opportunities. Nat. Rev. Cancer 2019, 19, 97–109. [Google Scholar] [CrossRef]
- Gunti, S.; Hoke, A.T.; Vu, K.P.; London Jr, N.R. Organoid and spheroid tumor models: Techniques and applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef]
- Schutgens, F.; Clevers, H. Human organoids: Tools for understanding biology and treating diseases. Annu. Rev. Pathol. Mech. Dis. 2020, 15, 211–234. [Google Scholar] [CrossRef]
- Puca, L.; Bareja, R.; Prandi, D.; Shaw, R.; Benelli, M.; Karthaus, W.R.; Hess, J.; Sigouros, M.; Donoghue, A.; Kossai, M. Patient derived organoids to model rare prostate cancer phenotypes. Nat. Commun. 2018, 9, 2404. [Google Scholar] [CrossRef]
- Neuhaus, J.; Rabien, A.; Reinhold, A.; Koehler, L.; Berndt-Paetz, M. 3D Tumor Models in Urology. Int. J. Mol. Sci. 2023, 24, 6232. [Google Scholar] [CrossRef]
- Okada, S.; Vaeteewoottacharn, K.; Kariya, R. Establishment of a patient-derived tumor xenograft model and application for precision cancer medicine. Chem. Pharm. Bull. 2018, 66, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Beshiri, M.L.; Tice, C.M.; Tran, C.; Nguyen, H.M.; Sowalsky, A.G.; Agarwal, S.; Jansson, K.H.; Yang, Q.; McGowen, K.M.; Yin, J. A PDX/Organoid Biobank of Advanced Prostate Cancers Captures Genomic and Phenotypic Heterogeneity for Disease Modeling and Therapeutic ScreeningAn Extensive PDX/Organoid Platform of Prostate Cancer Models. Clin. Cancer Res. 2018, 24, 4332–4345. [Google Scholar] [CrossRef] [PubMed]
- Gleave, A.M.; Ci, X.; Lin, D.; Wang, Y. A synopsis of prostate organoid methodologies, applications, and limitations. Prostate 2020, 80, 518–526. [Google Scholar] [CrossRef] [PubMed]
- Bessot, A.; Gunter, J.; Waugh, D.; Clements, J.A.; Hutmacher, D.W.; McGovern, J.; Bock, N. GelMA and Biomimetic Culture Allow the Engineering of Mineralized, Adipose, and Tumor Tissue Human Microenvironments for the Study of Advanced Prostate Cancer In Vitro and In Vivo. Adv. Healthc. Mater. 2023, 12, 2201701. [Google Scholar] [CrossRef]
- Cantin-Warren, L.; Guignard, R.; Cortez Ghio, S.; Larouche, D.; Auger, F.A.; Germain, L. Specialized living wound dressing based on the self-assembly approach of tissue engineering. J. Funct. Biomater. 2018, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Germain, L.; Larouche, D.; Nedelec, B.; Perreault, I.; Duranceau, L.; Bortoluzzi, P.; Beaudoin Cloutier, C.; Genest, H.; Caouette-Laberge, L.; Dumas, A. Autologous bilayered self-assembled skin substitutes (SASSs) as permanent grafts: A case series of 14 severely burned patients indicating clinical effectiveness. Eur Cell Mater 2018, 36, 128–141. [Google Scholar] [CrossRef]
- Chen, S.; Schoen, J. Air-liquid interface cell culture: From airway epithelium to the female reproductive tract. Reprod. Domest. Anim. 2019, 54, 38–45. [Google Scholar] [CrossRef]
- De Vuyst, E.; Charlier, C.; Giltaire, S.; De Glas, V.; De Rouvroit, C.L.; Poumay, Y. Reconstruction of normal and pathological human epidermis on polycarbonate filter. Epidermal Cells Methods Protoc. 2014, 1195, 191–201. [Google Scholar]
- Frankart, A.; Malaisse, J.; De Vuyst, E.; Minner, F.; de Rouvroit, C.L.; Poumay, Y. Epidermal morphogenesis during progressive in vitro 3 D reconstruction at the air–liquid interface. Exp. Dermatol. 2012, 21, 871–875. [Google Scholar] [CrossRef]
- Gordon, S.; Daneshian, M.; Bouwstra, J.; Caloni, F.; Constant, S.; Davies, D.E.; Dandekar, G.; Guzman, C.A.; Fabian, E.; Haltner, E. Non-animal models of epithelial barriers (skin, intestine and lung) in research, industrial applications and regulatory toxicology. Altex 2015, 32, 327–378. [Google Scholar] [CrossRef]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.; van den Bogaard, E.H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef] [PubMed]
- Gendreau, I.; Angers, L.; Jean, J.; Pouliot, R. Pigmented skin models: Understand the mechanisms of melanocytes. In Regenerative Medicine and Tissue Engineering; IntechOpen: London, UK, 2013. [Google Scholar]
- Moon, S.; Kim, D.H.; Shin, J.U. In vitro models mimicking immune response in the skin. Yonsei Med. J. 2021, 62, 969. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Neves, C.; Gibbs, S. Progress on reconstructed human skin models for allergy research and identifying contact sensitizers. Three Dimens. Hum. Organotypic Models Biomed. Res. 2021, 430, 103–129. [Google Scholar]
- Nestle, F.O.; Di Meglio, P.; Qin, J.-Z.; Nickoloff, B.J. Skin immune sentinels in health and disease. Nat. Rev. Immunol. 2009, 9, 679–691. [Google Scholar] [CrossRef] [PubMed]
- Michielon, E.; de Gruijl, T.D.; Gibbs, S. From simplicity to complexity in current melanoma models. Exp. Dermatol. 2022, 31, 1818–1836. [Google Scholar] [CrossRef]
- Rebecca, V.W.; Somasundaram, R.; Herlyn, M. Pre-clinical modeling of cutaneous melanoma. Nat. Commun. 2020, 11, 2858. [Google Scholar] [CrossRef]
- Randall, M.J.; Jüngel, A.; Rimann, M.; Wuertz-Kozak, K. Advances in the Biofabrication of 3D Skin in vitro: Healthy and Pathological Models. Front. Bioeng. Biotechnol. 2018, 6, 154. [Google Scholar] [CrossRef]
- Phang, S.J.; Basak, S.; Teh, H.X.; Packirisamy, G.; Fauzi, M.B.; Kuppusamy, U.R.; Neo, Y.P.; Looi, M.L. Advancements in Extracellular Matrix-Based Biomaterials and Biofabrication of 3D Organotypic Skin Models. ACS Biomater. Sci. Eng. 2022, 8, 3220–3241. [Google Scholar] [CrossRef]
- Malheiro, A.; Thon, M.; Lourenço, A.F.; Gamardo, A.S.; Chandrakar, A.; Gibbs, S.; Wieringa, P.; Moroni, L. A Humanized In Vitro Model of Innervated Skin for Transdermal Analgesic Testing. Macromol. Biosci. 2023, 23, 2200387. [Google Scholar] [CrossRef]
- Ackermann, K.; Borgia, S.L.; Korting, H.C.; Mewes, K.; Schäfer-Korting, M. The Phenion® full-thickness skin model for percutaneous absorption testing. Ski. Pharmacol. Physiol. 2010, 23, 105–112. [Google Scholar] [CrossRef]
- Rasmussen, C.; Gratz, K.; Liebel, F.; Southall, M.; Garay, M.; Bhattacharyya, S.; Simon, N.; Vander Zanden, M.; Van Winkle, K.; Pirnstill, J. The StrataTest® human skin model, a consistent in vitro alternative for toxicological testing. Toxicol. Vitro 2010, 24, 2021–2029. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, K.; Spiekstra, S.W.; Waaijman, T.; Scheper, R.J.; de Gruijl, T.D.; Gibbs, S. Technical advance: Langerhans cells derived from a human cell line in a full-thickness skin equivalent undergo allergen-induced maturation and migration. J. Leukoc. Biol. 2011, 90, 1027–1033. [Google Scholar] [CrossRef]
- Walters, R.M.; Gandolfi, L.; Mack, M.C.; Fevola, M.; Martin, K.; Hamilton, M.T.; Hilberer, A.; Barnes, N.; Wilt, N.; Nash, J.R. In vitro assessment of skin irritation potential of surfactant-based formulations by using a 3-D skin reconstructed tissue model and cytokine response. Altern. Lab. Anim. 2016, 44, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Morales, M.; Pérez, D.; Correa, L.; Restrepo, L. Evaluation of fibrin-based dermal-epidermal organotypic cultures for in vitro skin corrosion and irritation testing of chemicals according to OECD TG 431 and 439. Toxicol. Vitro 2016, 36, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.-Y.; Kim, N.-H.; Choi, W.-I.; Youm, Y.-H. Less keratinocyte-derived factors related to more keratinocyte apoptosis in depigmented than normally pigmented suction-blistered epidermis may cause passive melanocyte death in vitiligo. J. Investig. Dermatol. 2005, 124, 976–983. [Google Scholar] [CrossRef]
- Valyi-Nagy, I.; Murphy, G.; Mancianti, M.; Whitaker, D.; Herlyn, M. Phenotypes and interactions of human melanocytes and keratinocytes in an epidermal reconstruction model. Lab. Investig. A J. Tech. Methods Pathol. 1990, 62, 314–324. [Google Scholar]
- Cario-André, M.; Pain, C.; Taïeb, A.; Nikaido, O.; Gall, Y.; Ginestar, J. Studies on epidermis reconstructed with and without melanocytes: Melanocytes prevent sunburn cell formation but not appearance of DNA damaged cells in fair-skinned caucasians. J. Investig. Dermatol. 2000, 115, 193–199. [Google Scholar] [CrossRef][Green Version]
- Herlyn, M.; Berking, C. Human skin reconstruct models: A new application for studies of melanocyte and melanoma biology. Histol. Histopathol. 2001, 16, 669–674. [Google Scholar]
- Dror, S.; Sander, L.; Schwartz, H.; Sheinboim, D.; Barzilai, A.; Dishon, Y.; Apcher, S.; Golan, T.; Greenberger, S.; Barshack, I. Melanoma miRNA trafficking controls tumour primary niche formation. Nat. Cell Biol. 2016, 18, 1006–1017. [Google Scholar] [CrossRef]
- Kosten, I.J.; Spiekstra, S.W.; de Gruijl, T.D.; Gibbs, S. MUTZ-3 derived Langerhans cells in human skin equivalents show differential migration and phenotypic plasticity after allergen or irritant exposure. Toxicol. Appl. Pharmacol. 2015, 287, 35–42. [Google Scholar] [CrossRef]
- Facy, V.; Flouret, V.; Régnier, M.; Schmidt, R. Reactivity of Langerhans cells in human reconstructed epidermis to known allergens and UV radiation. Toxicol. Vitro 2005, 19, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Régnier, M.; Staquet, M.-J.; Schmitt, D.; Schimdt, R. Integration of Langerhans cells into a pigmented reconstructed human epidermis. J. Investig. Dermatol. 1997, 109, 510–512. [Google Scholar] [CrossRef] [PubMed]
- Ouwehand, K.; Oosterhoff, D.; Breetveld, M.; Scheper, R.J.; De Gruijl, T.D.; Gibbs, S. Irritant-induced migration of Langerhans cells coincides with an IL-10-dependent switch to a macrophage-like phenotype. J. Investig. Dermatol. 2011, 131, 418–425. [Google Scholar] [CrossRef]
- Kaur, A.; Ecker, B.L.; Douglass, S.M.; Kugel, C.H., III; Webster, M.R.; Almeida, F.V.; Somasundaram, R.; Hayden, J.; Ban, E.; Ahmadzadeh, H. Remodeling of the collagen matrix in aging skin promotes melanoma metastasis and affects immune cell motility. Cancer Discov. 2019, 9, 64–81. [Google Scholar] [CrossRef]
- Ronaldson-Bouchard, K.; Teles, D.; Yeager, K.; Tavakol, D.N.; Zhao, Y.; Chramiec, A.; Tagore, S.; Summers, M.; Stylianos, S.; Tamargo, M. A multi-organ chip with matured tissue niches linked by vascular flow. Nat. Biomed. Eng. 2022, 6, 351–371. [Google Scholar] [CrossRef]
- Wufuer, M.; Lee, G.; Hur, W.; Jeon, B.; Kim, B.J.; Choi, T.H.; Lee, S. Skin-on-a-chip model simulating inflammation, edema and drug-based treatment. Sci. Rep. 2016, 6, 37471. [Google Scholar] [CrossRef]
- Lee, S.; Jin, S.-P.; Kim, Y.K.; Sung, G.Y.; Chung, J.H.; Sung, J.H. Construction of 3D multicellular microfluidic chip for an in vitro skin model. Biomed. Microdevices 2017, 19, 22. [Google Scholar] [CrossRef]
- Mori, N.; Morimoto, Y.; Takeuchi, S. Skin integrated with perfusable vascular channels on a chip. Biomaterials 2017, 116, 48–56. [Google Scholar] [CrossRef]
- Abaci, H.E.; Guo, Z.; Coffman, A.; Gillette, B.; Lee, W.h.; Sia, S.K.; Christiano, A.M. Human skin constructs with spatially controlled vasculature using primary and iPSC-derived endothelial cells. Adv. Healthc. Mater. 2016, 5, 1800–1807. [Google Scholar] [CrossRef]
- Keenan, T.M.; Folch, A. Biomolecular gradients in cell culture systems. Lab Chip 2008, 8, 34–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasterok, S.; Gustafsson, A.; Gjörloff Wingren, A. Applications of Tumor Cells in an In Vitro 3D Environment. Appl. Sci. 2023, 13, 10349. https://doi.org/10.3390/app131810349
Hasterok S, Gustafsson A, Gjörloff Wingren A. Applications of Tumor Cells in an In Vitro 3D Environment. Applied Sciences. 2023; 13(18):10349. https://doi.org/10.3390/app131810349
Chicago/Turabian StyleHasterok, Sylwia, Anna Gustafsson, and Anette Gjörloff Wingren. 2023. "Applications of Tumor Cells in an In Vitro 3D Environment" Applied Sciences 13, no. 18: 10349. https://doi.org/10.3390/app131810349
APA StyleHasterok, S., Gustafsson, A., & Gjörloff Wingren, A. (2023). Applications of Tumor Cells in an In Vitro 3D Environment. Applied Sciences, 13(18), 10349. https://doi.org/10.3390/app131810349







