Osteoarthritis—The Role of Mesenchymal Stem Cells in Cartilage Regeneration
Abstract
:1. Introduction
2. Etiopathogenesis
3. Treatment of OA
3.1. Physical Measures
3.2. Drug Therapy
3.3. Surgical Intervention
4. MSC-Based Therapy for OA
4.1. General Characteristics
- The ICST has endorsed CD105 (endoglin), CD73 (ecto-5′ -nuclease), and CD90 (Thy-1) as positive and negative surface markers for MSCs. In contrast, MSCs lack the expression of hematological and endothelial markers such as CD45, CD34, CD14 or CD11b, CD79α or CD19, as well as human leukocyte antigen (HLA)-DR [128,154].
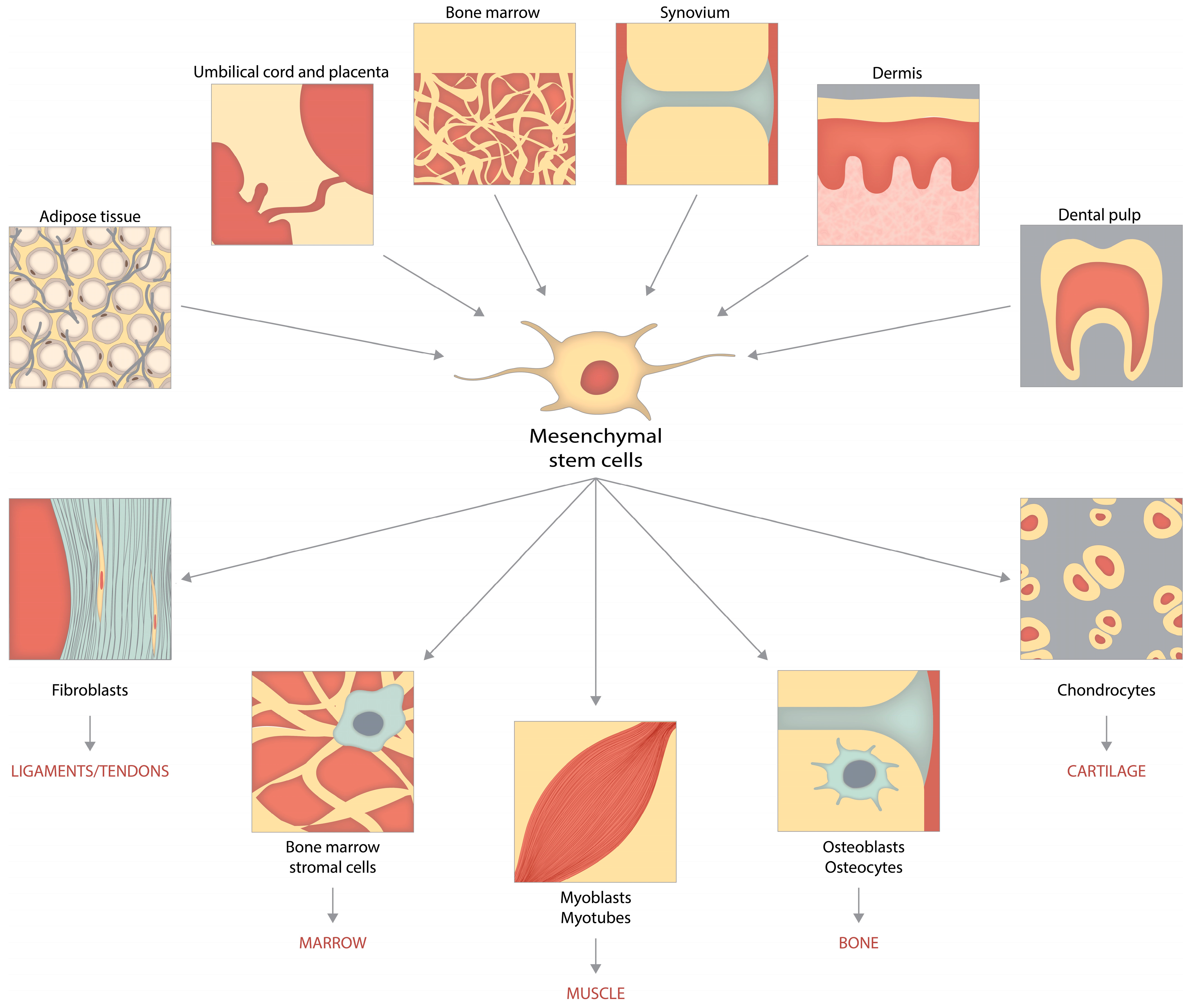
4.2. Sources of MSCs
4.2.1. BM-MSCs for OA Therapy
4.2.2. AD-MSCs for OA Therapy
4.2.3. UC-MSCs for OA Therapy
4.2.4. ESC-Induced MSCs (ESC-MSCs) for OA Treatment
4.2.5. SM-MSCs for OA Treatment
4.2.6. IPFP for OA Treatment
4.2.7. iPSCs for OA Treatment
4.3. Comparisons between Different Forms of MSCs
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lambova, S.; Müller-Ladner, U. Editorial: Osteoarthritis—Current Insights in Pathogenesis, Diagnosis and Treatment. Curr. Rheumatol. Rev. 2018, 14, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef] [PubMed]
- Abhishek, A.; Doherty, M. Diagnosis and Clinical Presentation of Osteoarthritis. Rheum. Dis. Clin. N. Am. 2013, 39, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Jo, C.; Chai, J.; Jeong, E.; Oh, S.; Shin, J.; Shim, H.; Yoon, K. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A 2-Year Follow-Up Study. Am. J. Sports Med. 2017, 45, 2774–2783. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Gong, T.; Jiang, Y.; Zhang, S.; Zhao, Y.; Wu, Q. Global, Regional, and National Prevalence and Disability-Adjusted Life-Years for Infertility in 195 Countries and Territories, 1990–2017: Results from a Global Burden of Disease Study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef]
- Blagojevic, M.; Jinks, C.; Jeffery, A.; Jordan, K. Risk Factors for Onset of Osteoarthritis of the Knee in Older Adults: A Systematic Review and Meta-Analysis. Osteoarthr. Cartil. 2010, 18, 24–33. [Google Scholar] [CrossRef]
- Carballo, C.; Nakagawa, Y.; Sekiya, I.; Rodeo, S. Basic Science of Articular Cartilage. Clin. Sports Med. 2017, 36, 413–425. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.; Agricola, R.; Price, A.; Vincent, T.; Weinans, H.; Carr, A. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- Loeser, R.; Goldring, S.; Scanzello, C.; Goldring, M. Osteoarthritis: A Disease of the Joint as an Organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef]
- Gignac, M.; Irvin, E.; Cullen, K.; Van Eerd, D.; Beaton, D.; Mahood, Q.; McLeod, C.; Backman, C. Men and Women’s Occupational Activities and the Risk of Developing Osteoarthritis of the Knee, Hip, or Hands: A Systematic Review and Recommendations for Future Research. Arthritis Care Res. 2020, 72, 378–396. [Google Scholar] [CrossRef]
- Ostrowska, M.; Maśliński, W.; Prochorec-Sobieszek, M.; Nieciecki, M.; Sudoł-Szopińska, I. Cartilage and Bone Damage in Rheumatoid Arthritis. Reumatol./Rheumatol. 2018, 56, 111–120. [Google Scholar] [CrossRef]
- Okikiade, A.; Osharode, A.; Oyewole, A.; Ogunesan, D.; Oladejo, D.; Oshobu, I.; Browne, K. Understanding the Role of Inflammation in Secondary Osteoarthritis. Asian J. Med. Health Res. 2022, 20, 60–74. [Google Scholar] [CrossRef]
- Lozada, C.J. Osteoarthritis. Available online: https://emedicine.medscape.com/article/330487-overview#a5 (accessed on 9 September 2023).
- Chen, D.; Shen, J.; Zhao, W.; Wang, T.; Han, L.; Hamilton, J.; Im, H. Osteoarthritis: Toward a Comprehensive Understanding of Pathological Mechanism. Bone Res. 2017, 5, 16044. [Google Scholar] [CrossRef] [PubMed]
- Vina, E.; Kwoh, C. Epidemiology of Osteoarthritis: Literature Update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.-N.; Zhu, S.-Y.; He, H.-C.; Yu, X.; Xu, Y.; He, C.-Q. Mesenchymal Stromal Cell-Based Therapy for Cartilage Regeneration in Knee Osteoarthritis. Stem Cell Res. Ther. 2022, 13, 14. [Google Scholar] [CrossRef]
- Shah, K.; Zhao, A.G.; Sumer, H. New Approaches to Treat Osteoarthritis with Mesenchymal Stem Cells. Stem Cells Int. 2018, 2018, 5373294. [Google Scholar] [CrossRef] [PubMed]
- Tiku, M.; Sabaawy, H. Cartilage Regeneration for Treatment of Osteoarthritis: A Paradigm for Nonsurgical Intervention. Ther. Adv. Musculoskelet. Dis. 2015, 7, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Felson, D. Osteoarthritis as a Disease of Mechanics. Osteoarthr. Cartil. 2013, 21, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Bastiaansen-Jenniskens, Y.; Koevoet, W.; de Bart, A.; van der Linden, J.; Zuurmond, A.; Weinans, H.; Verhaar, J.; van Osch, G.; DeGroot, J. Contribution of Collagen Network Features to Functional Properties of Engineered Cartilage. Osteoarthr. Cartil. 2008, 16, 359–366. [Google Scholar] [CrossRef]
- Nelson, A.; Allen, K.; Golightly, Y.; Goode, A.; Jordan, J. A Systematic Review of Recommendations and Guidelines for the Management of Osteoarthritis: The Chronic Osteoarthritis Management Initiative of the U.S. Bone and Joint Initiative. Semin. Arthritis Rheum. 2014, 43, 701–712. [Google Scholar] [CrossRef]
- Mehana, E.; Khafaga, A.; El-Blehi, S. The Role of Matrix Metalloproteinases in Osteoarthritis Pathogenesis: An Updated Review. Life Sci. 2019, 234, 116786. [Google Scholar] [CrossRef]
- Murphy, G. What Are the Roles of Metalloproteinases in Cartilage and Bone Damage? Ann. Rheum. Dis. 2005, 64, iv44–iv47. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.; Otero, M.; Plumb, D.; Dragomir, C.; Favero, M.; EI Hachem, K.; Hashimoto, K.; Roach, H.; Olivotto, E.; Borzì, R.; et al. Roles of Inflammatory and Anabolic Cytokines in Cartilage Metabolism: Signals and Multiple Effectors Converge Upon MMP-13 Regulation in Osteoarthritis. Eur. Cells Mater. 2011, 21, 202–220. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.; Bedi, A.; Rodeo, S. The Basic Science of Articular Cartilage: Structure, Composition, and Function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Imhof, H.; Sulzbacher, I.; Grampp, S.; Czerny, C.; Youssefzadeh, S.; Kainberger, F. Subchondral Bone and Cartilage Disease. Investig. Radiol. 2000, 35, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Kidd, B. Mechanisms of Pain in Osteoarthritis. HSS J. 2012, 8, 26–28. [Google Scholar] [CrossRef]
- Wang, H.; Bai, J.; He, B.; Hu, X.; Liu, D. Osteoarthritis and the Risk of Cardiovascular Disease: A Meta-Analysis of Observational Studies. Sci. Rep. 2016, 6, 39672. [Google Scholar] [CrossRef]
- Wallace, I.; Worthington, S.; Felson, D.; Jurmain, R.; Wren, K.; Maijanen, H.; Woods, R.; Lieberman, D. Knee Osteoarthritis Has Doubled in Prevalence Since the Mid-20th Century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef]
- Veronese, N.; Cereda, E.; Maggi, S.; Luchini, C.; Solmi, M.; Smith, T.; Denkinger, M.; Hurley, M.; Thompson, T.; Manzato, E.; et al. Osteoarthritis and Mortality: A Prospective Cohort Study and Systematic Review with Meta-Analysis. Semin. Arthritis Rheum. 2016, 46, 160–167. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Lee, D. Survival of Opening Versus Closing Wedge High Tibial Osteotomy: A Meta-Analysis. Sci. Rep. 2017, 7, 7296. [Google Scholar] [CrossRef]
- Wearing, S.; Hennig, E.; Byrne, N.; Steele, J.; Hills, A. Musculoskeletal Disorders Associated with Obesity: A Biomechanical Perspective. Obes. Rev. 2006, 7, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Musumeci, G.; Aiello, F.; Szychlinska, M.; Di Rosa, M.; Castrogiovanni, P.; Mobasheri, A. Osteoarthritis in the XXIst Century: Risk Factors and Behaviours That Influence Disease Onset and Progression. Int. J. Mol. Sci. 2015, 16, 6093–6112. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, B.; Katz, J.; Solomon, D.; Yelin, E.; Hunter, D.; Messier, S.; Suter, L.; Losina, E. Number of Persons with Symptomatic Knee Osteoarthritis in the US: Impact of Race and Ethnicity, Age, Sex, and Obesity. Arthritis Care Res. 2016, 68, 1743–1750. [Google Scholar] [CrossRef]
- Coaccioli, S.; Sarzi-Puttini, P.; Zis, P.; Rinonapoli, G.; Varrassi, G. Osteoarthritis: New Insight on Its Pathophysiology. J. Clin. Med. 2022, 11, 6013. [Google Scholar] [CrossRef] [PubMed]
- Reynard, L.; Barter, M. Osteoarthritis Year in Review 2019: Genetics, Genomics and Epigenetics. Osteoarthr. Cartil. 2020, 28, 275–284. [Google Scholar] [CrossRef]
- Tachmazidou, I.; Hatzikotoulas, K.; Southam, L.; Esparza-Gordillo, J.; Haberland, V.; Zheng, J.; Johnson, T.; Koprulu, M.; Zengini, E.; Steinberg, J.; et al. Identification of New Therapeutic Targets for Osteoarthritis through Genome-Wide Analyses of UK Biobank Data. Nat. Genet. 2019, 51, 230–236. [Google Scholar] [CrossRef]
- Loeser, R. Aging Processes and the Development of Osteoarthritis. Curr. Opin. Rheumatol. 2013, 25, 108–113. [Google Scholar] [CrossRef]
- Kelly, T.; Yang, W.; Chen, C.; Reynolds, K.; He, J. Global Burden of Obesity in 2005 and Projections to 2030. Int. J. Obes. 2008, 32, 1431–1437. [Google Scholar] [CrossRef]
- Conde, J.; Scotece, M.; Gómez, R.; Lopez, V.; Gómez-Reino, J.; Gualillo, O. Adipokines and Osteoarthritis: Novel Molecules Involved in the Pathogenesis and Progression of Disease. Arthritis 2011, 2011, 203901. [Google Scholar] [CrossRef]
- Eckel, R.; Grundy, S.; Zimmet, P. The Metabolic Syndrome. Lancet 2005, 365, 1415–1428. [Google Scholar] [CrossRef]
- Steenvoorden, M.; Huizinga, T.; Verzijl, N.; Bank, R.; Ronday, H.; Luning, H.; Lafeber, F.; Toes, R.; DeGroot, J. Activation of Receptor for Advanced Glycation End Products in Osteoarthritis Leads to Increased Stimulation of Chondrocytes and Synoviocytes. Semin. Arthritis Rheum. 2005, 54, 253–263. [Google Scholar] [CrossRef]
- Slemenda, C. Quadriceps Weakness and Osteoarthritis of the Knee. Ann. Intern. Med. 1997, 127, 97–104. [Google Scholar] [CrossRef]
- Daste, C.; Kirren, Q.; Akoum, J.; Lefèvre-Colau, M.; Rannou, F.; Nguyen, C. Physical Activity for Osteoarthritis: Efficiency and Review of Recommandations. Jt. Bone Spine 2021, 88, 105207. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.; Sprow, K.; Powell, K.; Buchner, D.; Bloodgood, B.; Piercy, K.; George, S.; Kraus, W. Effects of Physical Activity in Knee and Hip Osteoarthritis: A Systematic Umbrella Review. Med. Sci. Sports Exerc. 2019, 51, 1324–1339. [Google Scholar] [CrossRef] [PubMed]
- Simopoulos, A. An Increase in the Omega-6/Omega-3 Fatty Acid Ratio Increases the Risk for Obesity. Nutrients 2016, 8, 128. [Google Scholar] [CrossRef]
- Dai, Z.; Lu, N.; Niu, J.; Felson, D.; Zhang, Y. Dietary Fiber Intake in Relation to Knee Pain Trajectory. Arthritis Care Res. 2017, 69, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Roos, E. Joint Injury Causes Knee Osteoarthritis in Young Adults. Curr. Opin. Rheumatol. 2005, 17, 195–200. [Google Scholar] [CrossRef]
- Larsson, S.; Englund, M.; Struglics, A.; Lohmander, L. Interleukin-6 and Tumor Necrosis Factor Alpha in Synovial Fluid Are Associated with Progression of Radiographic Knee Osteoarthritis in Subjects with Previous Meniscectomy. Osteoarthr. Cartil. 2015, 23, 1906–1914. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, M.; Martel-Pelletier, J.; Lajeunesse, D.; Pelletier, J.; Fahmi, H. Role of Proinflammatory Cytokines in the Pathophysiology of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 7, 33–42. [Google Scholar] [CrossRef]
- Sokolove, J.; Lepus, C. Role of Inflammation in the Pathogenesis of Osteoarthritis: Latest Findings and Interpretations. Ther. Adv. Musculoskelet. Dis. 2013, 5, 77–94. [Google Scholar] [CrossRef]
- Melchiorri, C.; Meliconi, R.; Frizziero, L.; Silvestri, T.; Pulsatelli, L.; Mazzetti, I.; Borz, R.; Uguccioni, M.; Facchini, A. Enhanced and Coordinated In Vivo Expression of Inflammatory Cytokines and Nitric Oxide Synthase by Chondrocytes from Patients with Osteoarthritis. Arthritis Rheum. 1998, 41, 2165–2174. [Google Scholar] [CrossRef]
- Saklatvala, J. Tumour Necrosis Factor A Stimulates Resorption and Inhibits Synthesis of Proteoglycan in Cartilage. Nature 1986, 322, 547–549. [Google Scholar] [CrossRef]
- Lefebvre, V.; Peeters-Joris, C.; Vaes, G. Modulation by Interleukin 1 and Tumor Necrosis Factor α of Production of Collagenase, Tissue Inhibitor of Metalloproteinases and Collagen Types in Differentiated and Dedifferentiated Articular Chondrocytes. Biochim. Biophys. Acta Mol. Cell Res. 1990, 1052, 366–378. [Google Scholar] [CrossRef]
- Li, H.; Xie, S.; Qi, Y.; Li, H.; Zhang, R.; Lian, Y. TNF-A Increases the Expression of Inflammatory Factors in Synovial Fibroblasts by Inhibiting the PI3K/AKT Pathway in a Rat Model of Monosodium Iodoacetate-Induced Osteoarthritis. Exp. Ther. Med. 2018, 16, 4737–4744. [Google Scholar] [CrossRef] [PubMed]
- Alaaeddine, N.; Olee, T.; Hashimoto, S.; Creighton-Achermann, L.; Lotz, M. Production of the Chemokine RANTES by Articular Chondrocytes and Role in Cartilage Degradation. Arthritis Rheum. 2001, 44, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Homandberg, G.; Hui, F. Association of Proteoglycan Degradation with Catabolic Cytokine and Stromelysin Release from Cartilage Cultured with Fibronectin Fragments. Arch. Biochem. Biophys. 1996, 334, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Primorac, D.; Molnar, V.; Rod, E.; Jeleč, Ž.; Čukelj, F.; Matišić, V.; Vrdoljak, T.; Hudetz, D.; Hajsok, H.; Borić, I. Knee Osteoarthritis: A Review of Pathogenesis and State-of-the-Art Non-Operative Therapeutic Considerations. Genes 2020, 11, 854. [Google Scholar] [CrossRef]
- Alaaeddine, N.; Di Battista, J.; Pelletier, J.; Kiansa, K.; Cloutier, J.; Martel-Pelletier, J. Inhibition of Tumor Necrosis Factor α-Induced Prostaglandin E2 Production by the Antiinflammatory Cytokines Interleukin-4, Interleukin-10, and Interleukin-13 in Osteoarthritic Synovial Fibroblasts: Distinct Targeting in the Signaling Pathways. Arthritis Rheum. 1999, 42, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Porée, B.; Kypriotou, M.; Chadjichristos, C.; Beauchef, G.; Renard, E.; Legendre, F.; Melin, M.; Gueret, S.; Hartmann, D.; Malléin-Gerin, F.; et al. Interleukin-6 (IL-6) and/or Soluble IL-6 Receptor Down-Regulation of Human Type II Collagen Gene Expression in Articular Chondrocytes Requires a Decrease of Sp1·Sp3 Ratio and of the Binding Activity of Both Factors to the COL2A1 Promoter. J. Biol. Chem. 2008, 283, 4850–4865. [Google Scholar] [CrossRef]
- Chenoufi, H.; Diamant, M.; Rieneck, K.; Lund, B.; Stein, G.; Lian, J. Increased Mrna Expression and Protein Secretion of Interleukin-6 in Primary Human Osteoblasts Differentiated In Vitro from Rheumatoid and Osteoarthritic Bone. J. Cell Biochem. 2001, 81, 666–678. [Google Scholar] [CrossRef]
- Askari, A.; Naghizadeh, M.; Homayounfar, R.; Shahi, A.; Afsarian, M.; Paknahad, A.; Kennedy, D.; Ataollahi, M. Increased Serum Levels of IL-17A and IL-23 Are Associated with Decreased Vitamin D3 and Increased Pain in Osteoarthritis. PLoS ONE 2016, 11, e0164757. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P.; Blaney Davidson, E.; van den Berg, W. Bone Morphogenetic Proteins and Articular Cartilage. Osteoarthr. Cartil. 2010, 18, 735–741. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, X.; Goupille, P.; Beaulieu, A.; Burch, F.; Bensen, W.; Conrozier, T.; Loeuille, D.; Kivitz, A.; Silver, D.; Appleton, B. Intraarticular Injection of Anakinra in Osteoarthritis of the Knee: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Arthritis Rheum. 2009, 61, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Melrose, J.; Fuller, E.; Roughley, P.; Smith, M.; Kerr, B.; Hughes, C.; Caterson, B.; Little, C. Fragmentation of Decorin, Biglycan, Lumican and Keratocan Is Elevated in Degenerate Human Meniscus, Knee and Hip Articular Cartilages Compared with Age-Matched Macroscopically Normal and Control Tissues. Arthritis Res. Ther. 2008, 10, R79. [Google Scholar] [CrossRef] [PubMed]
- Midwood, K.; Sacre, S.; Piccinini, A.; Inglis, J.; Trebaul, A.; Chan, E.; Drexler, S.; Sofat, N.; Kashiwagi, M.; Orend, G.; et al. Tenascin-C Is an Endogenous Activator of Toll-Like Receptor 4 That Is Essential for Maintaining Inflammation in Arthritic Joint Disease. Nat. Med. 2009, 15, 774–780. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Chondrocyte Innate Immune Myd88-Dependent Signaling Drives Pro-Catabolic Effects of the Endogenous TLR2/TLR4 Ligands LMW-HA and HMGB1. Arthritis Rheum. 2010, 62, 2004–2012. [Google Scholar] [CrossRef]
- Lotz, M.; Loeser, R. Effects of Aging on Articular Cartilage Homeostasis. Bone 2012, 51, 241–248. [Google Scholar] [CrossRef]
- Bolduc, J.; Collins, J.; Loeser, R. Reactive Oxygen Species, Aging and Articular Cartilage Homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef]
- Loeser, R.; Collins, J.; Diekman, B. Ageing and the Pathogenesis of Osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, X.; Lotz, M.; Terkeltaub, R.; Liu-Bryan, R. Mitochondrial Biogenesis Is Impaired in Osteoarthritis Chondrocytes But Reversible via Peroxisome Proliferator-Activated Receptor Γ Coactivator 1A. Arthritis Rheum. 2015, 67, 2141–2153. [Google Scholar] [CrossRef]
- Hunter, D.; Felson, D. Osteoarthritis. BMJ 2006, 332, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Hochberg, M.; Altman, R.; April, K.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P. American College of Rheumatology 2012 Recommendations for the Use of Nonpharmacologic and Pharmacologic Therapies in Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2012, 64, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Bejarano, T.; Novo, M. Current Interventions in the Management of Knee Osteoarthritis. J. Pharm. Bioallied. Sci. 2013, 5, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Nuki, G.; Moskowitz, R.; Abramson, S.; Altman, R.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.; Croft, P.; Doherty, M.; et al. OARSI Recommendations for the Management of Hip and Knee Osteoarthritis. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef]
- Vincent, H.; Heywood, K.; Connelly, J.; Hurley, R. Obesity and Weight Loss in the Treatment and Prevention of Osteoarthritis. PM&R 2012, 4, S59–S67. [Google Scholar]
- Fransen, M.; McConnell, S.; Harmer, A.; Van der Esch, M.; Simic, M.; Bennell, K. Exercise for Osteoarthritis of the Knee: A Cochrane Systematic Review. Br. J. Sports Med. 2015, 49, 1554–1557. [Google Scholar] [CrossRef] [PubMed]
- Vincent, K.; Vincent, H. Resistance Exercise for Knee Osteoarthritis. PM&R 2012, 4, S45–S52. [Google Scholar]
- Latham, N.; Liu, C. Strength Training in Older Adults: The Benefits for Osteoarthritis. Clin. Geriatr. Med. 2010, 26, 445–459. [Google Scholar] [CrossRef]
- Thoumie, P.; Marty, M.; Avouac, B.; Pallez, A.; Vaumousse, A.; Pipet, L.; Monroche, A.; Graveleau, N.; Bonnin, A.; Amor, C.; et al. Effect of Unloading Brace Treatment on Pain and Function in Patients with Symptomatic Knee Osteoarthritis: The ROTOR Randomized Clinical Trial. Sci. Rep. 2018, 8, 10519. [Google Scholar] [CrossRef]
- Wilson, B.; Rankin, H.; Barnes, C. Long-Term Results of an Unloader Brace in Patients with Unicompartmental Knee Osteoarthritis. Orthopedics 2011, 34, e334–e337. [Google Scholar] [CrossRef]
- Cohen, E.; Lee, Y. A Mechanism-Based Approach to the Management of Osteoarthritis Pain. Curr. Osteoporos. Rep. 2015, 13, 399–406. [Google Scholar] [CrossRef] [PubMed]
- Balmaceda, C. Evolving Guidelines in the Use of Topical Nonsteroidal Anti-Inflammatory Drugs in the Treatment of Osteoarthritis. BMC Musculoskelet. Disord. 2014, 15, 27. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Robertson, W.; Zhao, J.; Chen, W.; Xu, J. Emerging Trend in the Pharmacotherapy of Osteoarthritis. Front. Endocrinol. 2019, 10, 431. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.; Bates, C.; Davidson, J.; Martin, K.; Hayes, P.; Simpson, K. Staggered Overdose Pattern and Delay to Hospital Presentation Are Associated with Adverse Outcomes Following Paracetamol-Induced Hepatotoxicity. Br. J. Clin. Pharmacol. 2012, 73, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Machado, G.; Maher, C.; Ferreira, P.; Pinheiro, M.; Lin, C.; Day, R.; McLachlan, A.; Ferreira, M. Efficacy and Safety of Paracetamol for Spinal Pain and Osteoarthritis: Systematic Review and Meta-Analysis of Randomised Placebo Controlled Trials. BMJ 2015, 350, h1225. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Moskowitz, R.; Nuki, G.; Abramson, S.; Altman, R.; Arden, N.; Bierma-Zeinstra, S.; Brandt, K.; Croft, P.; Doherty, M.; et al. OARSI Recommendations for the Management of Hip and Knee Osteoarthritis, Part II: OARSI Evidence-Based, Expert Consensus Guidelines. Osteoarthr. Cartil. 2008, 16, 137–162. [Google Scholar] [CrossRef]
- Manheimer, E.; Cheng, K.; Linde, K.; Lao, L.; Yoo, J.; Wieland, S.; van der Windt, D.; Berman, B.; Bouter, L. Acupuncture for Peripheral Joint Osteoarthritis. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef] [PubMed]
- Erickson, G.; Gimble, J.; Franklin, D.; Rice, H.; Awad, H.; Guilak, F. Chondrogenic Potential of Adipose Tissue-Derived Stromal Cells In Vitro and In Vivo. Biochem. Biophys. Res. Commun. 2002, 290, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Derry, S.; Conaghan, P.; Da Silva, J.; Wiffen, P.; Moore, R. Topical Nsaids for Chronic Musculoskeletal Pain in Adults. Cochrane Database Syst. Rev. 2016, 4, CD007400. [Google Scholar] [CrossRef] [PubMed]
- McAlindon, T.; Bannuru, R.; Sullivan, M.; Arden, N.; Berenbaum, F.; Bierma-Zeinstra, S.; Hawker, G.; Henrotin, Y.; Hunter, D.; Kawaguchi, H.; et al. OARSI Guidelines for the Non-Surgical Management of Knee Osteoarthritis. Osteoarthr. Cartil. 2014, 22, 363–388. [Google Scholar] [CrossRef]
- Dowell, D.; Haegerich, T.; Chou, R. CDC Guideline for Prescribing Opioids for Chronic Pain—United States, 2016. JAMA 2016, 315, 1624–1625. [Google Scholar] [CrossRef]
- Faúndez, J.; Cotoras, P.; Irarrázaval, S. Are Intraarticular Steroids Effective for Knee Osteoarthritis? Medwave 2016, 16, e6599. [Google Scholar] [CrossRef] [PubMed]
- Abou-Raya, S.; Abou-Raya, A.; Helmii, M. Duloxetine for the Management of Pain in Older Adults with Knee Osteoarthritis: Randomised Placebo-Controlled Trial. Age Ageing 2012, 41, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Mason, L.; Moore, R.; Derry, S.; Edwards, J.; McQuay, H. Systematic Review of Topical Capsaicin for the Treatment of Chronic Pain. BMJ 2004, 328, 991. [Google Scholar] [CrossRef]
- Patel, S.; Dhillon, M.; Aggarwal, S.; Marwaha, N.; Jain, A. Treatment with Platelet-Rich Plasma Is More Effective Than Placebo for Knee Osteoarthritis. Am. J. Sports Med. 2013, 41, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ma, F.; Pang, X.; Tang, B.; Lin, L. Synthesis of Chondroitin Sulfate Magnesium for Osteoarthritis Treatment. Carbohydr. Polym. 2019, 212, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ouyang, H.; Dass, C.; Xu, J. Current Research on Pharmacologic and Regenerative Therapies for Osteoarthritis. Bone Res. 2016, 4, 15040. [Google Scholar] [CrossRef]
- Hunter, D.; Pike, M.; Jonas, B.; Kissin, E.; Krop, J.; McAlindon, T. Phase 1 Safety and Tolerability Study of BMP-7 in Symptomatic Knee Osteoarthritis. BMC Musculoskelet. Disord. 2010, 11, 232. [Google Scholar] [CrossRef]
- Nguyen, C.; Boutron, I.; Baron, G.; Coudeyre, E.; Berenbaum, F.; Poiraudeau, S.; Rannou, F. Evolution of Pain at 3 Months by Oral Resveratrol in Knee Osteoarthritis (ARTHROL): Protocol for a Multicentre Randomised Double-Blind Placebo-Controlled Trial. BMJ Open 2017, 7, e017652. [Google Scholar] [CrossRef]
- Amirkhizi, F.; Ghoreishy, S.; Hamedi-Shahraki, S.; Asghari, S. Higher Dietary Phytochemical Index Is Associated with Lower Odds of Knee Osteoarthritis. Sci. Rep. 2022, 12, 9059. [Google Scholar] [CrossRef]
- Rönn, K.; Reischl, N.; Gautier, E.; Jacobi, M. Current Surgical Treatment of Knee Osteoarthritis. Arthritis 2011, 2011, 454873. [Google Scholar] [CrossRef]
- Reid, M.; Eccleston, C.; Pillemer, K. Management of Chronic Pain in Older Adults. BMJ 2015, 350, h532. [Google Scholar] [CrossRef]
- Moseley, J.; O’Malley, K.; Petersen, N.; Menke, T.; Brody, B.; Kuykendall, D.; Hollingsworth, J.; Ashton, C.; Wray, N. A Controlled Trial of Arthroscopic Surgery for Osteoarthritis of the Knee. N. Engl. J. Med. 2002, 347, 81–88. [Google Scholar] [CrossRef]
- de l’Escalopier, N.; Anract, P.; Biau, D. Surgical Treatments for Osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Goebel, L.; Orth, P.; Cucchiarini, M.; Madry, H. Subchondral Drilling for Articular Cartilage Repair: A Systematic Review of Translational Research. Dis. Models Mech. 2018, 11, dmm034280. [Google Scholar] [CrossRef]
- Brittberg, M.; Lindahl, A.; Nilsson, A.; Ohlsson, C.; Isaksson, O.; Peterson, L. Treatment of Deep Cartilage Defects in the Knee with Autologous Chondrocyte Transplantation. N. Engl. J. Med. 1994, 331, 889–895. [Google Scholar] [CrossRef] [PubMed]
- Medvedeva, E.; Grebenik, E.; Gornostaeva, S.; Telpuhov, V.; Lychagin, A.; Timashev, P.; Chagin, A. Repair of Damaged Articular Cartilage: Current Approaches and Future Directions. Int. J. Mol. Sci. 2018, 19, 2366. [Google Scholar] [CrossRef] [PubMed]
- Mistry, H.; Connock, M.; Pink, J.; Shyangdan, D.; Clar, C.; Royle, P.; Court, R.; Biant, L.; Metcalfe, A.; Waugh, N. Autologous Chondrocyte Implantation in the Knee: Systematic Review and Economic Evaluation. Health Technol. Assess. 2017, 21, 1–294. [Google Scholar] [CrossRef]
- Bijlsma, J.; Berenbaum, F.; Lafeber, F. Osteoarthritis: An Update with Relevance for Clinical Practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Pittenger, M.; Mackay, A.; Beck, S.; Jaiswal, R.; Douglas, R.; Mosca, J.; Moorman, M.; Simonetti, D.; Craig, S.; Marshak, D. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Caplan, A. Mesenchymal Stem Cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Lanzillotti, C.; De Mattei, M.; Mazziotta, C.; Taraballi, F.; Rotondo, J.; Tognon, M.; Martini, F. Long Non-Coding Rnas and Micrornas Interplay in Osteogenic Differentiation of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 2021, 9, 646032. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wu, J. TGF-Β/SMAD Signaling Regulation of Mesenchymal Stem Cells in Adipocyte Commitment. Stem Cell Res. Ther. 2020, 11, 41. [Google Scholar] [CrossRef] [PubMed]
- Testa, S.; Riera, C.; Fornetti, E.; Riccio, F.; Fuoco, C.; Bernardini, S.; Baldi, J.; Costantini, M.; Foddai, M.; Cannata, S.; et al. Skeletal Muscle-Derived Human Mesenchymal Stem Cells: Influence of Different Culture Conditions on Proliferative and Myogenic Capabilities. Front. Physiol. 2020, 11, 553198. [Google Scholar] [CrossRef] [PubMed]
- Badyra, B.; Sułkowski, M.; Milczarek, O.; Majka, M. Mesenchymal Stem Cells as a Multimodal Treatment for Nervous System Diseases. Stem Cells Transl. Med. 2020, 9, 1174–1189. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Rousche, K.; Tuan, R. Technology Insight: Adult Stem Cells in Cartilage Regeneration and Tissue Engineering. Nat. Clin. Pract. Rheumatol. 2006, 2, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Charbord, P. Bone Marrow Mesenchymal Stem Cells: Historical Overview and Concepts. Hum. Gene Ther. 2010, 21, 1045–1056. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, C.; Gu, J.; Wu, W.; Shen, Z.; Zhou, X.; Lu, H. A Randomized, Placebo-Controlled Trial of Human Umbilical Cord Blood Mesenchymal Stem Cell Infusion for Children with Cerebral Palsy. Cell Transplant. 2018, 27, 325–334. [Google Scholar] [CrossRef]
- Ding, D.; Chang, Y.; Shyu, W.; Lin, S. Human Umbilical Cord Mesenchymal Stem Cells: A New Era for Stem Cell Therapy. Cell Transplant. 2015, 24, 339–347. [Google Scholar] [CrossRef]
- Antoniadou, E.; David, A. Placental Stem Cells. Best. Pract. Res. Clin. Obstet. Gynaecol. 2016, 31, 13–29. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, A.; Yen, T.; Hong, H. Isolation of Mesenchymal Stem Cells from Human Alveolar Periosteum and Effects of Vitamin D on Osteogenic Activity of Periosteum-Derived Cells. J. Vis. Exp. 2018. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Ichinose, S.; Shinomiya, K.; Muneta, T. Suspended Cells from Trabecular Bone by Collagenase Digestion Become Virtually Identical to Mesenchymal Stem Cells Obtained from Marrow Aspirates. Blood 2004, 104, 2728–2735. [Google Scholar] [CrossRef]
- Minteer, D.; Young, M.; Lin, Y.; Over, P.; Rubin, J.; Gerlach, J.; Marra, K. Analysis of Type II Diabetes Mellitus Adipose-Derived Stem Cells for Tissue Engineering Applications. J. Tissue Eng. 2015, 6, 204173141557921. [Google Scholar] [CrossRef] [PubMed]
- Amemiya, M.; Tsuji, K.; Sekiya, I.; Muneta, T.; Koga, H. Characteristics and Usefulness of Synovial Fluid-Derived Stem Cells Compared with Synovium-Derived Stem Cells. Osteoarthr. Cartil. 2018, 26, S23–S24. [Google Scholar] [CrossRef]
- Hollands, P.; Aboyeji, D.; Orcharton, M. Dental Pulp Stem Cells in Regenerative Medicine. Br. Dent. J. 2018, 224, 747–750. [Google Scholar] [CrossRef]
- Saghahazrati, S.; Ayatollahi, S.; Kobarfard, F.; Minaei, B. The Protective Effects of Cultured Mesenchymal Stem Cells onto the Surface of Electrospun Poly-L-Lactide Acid Scaffolds Coated with Matricaria chamomilla L. Oil in Streptozotocin-Induced Diabetic Rabbits. Iran. Red Crescent Med. J. 2019, 21, e85247. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.; Caplan, A.; Goldberg, V.; Yoo, J. In Vitro chondrogenesis of Bone Marrow-Derived Mesenchymal Progenitor Cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Schmitt, B.; Ringe, J.; Häupl, T.; Notter, M.; Manz, R.; Burmester, G.; Sittinger, M.; Kaps, C. BMP2 Initiates Chondrogenic Lineage Development of Adult Human Mesenchymal Stem Cells in High-Density Culture. Differentiation 2003, 71, 567–577. [Google Scholar] [CrossRef]
- Indrawattana, N.; Chen, G.; Tadokoro, M.; Shann, L.; Ohgushi, H.; Tateishi, T.; Tanaka, J.; Bunyaratvej, A. Growth Factor Combination for Chondrogenic Induction from Human Mesenchymal Stem Cell. Biochem. Biophys. Res. Commun. 2004, 320, 914–919. [Google Scholar] [CrossRef]
- Iturriaga, L.; Hernáez-Moya, R.; Erezuma, I.; Dolatshahi-Pirouz, A.; Orive, G. Advances in Stem Cell Therapy for Cartilage Regeneration in Osteoarthritis. Expert Opin. Biol. Ther. 2018, 18, 883–896. [Google Scholar] [CrossRef]
- Singer, N.; Caplan, A. Mesenchymal Stem Cells: Mechanisms of Inflammation. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 457–478. [Google Scholar] [CrossRef] [PubMed]
- van der Kraan, P. Stem Cell Therapy in Osteoarthritis: A Step Too Far? BioDrugs 2013, 27, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Luque-Campos, N.; Contreras-López, R.; Jose Paredes-Martínez, M.; Torres, M.; Bahraoui, S.; Wei, M.; Espinoza, F.; Djouad, F.; Elizondo-Vega, R.; Luz-Crawford, P. Mesenchymal Stem Cells Improve Rheumatoid Arthritis Progression by Controlling Memory T Cell Response. Front. Immunol. 2019, 10, 798. [Google Scholar] [CrossRef]
- Fan, L.; Hu, C.; Chen, J.; Cen, P.; Wang, J.; Li, L. Interaction between Mesenchymal Stem Cells and B-Cells. Int. J. Mol. Sci. 2016, 17, 650. [Google Scholar] [CrossRef]
- Regmi, S.; Pathak, S.; Kim, J.; Yong, C.; Jeong, J. Mesenchymal Stem Cell Therapy for the Treatment of Inflammatory Diseases: Challenges, Opportunities, and Future Perspectives. Eur. J. Cell Biol. 2019, 98, 151041. [Google Scholar] [CrossRef]
- Jiang, W.; Xu, J. Immune Modulation by Mesenchymal Stem Cells. Cell Prolif. 2019, 53, e12712. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; Pittenger, M. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef]
- Beyth, S.; Borovsky, Z.; Mevorach, D.; Liebergall, M.; Gazit, Z.; Aslan, H.; Galun, E.; Rachmilewitz, J. Human Mesenchymal Stem Cells Alter Antigen-Presenting Cell Maturation and Induce T-Cell Unresponsiveness. Blood 2005, 105, 2214–2219. [Google Scholar] [CrossRef]
- Murphy, J.; Fink, D.; Hunziker, E.; Barry, F. Stem Cell Therapy in a Caprine Model of Osteoarthritis. Arthritis Rheum. 2003, 48, 3464–3474. [Google Scholar] [CrossRef]
- Wu, L.; Leijten, J.; Georgi, N.; Post, J.; van Blitterswijk, C.; Karperien, M. Trophic Effects of Mesenchymal Stem Cells Increase Chondrocyte Proliferation and Matrix Formation. Tissue Eng. Part A 2011, 17, 1425–1436. [Google Scholar] [CrossRef] [PubMed]
- Pak, J. Regeneration of Human Bones in Hip Osteonecrosis and Human Cartilage in Knee Osteoarthritis with Autologous Adipose-Tissue-Derived Stem Cells: A Case Series. J. Med. Case Rep. 2011, 5, 296. [Google Scholar] [CrossRef] [PubMed]
- Davatchi, F.; Sadeghi Abdollahi, B.; Mohyeddin, M.; Nikbin, B. Mesenchymal Stem Cell Therapy for Knee Osteoarthritis: 5 Years Follow-Up of Three Patients. Int. J. Rheum. Dis. 2015, 19, 219–225. [Google Scholar] [CrossRef]
- Wolfstadt, J.; Cole, B.; Ogilvie-Harris, D.; Viswanathan, S.; Chahal, J. Current Concepts. Sports Health 2014, 7, 38–44. [Google Scholar] [CrossRef]
- Filardo, G.; Madry, H.; Jelic, M.; Roffi, A.; Cucchiarini, M.; Kon, E. Mesenchymal Stem Cells for the Treatment of Cartilage Lesions: From Preclinical Findings to Clinical Application in Orthopaedics. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1717–1729. [Google Scholar] [CrossRef]
- De Windt, T.; Saris, D.; Slaper-Cortenbach, I.; van Rijen, M.; Gawlitta, D.; Creemers, L.; de Weger, R.; Dhert, W.; Vonk, L. Direct Cell–Cell Contact with Chondrocytes Is a Key Mechanism in Multipotent Mesenchymal Stromal Cell-Mediated Chondrogenesis. Tissue Eng. Part A 2015, 21, 2536–2547. [Google Scholar] [CrossRef]
- Mancuso, P.; Raman, S.; Glynn, A.; Barry, F.; Murphy, J. Mesenchymal Stem Cell Therapy for Osteoarthritis: The Critical Role of the Cell Secretome. Front. Bioeng. Biotechnol. 2019, 7, 9. [Google Scholar] [CrossRef]
- Ren, G.; Zhang, L.; Zhao, X.; Xu, G.; Zhang, Y.; Roberts, A.; Zhao, R.; Shi, Y. Mesenchymal Stem Cell-Mediated Immunosuppression Occurs via Concerted Action of Chemokines and Nitric Oxide. Cell Stem Cell 2008, 2, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Baksh, D.; Song, L.; Tuan, R. Adult Mesenchymal Stem Cells: Characterization, Differentiation, and Application in Cell and Gene Therapy. J. Cell Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, B.; Flaat, M.; Gagliardi, C.; Patel, B.; Ripoll, C. Adipose-Derived Stem Cells: Isolation, Expansion and Differentiation. Methods 2008, 45, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Bourin, P.; Bunnell, B.; Casteilla, L.; Dominici, M.; Katz, A.; March, K.; Redl, H.; Rubin, J.; Yoshimura, K.; Gimble, J. Stromal Cells from the Adipose Tissue-Derived Stromal Vascular Fraction and Culture Expanded Adipose Tissue-Derived Stromal/Stem Cells: A Joint Statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar] [PubMed]
- Yu, G.; Wu, X.; Dietrich, M.; Polk, P.; Scott, L.; Ptitsyn, A.; Gimble, J. Yield and Characterization of Subcutaneous Human Adipose-Derived Stem Cells by Flow Cytometric and Adipogenic Mrna Analyzes. Cytotherapy 2010, 12, 538–546. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Shi, Y.; Galipeau, J.; Krampera, M.; Leblanc, K.; Martin, I.; Nolta, J.; Phinney, D.; Sensebe, L. Mesenchymal Stem Versus Stromal Cells: International Society for Cell & Gene Therapy (ISCT®) Mesenchymal Stromal Cell Committee Position Statement on Nomenclature. Cytotherapy 2019, 21, 1019–1024. [Google Scholar] [PubMed]
- Saeed, H.; Ahsan, M.; Saleem, Z.; Iqtedar, M.; Islam, M.; Danish, Z.; Khan, A. Mesenchymal Stem Cells (MSCs) as Skeletal Therapeutics—An Update. J. Biomed. Sci. 2016, 23, 41. [Google Scholar] [CrossRef] [PubMed]
- Rajabzadeh, N.; Fathi, E.; Farahzadi, R. Stem Cell-Based Regenerative Medicine. Stem Cell Investig. 2019, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Farajkhoda, T. An Overview on Ethical Considerations in Stem Cell Research in Iran and Ethical Recommendations. Int. J. Reprod. Biomed. 2017, 15, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Lo, B.; Parham, L. Ethical Issues in Stem Cell Research. Endocr. Rev. 2009, 30, 204–213. [Google Scholar] [CrossRef]
- Shi, Y.; Inoue, H.; Wu, J.; Yamanaka, S. Induced Pluripotent Stem Cell Technology: A Decade of Progress. Nat. Rev. Drug Discov. 2016, 16, 115–130. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, L.; An, K.; Cai, J.; Li, G.; Yang, C.; Liu, H.; Du, F.; Han, X.; Zhang, Z.; et al. Lower Genomic Stability of Induced Pluripotent Stem Cells Reflects Increased Non-Homologous End Joining. Cancer Commun. 2018, 38, 49. [Google Scholar] [CrossRef]
- Serakinci, N.; Fahrioglu, U.; Christensen, R. Mesenchymal Stem Cells, Cancer Challenges and New Directions. Eur. J. Cancer 2014, 50, 1522–1530. [Google Scholar] [CrossRef]
- Centeno, C.; Markle, J.; Dodson, E.; Stemper, I.; Williams, C.; Hyzy, M.; Ichim, T.; Freeman, M. Treatment of Lumbar Degenerative Disc Disease-Associated Radicular Pain with Culture-Expanded Autologous Mesenchymal Stem Cells: A Pilot Study on Safety and Efficacy. J. Transl. Med. 2017, 15, 197. [Google Scholar] [CrossRef]
- Riecke, J.; Johns, K.; Cai, C.; Vahidy, F.; Parsha, K.; Furr-Stimming, E.; Schiess, M.; Savitz, S. A Meta-Analysis of Mesenchymal Stem Cells in Animal Models of Parkinson’s Disease. Stem Cells Dev. 2015, 24, 2082–2090. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Alexander, W. Haematopoietic Stem Cells: Past, Present and Future. Cell Death Discov. 2017, 3, 17002. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, R.; Wilkinson, A.; Nakauchi, H. Changing Concepts in Hematopoietic Stem Cells. Science 2018, 362, 895–896. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.; Gray, D.; Lomova, A.; Kohn, D. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell 2017, 21, 574–590. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.; Simmons, P. Mesenchymal Stem Cells: Revisiting History, Concepts, and Assays. Cell Stem Cell 2008, 2, 313–319. [Google Scholar] [CrossRef]
- Lindroos, B.; Suuronen, R.; Miettinen, S. The Potential of Adipose Stem Cells in Regenerative Medicine. Stem Cell Rev. Rep. 2010, 7, 269–291. [Google Scholar] [CrossRef]
- Meirelles, L.; Chagastelles, P.; Nardi, N. Mesenchymal Stem Cells Reside in Virtually All Post-Natal Organs and Tissues. J. Cell Sci. 2006, 119, 2204–2213. [Google Scholar] [CrossRef]
- Krampera, M.; Marconi, S.; Pasini, A.; Galiè, M.; Rigotti, G.; Mosna, F.; Tinelli, M.; Lovato, L.; Anghileri, E.; Andreini, A.; et al. Induction of Neural-Like Differentiation in Human Mesenchymal Stem Cells Derived from Bone Marrow, Fat, Spleen and Thymus. Bone 2007, 40, 382–390. [Google Scholar] [CrossRef]
- Steffenhagen, C.; Dechant, F.; Oberbauer, E.; Furtner, T.; Weidner, N.; Küry, P.; Aigner, L.; Rivera, F. Mesenchymal Stem Cells Prime Proliferating Adult Neural Progenitors toward an Oligodendrocyte Fate. Stem Cells Dev. 2012, 21, 1838–1851. [Google Scholar] [CrossRef]
- Jafarian, A.; Taghikhani, M.; Abroun, S.; Pourpak, Z.; Allahverdi, A.; Soleimani, M. Generation of High-Yield Insulin Producing Cells from Human Bone Marrow Mesenchymal Stem Cells. Mol. Biol. Rep. 2014, 41, 4783–4794. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.; Zakaria, M.; Refaie, A.; Abdel-Rahman, E.; Reda, A.; Ali, S.; Khater, S.; Ashamallah, S.; Ismail, A.; Ismail, H.; et al. From Human Mesenchymal Stem Cells to Insulin-Producing Cells: Comparison between Bone Marrow- and Adipose Tissue-Derived Cells. BioMed Res. Int. 2017, 2017, 3854232. [Google Scholar] [CrossRef]
- Kennea, N.; Waddington, S.; Chan, J.; O’Donoghue, K.; Yeung, D.; Taylor, D.; Al-Allaf, F.; Pirianov, G.; Themis, M.; Edwards, A.; et al. Differentiation of Human Fetal Mesenchymal Stem Cells into Cells with an Oligodendrocyte Phenotype. Cell Cycle 2009, 8, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Loo, S.; Wong, N. Advantages and Challenges of Stem Cell Therapy for Osteoarthritis (Review). Biomed. Rep. 2021, 15, 67. [Google Scholar] [CrossRef]
- Keating, A. Mesenchymal Stromal Cells: New Directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.; Youn Oh, J. Mesenchymal Stem/Stromal Cells (MSCs): Role as Guardians of Inflammation. Mol. Ther. 2012, 20, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Su, W.; Zhou, M.; Zheng, Y.; Fan, Y.; Wang, L.; Han, Z.; Kong, D.; Zhao, R.; Wu, J.; Xiang, R.; et al. Bioluminescence Reporter Gene Imaging Characterize Human Embryonic Stem Cell-Derived Teratoma Formation. J. Cell Biochem. 2011, 112, 840–848. [Google Scholar] [CrossRef]
- Glynn, S.; Busch, M.; Dodd, R.; Katz, L.; Stramer, S.; Klein, H.; Simmons, G.; Kleinman, S.; Shurin, S. Emerging Infectious Agents and the Nation’s Blood Supply: Responding to Potential Threats in the 21St Century. Transfusion 2012, 53, 438–454. [Google Scholar] [CrossRef]
- Theo, J. Regenerative Therapy in Osteoarthritis of the Knee. J. Musculoskelet. Disord. Treat. 2016, 2, 13. [Google Scholar] [CrossRef]
- Breitbach, M.; Bostani, T.; Roell, W.; Xia, Y.; Dewald, O.; Nygren, J.; Fries, J.; Tiemann, K.; Bohlen, H.; Hescheler, J.; et al. Potential Risks of Bone Marrow Cell Transplantation into Infarcted Hearts. Blood 2007, 110, 1362–1369. [Google Scholar] [CrossRef]
- Akay, I.; Oxmann, D.; Helfenstein, A.; Mentlein, R.; Schünke, M.; Hassenpflug, J.; Kurz, B. Tumor Risk by Tissue Engineering: Cartilaginous Differentiation of Mesenchymal Stem Cells Reduces Tumor Growth. Osteoarthr. Cartil. 2010, 18, 389–396. [Google Scholar] [CrossRef]
- Rai, V.; Dilisio, M.; Dietz, N.; Agrawal, D. Recent Strategies in Cartilage Repair: A Systemic Review of the Scaffold Development and Tissue Engineering. J. Biomed. Mater. Res. Part A 2017, 105, 2343–2354. [Google Scholar] [CrossRef] [PubMed]
- Somoza, R.; Welter, J.; Correa, D.; Caplan, A. Chondrogenic Differentiation of Mesenchymal Stem Cells: Challenges and Unfulfilled Expectations. Tissue Eng. Part B Rev. 2014, 20, 596–608. [Google Scholar] [CrossRef]
- Oryan, A.; Kamali, A.; Moshiri, A.; Baghaban Eslaminejad, M. Role of Mesenchymal Stem Cells in Bone Regenerative Medicine: What Is the Evidence? Cells Tissues Organs. 2017, 204, 59–83. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Sun, Y.; Wang, B.; Xiong, Y.; Lin, W.; Wei, Q.; Wang, H.; He, W.; Wang, B.; et al. Tissue Source Determines the Differentiation Potentials of Mesenchymal Stem Cells: A Comparative Study of Human Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue. Stem Cell Res. Ther. 2017, 8, 275. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Choi, Y.; Kim, H.; Kim, H. Comparison of Molecular Profiles of Human Mesenchymal Stem Cells Derived from Bone Marrow, Umbilical Cord Blood, Placenta and Adipose Tissue. Int. J. Mol. Med. 2015, 37, 115–125. [Google Scholar] [CrossRef]
- Tsuji, W. Adipose-Derived Stem Cells: Implications in Tissue Regeneration. World J. Stem Cells 2014, 6, 312–321. [Google Scholar] [CrossRef]
- Sakaguchi, Y.; Sekiya, I.; Yagishita, K.; Muneta, T. Comparison of Human Stem Cells Derived from Various Mesenchymal Tissues: Superiority of Synovium as a Cell Source. Arthritis Rheum. 2005, 52, 2521–2529. [Google Scholar] [CrossRef] [PubMed]
- Mochizuki, T.; Muneta, T.; Sakaguchi, Y.; Nimura, A.; Yokoyama, A.; Koga, H.; Sekiya, I. Higher Chondrogenic Potential of Fibrous Synovium– and Adipose Synovium–Derived Cells Compared with Subcutaneous Fat–Derived Cells: Distinguishing Properties of Mesenchymal Stem Cells in Humans. Arthritis Rheum. 2006, 54, 843–853. [Google Scholar] [CrossRef]
- Volarevic, V.; Gazdic, M.; Simovic Markovic, B.; Jovicic, N.; Djonov, V.; Arsenijevic, N. Mesenchymal Stem Cell-Derived Factors: Immuno-Modulatory Effects and Therapeutic Potential. BioFactors 2017, 43, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of Knee Osteoarthritis with Autologous Mesenchymal Stem Cells. Transplantation 2013, 95, 1535–1541. [Google Scholar] [CrossRef]
- Wong, K.; Lee, K.; Tai, B.; Law, P.; Lee, E.; Hui, J. Injectable Cultured Bone Marrow–Derived Mesenchymal Stem Cells in Varus Knees with Cartilage Defects Undergoing High Tibial Osteotomy: A Prospective, Randomized Controlled Clinical Trial with 2 Years’ Follow-Up. Arthroscopy 2013, 29, 2020–2028. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, H.; Chen, X.; Peng, Y.; Huang, W.; Li, X.; Li, G.; Xia, W.; Sun, Q.; Xiang, A. Human Mesenchymal Stromal Cells Enhance the Immunomodulatory Function of CD8+CD28− Regulatory T Cells. Cell. Mol. Immunol. 2014, 12, 708–718. [Google Scholar] [CrossRef]
- Nauta, A.; Fibbe, W. Immunomodulatory Properties of Mesenchymal Stromal Cells. Blood 2007, 110, 3499–3506. [Google Scholar] [CrossRef] [PubMed]
- Freitag, J.; Bates, D.; Boyd, R.; Shah, K.; Barnard, A.; Huguenin, L.; Tenen, A. Mesenchymal Stem Cell Therapy in the Treatment of Osteoarthritis: Reparative Pathways, Safety and Efficacy—A Review. BMC Musculoskelet. Disord. 2016, 17, 230. [Google Scholar] [CrossRef]
- Gupta, P.; Das, A.; Chullikana, A.; Majumdar, A. Mesenchymal Stem Cells for Cartilage Repair in Osteoarthritis. Stem Cell Res. Ther. 2012, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Shen, J.; Jin, H.; Im, H.; Sandy, J.; Chen, D. Recent Progress in Understanding Molecular Mechanisms of Cartilage Degeneration during Osteoarthritis. Ann. N. Y. Acad. Sci. 2011, 1240, 61–69. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, Y.; Li, X.; Fu, Q. Mechanisms Underlying the Protective Effects of Mesenchymal Stem Cell-Based Therapy. Cell Mol. Life Sci. 2020, 77, 2771–2794. [Google Scholar] [CrossRef] [PubMed]
- Mohamed-Ahmed, S.; Fristad, I.; Lie, S.; Suliman, S.; Mustafa, K.; Vindenes, H.; Idris, S. Adipose-Derived and Bone Marrow Mesenchymal Stem Cells: A Donor-Matched Comparison. Stem Cell Res. Ther. 2018, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Madry, H.; Gao, L.; Eichler, H.; Orth, P.; Cucchiarini, M. Bone Marrow Aspirate Concentrate-Enhanced Marrow Stimulation of Chondral Defects. Stem Cells Int. 2017, 2017, 1609685. [Google Scholar] [CrossRef]
- Haynesworth, S.; Goshima, J.; Goldberg, V.; Caplan, A. Characterization of Cells with Osteogenic Potential from Human Marrow. Bone 1992, 13, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wakitani, S.; Imoto, K.; Yamamoto, T.; Saito, M.; Murata, N.; Yoneda, M. Human Autologous Culture Expanded Bone Marrow Mesenchymal Cell Transplantation for Repair of Cartilage Defects in Osteoarthritic Knees. Osteoarthr. Cartil. 2002, 10, 199–206. [Google Scholar] [CrossRef] [PubMed]
- Busse, D. Increased Knee Cartilage Volume Indegenerative Joint Disease Usingpercutaneously Implanted, Autologousmesenchymal Stem Cells. Pain Physician 2008, 11, 343–353. [Google Scholar] [CrossRef]
- Centeno, C.; Schultz, J.; Cheever, M.; Robinson, B.; Freeman, M.; Marasco, W. Safety and Complications Reporting on the Re-Implantation of Culture-Expanded Mesenchymal Stem Cells Using Autologous Platelet Lysate Technique. Curr. Stem Cell Res. Ther. 2010, 5, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Vega, A.; Martín-Ferrero, M.; Del Canto, F.; Alberca, M.; García, V.; Munar, A.; Orozco, L.; Soler, R.; Fuertes, J.; Huguet, M.; et al. Treatment of Knee Osteoarthritis with Allogeneic Bone Marrow Mesenchymal Stem Cells. Transplantation 2015, 99, 1681–1690. [Google Scholar] [CrossRef]
- Davatchi, F.; Abdollahi, B.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal Stem Cell Therapy for Knee Osteoarthritis. Preliminary Report of Four Patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef]
- Soler, R.; Orozco, L.; Munar, A.; Huguet, M.; López, R.; Vives, J.; Coll, R.; Codinach, M.; Garcia-Lopez, J. Final Results of a Phase I–II Trial Using Ex Vivo Expanded Autologous Mesenchymal Stromal Cells for the Treatment of Osteoarthritis of the Knee Confirming Safety and Suggesting Cartilage Regeneration. Knee 2016, 23, 647–654. [Google Scholar] [CrossRef]
- Vangsness, C.; Farr, J.; Boyd, J.; Dellaero, D.; Mills, C.; LeRoux-Williams, M. Adult Human Mesenchymal Stem Cells Delivered via Intra-Articular Injection to the Knee Following Partial Medial Meniscectomy. J. Bone Jt. Surg. 2014, 96, 90–98. [Google Scholar] [CrossRef]
- Gupta, P.; Chullikana, A.; Rengasamy, M.; Shetty, N.; Pandey, V.; Agarwal, V.; Wagh, S.; Vellotare, P.; Damodaran, D.; Viswanathan, P.; et al. Efficacy and Safety of Adult Human Bone Marrow-Derived, Cultured, Pooled, Allogeneic Mesenchymal Stromal Cells (Stempeucel®): Preclinical and Clinical Trial in Osteoarthritis of the Knee Joint. Arthritis Res. Ther. 2016, 18, 301. [Google Scholar] [CrossRef]
- Chahal, J.; Gómez-Aristizábal, A.; Shestopaloff, K.; Bhatt, S.; Chaboureau, A.; Fazio, A.; Chisholm, J.; Weston, A.; Chiovitti, J.; Keating, A.; et al. Bone Marrow Mesenchymal Stromal Cell Treatment in Patients with Osteoarthritis Results in Overall Improvement in Pain and Symptoms and Reduces Synovial Inflammation. Stem Cells Transl. Med. 2019, 8, 746–757. [Google Scholar] [CrossRef]
- Zuk, P.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.; Katz, A.; Benhaim, P.; Lorenz, H.; Hedrick, M. Multilineage Cells from Human Adipose Tissue: Implications for Cell-Based Therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lee, M.; Chen, C.; Chuang, S.; Chang, L.; Ho, M.; Hung, S.; Fu, Y.; Wang, Y.; Wang, H.; et al. Proliferation and Differentiation Potential of Human Adipose-Derived Mesenchymal Stem Cells Isolated from Elderly Patients with Osteoporotic Fractures. J. Cell Mol. Med. 2012, 16, 582–592. [Google Scholar] [CrossRef]
- Jin, Y.; Lee, J. Mesenchymal Stem Cell Therapy for Bone Regeneration. Clin. Orthop. Surg. 2018, 10, 271–278. [Google Scholar] [CrossRef]
- Nepali, S.; Park, M.; Lew, H.; Kim, O. Comparative Analysis of Human Adipose-Derived Mesenchymal Stem Cells from Orbital and Abdominal Fat. Stem Cells Int. 2018, 2018, 3932615. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.; Dennis, J. Mesenchymal Stem Cells as Trophic Mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Mathieu, G.; Poignard, A.; Manicom, O.; Beaujean, F.; Rouard, H. Percutaneous Autologous Bone-Marrow Grafting for Nonunions. J. Bone Jt. Surg. 2006, 88, 322–327. [Google Scholar] [CrossRef]
- Centeno, C.; Pitts, J.; Al-Sayegh, H.; Freeman, M. Efficacy of Autologous Bone Marrow Concentrate for Knee Osteoarthritis with and without Adipose Graft. BioMed Res. Int. 2014, 2014, 370621. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, S.; Kazmerchak, S.; Heckman, M.; Zubair, A.; O’Connor, M. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2016, 45, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Calabrese, C.; De Angelis, B.; Pizzicannella, J.; Kothari, A.; Garcovich, S. Impact of the Different Preparation Methods to Obtain Human Adipose-Derived Stromal Vascular Fraction Cells (AD-SVFs) and Human Adipose-Derived Mesenchymal Stem Cells (AD-MSCs): Enzymatic Digestion Versus Mechanical Centrifugation. Int. J. Mol. Sci. 2019, 20, 5471. [Google Scholar] [CrossRef]
- Patrikoski, M.; Juntunen, M.; Boucher, S.; Campbell, A.; Vemuri, M.; Mannerström, B.; Miettinen, S. Development of Fully Defined Xeno-Free Culture System for the Preparation and Propagation of Cell Therapy-Compliant Human Adipose Stem Cells. Stem Cell Res. Ther. 2013, 4, 27. [Google Scholar] [CrossRef]
- Van Pham, P.; Bui, K.; Duong, T.; Nguyen, N.; Nguyen, T.; Le, V.; Mai, V.; Phan, N.; Le, D.; Ngoc, N. Symptomatic Knee Osteoarthritis Treatment Using Autologous Adipose Derived Stem Cells and Platelet-Rich Plasma: A Clinical Study. Biomed. Res. Ther. 2014, 1, 2. [Google Scholar] [CrossRef]
- Jo, C.; Lee, Y.; Shin, W.; Kim, H.; Chai, J.; Jeong, E.; Kim, J.; Shim, H.; Shin, J.; Shin, I.; et al. Intra-Articular Injection of Mesenchymal Stem Cells for the Treatment of Osteoarthritis of the Knee: A Proof-of-Concept Clinical Trial. Stem Cells 2014, 32, 1254–1266. [Google Scholar] [CrossRef]
- Pers, Y.; Rackwitz, L.; Ferreira, R.; Pullig, O.; Delfour, C.; Barry, F.; Sensebe, L.; Casteilla, L.; Fleury, S.; Bourin, P.; et al. Adipose Mesenchymal Stromal Cell-Based Therapy for Severe Osteoarthritis of the Knee: A Phase I Dose-Escalation Trial. Stem Cells Transl. Med. 2016, 5, 847–856. [Google Scholar] [CrossRef]
- Fodor, P.; Paulseth, S. Adipose Derived Stromal Cell (ADSC) Injections for Pain Management of Osteoarthritis in the Human Knee Joint. Aesthetic Surg. J. 2015, 36, 229–236. [Google Scholar] [CrossRef]
- Koh, Y.; Kwon, O.; Kim, Y.; Choi, Y.; Tak, D. Adipose-Derived Mesenchymal Stem Cells with Microfracture Versus Microfracture Alone: 2-Year Follow-Up of a Prospective Randomized Trial. Arthroscopy 2016, 32, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Kubosch, E.; Lang, G.; Furst, D.; Kubosch, D.; Izadpanah, K.; Rolauffs, B.; Sudkamp, N.; Schmal, H. The Potential for Synovium-Derived Stem Cells in Cartilage Repair. Curr. Stem Cell Res. Ther. 2018, 13, 174–184. [Google Scholar] [CrossRef] [PubMed]
- Hurley, E.; Yasui, Y.; Gianakos, A.; Seow, D.; Shimozono, Y.; Kerkhoffs, G.; Kennedy, J. Limited Evidence for Adipose-Derived Stem Cell Therapy on the Treatment of Osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 3499–3507. [Google Scholar] [CrossRef]
- Koh, Y.; Jo, S.; Kwon, O.; Suh, D.; Lee, S.; Park, S.; Choi, Y. Mesenchymal Stem Cell Injections Improve Symptoms of Knee Osteoarthritis. Arthroscopy 2013, 29, 748–755. [Google Scholar] [CrossRef]
- Kim, J.; Jo, C.; Kim, H.; Hwang, Y. Comparison of Immunological Characteristics of Mesenchymal Stem Cells from the Periodontal Ligament, Umbilical Cord, and Adipose Tissue. Stem Cells Int. 2018, 2018, 8429042. [Google Scholar] [CrossRef]
- Lee, W.; Kim, H.; Kim, K.; Kim, G.; Jin, W. Intra-Articular Injection of Autologous Adipose Tissue-Derived Mesenchymal Stem Cells for the Treatment of Knee Osteoarthritis: A Phase IIb, Randomized, Placebo-Controlled Clinical Trial. Stem Cells Transl. Med. 2019, 8, 504–511. [Google Scholar] [CrossRef]
- Yokota, N.; Hattori, M.; Ohtsuru, T.; Otsuji, M.; Lyman, S.; Shimomura, K.; Nakamura, N. Comparative Clinical Outcomes After Intra-Articular Injection with Adipose-Derived Cultured Stem Cells or Noncultured Stromal Vascular Fraction for the Treatment of Knee Osteoarthritis. Am. J. Sports Med. 2019, 47, 2577–2583. [Google Scholar] [CrossRef]
- Ilic, D.; Miere, C.; Lazic, E. Umbilical Cord Blood Stem Cells: Clinical Trials in Non-Hematological Disorders. Br. Med. Bull. 2012, 102, 43–57. [Google Scholar] [CrossRef]
- Nagamura-Inoue, T. Umbilical Cord-Derived Mesenchymal Stem Cells: Their Advantages and Potential Clinical Utility. World J. Stem Cells 2014, 6, 195–202. [Google Scholar] [CrossRef]
- Chen, J.; Mou, X.; Du, X.; Xiang, C. Comparative Analysis of Biological Characteristics of Adult Mesenchymal Stem Cells with Different Tissue Origins. Asian Pac. J. Trop. Med. 2015, 8, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Seo, D.; Lee, S.; Lee, S.; An, G.; Ahn, H.; Kwon, D.; Seo, K.; Kang, K. Conditioned Media from Human Umbilical Cord Blood-Derived Mesenchymal Stem Cells Stimulate Rejuvenation Function in Human Skin. Biochem. Biophys. Rep. 2018, 16, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Klontzas, M.; Kenanidis, E.; Heliotis, M.; Tsiridis, E.; Mantalaris, A. Bone and Cartilage Regeneration with the Use of Umbilical Cord Mesenchymal Stem Cells. Expert Opin. Biol. Ther. 2015, 15, 1541–1552. [Google Scholar] [CrossRef] [PubMed]
- Stanko, P.; Kaiserova, K.; Altanerova, V.; Altaner, C. Comparison of Human Mesenchymal Stem Cells Derived from Dental Pulp, Bone Marrow, Adipose Tissue, and Umbilical Cord Tissue by Gene Expression. Biomed. Pap. 2014, 158, 373–377. [Google Scholar] [CrossRef]
- Schmelzer, E.; McKeel, D.; Gerlach, J. Characterization of Human Mesenchymal Stem Cells from Different Tissues and Their Membrane Encasement for Prospective Transplantation Therapies. BioMed Res. Int. 2019, 2019, 6376271. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, Y.; Koh, Y. Intra-Articular Injection of Human Synovium-Derived Mesenchymal Stem Cells in Beagles with Surgery-Induced Osteoarthritis. Knee 2021, 28, 159–168. [Google Scholar] [CrossRef]
- Liu, C.; Yang, Y.; He, G. Efficacy and Safety of Umbilical Cord-Mesenchymal Stem Cells Transplantation for Treating Osteoarthritis. Osteoarthr. Cartil. 2017, 25, S389. [Google Scholar] [CrossRef]
- Park, Y.; Ha, C.; Lee, C.; Yoon, Y.; Park, Y. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2016, 6, 613–621. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.; He, P.; Slukvin, I.; Thomson, J. Human Embryonic Stem Cells Reprogram Myeloid Precursors Following Cell–Cell Fusion. Stem Cells 2006, 24, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.; Nishikawa, S.; Nishikawa, S.; Muguruma, K.; et al. A ROCK Inhibitor Permits Survival of Dissociated Human Embryonic Stem Cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Hong, Y.; Yao, D.; Chaudhry, G. Compression Induced Chondrogenic Differentiation of Embryonic Stem Cells in Three-Dimensional Polydimethylsiloxane Scaffolds. Tissue Eng. Part A 2017, 23, 426–435. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, X.; Liang, Y.; Gu, H.; Song, K.; Zou, X.; Zhou, G. Repair of Cartilage Defects in Osteoarthritis Rats with Induced Pluripotent Stem Cell Derived Chondrocytes. BMC Biotechnol. 2016, 16, 78. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Zhao, B.; Niu, X.; Hu, B.; Li, Q.; Zhang, J.; Ding, J.; Chen, Y.; Wang, Y. Comparison of Exosomes Secreted by Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells and Synovial Membrane-Derived Mesenchymal Stem Cells for the Treatment of Osteoarthritis. Stem Cell Res. Ther. 2017, 8, 64. [Google Scholar] [CrossRef]
- Yamashita, A.; Morioka, M.; Yahara, Y.; Okada, M.; Kobayashi, T.; Kuriyama, S.; Matsuda, S.; Tsumaki, N. Generation of Scaffoldless Hyaline Cartilaginous Tissue from Human iPSCs. Stem Cell Rep. 2015, 4, 404–418. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Uchida, S.; Utsunomiya, H.; Hatakeyama, A.; Nakashima, H.; Chang, A.; Sekiya, I.; Sakai, A. Synovial Mesenchymal Stem Cells Derived from the Cotyloid Fossa Synovium Have Higher Self-Renewal and Differentiation Potential Than Those from the Paralabral Synovium in the Hip Joint. Am. J. Sports Med. 2018, 46, 2942–2953. [Google Scholar] [CrossRef]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial Membrane Mesenchymal Stem Cells: Past Life, Current Situation, and Application in Bone and Joint Diseases. Stem Cell Res. Ther. 2020, 11, 381. [Google Scholar] [CrossRef]
- Ogata, Y.; Mabuchi, Y.; Yoshida, M.; Suto, E.; Suzuki, N.; Muneta, T.; Sekiya, I.; Akazawa, C. Purified Human Synovium Mesenchymal Stem Cells as a Good Resource for Cartilage Regeneration. PLoS ONE 2015, 10, e0129096. [Google Scholar] [CrossRef]
- Sasaki, A.; Mizuno, M.; Ozeki, N.; Katano, H.; Otabe, K.; Tsuji, K.; Koga, H.; Mochizuki, M.; Sekiya, I. Canine Mesenchymal Stem Cells from Synovium Have a Higher Chondrogenic Potential Than Those from Infrapatellar Fat Pad, Adipose Tissue, and Bone Marrow. PLoS ONE 2018, 13, e0202922. [Google Scholar] [CrossRef]
- Ozeki, N.; Muneta, T.; Koga, H.; Nakagawa, Y.; Mizuno, M.; Tsuji, K.; Mabuchi, Y.; Akazawa, C.; Kobayashi, E.; Matsumoto, K.; et al. Not Single but Periodic Injections of Synovial Mesenchymal Stem Cells Maintain Viable Cells in Knees and Inhibit Osteoarthritis Progression in Rats. Osteoarthr. Cartil. 2016, 24, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, S.; Nicolau, D.V.; Langford, B.; Mahdi, M.; Jeffers, H.; Mwasuku, C.; Krassowska, K.; Fox, R.; Binnian, I.; Glover, V.; et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): A phase 2, open-label, randomised controlled trial. Lancet Respir. Med. 2021, 9, 763–772, Erratum in Lancet Respir. Med. 2021, 9, e55. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.; Choi, Y. Infrapatellar Fat Pad-Derived Mesenchymal Stem Cell Therapy for Knee Osteoarthritis. Knee 2012, 19, 902–907. [Google Scholar] [CrossRef]
- Chuckpaiwong, B.; Charles, H.; Kraus, V.; Guilak, F.; Nunley, J. Age-Associated Increases in the Size of the Infrapatellar Fat Pad in Knee Osteoarthritis as Measured by 3T MRI. J. Orthop. Res. 2010, 28, 1149–1154. [Google Scholar] [CrossRef]
- Koga, H.; Muneta, T.; Nagase, T.; Nimura, A.; Ju, Y.; Mochizuki, T.; Sekiya, I. Comparison of Mesenchymal Tissues-Derived Stem Cells for In Vivo Chondrogenesis: Suitable Conditions for Cell Therapy of Cartilage Defects in Rabbit. Cell Tissue Res. 2008, 333, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wickham, M.; Erickson, G.; Gimble, J.; Vail, T.; Guilak, F. Multipotent Stromal Cells Derived from the Infrapatellar Fat Pad of the Knee. Clin. Orthop. Relat. Res. 2003, 412, 196–212. [Google Scholar] [CrossRef]
- Ye, K.; Felimban, R.; Traianedes, K.; Moulton, S.; Wallace, G.; Chung, J.; Quigley, A.; Choong, P.; Myers, D. Chondrogenesis of Infrapatellar Fat Pad Derived Adipose Stem Cells in 3D Printed Chitosan Scaffold. PLoS ONE 2014, 9, e99410. [Google Scholar] [CrossRef]
- Toghraie, F.; Chenari, N.; Gholipour, M.; Faghih, Z.; Torabinejad, S.; Dehghani, S.; Ghaderi, A. Treatment of Osteoarthritis with Infrapatellar Fat Pad Derived Mesenchymal Stem Cells in Rabbit. Knee 2011, 18, 71–75. [Google Scholar] [CrossRef]
- Chen, W.; Lin, C.; Huang, C.; Hsu, W.; Lee, C.; Ou, K.; Dubey, N.; Deng, W. Functional Recovery in Osteoarthritic Chondrocytes through Hyaluronic Acid and Platelet-Rich Plasma–Inhibited Infrapatellar Fat Pad Adipocytes. Am. J. Sports Med. 2016, 44, 2696–2705. [Google Scholar] [CrossRef]
- Neri, S.; Guidotti, S.; Lilli, N.; Cattini, L.; Mariani, E. Infrapatellar Fat Pad-Derived Mesenchymal Stromal Cells from Osteoarthritis Patients: In Vitro Genetic Stability and Replicative Senescence. J. Orthop. Res. 2016, 35, 1029–1037. [Google Scholar] [CrossRef]
- Huang, S.; Song, X.; Li, T.; Xiao, J.; Chen, Y.; Gong, X.; Zeng, W.; Yang, L.; Chen, C. Pellet Coculture of Osteoarthritic Chondrocytes and Infrapatellar Fat Pad-Derived Mesenchymal Stem Cells with Chitosan/Hyaluronic Acid Nanoparticles Promotes Chondrogenic Differentiation. Stem Cell Res. Ther. 2017, 8, 264. [Google Scholar] [CrossRef]
- Spasovski, D.; Spasovski, V.; Baščarević, Z.; Stojiljković, M.; Vreća, M.; Anđelković, M.; Pavlović, S. Intra-Articular Injection of Autologous Adipose-Derived Mesenchymal Stem Cells in the Treatment of Knee Osteoarthritis. J. Gene Med. 2018, 20, e3002. [Google Scholar] [CrossRef]
- Saito, T.; Yano, F.; Mori, D.; Ohba, S.; Hojo, H.; Otsu, M.; Eto, K.; Nakauchi, H.; Tanaka, S.; Chung, U.; et al. Generation of Col2a1-EGFP iPS Cells for Monitoring Chondrogenic Differentiation. PLoS ONE 2013, 8, e74137. [Google Scholar] [CrossRef]
- Guzzo, R.; O’Sullivan, M. Human Pluripotent Stem Cells: Advances in Chondrogenic Differentiation and Articular Cartilage Regeneration. Mol. Biol. Rep. 2016, 2, 113–122. [Google Scholar] [CrossRef]
- Kuroda, T.; Yasuda, S.; Kusakawa, S.; Hirata, N.; Kanda, Y.; Suzuki, K.; Takahashi, M.; Nishikawa, S.; Kawamata, S.; Sato, Y. Highly Sensitive In Vitro Methods for Detection of Residual Undifferentiated Cells in Retinal Pigment Epithelial Cells Derived from Human iPS Cells. PLoS ONE 2012, 7, e37342. [Google Scholar] [CrossRef]
- Rezuş, E.; Burlui, A.; Cardoneanu, A.; Macovei, L.A.; Tamba, B.I.; Rezuş, C. from Pathogenesis to Therapy in Knee Osteoarthritis: Bench-to-Bedside. Int. J. Mol. Sci. 2021, 22, 2697. [Google Scholar] [CrossRef] [PubMed]
- Buhrmann, C.; Mobasheri, A.; Matis, U.; Shakibaei, M. Curcumin Mediated Suppression of Nuclear Factor-Κb Promotes Chondrogenic Differentiation of Mesenchymal Stem Cells in a High-Density Co-Culture Microenvironment. Arthritis Rheum. 2010, 12, R127. [Google Scholar] [CrossRef] [PubMed]
- Giorgino, R.; Albano, D.; Fusco, S.; Peretti, G.M.; Mangiavini, L.; Messina, C. Knee Osteoarthritis: Epidemiology, Pathogenesis, and Mesenchymal Stem Cells: What Else Is New? An Update. Int. J. Mol. Sci. 2023, 24, 6405. [Google Scholar] [CrossRef]
- Muthu, S.; Patil, S.C.; Jeyaraman, N.; Jeyaraman, M.; Gangadaran, P.; Rajendran, R.L.; Oh, E.J.; Khanna, M.; Chung, H.Y.; Ahn, B.-C. Comparative Effectiveness of Adipose-Derived Mesenchymal Stromal Cells in the Management of Knee Osteoarthritis: A Meta-Analysis. World J. Orthop. 2023, 14, 23–41. [Google Scholar] [CrossRef]
- Lv, Z.; Cai, X.; Bian, Y.; Wei, Z.; Zhu, W.; Zhao, X.; Weng, X. Advances in Mesenchymal Stem Cell Therapy for Osteoarthritis: From Preclinical and Clinical Perspectives. Bioengineering 2023, 10, 195. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Administration | Therapeutic Effect in OA |
|---|---|---|
| Physical measures | lifestyle change, weight loss, adapted exercise, braces | |
| Pharmacological options | ||
| Acetaminophen | systemic | ↓ pain |
| NSAIDs | systemic, topical | ↓ pain, ↓ inflammation |
| Opioids | systemic | ↓ pain |
| Glucocorticoids | intra-articular injection | ↓ pain, ↓ inflammation |
| Duloxetine | systemic | ↓ pain |
| Capsaicin | topical | ↓ pain |
| PRP | intra-articular injection | ↓ pain, ↓ inflammation, ↑ tissue regeneration, ↑ function |
| Hyaluronic acid | intra-articular injection | ↓ pain, ↑ function |
| Glucosamine and chondroitin sulfate | systemic | ↑ function |
| MSCs | intra-articular injection | ↑ bone and cartilage regeneration, ↓ pain, ↑ function |
| Surgical intervention | ||
| Joint debridement | arthrotomy, arthroscopy | ↓ pain, ↑ function |
| Marrow stimulation methods | subchondral drilling | ↑ cartilage repair |
| Osteotomy | ↓ pain, ↓ degenerative process | |
| Joint lavage | arthroscopy | ↓ pain, ↓ inflammation |
| Arthrodesis | ↓ pain | |
| Autologous chondrocyte implantation | ↓ pain, ↑ cartilage repair | |
| Joint replacement | ↓ pain, ↑ function | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gherghel, R.; Macovei, L.A.; Burlui, M.-A.; Cardoneanu, A.; Rezus, I.-I.; Mihai, I.R.; Rezus, E. Osteoarthritis—The Role of Mesenchymal Stem Cells in Cartilage Regeneration. Appl. Sci. 2023, 13, 10617. https://doi.org/10.3390/app131910617
Gherghel R, Macovei LA, Burlui M-A, Cardoneanu A, Rezus I-I, Mihai IR, Rezus E. Osteoarthritis—The Role of Mesenchymal Stem Cells in Cartilage Regeneration. Applied Sciences. 2023; 13(19):10617. https://doi.org/10.3390/app131910617
Chicago/Turabian StyleGherghel, Robert, Luana Andreea Macovei, Maria-Alexandra Burlui, Anca Cardoneanu, Ioana-Irina Rezus, Ioana Ruxandra Mihai, and Elena Rezus. 2023. "Osteoarthritis—The Role of Mesenchymal Stem Cells in Cartilage Regeneration" Applied Sciences 13, no. 19: 10617. https://doi.org/10.3390/app131910617





