Epicardial Adipose Tissue Changes during Statin Administration in Relation to the Body Mass Index: A Longitudinal Cardiac CT Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiac CT Scanning Protocol
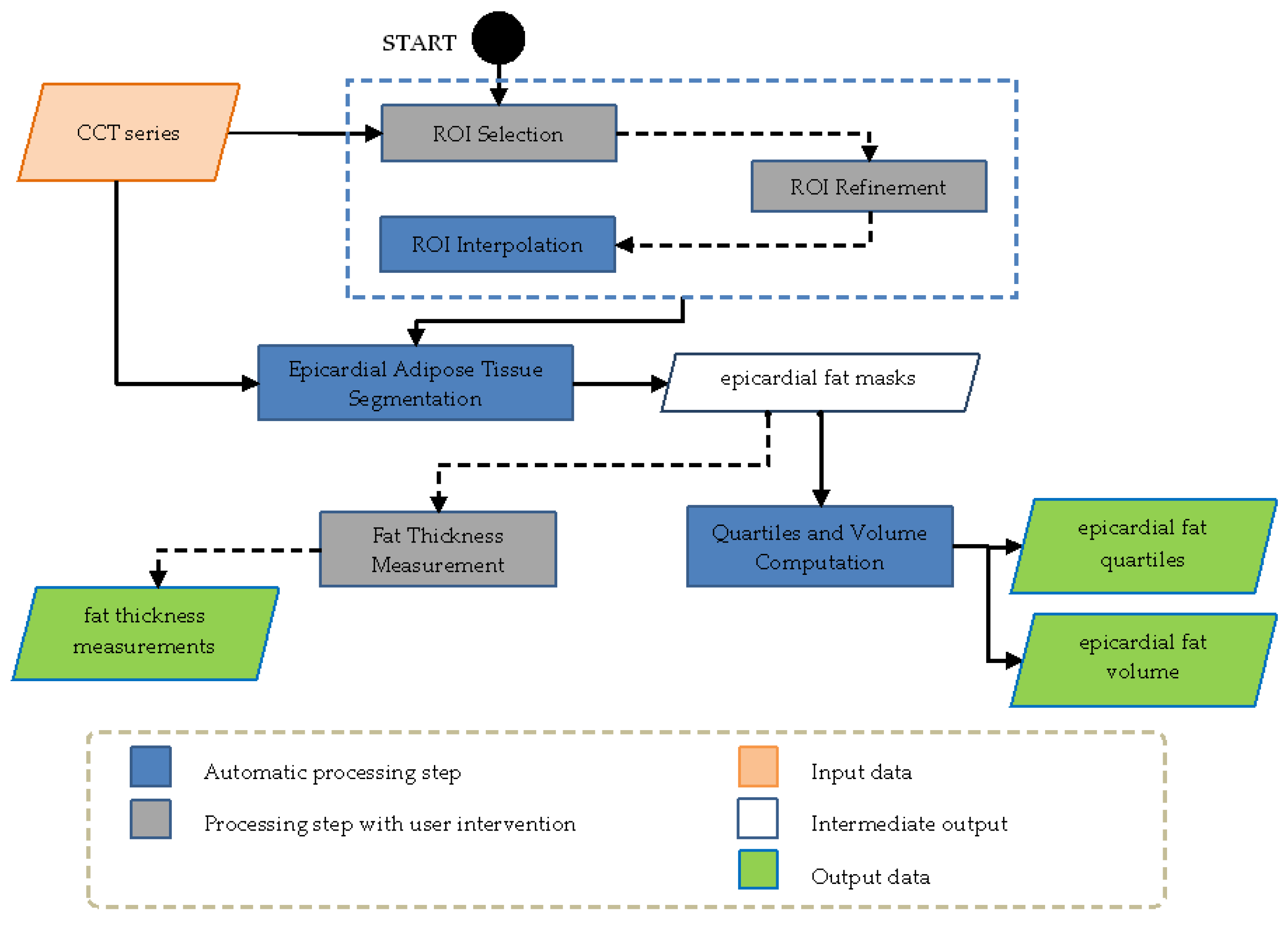
2.3. Image Analysis
- ROI Selection: after loading the CCT series, the operator selects the regions containing the heart trough free hand or polygonal lasso strategies, this procedure was implemented and ameliorated by receiving clinician’s feedback.
- ROI Interpolation: after reference ROIs are selected by the operator, the system interpolates the missing ROIs in the other slices included in the volume of interest. This procedure made it possible to reduce manual effort of the operator.
- ROI Refinement: This option allowed the operator to refine one or more interpolated ROIs. After a single slice refinement, the modification was propagated to the ROIs next to the selected slice with a further automatic interpolation. This step ensured shape and spatial continuity among refined and unrefined ROIs.
- Epicardial Adipose Tissue Segmentation: Segmentation was applied only to the volume inside the ROI using an efficient double-threshold algorithm, analyzing the voxels having HU values in the selected range. In general, this range is kept fixed, but it can be modified according to the different CT scanners or acquisition thickness protocols.
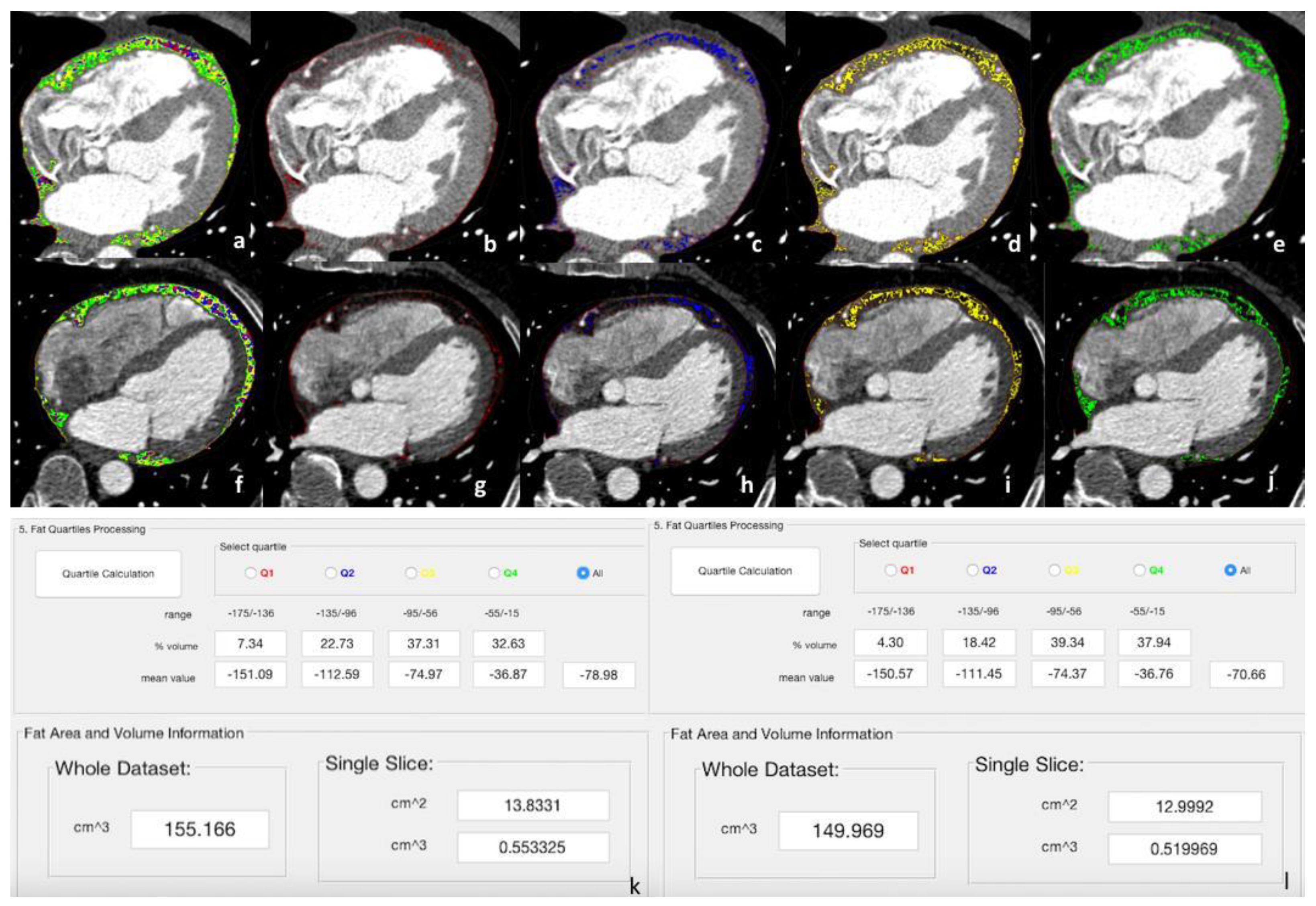
- Quartiles and Volume Computation: the fat quartiles were computed considering the range of interest in terms of Hounsfield Units (HU) and volume. In addition, the mean HU value, the percentage, and the volume (overall and of each quartile) were calculated.
2.4. Statistical Analysis
3. Results
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sacks, H.S.; Fain, J.N. Human Epicardial Adipose Tissue: A Review. Am. Heart J. 2007, 153, 907–917. [Google Scholar] [CrossRef]
- Iacobellis, G.; Corradi, D.; Sharma, A.M. Epicardial Adipose Tissue: Anatomic, Biomolecular and Clinical Relationships with the Heart. Nat. Clin. Pract. Cardiovasc. Med. 2005, 2, 536–543. [Google Scholar] [CrossRef]
- Chen, X.; Jiao, Z.; Wang, L.; Sun, Z.; Wei, Y.; Wang, X.; Xia, D. Roles of Human Epicardial Adipose Tissue in Coronary Artery Atherosclerosis. J. Huazhong Univ. Sci. Technolog. Med. Sci. 2010, 30, 589–593. [Google Scholar] [CrossRef]
- Fain, J.N.; Sacks, H.S.; Bahouth, S.W.; Tichansky, D.S.; Madan, A.K.; Cheema, P.S. Human Epicardial Adipokine Messenger RNAs: Comparisons of Their Expression in Substernal, Subcutaneous, and Omental Fat. Metabolism 2010, 59, 1379–1386. [Google Scholar] [CrossRef]
- Karastergiou, K.; Evans, I.; Ogston, N.; Miheisi, N.; Nair, D.; Kaski, J.-C.; Jahangiri, M.; Mohamed-Ali, V. Epicardial Adipokines in Obesity and Coronary Artery Disease Induce Atherogenic Changes in Monocytes and Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1340–1346. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Eringa, E.; Stehouwer, C.D.A. “Vasocrine” Signalling from Perivascular Fat: A Mechanism Linking Insulin Resistance to Vascular Disease. Lancet 2005, 365, 1817–1820. [Google Scholar] [CrossRef]
- Iacobellis, G.; Ribaudo, M.C.; Assael, F.; Vecci, E.; Tiberti, C.; Zappaterreno, A.; Di Mario, U.; Leonetti, F. Echocardiographic Epicardial Adipose Tissue Is Related to Anthropometric and Clinical Parameters of Metabolic Syndrome: A New Indicator of Cardiovascular Risk. J. Clin. Endocrinol. Metab. 2003, 88, 5163–5168. [Google Scholar] [CrossRef]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human Epicardial Adipose Tissue Is a Source of Inflammatory Mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Khan, S.S.; Ning, H.; Wilkins, J.T.; Allen, N.; Carnethon, M.; Berry, J.D.; Sweis, R.N.; Lloyd-Jones, D.M. Association of Body Mass Index With Lifetime Risk of Cardiovascular Disease and Compression of Morbidity. JAMA Cardiol. 2018, 3, 280–287. [Google Scholar] [CrossRef]
- Powell-Wiley, T.M.; Poirier, P.; Burke, L.E.; Després, J.-P.; Gordon-Larsen, P.; Lavie, C.J.; Lear, S.A.; Ndumele, C.E.; Neeland, I.J.; Sanders, P.; et al. Obesity and Cardiovascular Disease: A Scientific Statement from the American Heart Association. Circulation 2021, 143, e984–e1010. [Google Scholar] [CrossRef]
- Kavalipati, N.; Shah, J.; Ramakrishan, A.; Vasnawala, H. Pleiotropic Effects of Statins. Indian J. Endocrinol. Metab. 2015, 19, 554–562. [Google Scholar]
- La Grutta, L.; Toia, P.; Farruggia, A.; Albano, D.; Grassedonio, E.; Palmeri, A.; Maffei, E.; Galia, M.; Vitabile, S.; Cademartiri, F.; et al. Quantification of Epicardial Adipose Tissue in Coronary Calcium Score and CT Coronary Angiography Image Data Sets: Comparison of Attenuation Values, Thickness and Volumes. Br. J. Radiol. 2016, 89, 20150773. [Google Scholar] [CrossRef]
- Militello, C.; Rundo, L.; Toia, P.; Conti, V.; Russo, G.; Filorizzo, C.; Maffei, E.; Cademartiri, F.; La Grutta, L.; Midiri, M.; et al. A Semi-Automatic Approach for Epicardial Adipose Tissue Segmentation and Quantification on Cardiac CT Scans. Comput. Biol. Med. 2019, 114, 103424. [Google Scholar] [CrossRef]
- Maffei, E.; Martini, C.; Rossi, A.; Mollet, N.; Lario, C.; Castiglione Morelli, M.; Clemente, A.; Gentile, G.; Arcadi, T.; Seitun, S.; et al. Diagnostic Accuracy of Second-Generation Dual-Source Computed Tomography Coronary Angiography with Iterative Reconstructions: A Real-World Experience. Radiol. Med. 2012, 117, 725–738. [Google Scholar] [CrossRef]
- Acquisition and Reconstruction Techniques for Coronary CT Angiography. Siemens Healthineers Scanner Platforms. Available online: https://cdn-corpweb.heartflow.com/assets/docs/Siemens-Cardiac-CCTA-Protocol_042617/Siemens-Cardiac-CCTA-Protocol_042617.html (accessed on 7 September 2023).
- Yoshizumi, T.; Nakamura, T.; Yamane, M.; Islam, A.H.; Menju, M.; Yamasaki, K.; Arai, T.; Kotani, K.; Funahashi, T.; Yamashita, S.; et al. Abdominal Fat: Standardized Technique for Measurement at CT. Radiology 1999, 211, 283–286. [Google Scholar] [CrossRef]
- Christensen, R.H.; von Scholten, B.J.; Hansen, C.S.; Jensen, M.T.; Vilsbøll, T.; Rossing, P.; Jørgensen, P.G. Epicardial Adipose Tissue Predicts Incident Cardiovascular Disease and Mortality in Patients with Type 2 Diabetes. Cardiovasc. Diabetol. 2019, 18, 114. [Google Scholar] [CrossRef]
- Iacobellis, G.; Barbaro, G. The Double Role of Epicardial Adipose Tissue as pro- and Anti-Inflammatory Organ. Horm. Metab. Res. 2008, 40, 442–445. [Google Scholar] [CrossRef]
- Marwan, M.; Achenbach, S. Quantification of Epicardial Fat by Computed Tomography: Why, When and How? J. Cardiovasc. Comput. Tomogr. 2013, 7, 3–10. [Google Scholar] [CrossRef]
- Milanese, G.; Silva, M.; Bruno, L.; Goldoni, M.; Benedetti, G.; Rossi, E.; Ferrari, C.; Grutta, L.L.; Maffei, E.; Toia, P.; et al. Quantification of Epicardial Fat with Cardiac CT Angiography and Association with Cardiovascular Risk Factors in Symptomatic Patients: From the ALTER-BIO (Alternative Cardiovascular Bio-Imaging Markers) Registry. Diagn. Interv. Radiol. 2019, 25, 35–41. [Google Scholar] [CrossRef]
- Taha, D.A.; El Shafey, R.A.A.; Hamesa, M.F.; Abu-Dewan, K.A.E.-W.; Nagy, H.A. Relationship between Epicardial Fat Volume Measured by Multi-Detector Computed Tomography and Coronary Artery Disease. Egypt J. Radiol. Nucl. Med. 2021, 52, 235. [Google Scholar] [CrossRef]
- Ueno, K.; Anzai, T.; Jinzaki, M.; Yamada, M.; Jo, Y.; Maekawa, Y.; Kawamura, A.; Yoshikawa, T.; Tanami, Y.; Sato, K.; et al. Increased Epicardial Fat Volume Quantified by 64-Multidetector Computed Tomography Is Associated with Coronary Atherosclerosis and Totally Occlusive Lesions. Circ. J. 2009, 73, 1927–1933. [Google Scholar] [CrossRef]
- Förstermann, U.; Li, H. Therapeutic Effect of Enhancing Endothelial Nitric Oxide Synthase (eNOS) Expression and Preventing eNOS Uncoupling. Br. J. Pharmacol. 2011, 164, 213–223. [Google Scholar] [CrossRef]
- Sizar, O.; Khare, S.; Jamil, R.T.; Talati, R. Statin Medications. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2023. [Google Scholar]
- Grassi, D.; Desideri, G.; Ferri, C. Cardiovascular Risk and Endothelial Dysfunction: The Preferential Route for Atherosclerosis. Curr. Pharm. Biotechnol. 2011, 12, 1343–1353. [Google Scholar] [CrossRef]
- Bedi, O.; Dhawan, V.; Sharma, P.L.; Kumar, P. Pleiotropic Effects of Statins: New Therapeutic Targets in Drug Design. Naunyn. Schmiedebergs. Arch. Pharmacol. 2016, 389, 695–712. [Google Scholar] [CrossRef]
- Park, J.-H.; Park, Y.S.; Kim, Y.J.; Lee, I.S.; Kim, J.H.; Lee, J.-H.; Choi, S.W.; Jeong, J.-O.; Seong, I.-W. Effects of Statins on the Epicardial Fat Thickness in Patients with Coronary Artery Stenosis Underwent Percutaneous Coronary Intervention: Comparison of Atorvastatin with Simvastatin/ezetimibe. J. Cardiovasc. Ultrasound 2010, 18, 121–126. [Google Scholar] [CrossRef]
- Alexopoulos, N.; Melek, B.H.; Arepalli, C.D.; Hartlage, G.-R.; Chen, Z.; Kim, S.; Stillman, A.E.; Raggi, P. Effect of Intensive versus Moderate Lipid-Lowering Therapy on Epicardial Adipose Tissue in Hyperlipidemic Post-Menopausal Women: A Substudy of the BELLES Trial (Beyond Endorsed Lipid Lowering with EBT Scanning). J. Am. Coll. Cardiol. 2013, 61, 1956–1961. [Google Scholar] [CrossRef]
- Parisi, V.; Petraglia, L.; D’Esposito, V.; Cabaro, S.; Rengo, G.; Caruso, A.; Grimaldi, M.G.; Baldascino, F.; De Bellis, A.; Vitale, D.; et al. Statin Therapy Modulates Thickness and Inflammatory Profile of Human Epicardial Adipose Tissue. Int. J. Cardiol. 2019, 274, 326–330. [Google Scholar] [CrossRef]
- Ghigliotti, G.; Barisione, C.; Garibaldi, S.; Fabbi, P.; Brunelli, C.; Spallarossa, P.; Altieri, P.; Rosa, G.; Spinella, G.; Palombo, D.; et al. Adipose Tissue Immune Response: Novel Triggers and Consequences for Chronic Inflammatory Conditions. Inflammation 2014, 37, 1337–1353. [Google Scholar] [CrossRef]
- Vyas, V.; Blythe, H.; Wood, E.G.; Sandhar, B.; Sarker, S.-J.; Balmforth, D.; Ambekar, S.G.; Yap, J.; Edmondson, S.J.; Di Salvo, C.; et al. Obesity and Diabetes Are Major Risk Factors for Epicardial Adipose Tissue Inflammation. JCI Insight 2021, 6, 145495. [Google Scholar] [CrossRef]
- Raggi, P.; Gadiyaram, V.; Zhang, C.; Chen, Z.; Lopaschuk, G.; Stillman, A.E. Statins Reduce Epicardial Adipose Tissue Attenuation Independent of Lipid Lowering: A Potential Pleiotropic Effect. J. Am. Heart Assoc. 2019, 8, e013104. [Google Scholar] [CrossRef]
- Nerlekar, N.; Thakur, U.; Lin, A.; Koh, J.Q.S.; Potter, E.; Liu, D.; Muthalaly, R.G.; Rashid, H.N.; Cameron, J.D.; Dey, D.; et al. The Natural History of Epicardial Adipose Tissue Volume and Attenuation: A Long-Term Prospective Cohort Follow-up Study. Sci. Rep. 2020, 10, 7109. [Google Scholar] [CrossRef]
- Agnese, M.; Toia, P.; Sollami, G.; Militello, C.; Rundo, L.; Vitabile, S.; Maffei, E.; Agnello, F.; Gagliardo, C.; Grassedonio, E.; et al. Epicardial and Thoracic Subcutaneous Fat Texture Analysis in Patients Undergoing Cardiac CT. Heliyon 2023, 9, e15984. [Google Scholar] [CrossRef]
- Liu, Z.; Deng, Y.; Wang, X.; Liu, X.; Zheng, X.; Sun, G.; Zhen, Y.; Liu, M.; Ye, Z.; Wen, J.; et al. Radiomics Signature of Epicardial Adipose Tissue for Predicting Postoperative Atrial Fibrillation after Pulmonary Endarterectomy. Front. Cardiovasc. Med. 2022, 9, 1046931. [Google Scholar] [CrossRef]
- Kolossváry, M.; Karády, J.; Kikuchi, Y.; Ivanov, A.; Schlett, C.L.; Lu, M.T.; Foldyna, B.; Merkely, B.; Aerts, H.J.; Hoffmann, U.; et al. Radiomics versus Visual and Histogram-Based Assessment to Identify Atheromatous Lesions at Coronary CT Angiography: An Ex Vivo Study. Radiology 2019, 293, 89–96. [Google Scholar] [CrossRef]

| Parameter | Value |
|---|---|
| Age (years) (mean ± SD) | 62 ± 10 |
| Male (%) | 68 (72%) |
| Family history (%) | 51 (54%) |
| Smoking habit (%) | 43 (45%) |
| Hypertension (%) | 63 (66%) |
| Dyslipidemia (%) | 62 (65%) |
| Diabetes (%) | 13 (14%) |
| Obesity (BMI > 30) (%) | 24 (25%) |
| Anatomical Landmarks | Attenuation Values (HU) | ||
|---|---|---|---|
| First CCT | Second CCT | p-Value § | |
| Antero-superior mediastinum | −96.20 ± 24.4 | −93.39 ± 26.9 | 0.240 |
| Proximal RCA | −65.41 ± 28.5 | −61.64 ± 20.6 | 0.249 |
| Left main | −57.50 ± 32.6 | −60.66 ± 31.3 | 0.354 |
| Interventricular artery | −66.72 ± 31.5 | −68.59 ± 31.2 | 0.599 |
| Circumflex artery | −60.97 ± 28.4 | −58.6 ± 28.5 | 0.382 |
| Posterior mediastinum | −92.74 ± 31.5 | −84.99 ± 31.4 | 0.054 |
| Overall EAT | −78.18 ± 5.3 | −75.59 ± 7.0 | <0.001 |
| Liver | 64.43 ± 16.7 | 61.85 ± 23.3 | 0.323 |
| Spleen | 97.32 ± 44.8 | 100.13 ± 53.8 | 0.686 |
| Thorax subcutaneous adipose tissue | −104.13 ± 30.3 | −107.14 ± 14.6 | 0.369 |
| Abdomen subcutaneous adipose tissue | −99.40 ± 21.0 | −100.22 ± 15.9 | 0.744 |
| Intercostal muscle | 39.58 ± 26.0 | 41.02 ± 22.5 | 0.643 |
| Intra-abdominal fat | −103.94 ± 24.6 | −100.05 ± 31.3 | 0.350 |
| Anatomical Landmarks | Thickness Values (mm) | ||
|---|---|---|---|
| First CCT | Second CCT | p-Value § | |
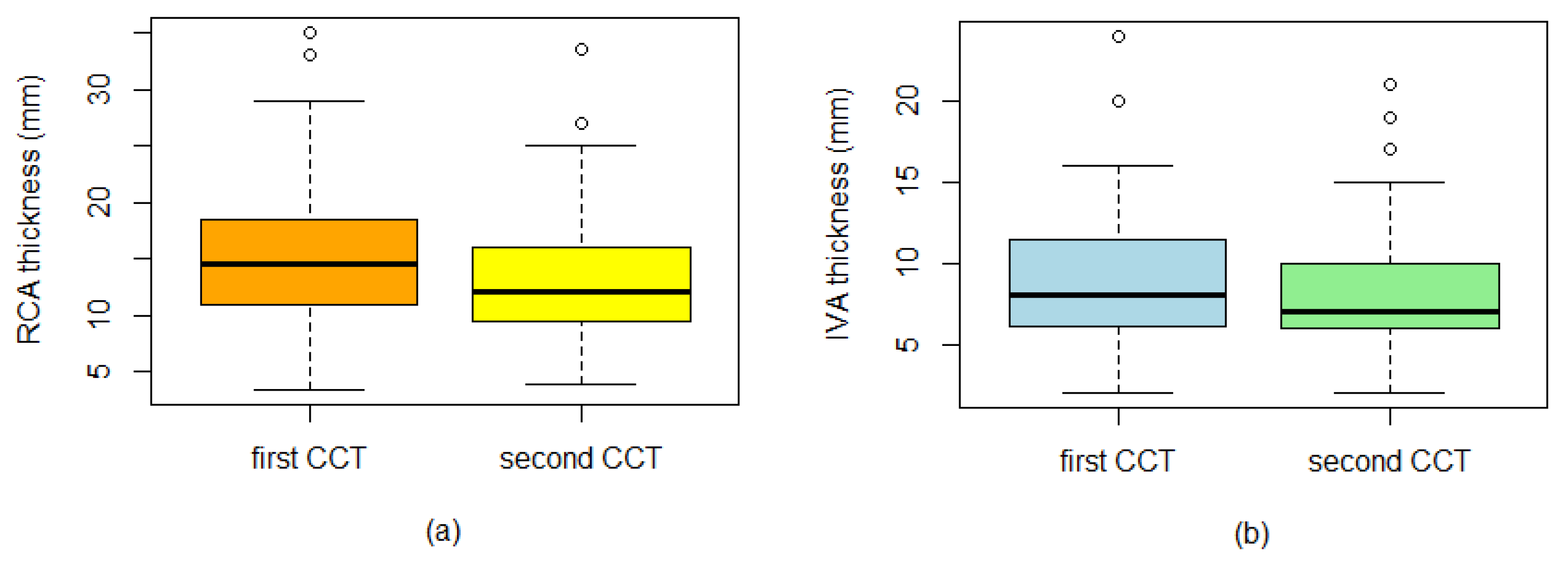
| Interventricular artery | 9.13 ± 3.9 | 8.22 ± 3.7 | 0.001 |
| Circumflex artery | 4.84 ± 2.6 | 4.76 ± 3.0 | 0.711 |
| Right coronary artery | 14.94 ± 5.8 | 13.26 ± 5.2 | 0.001 |
| Superior interventricular groove | 7.33 ± 2.9 | 6.58 ± 2.6 | <0.001 |
| Inferior interventricular groove | 1.86 ± 0.6 | 1.81 ± 1.2 | 0.663 |
| Right ventricular free wall basal tract | 4.05 ± 1.7 | 3.88 ± 2.0 | 0.195 |
| Right ventricular free wall medium tract | 5.01 ± 2.0 | 4.54 ± 2.0 | 0.002 |
| Right ventricular free wall apical tract | 3.09 ± 1.8 | 2.66 ± 1.7 | 0.001 |
| Posterior epicardial fat | 2.92 ±1.4 | 2.62 ± 1.2 | 0.002 |
| Subcutaneous thoracic fat thickness | 12.44 ± 5.1 | 11.0 ± 4.9 | 0.002 |
| First CCT | Fat % Mean (SD) p-Value | Second CCT | Fat % Mean (SD) p-Value |
|---|---|---|---|
| Q1 | Q1 | ||
| Normal weight (BMI ≤ 24.9) Overweight (BMI 25–29.9) Obese I (BMI 30–34.9) Obese II (BMI ≥ 35) | 6.68 (1.8) 6.88 (1.8) 7.66 (1.8) 10.29 (2.8) | Normal weight (BMI ≤ 24.9) Overweight (BMI 25–29.9) Obese I (BMI 30–34.9) Obese II (BMI ≥ 35) | 5.97 (2.6) 6.01 (2.9) 7.28 (2.3) 9.14 (2.1) |
| <0.001 § | 0.015 § | ||
| Q2 | Q2 | ||
| Normal weight (BMI ≤ 24.9) Overweight (BMI 25–29.9) Obese I (BMI 30–34.9) Obese II (BMI ≥ 35) | 22.02 (3.4) 23.47 (3.3) 23.75 (3.9) 24.0 (2.9) | Normal weight (BMI ≤ 24.9) Overweight (BMI 25–29.9) Obese I (BMI 30–34.9) Obese II (BMI ≥ 35) | 19.88 (3.9) 21.66 (4.2) 22.57 (3.4) 22.14 (4.3) |
| 0.214 § | 0.110 § | ||
| Q3 | Q3 | ||
| Normal weight (BMI ≤ 24.9) Overweight (BMI 25–29.9) Obese I (BMI 30–34.9) Obese II (BMI ≥ 35) | 33.73 (20.7) 37.96 (2.03) 37.0 (1.17) 33.3 (4.0) | Normal weight (BMI ≤ 24.9) Overweight (BMI 25–29.9) Obese I (BMI 30–34.9) Obese II (BMI ≥ 35) | 37.57 (3.0) 38.88 (2.9) 36.62 (3.2) 34.57 (1.9) |
| 0.440 § | 0.001 § | ||
| Q4 | Q4 | ||
| Normal weight (BMI ≤ 24.9) Overweight (BMI 25–29.9) Obese I (BMI 30–34.9) Obese II (BMI ≥ 35) | 31.37 (13.7) 31.62 (4.4) 31.78 (5.4) 30.86 (4.9) | Normal weight (BMI ≤ 24.9) Overweight (BMI 25–29.9) Obese I (BMI 30–34.9) Obese II (BMI ≥ 35) | 36.62 (5.0) 33.97 (5.1) 36.38 (16.6) 34.14 (6.5) |
| 0.995 § | 0.043 §§ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toia, P.; La Grutta, L.; Vitabile, S.; Punzo, B.; Cavaliere, C.; Militello, C.; Rundo, L.; Matranga, D.; Filorizzo, C.; Maffei, E.; et al. Epicardial Adipose Tissue Changes during Statin Administration in Relation to the Body Mass Index: A Longitudinal Cardiac CT Study. Appl. Sci. 2023, 13, 10709. https://doi.org/10.3390/app131910709
Toia P, La Grutta L, Vitabile S, Punzo B, Cavaliere C, Militello C, Rundo L, Matranga D, Filorizzo C, Maffei E, et al. Epicardial Adipose Tissue Changes during Statin Administration in Relation to the Body Mass Index: A Longitudinal Cardiac CT Study. Applied Sciences. 2023; 13(19):10709. https://doi.org/10.3390/app131910709
Chicago/Turabian StyleToia, Patrizia, Ludovico La Grutta, Salvatore Vitabile, Bruna Punzo, Carlo Cavaliere, Carmelo Militello, Leonardo Rundo, Domenica Matranga, Clarissa Filorizzo, Erica Maffei, and et al. 2023. "Epicardial Adipose Tissue Changes during Statin Administration in Relation to the Body Mass Index: A Longitudinal Cardiac CT Study" Applied Sciences 13, no. 19: 10709. https://doi.org/10.3390/app131910709
APA StyleToia, P., La Grutta, L., Vitabile, S., Punzo, B., Cavaliere, C., Militello, C., Rundo, L., Matranga, D., Filorizzo, C., Maffei, E., Galia, M., Midiri, M., Lagalla, R., Saba, L., Bossone, E., & Cademartiri, F. (2023). Epicardial Adipose Tissue Changes during Statin Administration in Relation to the Body Mass Index: A Longitudinal Cardiac CT Study. Applied Sciences, 13(19), 10709. https://doi.org/10.3390/app131910709












