Review on Surface Modification of SnO2 Electron Transport Layer for High-Efficiency Perovskite Solar Cells
Abstract
:1. Introduction
2. The Role of Surface Modification
3. Importance of Surface Modification of SnO2 ETL
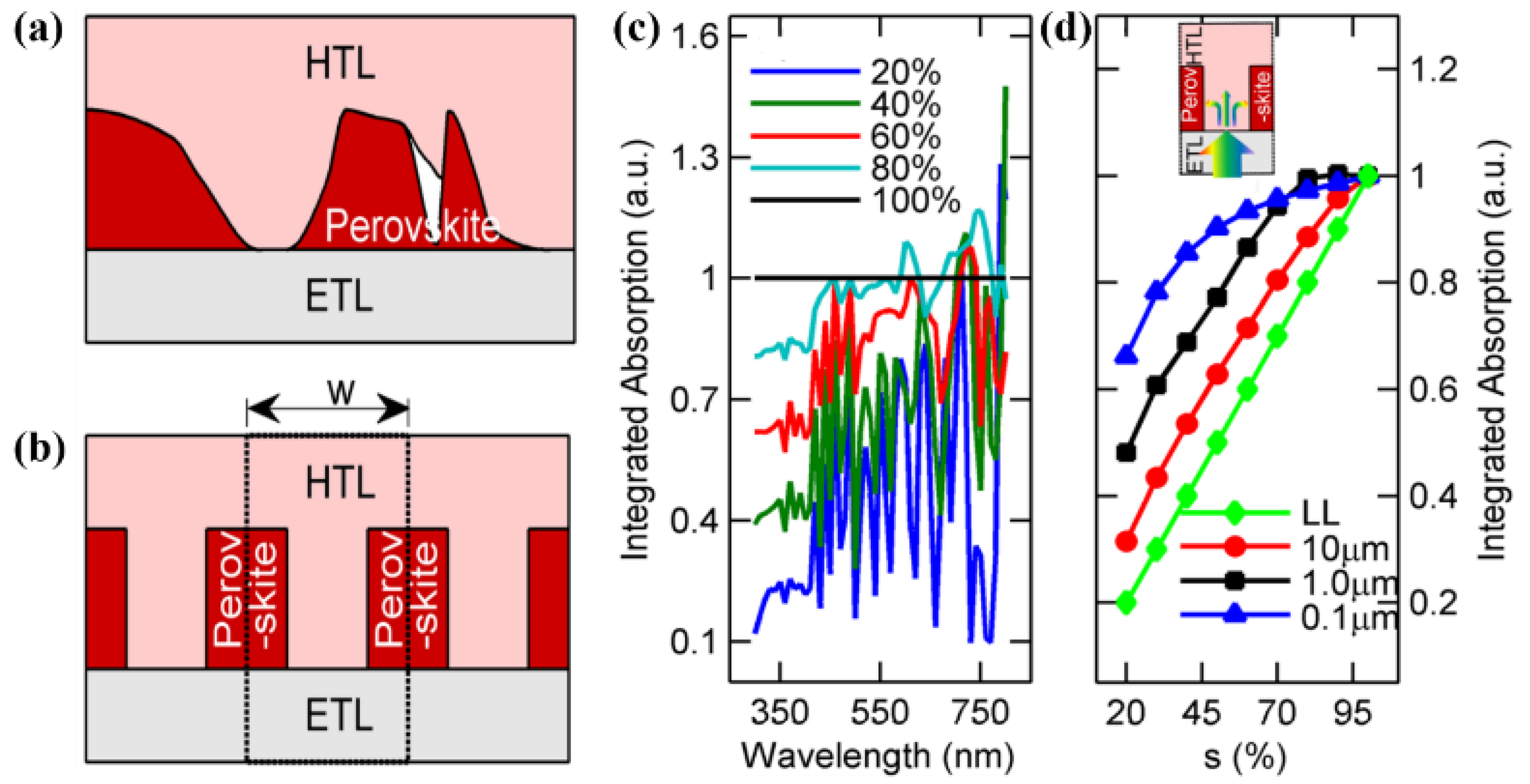
3.1. Pinholes
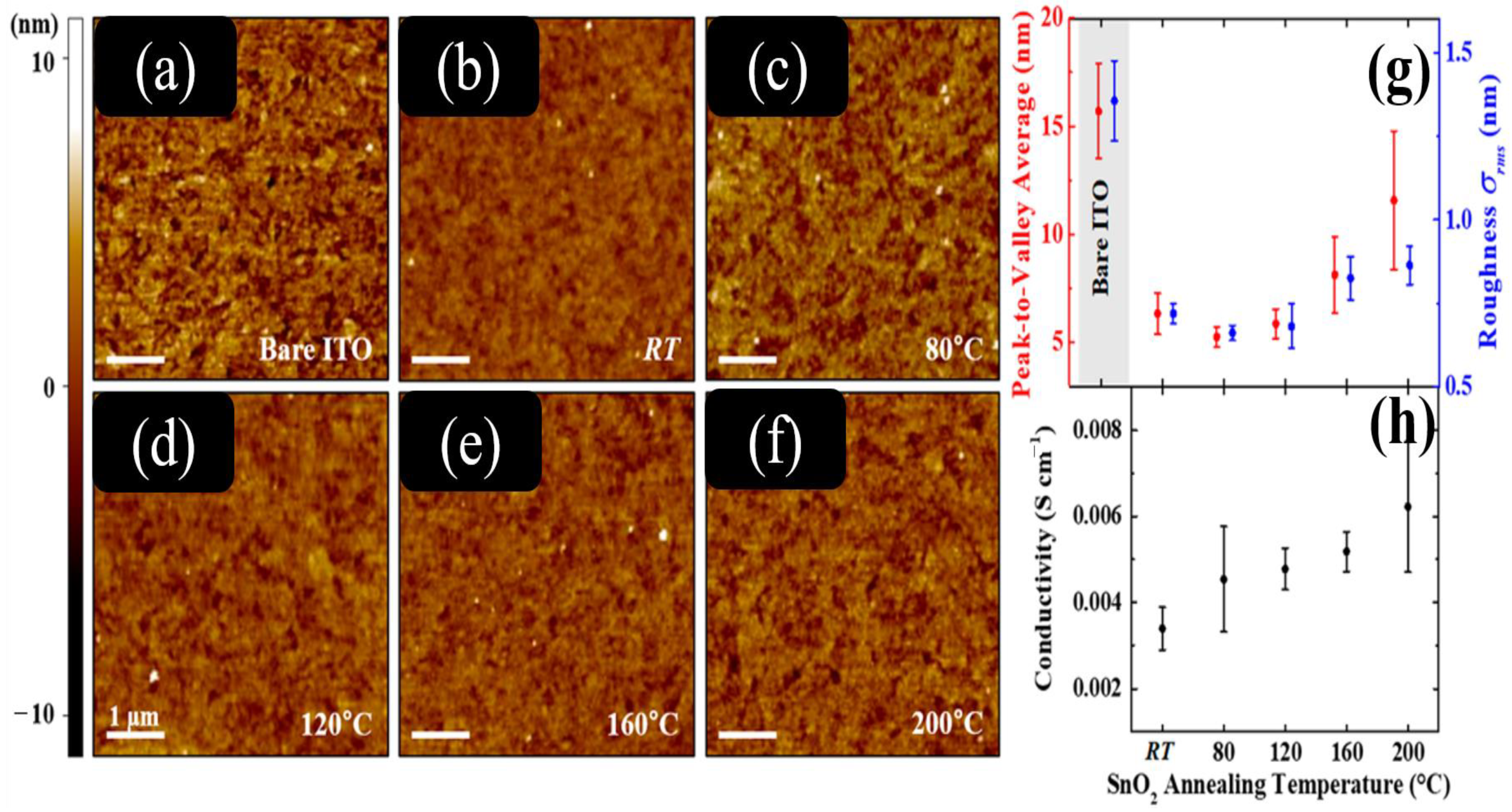
3.2. Surface Roughness
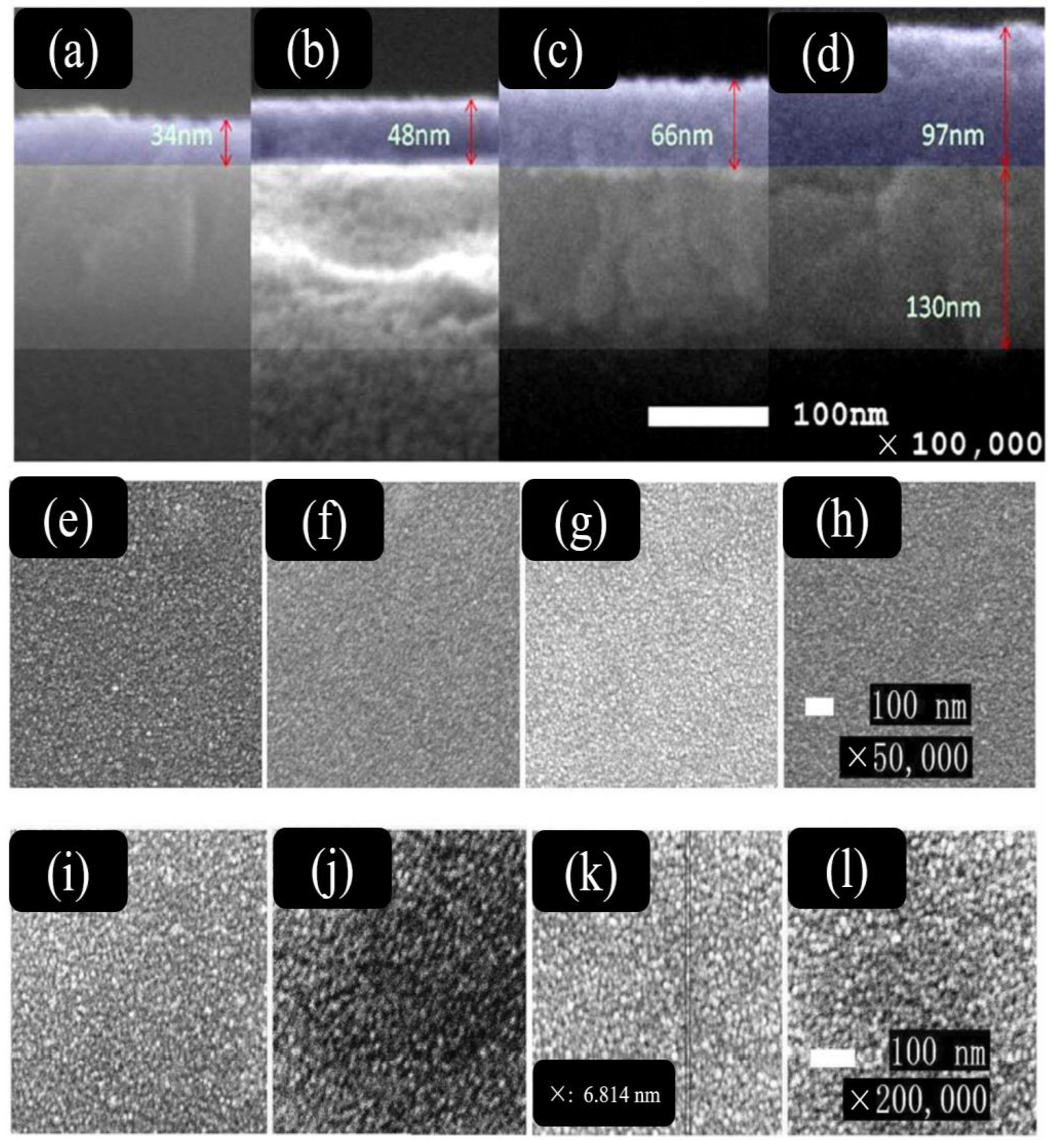
3.3. Thickness
4. Interface Design Rules for Efficient SnO2 ETL
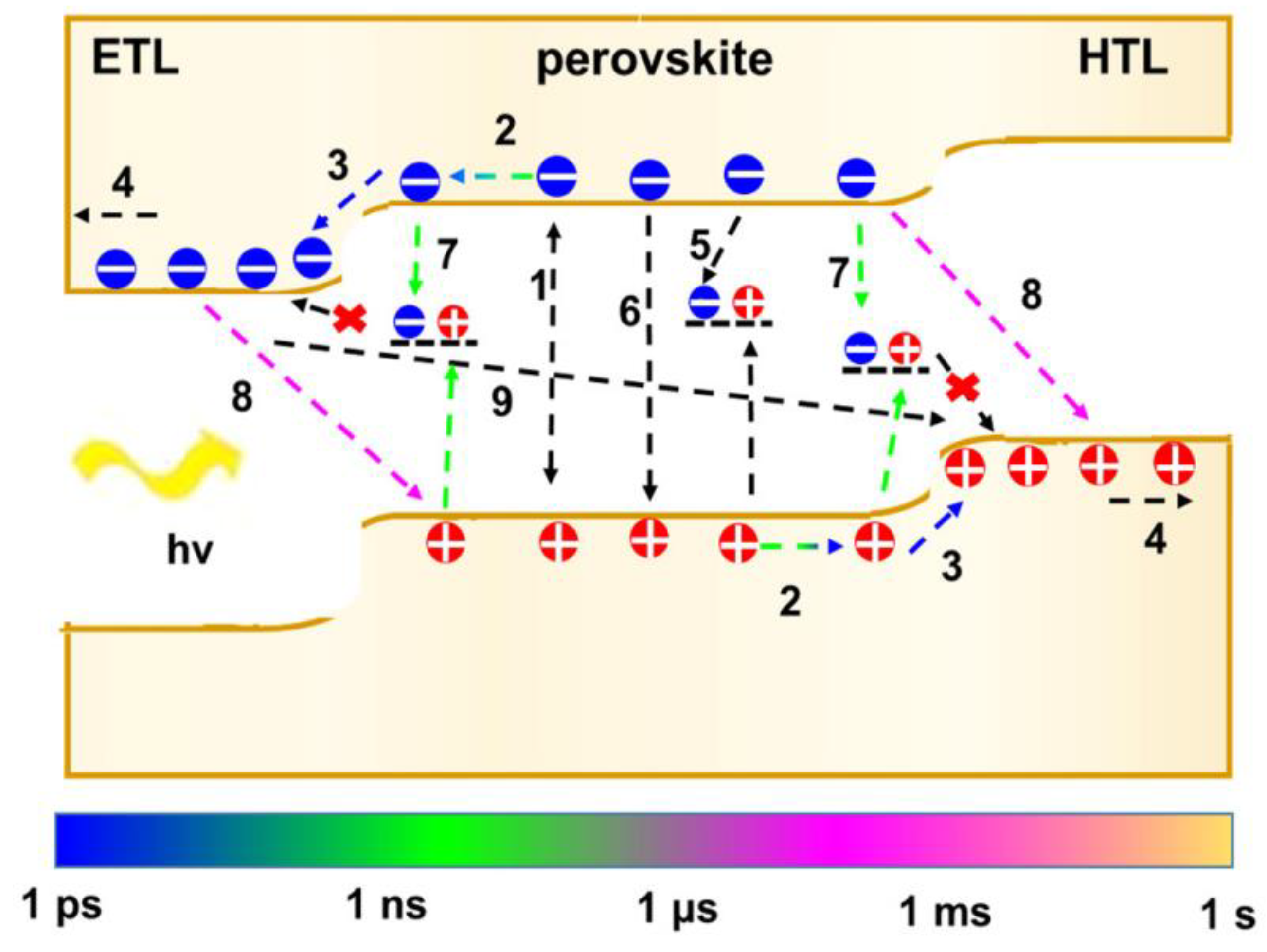
4.1. Charge Carrier Transport
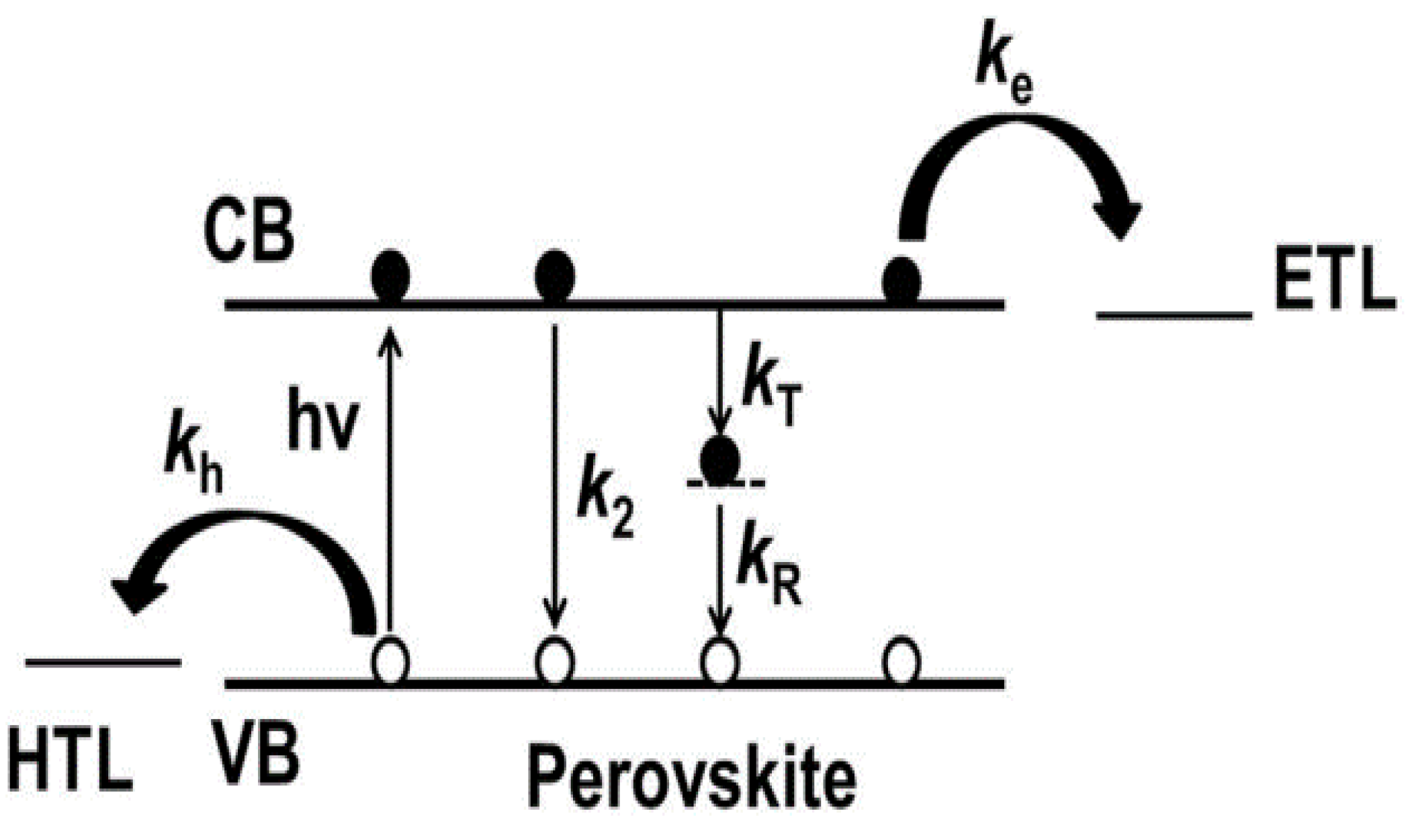
4.2. Non-Radiative Recombination
4.3. Surface Trap
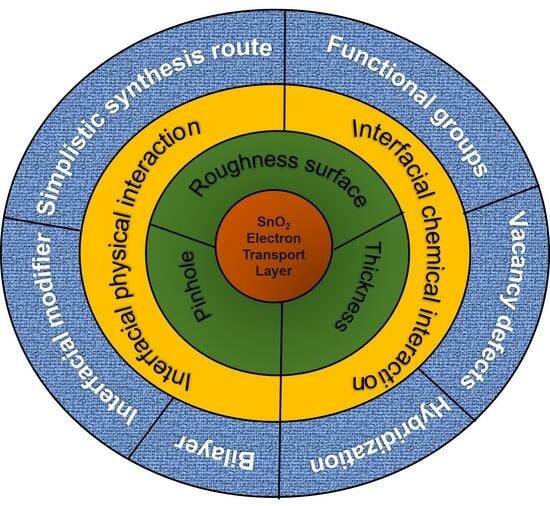
5. Surface Engineering of SnO2 ETL
5.1. Interfacial Physical Interaction
5.1.1. Simplistic Synthesis Route
5.1.2. Interface Modifier
5.1.3. Bilayer
5.2. Interfacial Chemical Interaction
5.2.1. Functional Groups
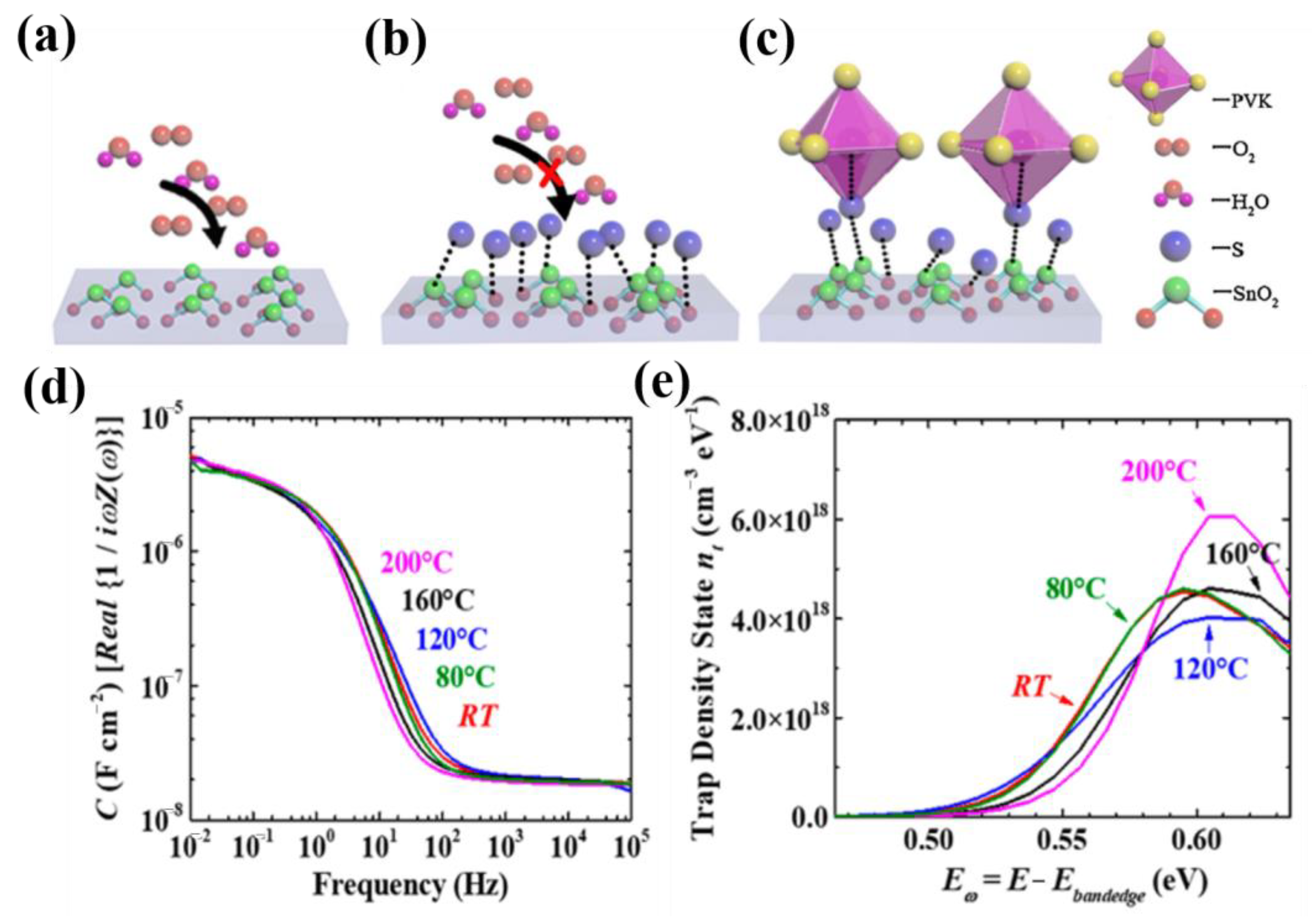
5.2.2. Vacancy Defects
5.2.3. Hybridization
6. Outlook and Perspective
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tao, M.; Fthenakis, V.; Ebin, B.; Steenari, B.-M.; Butler, E.; Sinha, P.; Corkish, R.; Wambach, K.; Simon, E.S. Major challenges and opportunities in silicon solar module recycling. Prog. Photovolt. Res. Appl. 2020, 28, 1077–1088. [Google Scholar] [CrossRef]
- Wang, X.; Tian, X.; Chen, X.; Ren, L.; Geng, C. A review of end-of-life crystalline silicon solar photovoltaic panel recycling technology. Sol. Energy Mater. Sol. Cells 2022, 248, 111976. [Google Scholar] [CrossRef]
- Shao, S.; Loi, M.A. The Role of the Interfaces in Perovskite Solar Cells. Adv. Mater. Interfaces 2020, 7, 1901469. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Mei, A.; Jiang, Y.; Hou, X.; Wang, Q.; Du, K.; Rong, Y.; Zhou, Y.; Xu, G.; et al. Improved Performance of Printable Perovskite Solar Cells with Bifunctional Conjugated Organic Molecule. Adv. Mater. 2018, 30, 1705786. [Google Scholar] [CrossRef] [PubMed]
- Park, N.-G. Perovskite solar cells: An emerging photovoltaic technology. Mater. Today 2015, 18, 65–72. [Google Scholar] [CrossRef]
- Xing, G.; Mathews, N.; Sun, S.; Lim, S.S.; Lam, Y.M.; Grätzel, M.; Mhaisalkar, S.; Sum, T.C. Long-Range Balanced Electron- and Hole-Transport Lengths in Organic-Inorganic CH3NH3PbI3. Science 2013, 342, 344–347. [Google Scholar] [CrossRef]
- Shi, D.; Adinolfi, V.; Comin, R.; Yuan, M.; Alarousu, E.; Buin, A.; Chen, Y.; Hoogland, S.; Rothenberger, A.; Katsiev, K.; et al. Low trap-state density and long carrier diffusion in organolead trihalide perovskite single crystals. Science 2015, 347, 519–522. [Google Scholar] [CrossRef]
- Stranks, S.D.; Eperon, G.E.; Grancini, G.; Menelaou, C.; Alcocer, M.J.P.; Leijtens, T.; Herz, L.M.; Petrozza, A.; Snaith, H.J. Electron-Hole Diffusion Lengths Exceeding 1 Micrometer in an Organometal Trihalide Perovskite Absorber. Science 2013, 342, 341–344. [Google Scholar] [CrossRef]
- Herz, L. Charge-carrier mobilities in metal halide perovskites: Fundamental mechanisms and limits. ACS Energy Lett. 2017, 2, 1539–1548. [Google Scholar] [CrossRef]
- Miyata, A.; Mitioglu, A.; Plochocka, P.; Portugall, O.; Wang, J.T.-W.; Stranks, S.D.; Snaith, H.J.; Nicholas, R.J. Direct measurement of the exciton binding energy and effective masses for charge carriers in organic—Inorganic tri-halide perovskites. Nat. Phys. 2015, 11, 582–587. [Google Scholar] [CrossRef]
- Even, J.; Pedesseau, L.; Katan, C. Analysis of Multivalley and Multibandgap Absorption and Enhancement of Free Carriers Related to Exciton Screening in Hybrid Perovskites. J. Phys. Chem. C 2014, 118, 11566–11572. [Google Scholar] [CrossRef]
- Hirasawa, M.; Ishihara, T.; Goto, T.; Uchida, K.; Miura, N. Magnetoabsorption of the lowest exciton in perovskite-type compound (CH3NH3)PbI3. Phys. B Condens. Matter 1994, 201, 427–430. [Google Scholar] [CrossRef]
- Tanaka, K.; Takahashi, T.; Ban, T.; Kondo, T.; Uchida, K.; Miura, N. Comparative study on the excitons in lead-halide-based perovskite-type crystals CH3NH3PbBr3 CH3NH3PbI3. Solid State Commun. 2003, 127, 619–623. [Google Scholar] [CrossRef]
- Wang, R.; Xue, J.; Wang, K.-L.; Wang, Z.-K.; Luo, Y.; Fenning, D.; Xu, G.; Nuryyeva, S.; Huang, T.; Zhao, Y.; et al. Constructive molecular configurations for surface-defect passivation of perovskite photovoltaics. Science 2019, 366, 1509–1513. [Google Scholar] [CrossRef]
- Min, H.; Kim, M.; Lee, S.-U.; Kim, H.; Kim, G.; Choi, K.; Lee, J.H.; Seok, S.I. Efficient, stable solar cells by using inherent bandgap of α-phase formamidinium lead iodide. Science 2019, 366, 749–753. [Google Scholar] [CrossRef]
- Yang, S.; Chen, S.; Mosconi, E.; Fang, Y.; Xiao, X.; Wang, C.; Zhou, Y.; Yu, Z.; Zhao, J.; Gao, Y.; et al. Stabilizing halide perovskite surfaces for solar cell operation with wide-bandgap lead oxysalts. Science 2019, 365, 473–478. [Google Scholar] [CrossRef]
- Xue, J.; Wang, R.; Wang, K.-L.; Wang, Z.-K.; Yavuz, I.; Wang, Y.; Yang, Y.; Gao, X.; Huang, T.; Nuryyeva, S.; et al. Crystalline Liquid-like Behavior: Surface-Induced Secondary Grain Growth of Photovoltaic Perovskite Thin Film. J. Am. Chem. Soc. 2019, 141, 13948–13953. [Google Scholar] [CrossRef]
- Tan, H.; Jain, A.; Voznyy, O.; Lan, X.; de Arquer, F.P.G.; Fan, J.Z.; Quintero-Bermudez, R.; Yuan, M.; Zhang, B.; Zhao, Y.; et al. Efficient and stable solution-processed planar perovskite solar cells via contact passivation. Science 2017, 355, 722–726. [Google Scholar] [CrossRef]
- Kim, H.-S.; Lee, C.-R.; Im, J.-H.; Lee, K.-B.; Moehl, T.; Marchioro, A.; Moon, S.-J.; Humphry-Baker, R.; Yum, J.-H.; Moser, J.E.; et al. Lead Iodide Perovskite Sensitized All-Solid-State Submicron Thin Film Mesoscopic Solar Cell with Efficiency Exceeding 9%. Sci. Rep. 2012, 2, 591. [Google Scholar] [CrossRef]
- Kojima, A.; Teshima, K.; Shirai, Y.; Miyasaka, T. Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells. J. Am. Chem. Soc. 2009, 131, 6050–6051. [Google Scholar] [CrossRef]
- Yang, S.; Liu, Y.; Lian, X.; Chu, J. Inactive impurity stabilizes the highly efficient perovskite photovoltaics. Joule 2022, 6, 2248–2250. [Google Scholar] [CrossRef]
- Green, M.A.; Ho-Baillie, A.; Snaith, H.J. The emergence of perovskite solar cells. Nat. Photonics 2014, 8, 506–514. [Google Scholar] [CrossRef]
- Shao, S.; Dong, J.; Duim, H.; Brink, G.H.T.; Blake, G.R.; Portale, G.; Loi, M.A. Enhancing the crystallinity and perfecting the orientation of formamidinium tin iodide for highly efficient Sn-based perovskite solar cells. Nano Energy 2019, 60, 810–816. [Google Scholar] [CrossRef]
- Deng, Y.; Peng, E.; Shao, Y.; Xiao, Z.; Dong, Q.; Huang, J. Scalable fabrication of efficient organolead trihalide perovskite solar cells with doctor-bladed active layers. Energy Environ. Sci. 2015, 8, 1544–1550. [Google Scholar] [CrossRef]
- Hwang, K.; Jung, Y.; Heo, Y.; Scholes, F.; Watkins, S.; Subbiah, J.J.A.M.; Jones, D.J.; Kim, D.-Y.; Vak, D. Toward large scale roll-to-roll production of fully printed perovskite solar cells. Adv. Mater. 2015, 27, 283. [Google Scholar] [CrossRef]
- Razza, S.; Castro-Hermosa, S.; Di Carlo, A.; Brown, T.M. Research Update: Large-area deposition, coating, printing, and processing techniques for the upscaling of perovskite solar cell technology. APL Mater. 2016, 4, 091508. [Google Scholar] [CrossRef]
- Ball, J.M.; Stranks, S.D.; Hörantner, M.T.; Hüttner, S.; Zhang, W.; Crossland, E.J.W.; Ramirez, I.; Riede, M.; Johnston, M.B.; Friend, R.H.; et al. Optical properties and limiting photocurrent of thin-film perovskite solar cells. Energy Environ. Sci. 2015, 8, 602–609. [Google Scholar] [CrossRef]
- Brittman, S.; Adhyaksa, G.W.P.; Garnett, E.C. The expanding world of hybrid perovskites: Materials properties and emerging applications. MRS Commun. 2015, 5, 7–26. [Google Scholar] [CrossRef]
- Gonzalez-Pedro, V.; Juarez-Perez, E.J.; Arsyad, W.-S.; Barea, E.M.; Fabregat-Santiago, F.; Mora-Sero, I.; Bisquert, J. General Working Principles of CH3NH3PbX3 Perovskite Solar Cells. Nano Lett. 2014, 14, 888–893. [Google Scholar] [CrossRef]
- Dkhili, M.; Lucarelli, G.; De Rossi, F.; Taheri, B.; Hammedi, K.; Ezzaouia, H.; Brunetti, F.; Brown, T.M. Attributes of High-Performance Electron Transport Layers for Perovskite Solar Cells on Flexible PET versus on Glass. ACS Appl. Energy Mater. 2022, 5, 4096–4107. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-B.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef]
- Yang, G.; Tao, H.; Qin, P.; Ke, W.; Fang, G. Recent progress in electron transport layers for efficient perovskite solar cells. J. Mater. Chem. A 2016, 4, 3970–3990. [Google Scholar] [CrossRef]
- Mosconi, E.; Grancini, G.; Roldán-Carmona, C.; Gratia, P.; Zimmermann, I.; Nazeeruddin, M.K.; De Angelis, F. Enhanced TiO2/MAPbI3 Electronic Coupling by Interface Modification with PbI2. Chem. Mater. 2016, 28, 3612–3615. [Google Scholar] [CrossRef]
- Dong, Q.; Li, J.; Shi, Y.; Chen, M.; Ono, L.K.; Zhou, K.; Zhang, C.; Qi, Y.; Zhou, Y.; Padture, N.P.; et al. Improved SnO2 Electron Transport Layers Solution-Deposited at Near Room Temperature for Rigid or Flexible Perovskite Solar Cells with High Efficiencies. Adv. Energy Mater. 2019, 9, 1900834. [Google Scholar] [CrossRef]
- Roose, B.; Baena, J.-P.C.; Gödel, K.C.; Graetzel, M.; Hagfeldt, A.; Steiner, U.; Abate, A. Mesoporous SnO2 electron selective contact enables UV-stable perovskite solar cells. Nano Energy 2016, 30, 517–522. [Google Scholar] [CrossRef]
- Leijtens, T.; Eperon, G.E.; Pathak, S.; Abate, A.; Lee, M.M.; Snaith, H.J. Overcoming ultraviolet light instability of sensitized TiO2 with meso-superstructured organometal tri-halide perovskite solar cells. Nat. Commun. 2013, 4, 2885. [Google Scholar] [CrossRef]
- Kim, H.-S.; Im, S.H.; Park, N.-G. Organolead Halide Perovskite: New Horizons in Solar Cell Research. J. Phys. Chem. C 2014, 118, 5615–5625. [Google Scholar] [CrossRef]
- Yella, A.; Heiniger, L.-P.; Gao, P.; Nazeeruddin, M.K.; Grätzel, M. Nanocrystalline Rutile Electron Extraction Layer Enables Low-Temperature Solution Processed Perovskite Photovoltaics with 13.7% Efficiency. Nano Lett. 2014, 14, 2591–2596. [Google Scholar] [CrossRef]
- Ogomi, Y.; Morita, A.; Tsukamoto, S.; Saitho, T.; Fujikawa, N.; Shen, Q.; Toyoda, T.; Yoshino, K.; Pandey, S.S.; Ma, T.; et al. CH3NH3SnxPb(1−x)I3 Perovskite Solar Cells Covering up to 1060 nm. J. Phys. Chem. Lett. 2014, 5, 1004–1011. [Google Scholar] [CrossRef]
- Liu, D.; Kelly, T.L. Perovskite solar cells with a planar heterojunction structure prepared using room-temperature solution processing techniques. Nat. Photonics 2014, 8, 133–138. [Google Scholar] [CrossRef]
- Aldakov, D.; Reiss, P. Safer-by-Design Fluorescent Nanocrystals: Metal Halide Perovskites vs. Semiconductor Quantum Dots. J. Phys. Chem. C 2019, 123, 12527–12541. [Google Scholar] [CrossRef]
- Elumalai, N.K.; Mahmud, M.A.; Wang, D.; Uddin, A. Perovskite Solar Cells: Progress and Advancements. Energies 2016, 9, 861. [Google Scholar] [CrossRef]
- Tao, S.; Schmidt, I.; Brocks, G.; Jiang, J.; Tranca, I.; Meerholz, K.; Olthof, S. Absolute energy level positions in tin- and lead-based halide perovskites. Nat. Commun. 2019, 10, 2560. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Shi, Y.; Zhang, C.; Wu, Y.; Wang, L. Energetically favored formation of SnO2 nanocrystals as electron transfer layer in perovskite solar cells with high efficiency exceeding 19%. Nano Energy 2017, 40, 336–344. [Google Scholar] [CrossRef]
- Myung, C.W.; Lee, G.; Kim, K.S. La-doped BaSnO3 electron transport layer for perovskite solar cells. J. Mater. Chem. A 2018, 6, 23071–23077. [Google Scholar] [CrossRef]
- Baena, J.P.C.; Steier, L.; Tress, W.; Saliba, M.; Neutzner, S.; Matsui, T.; Giordano, F.; Jacobsson, T.J.; Kandada, A.R.S.; Zakeeruddin, S.M.; et al. Highly efficient planar perovskite solar cells through band alignment engineering. Energy Environ. Sci. 2015, 8, 2928–2934. [Google Scholar] [CrossRef]
- Batzill, M.; Diebold, U. The surface and materials science of tin oxide. Prog. Surf. Sci. 2005, 79, 47–154. [Google Scholar] [CrossRef]
- Liu, Q.; Qin, M.-C.; Ke, W.-J.; Zheng, X.-L.; Chen, Z.; Qin, P.-L.; Xiong, L.-B.; Lei, H.-W.; Wan, J.-W.; Wen, J.; et al. Enhanced Stability of Perovskite Solar Cells with Low-Temperature Hydrothermally Grown SnO2 Electron Transport Layers. Adv. Funct. Mater. 2016, 26, 6069–6075. [Google Scholar] [CrossRef]
- Tiwana, P.; Docampo, P.; Johnston, M.B.; Snaith, H.J.; Herz, L.M. Electron Mobility and Injection Dynamics in Mesoporous ZnO, SnO2, and TiO2 Films Used in Dye-Sensitized Solar Cells. ACS Nano 2011, 5, 5158–5166. [Google Scholar] [CrossRef]
- Xiong, L.; Qin, M.; Chen, C.; Wen, J.; Yang, G.; Guo, Y.; Ma, J.; Zhang, Q.; Qin, P.; Li, S.; et al. Fully High-Temperature-Processed SnO2 as Blocking Layer and Scaffold for Efficient, Stable, Hysteresis-Free Mesoporous Perovskite Solar Cells. Adv. Funct. Mater. 2018, 28, 1706276. [Google Scholar] [CrossRef]
- Yang, Y.; Pham, N.D.; Yao, D.; Fan, L.; Hoang, M.T.; Tiong, V.T.; Wang, Z.; Zhu, H.; Wang, H. Interface Engineering to Eliminate Hysteresis of Carbon-Based Planar Heterojunction Perovskite Solar Cells via CuSCN Incorporation. ACS Appl. Mater. Interfaces 2019, 11, 28431–28441. [Google Scholar] [CrossRef]
- Yang, Y.; Hoang, M.T.; Yao, D.; Pham, N.D.; Tiong, V.T.; Wang, X.; Wang, H. Spiro-OMeTAD or CuSCN as a preferable hole transport material for carbon-based planar perovskite solar cells. J. Mater. Chem. A 2020, 8, 12723–12734. [Google Scholar] [CrossRef]
- Yang, Y.; Hoang, M.T.; Yao, D.; Pham, N.D.; Tiong, V.T.; Wang, X.; Sun, W.; Wang, H. High performance carbon-based planar perovskite solar cells by hot-pressing approach. Sol. Energy Mater. Sol. Cells 2020, 210, 110517. [Google Scholar] [CrossRef]
- Zhang, P.; Wu, J.; Zhang, T.; Wang, Y.; Liu, D.; Chen, H.; Ji, L.; Liu, C.; Ahmad, W.; Chen, Z.D.; et al. Perovskite Solar Cells with ZnO Electron-Transporting Materials. Adv. Mater. 2018, 30, 1703737. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Meng, Q.; Zhang, L.; Han, C.; Gao, H.; Zhang, Y.; Yan, H. SnO2-based electron transporting layer materials for perovskite solar cells: A review of recent progress. J. Energy Chem. 2019, 35, 144–167. [Google Scholar] [CrossRef]
- Huang, S.; Li, P.; Wang, J.; Huang, J.C.-C.; Xue, Q.; Fu, N. Modification of SnO2 electron transport Layer: Brilliant strategies to make perovskite solar cells stronger. Chem. Eng. J. 2022, 439, 135687. [Google Scholar] [CrossRef]
- Ke, W.; Zhao, D.; Cimaroli, A.J.; Grice, C.R.; Qin, P.; Liu, Q.; Xiong, L.; Yan, Y.; Fang, G. Effects of annealing temperature of tin oxide electron selective layers on the performance of perovskite solar cells. J. Mater. Chem. A 2015, 3, 24163–24168. [Google Scholar] [CrossRef]
- Liu, Z.; Chen, Q.; Hong, Z.; Zhou, H.; Xu, X.; De Marco, N.; Sun, P.; Zhao, Z.; Cheng, Y.-B.; Yang, Y. Low-Temperature TiOx Compact Layer for Planar Heterojunction Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 11076–11083. [Google Scholar] [CrossRef]
- Huang, X.; Hu, Z.; Xu, J.; Wang, P.; Wang, L.; Zhang, J.; Zhu, Y. Low-temperature processed SnO2 compact layer by incorporating TiO2 layer toward efficient planar heterojunction perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 164, 87–92. [Google Scholar] [CrossRef]
- Rao, H.-S.; Chen, B.-X.; Li, W.-G.; Xu, Y.-F.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. Improving the Extraction of Photogenerated Electrons with SnO2 Nanocolloids for Efficient Planar Perovskite Solar Cells. Adv. Funct. Mater. 2015, 25, 7200–7207. [Google Scholar] [CrossRef]
- O’Regan, B.C.; Durrant, J.R.; Sommeling, P.M.; Bakker, N.J. Influence of the TiCl4 Treatment on Nanocrystalline TiO2 Films in Dye-Sensitized Solar Cells. 2. Charge Density, Band Edge Shifts, and Quantification of Recombination Losses at Short Circuit. J. Phys. Chem. C 2007, 111, 14001–14010. [Google Scholar] [CrossRef]
- Hutter, E.M.; Hofman, J.-J.; Petrus, M.L.; Moes, M.; Abellón, R.D.; Docampo, P.; Savenije, T.J. Savenije, Charge Transfer from Methylammonium Lead Iodide Perovskite to Organic Transport Materials: Efficiencies, Transfer Rates, and Interfacial Recombination. Adv. Energy Mater. 2017, 7, 1602349. [Google Scholar] [CrossRef]
- Hutter, E.M.; Eperon, G.E.; Stranks, S.D.; Savenije, T.J. Savenije, Charge Carriers in Planar and Meso-Structured Organic–Inorganic Perovskites: Mobilities, Lifetimes, and Concentrations of Trap States. J. Phys. Chem. Lett. 2015, 6, 3082–3090. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.-H.; Raissa, R.; Abdu-Aguye, M.; Adjokatse, S.; Blake, G.R.; Even, J.; Loi, M.A. Photophysics of Organic–Inorganic Hybrid Lead Iodide Perovskite Single Crystals. Adv. Funct. Mater. 2015, 25, 2378–2385. [Google Scholar] [CrossRef]
- Shao, S.; Abdu-Aguye, M.; Qiu, L.; Lai, L.-H.; Liu, J.; Adjokatse, S.; Jahani, F.; Kamminga, M.E.; Brink, G.H.T.; Palstra, T.T.M.; et al. Elimination of the light soaking effect and performance enhancement in perovskite solar cells using a fullerene derivative. Energy Environ. Sci. 2016, 9, 2444–2452. [Google Scholar] [CrossRef]
- Unger, E.L.; Hoke, E.T.; Bailie, C.D.; Nguyen, W.H.; Bowring, A.R.; Heumüller, T.; Christoforo, M.G.; McGehee, M.D. Hysteresis and transient behavior in current–voltage measurements of hybrid-perovskite absorber solar cells. Energy Environ. Sci. 2014, 7, 3690–3698. [Google Scholar] [CrossRef]
- Zhao, C.; Chen, B.; Qiao, X.; Luan, L.; Lu, K.; Hu, B. Revealing Underlying Processes Involved in Light Soaking Effects and Hysteresis Phenomena in Perovskite Solar Cells. Adv. Energy Mater. 2015, 5, 1500279. [Google Scholar] [CrossRef]
- Agarwal, S.; Nair, P.R. Pinhole induced efficiency variation in perovskite solar cells. J. Appl. Phys. 2017, 122, 163104. [Google Scholar] [CrossRef]
- Chaudhary, S.; Yadav, V.; Negi, C.M.S.; Gupta, S.K. Active layer thickness dependence of optoelectronic performance in CH3NH3PbI3 perovskite-based planar heterojunction photodiodes. Opt. Mater. 2020, 106, 109960. [Google Scholar] [CrossRef]
- Luan, Y.; Yi, X.; Mao, P.; Wei, Y.; Zhuang, J.; Chen, N.; Lin, T.; Li, C.; Wang, J. High-Performance Planar Perovskite Solar Cells with Negligible Hysteresis Using 2,2,2-Trifluoroethanol-Incorporated SnO2. iScience 2019, 16, 433–441. [Google Scholar] [CrossRef]
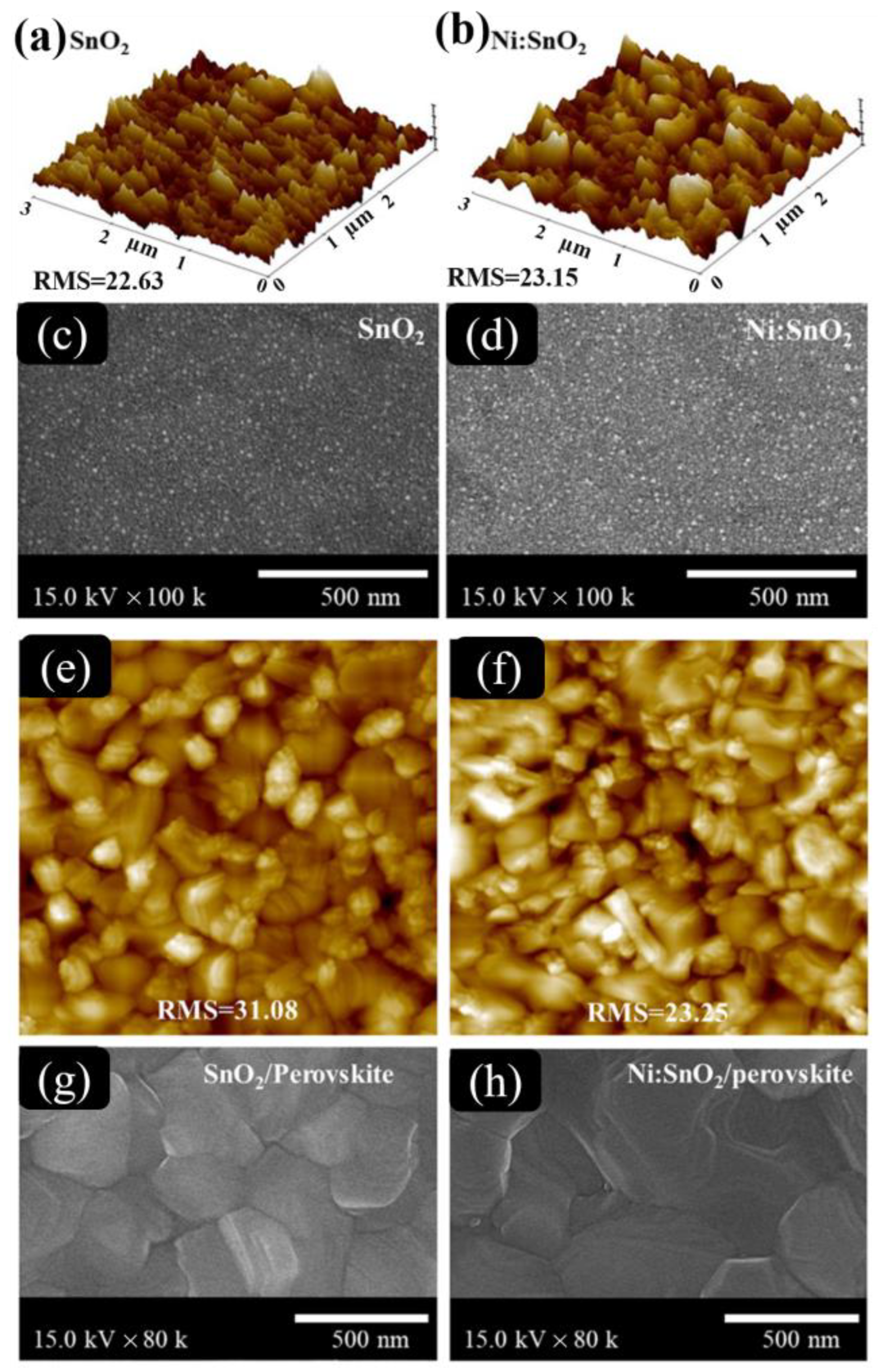
- Quy, H.V.; Bark, C.W. Ni-Doped SnO2 as an Electron Transport Layer by a Low-Temperature Process in Planar Perovskite Solar Cells. ACS Omega 2022, 7, 22256–22262. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; He, A.; Xie, S.; Yang, X.; Wu, L. Prospect of SnO2 Electron Transport Layer Deposited by Ultrasonic Spraying. Energies 2022, 15, 3211. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, F.; Chi, D.; Shi, K.; Huang, S. High-efficiency perovskite solar cells with poly(vinylpyrrolidone)-doped SnO2 as an electron transport layer. Mater. Adv. 2020, 1, 617–624. [Google Scholar] [CrossRef]
- Bi, H.; Zuo, X.; Liu, B.; He, D.; Bai, L.; Wang, W.; Li, X.; Xiao, Z.; Sun, K.; Song, Q.; et al. Multifunctional organic ammonium salt-modified SnO2 nanoparticles toward efficient and stable planar perovskite solar cells. J. Mater. Chem. A 2021, 9, 3940–3951. [Google Scholar] [CrossRef]
- Jeyakumar, R.; Bag, A.; Nekovei, R.; Radhakrishnan, R. Influence of Electron Transport Layer (TiO2) Thickness and Its Doping Density on the Performance of CH3NH3PbI3-Based Planar Perovskite Solar Cells. J. Electron. Mater. 2020, 49, 3533–3539. [Google Scholar] [CrossRef]
- Lu, H.; Ma, Y.; Gu, B.; Tian, W.; Li, L. Identifying the optimum thickness of electron transport layers for highly efficient perovskite planar solar cells. J. Mater. Chem. A 2015, 3, 16445–16452. [Google Scholar] [CrossRef]
- Hui, W.; Yang, Y.; Xu, Q.; Gu, H.; Feng, S.; Su, Z.; Zhang, M.; Wang, J.; Li, X.; Fang, J.; et al. Red-Carbon-Quantum-Dot-Doped SnO2 Composite with Enhanced Electron Mobility for Efficient and Stable Perovskite Solar Cells. Adv. Mater. 2020, 32, 1906374. [Google Scholar] [CrossRef]
- Ke, W.; Fang, G.; Liu, Q.; Xiong, L.; Qin, P.; Tao, H.; Wang, J.; Lei, H.; Li, B.; Wan, J.; et al. Low-Temperature Solution-Processed Tin Oxide as an Alternative Electron Transporting Layer for Efficient Perovskite Solar Cells. J. Am. Chem. Soc. 2015, 137, 6730–6733. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, D.; Grice, C.R.; Liao, W.; Yu, Y.; Cimaroli, A.; Shrestha, N.; Roland, P.J.; Chen, J.; Yu, Z.; et al. Low-temperature plasma-enhanced atomic layer deposition of tin oxide electron selective layers for highly efficient planar perovskite solar cells. J. Mater. Chem. A 2016, 4, 12080–12087. [Google Scholar] [CrossRef]
- Yeom, E.J.; Shin, S.S.; Yang, W.S.; Lee, S.J.; Yin, W.; Kim, D.; Noh, J.H.; Ahn, T.K.; Seok, S.I. Controllable synthesis of single crystalline Sn-based oxides and their application in perovskite solar cells. J. Mater. Chem. A 2017, 5, 79–86. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, C.; Ke, W.; Gorman, B.P.; Ye, J.; Jiang, C.-S.; Yan, Y.; Al-Jassim, M.M. Junction Quality of SnO2-Based Perovskite Solar Cells Investigated by Nanometer-Scale Electrical Potential Profiling. ACS Appl. Mater. Interfaces 2017, 9, 38373–38380. [Google Scholar] [CrossRef]
- Anaraki, E.H.; Kermanpur, A.; Steier, L.; Domanski, K.; Matsui, T.; Tress, W.; Saliba, M.; Abate, A.; Grätzel, M.; Hagfeldt, A.; et al. Highly efficient and stable planar perovskite solar cells by solution-processed tin oxide. Energy Environ. Sci. 2016, 9, 3128–3134. [Google Scholar] [CrossRef]
- Wang, C.; Guan, L.; Zhao, D.; Yu, Y.; Grice, C.R.; Song, Z.; Awni, R.A.; Chen, J.; Wang, J.; Zhao, X.; et al. Water Vapor Treatment of Low-Temperature Deposited SnO2 Electron Selective Layers for Efficient Flexible Perovskite Solar Cells. ACS Energy Lett. 2017, 2, 2118–2124. [Google Scholar] [CrossRef]
- Li, F.; Xu, M.; Ma, X.; Shen, L.; Zhu, L.; Weng, Y.; Yue, G.; Tan, F.; Chen, C. UV Treatment of Low-Temperature Processed SnO2 Electron Transport Layers for Planar Perovskite Solar Cells. Nanoscale Res. Lett. 2018, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-H.; Seo, J.-Y.; Lee, S.; Shin, H.; Park, N.-G. Solution-processed SnO2 thin film for a hysteresis-free planar perovskite solar cell with a power conversion efficiency of 19.2%. J. Mater. Chem. A 2017, 5, 24790–24803. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, J.; Guo, P.; Liu, X.; Guo, Q.; Liu, Q.; Luo, H. Low-temperature sintered SnO2 electron transport layer for efficient planar perovskite solar cells. J. Mater. Sci. Mater. Electron. 2018, 29, 13138–13147. [Google Scholar] [CrossRef]
- Chen, J.-Y.; Chueh, C.-C.; Zhu, Z.; Chen, W.-C.; Jen, A.K.Y. Low-temperature electrodeposited crystalline SnO2 as an efficient electron-transporting layer for conventional perovskite solar cells. Sol. Energy Mater. Sol. Cells 2017, 164, 47–55. [Google Scholar] [CrossRef]
- Muthukrishnan, A.P.; Lee, J.; Kim, J.; Kim, C.S.; Jo, S. Low-temperature solution-processed SnO2 electron transport layer modified by oxygen plasma for planar perovskite solar cells. RSC Adv. 2022, 12, 4883–4890. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, Z.; Snaith, H.J.; Johnston, M.B.; Herz, L.M. Hybrid Perovskites: Prospects for Concentrator Solar Cells. Adv. Sci. 2018, 5, 1700792. [Google Scholar] [CrossRef]
- Lee, H.S.; Kim, M.K.; Pae, S.R.; Kim, D.; Seo, H.; Boonmongkolras, P.; Gereige, I.; Park, S.; Shin, B. Tuning the wettability of the blade enhances solution-sheared perovskite solar cell performance. Nano Energy 2020, 74, 104830. [Google Scholar] [CrossRef]
- Feleki, B.T.; Chandrashekar, S.; Bouwer, R.K.M.; Wienk, M.M.; Janssen, R.A.J. Development of a Perovskite Solar Cell Architecture for Opaque Substrates. Sol. RRL 2020, 4, 2000385. [Google Scholar] [CrossRef]
- Shirayama, M.; Kadowaki, H.; Miyadera, T.; Sugita, T.; Tamakoshi, M.; Kato, M.; Fujiseki, T.; Murata, D.; Hara, S.; Murakami, T.N.; et al. Optical Transitions in Hybrid Perovskite Solar Cells: Ellipsometry. Density Functional Theory, and Quantum Efficiency Analyses for CH3NH3PbI3. Phys. Rev. Appl. 2016, 5, 014012. [Google Scholar] [CrossRef]
- Contreras-Bernal, L.; Ramos-Terrón, S.; Riquelme, A.; Boix, P.P.; Idígoras, J.; Mora-Seró, I.; Anta, J.A. Impedance analysis of perovskite solar cells: A case study. J. Mater. Chem. A 2019, 7, 12191–12200. [Google Scholar] [CrossRef]
- Pan, H.; Shao, H.; Zhang, X.L.; Shen, Y.; Wang, M. Interface engineering for high-efficiency perovskite solar cells. J. Appl. Phys. 2021, 129, 130904. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, H.; Fang, Y.; Stieg, A.Z.; Song, T.-B.; Wang, H.-H.; Xu, X.; Liu, Y.; Lu, S.; You, J.; et al. The optoelectronic role of chlorine in CH3NH3PbI3(Cl)-based perovskite solar cells. Nat. Commun. 2015, 6, 7269. [Google Scholar] [CrossRef]
- Yu, M.; Wang, H.-Y.; Hao, M.-Y.; Qin, Y.; Fu, L.-M.; Zhang, J.-P.; Ai, X.-C. Power output and carrier dynamics studies of perovskite solar cells under working conditions. Phys. Chem. Chem. Phys. 2017, 19, 19922–19927. [Google Scholar] [CrossRef]
- Bisquert, J.; Zaban, A.; Greenshtein, M.; Mora-Seró, I. Determination of Rate Constants for Charge Transfer and the Distribution of Semiconductor and Electrolyte Electronic Energy Levels in Dye-Sensitized Solar Cells by Open-Circuit Photovoltage Decay Method. J. Am. Chem. Soc. 2004, 126, 13550–13559. [Google Scholar] [CrossRef]
- Huo, M.-M.; Hu, R.; Zhang, Q.-S.; Chen, S.; Gao, X.; Zhang, Y.; Yan, W.; Wang, Y. Morphology and carrier non-geminate recombination dynamics regulated by solvent additive in polymer/fullerene solar cells. RSC Adv. 2020, 10, 23128–23135. [Google Scholar] [CrossRef]
- Kim, S.; Zhang, F.; Tong, J.; Chen, X.; Enkhbayar, E.; Zhu, K.; Kim, J. Effects of potassium treatment on SnO2 electron transport layers for improvements of perovskite solar cells. Sol. Energy 2022, 233, 353–362. [Google Scholar] [CrossRef]
- Kim, J.; Kim, K.S.; Myung, C.W. Efficient electron extraction of SnO2 electron transport layer for lead halide perovskite solar cell. npj Comput. Mater. 2020, 6, 100. [Google Scholar] [CrossRef]
- Hu, W.; Yang, S.; Yang, S. Surface Modification of TiO2 for Perovskite Solar Cells. Trends Chem. 2020, 2, 148–162. [Google Scholar] [CrossRef]
- Park, J.-W.; Baik, H.-K.; Lim, T.; Ju, S. Threshold voltage control of oxide nanowire transistors using nitrogen plasma treatment. Appl. Phys. Lett. 2010, 97, 203508. [Google Scholar] [CrossRef]
- Shockley, W.; Queisser, H.J. Detailed Balance Limit of Efficiency of p-n Junction Solar Cells. J. Appl. Phys. 1961, 32, 510–519. [Google Scholar] [CrossRef]
- Liu, Z.; Krückemeier, L.; Krogmeier, B.; Klingebiel, B.; Márquez, J.A.; Levcenko, S.; Öz, S.; Mathur, S.; Rau, U.; Unold, T.; et al. Open-Circuit Voltages Exceeding 1.26 V in Planar Methylammonium Lead Iodide Perovskite Solar Cells. ACS Energy Lett. 2019, 4, 110–117. [Google Scholar] [CrossRef]
- Buin, A.; Pietsch, P.; Xu, J.; Voznyy, O.; Ip, A.H.; Comin, R.; Sargent, E.H. Materials Processing Routes to Trap-Free Halide Perovskites. Nano Lett. 2014, 14, 6281–6286. [Google Scholar] [CrossRef]
- Xie, L.; Chen, J.; Vashishtha, P.; Zhao, X.; Shin, G.S.; Mhaisalkar, S.G.; Park, N.-G. Importance of Functional Groups in Cross-Linking Methoxysilane Additives for High-Efficiency and Stable Perovskite Solar Cells. ACS Energy Lett. 2019, 4, 2192–2200. [Google Scholar] [CrossRef]
- Davies, C.L.; Filip, M.R.; Patel, J.B.; Crothers, T.W.; Verdi, C.; Wright, A.D.; Milot, R.L.; Giustino, F.; Johnston, M.B.; Herz, L.M. Bimolecular recombination in methylammonium lead triiodide perovskite is an inverse absorption process. Nat. Commun. 2018, 9, 293. [Google Scholar] [CrossRef]
- Heo, S.; Seo, G.; Lee, Y.; Lee, D.; Seol, M.; Lee, J.; Park, J.-B.; Kim, K.; Yun, D.-J.; Kim, Y.S.; et al. Deep level trapped defect analysis in CH3NH3PbI3 perovskite solar cells by deep level transient spectroscopy. Energy Environ. Sci. 2017, 10, 1128–1133. [Google Scholar] [CrossRef]
- Li, Y.; Cooper, J.K.; Liu, W.; Sutter-Fella, C.M.; Amani, M.; Beeman, J.W.; Javey, A.; Ager, J.W.; Liu, Y.; Toma, F.M.; et al. Defective TiO2 with high photoconductive gain for efficient and stable planar heterojunction perovskite solar cells. Nat. Commun. 2016, 7, 12446. [Google Scholar] [CrossRef]
- Agiorgousis, M.L.; Sun, Y.-Y.; Zeng, H.; Zhang, S. Strong Covalency-Induced Recombination Centers in Perovskite Solar Cell Material CH3NH3PbI3. J. Am. Chem. Soc. 2014, 136, 14570–14575. [Google Scholar] [CrossRef]
- Nie, W.; Blancon, J.-C.; Neukirch, A.J.; Appavoo, K.; Tsai, H.; Chhowalla, M.; Alam, M.A.; Sfeir, M.Y.; Katan, C.; Even, J.; et al. Light-activated photocurrent degradation and self-healing in perovskite solar cells. Nat. Commun. 2016, 7, 11574. [Google Scholar] [CrossRef]
- Park, S.Y.; Zhu, K. Advances in SnO2 for Efficient and Stable n-i-p Perovskite Solar Cells. Adv. Mater. 2022, 34, e2110438. [Google Scholar] [CrossRef] [PubMed]
- Ai, Y.; Liu, W.; Shou, C.; Yan, J.; Li, N.; Yang, Z.; Song, W.; Yan, B.; Sheng, J.; Ye, J. SnO2 surface defects tuned by (NH4)2S for high-efficiency perovskite solar cells. Sol. Energy 2019, 194, 541–547. [Google Scholar] [CrossRef]
- Gong, W.; Guo, H.; Zhang, H.; Yang, J.; Chen, H.; Wang, L.; Hao, F.; Niu, X. Chlorine-doped SnO2 hydrophobic surfaces for large grain perovskite solar cells. J. Mater. Chem. C 2020, 8, 11638–11646. [Google Scholar] [CrossRef]
- Walter, T.; Herberholz, R.; Müller, C.; Schock, H.W. Determination of defect distributions from admittance measurements and application to Cu(In,Ga)Se2 based heterojunctions. J. Appl. Phys. 1996, 80, 4411–4420. [Google Scholar] [CrossRef]
- Lee, J.-W.; Kim, D.-H.; Kim, H.-S.; Seo, S.-W.; Cho, S.M.; Park, N.-G. Formamidinium and Cesium Hybridization for Photo- and Moisture-Stable Perovskite Solar Cell. Adv. Energy Mater. 2015, 5, 1501310. [Google Scholar] [CrossRef]
- Prochowicz, D.; Yadav, P.; Saliba, M.; Saski, M.; Zakeeruddin, S.M.; Lewiński, J.; Grätzel, M. Reduction in the Interfacial Trap Density of Mechanochemically Synthesized MAPbI3. ACS Appl. Mater. Interfaces 2017, 9, 28418–28425. [Google Scholar] [CrossRef]
- Samiee, M.; Konduri, S.; Ganapathy, B.; Kottokkaran, R.; Abbas, H.A.; Kitahara, A.; Joshi, P.; Zhang, L.; Noack, M.; Dalal, V. Defect density and dielectric constant in perovskite solar cells. Appl. Phys. Lett. 2014, 105, 153502. [Google Scholar] [CrossRef]
- Yun, A.J.; Kim, J.; Hwang, T.; Park, B. Origins of Efficient Perovskite Solar Cells with Low-Temperature Processed SnO2 Electron Transport Layer. ACS Appl. Energy Mater. 2019, 2, 3554–3560. [Google Scholar] [CrossRef]
- Guo, X.; Du, J.; Lin, Z.; Su, J.; Feng, L.; Zhang, J.; Hao, Y.; Chang, J. Enhanced efficiency and stability of planar perovskite solar cells using SnO2:InCl3 electron transport layer through synergetic doping and passivation approaches. Chem. Eng. J. 2021, 407, 127997. [Google Scholar] [CrossRef]
- Chen, J.; Zhang, J.; Huang, C.; Bi, Z.; Xu, X.; Yu, H. SnO2/2D-Bi2O2Se new hybrid electron transporting layer for efficient and stable perovskite solar cells. Chem. Eng. J. 2021, 410, 128436. [Google Scholar] [CrossRef]
- Kamei, M.; Takao, Y.; Eriguchi, K.; Ono, K. Effects of plasma-induced charging damage on random telegraph noise in metal–oxide–semiconductor field-effect transistors with SiO2 and high-k gate dielectrics. Jpn. J. Appl. Phys. 2014, 53, 03DF02. [Google Scholar] [CrossRef]
- Eriguchi, K.; Nakakubo, Y.; Matsuda, A.; Kamei, M.; Ohta, H.; Nakagawa, H.; Hayashi, S.; Noda, S.; Ishikawa, K.; Yoshimaru, M.; et al. A new framework for performance prediction of advanced MOSFETs with plasma-induced recess structure and latent defect site. In Proceedings of the 2008 IEEE International Electron Devices Meeting, San Francisco, CA, USA, 15–17 December 2008; pp. 1–4. [Google Scholar]
- Yang, D.; Yang, R.; Wang, K.; Wu, C.; Zhu, X.; Feng, J.; Ren, X.; Fang, G.; Priya, S.; Liu, S. High efficiency planar-type perovskite solar cells with negligible hysteresis using EDTA-complexed SnO2. Nat. Commun. 2018, 9, 3239. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Lin, P.; Fu, N.; Sun, K.; Ye, M.; Liu, C.; Zhou, X.; Shu, L.; Hao, X.; Xu, B.; et al. Ionic liquid modified SnO2 nanocrystals as a robust electron transporting layer for efficient planar perovskite solar cells. J. Mater. Chem. A 2018, 6, 22086–22095. [Google Scholar] [CrossRef]
- Park, H.H.; Heasley, R.; Sun, L.; Steinmann, V.; Jaramillo, R.; Hartman, K.; Chakraborty, R.; Sinsermsuksakul, P.; Chua, D.; Buonassisi, T.; et al. Co-optimization of SnS absorber and Zn(O,S) buffer materials for improved solar cells. Prog. Photovolt. Res. Appl. 2015, 23, 901–908. [Google Scholar] [CrossRef]
- Dagar, J.; Castro-Hermosa, S.; Lucarelli, G.; Cacialli, F.; Brown, T.M. Highly efficient perovskite solar cells for light harvesting under indoor illumination via solution processed SnO2/MgO composite electron transport layers. Nano Energy 2018, 49, 290–299. [Google Scholar] [CrossRef]
- Kam, M.; Zhang, Q.; Zhang, D.; Fan, Z. Room-Temperature Sputtered SnO2 as Robust Electron Transport Layer for Air-Stable and Efficient Perovskite Solar Cells on Rigid and Flexible Substrates. Sci. Rep. 2019, 9, 6963. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Bu, T.; Yang, S.; Xiao, J.; Peng, Y.; Li, W.; Zhong, J.; Ku, Z.; Cheng, Y.-B.; et al. Room-temperature synthesized SnO2 electron transport layers for efficient perovskite solar cells. RSC Adv. 2019, 9, 9946–9950. [Google Scholar] [CrossRef]
- Meymian, M.R.Z.; Keshtmand, R. Surface Modification of the SnO2 Layer Using UV-Ozone in a Perovskite Solar Cell with a Planar Structure. Iran. J. Mater. Sci. 2021, 18, 1–9. [Google Scholar]
- Huang, L.; Sun, X.; Li, C.; Xu, J.; Xu, R.; Du, Y.; Ni, J.; Cai, H.; Li, J.; Hu, Z.; et al. UV-Sintered Low-Temperature Solution-Processed SnO2 as Robust Electron Transport Layer for Efficient Planar Heterojunction Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2017, 9, 21909–21920. [Google Scholar] [CrossRef]
- Jung, K.; Kim, D.H.; Kim, J.; Ko, S.; Choi, J.W.; Kim, K.C.; Lee, S.-G.; Lee, M.-J. Influence of a UV-ozone treatment on amorphous SnO2 electron selective layers for highly efficient planar MAPbI3 perovskite solar cells. J. Mater. Sci. Technol. 2020, 59, 195–202. [Google Scholar] [CrossRef]
- Lu, D.; Zhang, W.; Kloo, L.; Belova, L. Inkjet-Printed Electron Transport Layers for Perovskite Solar Cells. Materials 2021, 14, 7525. [Google Scholar] [CrossRef] [PubMed]
- Bu, T.; Li, J.; Zheng, F.; Chen, W.; Wen, X.; Ku, Z.; Peng, Y.; Zhong, J.; Cheng, Y.-B.; Huang, F. Universal passivation strategy to slot-die printed SnO2 for hysteresis-free efficient flexible perovskite solar module. Nat. Commun. 2018, 9, 4609. [Google Scholar] [CrossRef] [PubMed]
- Keshtmand, R.; Zamani-Meymian, M.R.; Taghavinia, N. Improving the performance of planar perovskite solar cell using NH4Cl treatment of SnO2 as electron transport layer. Surf. Interfaces 2022, 28, 101596. [Google Scholar] [CrossRef]
- Xu, C.; Liu, Z.; Sun, Q.; Lee, E.-C. Morphology control of SnO2 layer by solvent engineering for efficient perovskite solar cells. Sol. Energy 2021, 214, 280–287. [Google Scholar] [CrossRef]
- Hoang, M.T.; Yang, Y.; Chiu, W.H.; Yu, Y.; Pham, N.D.; Moonie, P.; Koplick, A.; Tulloch, G.; Martens, W.; Wang, H. Unraveling the Mechanism of Alkali Metal Fluoride Post-Treatment of SnO2 for Efficient Planar Perovskite Solar Cells. Small Methods 2023, 2300431. [Google Scholar] [CrossRef]
- Jindra, J. The Preparation of Crystalline Lithium Fluoride by Controlled Precipitation. Krist. Und Tech. 1969, 4, 69–75. [Google Scholar] [CrossRef]
- Fokin, V.M.; Souza, G.P.; Zanotto, E.D.; Lumeau, J.; Glebova, L.; Glebov, L.B. Sodium Fluoride Solubility and Crystallization in Photo-Thermo-Refractive Glass. J. Am. Ceram. Soc. 2010, 93, 716–721. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Yu, M.; Zhong, J.; Zhou, Q.; Qiu, J.; Zhang, X. SnO2 surface halogenation to improve photovoltaic performance of perovskite solar cells. CCS Chem. 2021, 37, 200603. [Google Scholar]
- Shi, Z.; Zhou, D.; Wu, Y.; Pan, G.; Xu, W.; Wang, N.; Liu, S.; Sun, R.; Liu, L.; Zhuang, X.; et al. Dual interfacial engineering to improve ultraviolet and near-infrared light harvesting for efficient and stable perovskite solar cells. Chem. Eng. J. 2022, 435, 134792. [Google Scholar] [CrossRef]
- Ai, Y.; Zhang, Y.; Song, J.; Kong, T.; Li, Y.; Xie, H.; Bi, D. In Situ Perovskitoid Engineering at SnO2 Interface toward Highly Efficient and Stable Formamidinium Lead Triiodide Perovskite Solar Cells. J. Phys. Chem. Lett. 2021, 12, 10567–10573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Wang, L.; Huang, J.; Miao, X.; Sun, L.; Hua, Y.; Wang, Y. Amino-capped zinc oxide modified tin oxide electron transport layer for efficient perovskite solar cells. Cell Rep. Phys. Sci. 2021, 2, 100590. [Google Scholar] [CrossRef]
- Jia, J.; Qian, C.; Dong, Y.; Li, Y.F.; Wang, H.; Ghoussoub, M.; Butler, K.T.; Walsh, A.; Ozin, G.A. Heterogeneous catalytic hydrogenation of CO2 by metal oxides: Defect engineering-perfecting imperfection. Chem. Soc. Rev. 2017, 46, 4631–4644. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Chen, K.; Chen, Q.; Zhou, Y.; Chen, N.; Li, Y. Fullerene Derivative-Modified SnO2 Electron Transport Layer for Highly Efficient Perovskite Solar Cells with Efficiency over 21%. ACS Appl. Mater. Interfaces 2019, 11, 33825–33834. [Google Scholar] [CrossRef]
- Yang, G.; Wang, C.; Lei, H.; Zheng, X.; Qin, P.; Xiong, L.; Zhao, X.; Yan, Y.; Fang, G. Interface engineering in planar perovskite solar cells: Energy level alignment, perovskite morphology control and high performance achievement. J. Mater. Chem. A 2017, 5, 1658–1666. [Google Scholar] [CrossRef]
- Zhou, Y.; Fuentes-Hernandez, C.; Shim, J.; Meyer, J.; Giordano, A.J.; Li, H.; Winget, P.; Papadopoulos, T.; Cheun, H.; Kim, J.; et al. A Universal Method to Produce Low–Work Function Electrodes for Organic Electronics. Science 2012, 336, 327–332. [Google Scholar] [CrossRef]
- Bulliard, X.; Ihn, S.-G.; Yun, S.; Kim, Y.; Choi, D.; Choi, J.-Y.; Kim, M.; Sim, M.; Park, J.-H.; Choi, W.; et al. Enhanced Performance in Polymer Solar Cells by Surface Energy Control. Adv. Funct. Mater. 2010, 20, 4381–4387. [Google Scholar] [CrossRef]
- Liu, K.; Chen, S.; Wu, J.; Zhang, H.; Qin, M.; Lu, X.; Tu, Y.; Meng, Q.; Zhan, X. Fullerene derivative anchored SnO2 for high-performance perovskite solar cells. Energy Environ. Sci. 2018, 11, 3463–3471. [Google Scholar] [CrossRef]
- Zhao, X.; Tao, L.; Li, H.; Huang, W.; Sun, P.; Liu, J.; Liu, S.; Sun, Q.; Cui, Z.; Sun, L.; et al. Efficient Planar Perovskite Solar Cells with Improved Fill Factor via Interface Engineering with Graphene. Nano Lett. 2018, 18, 2442–2449. [Google Scholar] [CrossRef]
- Hernandez, Y.; Nicolosi, V.; Lotya, M.; Blighe, F.M.; Sun, Z.; De, S.; McGovern, I.T.; Holland, B.; Byrne, M.; Gun, Y.K.; et al. High-yield production of graphene by liquid-phase exfoliation of graphite. Nat. Nanotechnol. 2008, 3, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Jiang, E.; Ai, Y.; Yan, J.; Li, N.; Lin, L.; Wang, Z.; Shou, C.; Yan, B.; Zeng, Y.; Sheng, J.; et al. Phosphate-Passivated SnO2 Electron Transport Layer for High-Performance Perovskite Solar Cells. ACS Appl. Mater. Interfaces 2019, 11, 36727–36734. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Zhang, H.; Tong, X.; Hou, X.; Li, F.; Du, Y.; Wang, S.; Zhang, Q.; Liu, P.; Zhao, X. Interfacial Engineering via Self-Assembled Thiol Silane for High Efficiency and Stability Perovskite Solar Cells. Sol. RRL 2021, 5, 2100128. [Google Scholar] [CrossRef]
- Chen, Q.; Peng, C.; Du, L.; Hou, T.; Yu, W.; Chen, D.; Shu, H.; Huang, D.; Zhou, X.; Zhang, J.; et al. Synergy of mesoporous SnO2 and RbF modification for high-efficiency and stable perovskite solar cells. J. Energy Chem. 2022, 66, 250–259. [Google Scholar] [CrossRef]
- Wang, Z.; Kamarudin, M.A.; Huey, N.C.; Yang, F.; Pandey, M.; Kapil, G.; Ma, T.; Hayase, S. Interfacial Sulfur Functionalization Anchoring SnO2 and CH3NH3PbI3 for Enhanced Stability and Trap Passivation in Perovskite Solar Cells. ChemSusChem 2018, 11, 3941–3948. [Google Scholar] [CrossRef]
- Hong, J.A.; Jung, E.D.; Yu, J.C.; Kim, D.W.; Nam, Y.S.; Oh, I.; Lee, E.; Yoo, J.-W.; Cho, S.; Song, M.H. Improved Efficiency of Perovskite Solar Cells Using a Nitrogen-Doped Graphene-Oxide-Treated Tin Oxide Layer. ACS Appl. Mater. Interfaces 2020, 12, 2417–2423. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yuan, J.; Xi, J.; Du, J.; Tian, J. Multiple-Function Surface Engineering of SnO2 Nanoparticles to Achieve Efficient Perovskite Solar Cells. J. Phys. Chem. Lett. 2021, 12, 9142–9148. [Google Scholar] [CrossRef]
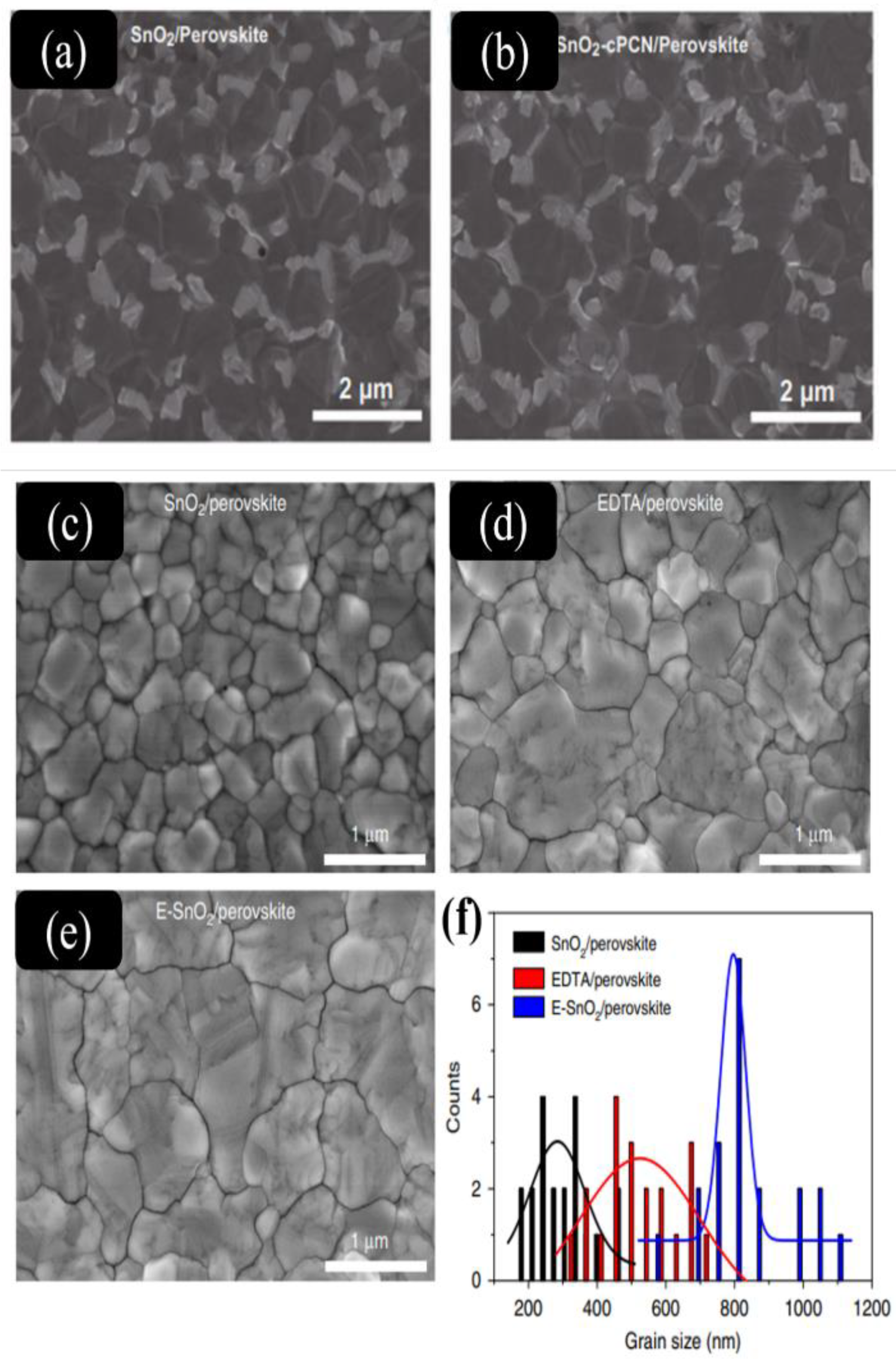
- Li, Z.; Gao, Y.; Zhang, Z.; Xiong, Q.; Deng, L.; Li, X.; Zhou, Q.; Fang, Y.; Gao, P. cPCN-Regulated SnO2 Composites Enables Perovskite Solar Cell with Efficiency Beyond 23%. Nano-Micro Lett. 2021, 13, 101. [Google Scholar] [CrossRef]
- Tu, B.; Shao, Y.; Chen, W.; Wu, Y.; Li, X.; He, Y.; Li, J.; Liu, F.; Zhang, Z.; Lin, Y.; et al. Novel Molecular Doping Mechanism for n-Doping of SnO2 via Triphenylphosphine Oxide and Its Effect on Perovskite Solar Cells. Adv. Mater. 2019, 31, 1805944. [Google Scholar] [CrossRef]
- Liu, P.; Wang, W.; Liu, S.; Yang, H.; Shao, Z. Fundamental Understanding of Photocurrent Hysteresis in Perovskite Solar Cells. Adv. Energy Mater. 2019, 9, 1803017. [Google Scholar] [CrossRef]



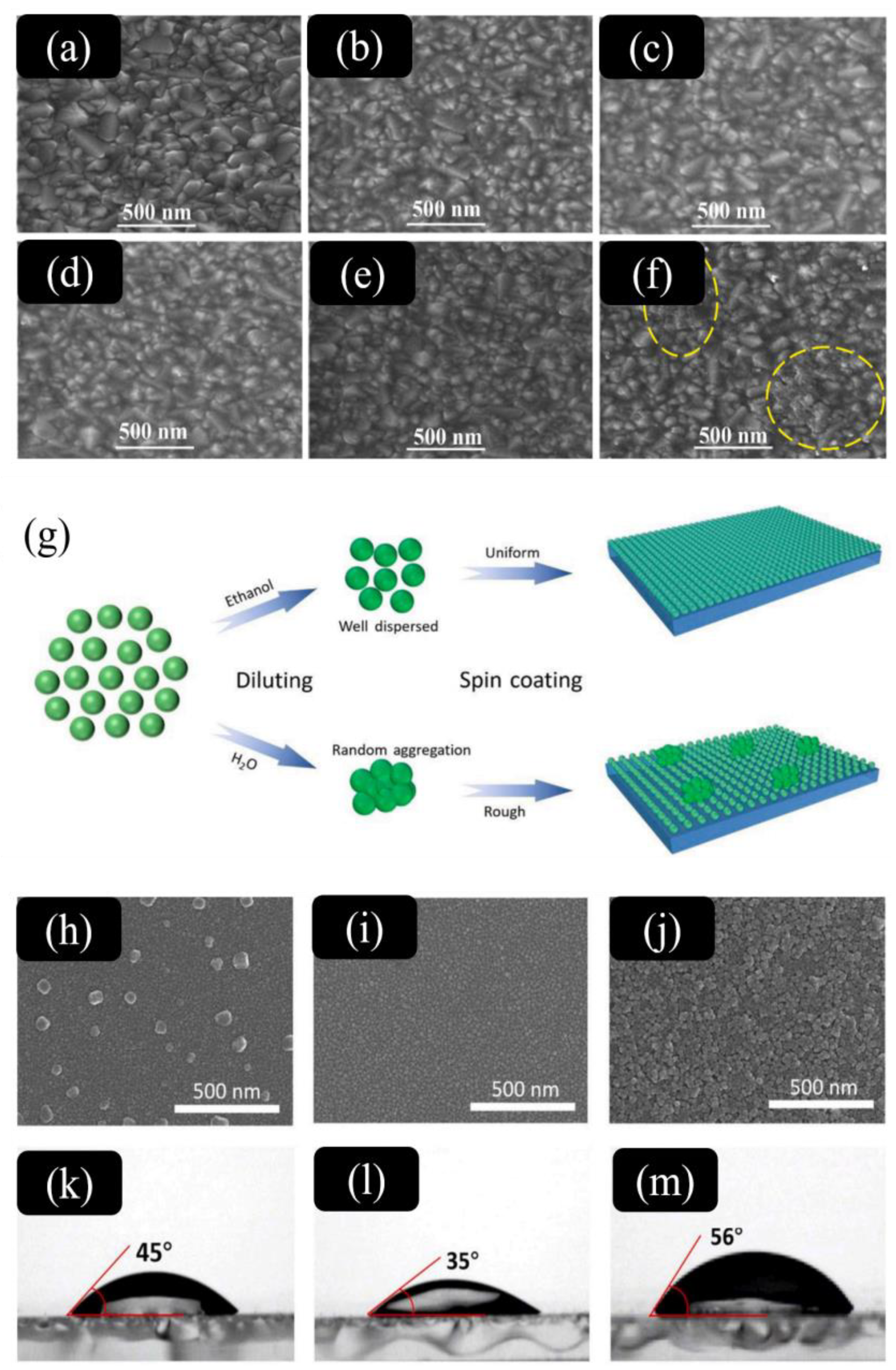
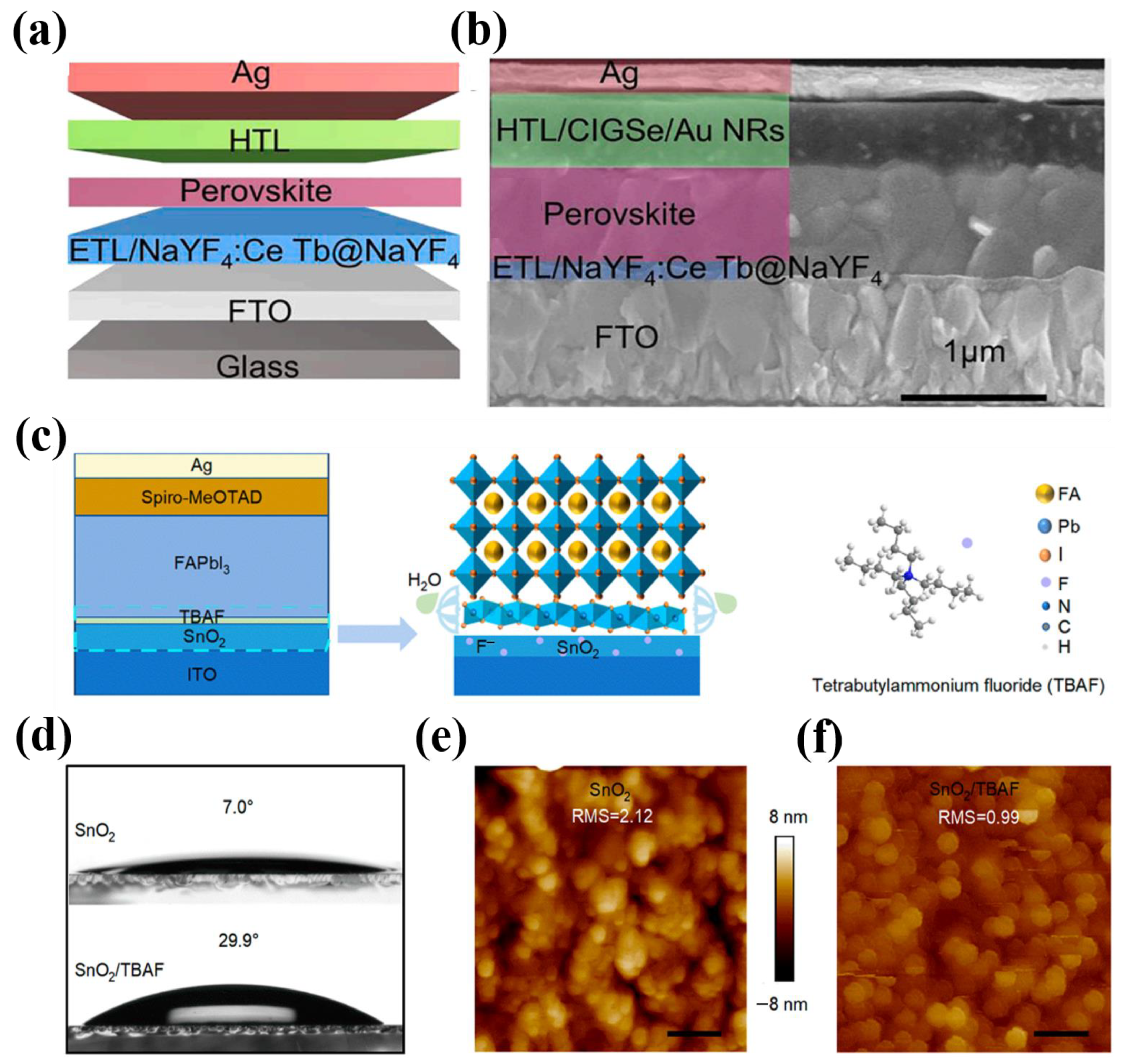
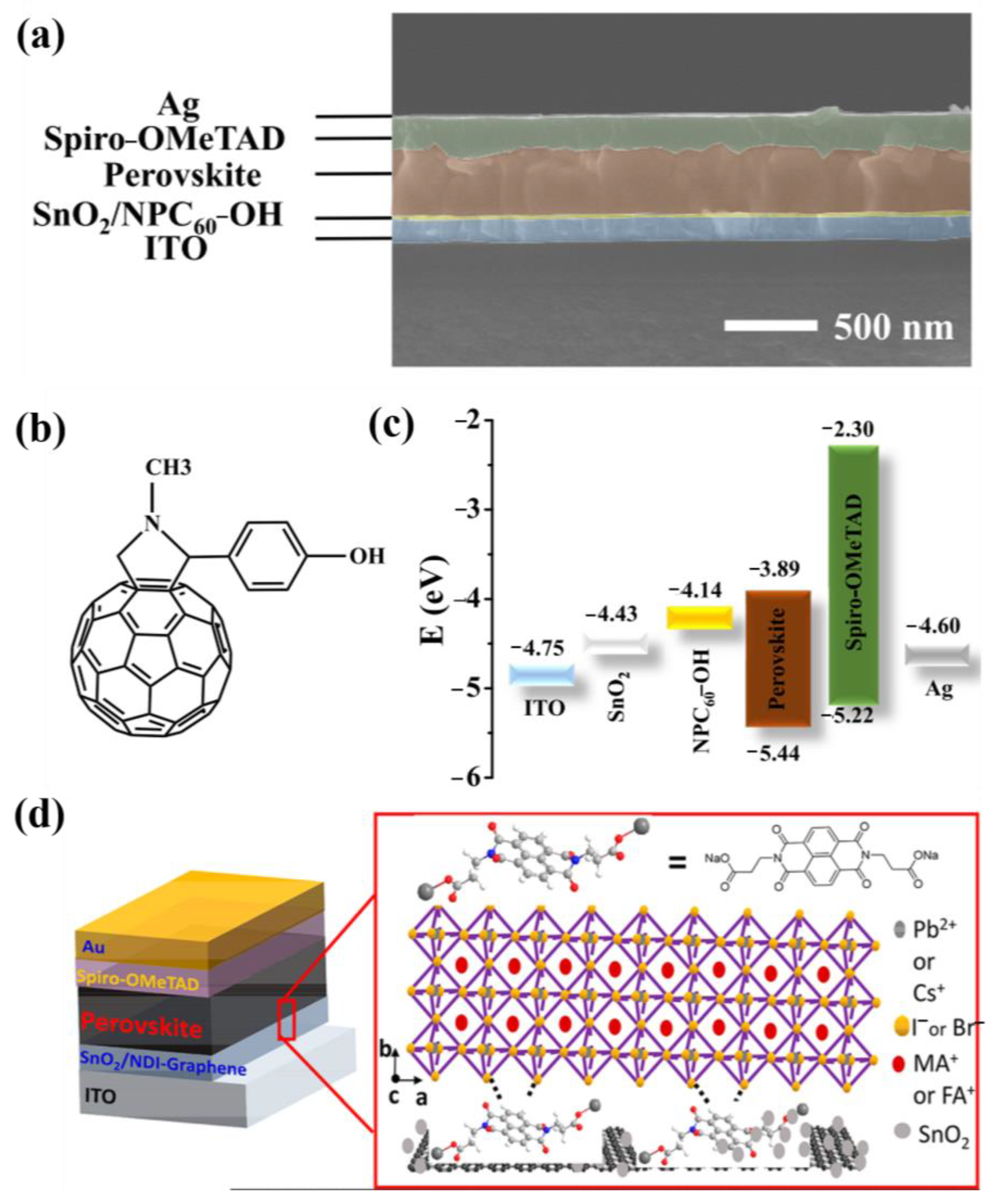
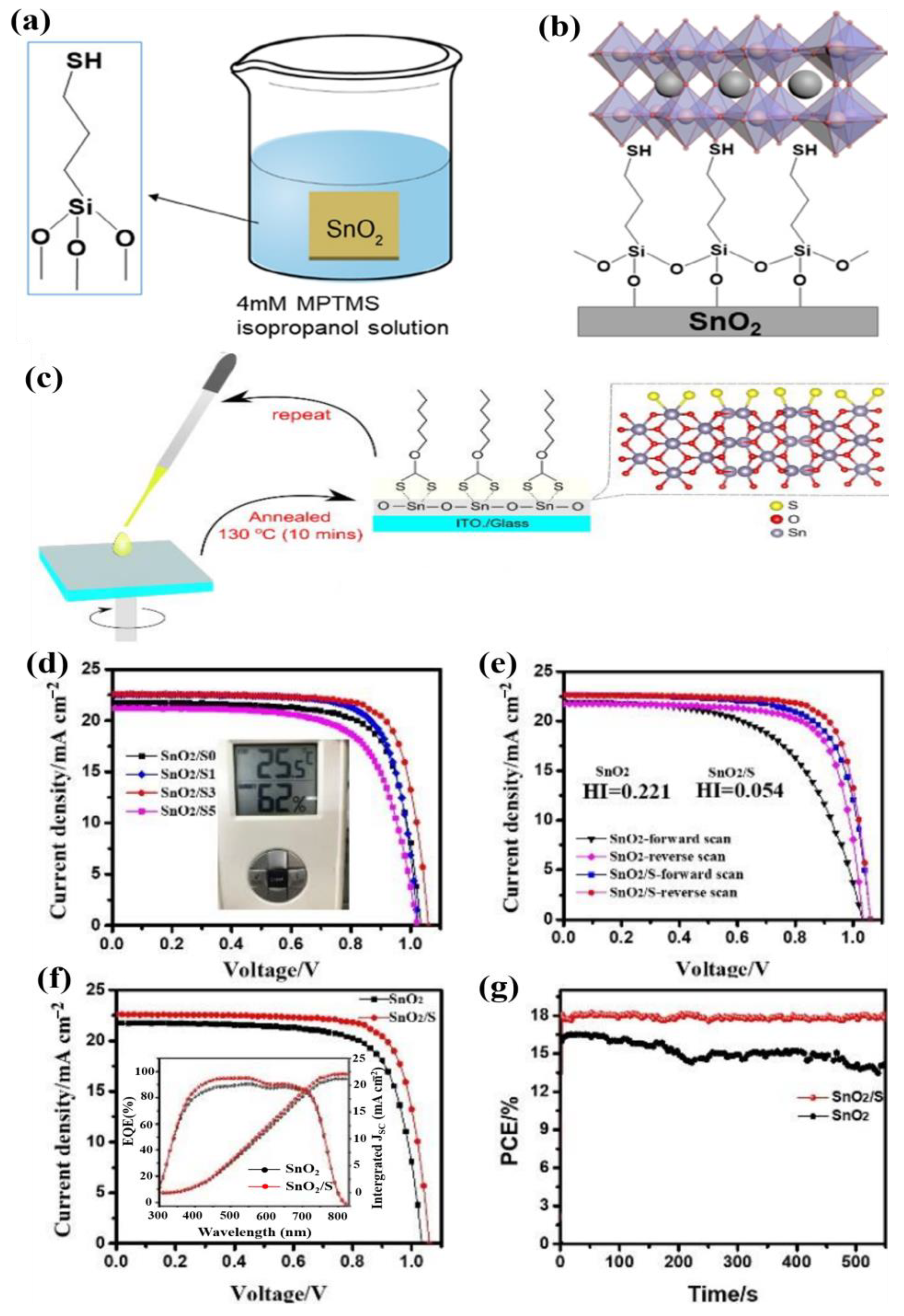

| TiO2 | ZnO | SnO2 | |
|---|---|---|---|
| Crystal structure | Rutile, anatase, brookite | Rocksalt, zinc blende, wutzite | Rutile |
| Energy band gap (eV) | 3.0–3.2 | 3.2–3.3 | 3.50–4.0 |
| Surface work function (eV) | 4.5–5.0 | 4.45–5.30 | 4.71–5.33 |
| Electron mobility (cm2 V s−1) | 0.1–4.0 | Bulk ZnO: 205–300; Nanowire: 1000 | 240 |
| Refractive index | 2.5 | 2.0 | 2.0 |
| Electron effective mass (m*) | 9 | 0.26 | 0.3 |
| Relative dielectric constant | 170 | 8.5 | 9.6 |
| Electron diffusion coefficient (cm2 s−1) | Bulk TiO2: 0.5; nanoparticle: 10−8–10−4 | Bulk ZnO: 5.2; nanoparticle film: 1.7 × 10−4 | nanoparticle film: 6.22 × 10−6 |
| Surface Modification | Device Structure | Jsc (mA cm−2) | Voc (V) | FF | PCE (%) | Ref. |
|---|---|---|---|---|---|---|
| Simplistic synthesis route | ITO/SnO2-RT/PVSK/Spiro OMeTAD/Au | 21.8 | 1.084 | 0.767 | 18.1 | [119] |
| ITO/SnO2-120 °C/PVSK/Spiro OMeTAD/Au | 22.0 | 1.098 | 0.785 | 19.0 | ||
| FTO/spin-coated SnO2/MAPbI3/Spiro OMeTAD/Au | 21.8 | 0.901 | 0.549 | 10.78 | [128] | |
| FTO/sputtered SnO2/MAPbI3/Spiro OMeTAD/Au | 22.9 | 0.965 | 0.580 | 12.82 | ||
| FTO/SnO2-water/PVSK/Spiro-OMeTAD/Au | 21.6 | 1.119 | 0.740 | 17.87 | [129] | |
| FTO/SnO2-butyl acetate/PVSK/Spiro-OMeTAD/Au | 22.3 | 1.132 | 0.760 | 19.22 | ||
| FTO/SnO2-UVO (0 min)/PVSK/CIS/Au | 12.2 | 0.860 | 0.411 | 4.34 | [130] | |
| FTO/SnO2-UVO (30 min)/PVSK/CIS/Au | 20.39 | 0.920 | 0.583 | 10.96 | ||
| FTO/inkjet-printed SnO2/PVSK/Spiro-OMeTAD/Au | 21.1 | 1.100 | 0.750 | 17.37 | [133] | |
| FTO/SnO2 NCs/CsFAMA/Spiro-OMeTAD/Au | 22.5 | 1.099 | 0.780 | 19.27 | [134] | |
| FTO/SnO2 NCs-KOH/CsFAMA/Spiro-OMeTAD/Au | 22.6 | 1.148 | 0.790 | 20.50 | ||
| Interface modifier | FTO/SnO2/PVSK/CIS/Au | 19.9 | 1.010 | 0.600 | 12.95 | [135] |
| FTO/SnO2-NH4Cl/PVSK/CIS/Au | 20.1 | 1.150 | 0.710 | 16.79 | ||
| FTO/SnO2-water/PVSK/Spiro-OMeTAD/Au | 20.8 | 1.080 | 0.680 | 15.27 | [136] | |
| FTO/SnO2 -ethanol/PVSK/Spiro-OMeTAD/Au | 21.8 | 1.110 | 0.780 | 18.84 | ||
| ITO/SnO2/PVSK/Spiro-OMeTAD/Au | 22.2 | 1.120 | 0.690 | 17.15 | [140] | |
| ITO/SnO2-TPAC/PVSK/Spiro-OMeTAD/Au | 23.11 | 1.160 | 0.715 | 19.17 | ||
| Bilayer | ITO/SnO2/PVSK/Spiro-OMeTAD/Au | 22.5 | 1.157 | 0.760 | 19.78 | [141] |
| ITO/SnO2-NaYF4:Ce,Tb@NaYF4/PVSK/Spiro-OMeTAD/Au | 23.4 | 1.159 | 0.761 | 20.64 | ||
| ITO/SnO2/FAPbI3/Spiro-OMeTAD/Au | 24.8 | 1.060 | 0.750 | 20.10 | [142] | |
| ITO/SnO2-TBAF/FAPbI3/Spiro-OMeTAD/Au | 25.4 | 1.130 | 0.760 | 23.10 | ||
| Functional groups | FTO/SnO2/PVSK/Spiro-OMeTAD/Au | 24.1 | 1.080 | 0.706 | 18.45 | [143] |
| FTO/NH2-ZnO@SnO2/PVSK/Spiro-OMeTAD/Au | 25.1 | 1.139 | 0.787 | 22.52 | ||
| FTO/SnO2/PVSK/Spiro-OMeTAD/Ag | 22.7 | 1.110 | 0.759 | 19.04 | [145] | |
| FTO/SnO2 -NPC60-OH/PVSK/Spiro-OMeTAD/Ag | 23.4 | 1.130 | 0.807 | 21.39 | ||
| FTO/SnO2/PVSK/Spiro-OMeTAD/Au | 22.0 | 1.108 | 0.781 | 19.01 | [149] | |
| FTO/SnO2 -C9/PVSK/Spiro-OMeTAD/Au | 22.8 | 1.120 | 0.784 | 20.05 | ||
| ITO/SnO2/PVSK/Spiro-OMeTAD/Au | 23.2 | 1.097 | 0.746 | 18.99 | [150] | |
| FTO/SnO2-NDI graphene/PVSK/Spiro-OMeTAD/Au | 22.7 | 1.084 | 0.821 | 20.16 | ||
| FTO/SnO2/MAPbI3/Spiro-OMeTAD/Au | 20.8 | 1.065 | 0.662 | 14.69 | [146] | |
| FTO/SnO2-SAM/MAPbI3/Spiro-OMeTAD/Au | 21.2 | 1.160 | 0.692 | 17.03 | ||
| ITO/SnO2/PVSK/Spiro-OMeTAD/Ag | 21.8 | 1.150 | 0.732 | 18.41 | [152] | |
| ITO/P-SnO2/PVSK/Spiro-OMeTAD/Ag | 22.6 | 1.140 | 0.767 | 19.72 | ||
| FTO/SnO2/PVSK/Spiro-OMeTAD/Au | 22.1 | 1.054 | 0.713 | 16.62 | [153] | |
| FTO/SnO2-MPTMS/PVSK/Spiro-OMeTAD/Au | 22.8 | 1.105 | 0.746 | 18.75 | ||
| ITO/SnO2/PVSK/Spiro-OMeTAD/Ag | 23.7 | 1.113 | 0.778 | 20.1 | [154] | |
| ITO/SnO2-S/PVSK/Spiro-OMeTAD/Ag | 24.5 | 1.148 | 0.821 | 22.72 | ||
| ITO/SnO2/MAPbI3/Spiro-OMeTAD/Au | 21.2 | 1.030 | 0.587 | 13.30 | [155] | |
| ITO/SnO2-RbF/MAPbI3/Spiro-OMeTAD/Au | 22.6 | 1.060 | 0.769 | 18.41 | ||
| Vacancy defects | ITO/SnO2/PVSK/Spiro-OMeTAD/Au | 19.2 | 1.110 | 0.690 | 14.70 | [156] |
| ITO/SnO2-NGO/PVSK/Spiro-OMeTAD/Au | 19.3 | 1.170 | 0.745 | 16.54 | ||
| ITO/SnO2/PVSK/Spiro-OMeTAD/Ag | 23.9 | 1.060 | 0.741 | 18.85 | [157] | |
| ITO/TBAI-SnO2/PVSK/Spiro-OMeTAD/Ag | 24.6 | 1.150 | 0.771 | 21.71 | ||
| ITO/SnO2/PVSK/Spiro-OMeTAD/Ag | 22.4 | 1.130 | 0.734 | 18.67 | [113] | |
| ITO/SnO2-(NH4)2S/PVSK/Spiro-OMeTAD/Ag | 22.9 | 1.150 | 0.759 | 20.03 | ||
| Hybridization | ITO/SnO2/PVSK/Spiro-OMeTAD/Ag | 23.4 | 1.110 | 0.820 | 21.30 | [158] |
| ITO/SnO2-cPCN/PVSK/Spiro-OMeTAD/Ag | 24.9 | 1.126 | 0.825 | 23.17 | ||
| ITO/SnO2/PVSK/Spiro-OMeTAD/Au | 24.4 | 1.079 | 0.722 | 19.01 | [159] | |
| ITO/SnO2-TPPO/PVSK/Spiro-OMeTAD/Au | 24.3 | 1.106 | 0.770 | 20.69 | ||
| ITO/SnO2/PVSK/Spiro-OMeTAD/Au | 22.8 | 1.100 | 0.755 | 18.93 | [124] | |
| ITO/SnO2-EDTA/PVSK/Spiro-OMeTAD/Au | 24.6 | 1.110 | 0.792 | 21.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoang Huy, V.P.; Bark, C.-W. Review on Surface Modification of SnO2 Electron Transport Layer for High-Efficiency Perovskite Solar Cells. Appl. Sci. 2023, 13, 10715. https://doi.org/10.3390/app131910715
Hoang Huy VP, Bark C-W. Review on Surface Modification of SnO2 Electron Transport Layer for High-Efficiency Perovskite Solar Cells. Applied Sciences. 2023; 13(19):10715. https://doi.org/10.3390/app131910715
Chicago/Turabian StyleHoang Huy, Vo Pham, and Chung-Wung Bark. 2023. "Review on Surface Modification of SnO2 Electron Transport Layer for High-Efficiency Perovskite Solar Cells" Applied Sciences 13, no. 19: 10715. https://doi.org/10.3390/app131910715
APA StyleHoang Huy, V. P., & Bark, C.-W. (2023). Review on Surface Modification of SnO2 Electron Transport Layer for High-Efficiency Perovskite Solar Cells. Applied Sciences, 13(19), 10715. https://doi.org/10.3390/app131910715