Evaluation of In Vitro Antimicrobial Activity of Bioactive Compounds and the Effect of Allyl-Isothiocyanate on Chicken Meat Quality under Refrigerated Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Raw Meat Samples and Experimental Design
2.2. Physicochemical Properties of Chicken Meat
2.2.1. Measurement of pH
2.2.2. Color Measurement
- -
- Chroma: C* = [(a*)2 + (b*)2]1/2.
- -
- Hue angle: h* = tan−1 (arctangent) (b*/a*).
2.2.3. Measurement of Water Holding Capacity (WHC)
2.2.4. Determination of Metmyoglobin, Deoxymyoglobin, and Oxymyoglobin Pigments
- % MetMb = (−0.159R1 − 0.085R2 + 1.262R3 − 0.520) ∗ 100
- % DeoMb = (−0.543R1 + 1.594R2 + 0.552R3 − 1.329) ∗ 100
- % OxyMb = (0.722R1 − 1.432R2 − 1.659R3 + 2.599) ∗ 100
- *R1 = A582/A557, R2 = A557/A525, and R3 = A503/A525
2.3. Determinations of Thiobarbituric Acid Reactive Substances (TBARS)
2.4. Microbiological Properties
2.4.1. In Vitro Antimicrobial Activity of BACs
2.4.2. Disc Diffusion Assay
2.4.3. Minimum Inhibitory Concentration (MIC)—Micro-Dilution Method
2.5. Determination of Aerobic Mesophilic Counts (AMCs), Pseudomonas lundensis, Listeria monocytogenes, and Salmonella Typhimurium in Meat
2.5.1. Preparation of Bacterial Strains and Inocula
2.5.2. Bacterial Inoculation on Chicken Meat
2.5.3. Microbial Enumeration
2.6. Electronic Nose Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of the In Vitro Antimicrobial Activity of BACs
3.1.1. Disc Diffusion Assay
3.1.2. Minimum Inhibitory Concentration
3.2. Physicochemical Properties of Chicken Meat
3.2.1. pH of the Meat
3.2.2. Color Values
3.2.3. Water Holding Capacity
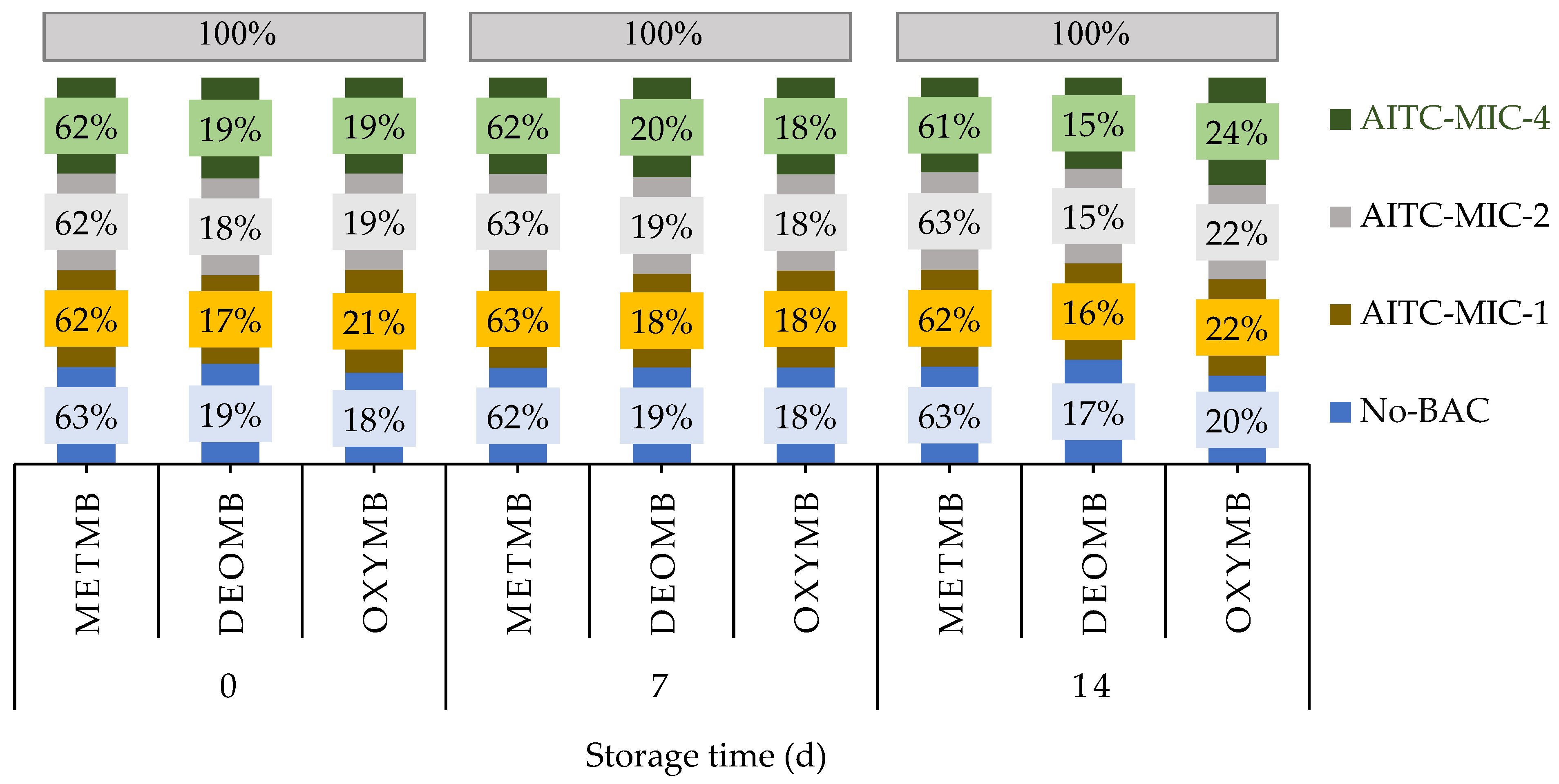
3.2.4. Meat Pigments (Metmyoglobin, Deoxymyoglobin, and Oxymyoglobin)
3.3. Effect of AITC on the TBARS Values of Chicken Meat.
3.4. Effect of AITC on the Microbiological Properties of Chicken Meat
3.5. Effect of AITC on the Smell Detection by Electronic Nose in Chicken Meat
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| MIC-1: | 0.0088 g AITC | in 1000 | g final mixture | ||
| if | 0.0088 g AITC | In 1000 | g final mixture | let 1 mL AITC is 1 g AITC | |
| then | 0.00088 g AITC | In 100 | g final mixture | ||
| AITC | 0.00088 g | ||||
| + | ethanol | 0.0044 g | |||
| AITC with ethanol | 0.00528 g | ||||
| + | Distillate water | 4.99384 g | |||
| AITC + Distillate water + ethanol | 5 g | 5% of total weight | |||
| + | meat | 95 g | 95% of total weight | ||
| 100 g | Total weight | ||||
| The concentration of AITC was two times higher in MIC-2 and four times higher in MIC-4. | |||||

References
- Chai, H.-E.; Sheen, S. Effect of High Pressure Processing, Allyl Isothiocyanate, and Acetic Acid Stresses on Salmonella Survivals, Storage, and Appearance Color in Raw Ground Chicken Meat. Food Control 2021, 123, 107784. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Guo, X. Effects of Antimicrobial and Antioxidant Activities of Spice Extracts on Raw Chicken Meat Quality. Food Sci. Hum. Wellness 2016, 5, 39–48. [Google Scholar] [CrossRef]
- Shin, J.; Harte, B.; Ryser, E.; Selke, S. Active Packaging of Fresh Chicken Breast, with Allyl Isothiocyanate (AITC) in Combination with Modified Atmosphere Packaging (MAP) to Control the Growth of Pathogens. J. Food Sci. 2010, 75, M65–M71. [Google Scholar] [CrossRef] [PubMed]
- Karabagias, I.; Badeka, A.; Kontominas, M.G. Shelf Life Extension of Lamb Meat Using Thyme or Oregano Essential Oils and Modified Atmosphere Packaging. Meat Sci. 2011, 88, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Šojić, B.; Tomović, V.; Jokanović, M.; Ikonić, P.; Džinić, N.; Kocić-Tanackov, S.; Popović, L.; Tasić, T.; Savanović, J.; Šojić, N.Ž. Antioxidant Activity of Juniperus communis L. Essential Oil in Cooked Pork Sausages. Czech J. Food Sci. 2017, 35, 189–193. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Nobile, M.A.D. Food Applications of Natural Antimicrobial Compounds. Front. Microbiol. 2012, 3. [Google Scholar] [CrossRef]
- Sant’Ana, A.S.; Igarashi, M.C.; Landgraf, M.; Destro, M.T.; Franco, B.D.G.M. Prevalence, Populations and Pheno- and Genotypic Characteristics of Listeria Monocytogenes Isolated from Ready-to-Eat Vegetables Marketed in São Paulo, Brazil. Int. J. Food Microbiol. 2012, 155, 1–9. [Google Scholar] [CrossRef]
- Cegiełka, A.; Chmiel, M.; Hać-Szymańczuk, E.; Pietrzak, D. Evaluation of the Effect of Sage (Salvia officinalis L.) Preparations on Selected Quality Characteristics of Vacuum-Packed Chicken Meatballs Containing Mechanically Separated Meat. Appl. Sci. 2022, 12, 12890. [Google Scholar] [CrossRef]
- FAO. Fao-World Livestock 2011—Livestock in Food Security. Available online: http://www.fao.org/3/i2373e/i2373e00.htm (accessed on 8 May 2020).
- Sampels, S. Oxidation and Antioxidants in Fish and Meat from Farm to Fork. Food Ind. 2013. Chapter 6. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, W.G.; Lee, E.J.; Ma, C.W.; Ahn, D.U. Effects of Diet, Packaging, and Irradiation on Protein Oxidation, Lipid Oxidation, and Color of Raw Broiler Thigh Meat during Refrigerated Storage. Poult. Sci. 2011, 90, 1348–1357. [Google Scholar] [CrossRef]
- Kanner, J. Oxidative Processes in Meat and Meat Products: Quality Implications. Meat Sci. 1994, 36, 169–189. [Google Scholar] [CrossRef] [PubMed]
- Dave, D.; Ghaly, A.E. Meat Spoilage Mechanisms and Preservation Techniques: A Critical Review. Am. J. Agric. Biol. Sci. 2011, 6, 486–510. [Google Scholar]
- Karanth, S.; Feng, S.; Patra, D.; Pradhan, A.K. Linking Microbial Contamination to Food Spoilage and Food Waste: The Role of Smart Packaging, Spoilage Risk Assessments, and Date Labeling. Front. Microbiol. 2023, 14, 1198124. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, D.; Delgado, S.; Vázquez-Sánchez, D.; Martínez, B.; Cabo, M.L.; Rodríguez, A.; Herrera, J.J.; García, P. Incidence of Staphylococcus Aureus and Analysis of Associated Bacterial Communities on Food Industry Surfaces. Appl. Environ. Microbiol. 2012, 78, 8547–8554. [Google Scholar] [CrossRef] [PubMed]
- Fasseas, M.K.; Mountzouris, K.C.; Tarantilis, P.A.; Polissiou, M.; Zervas, G. Antioxidant Activity in Meat Treated with Oregano and Sage Essential Oils. Food Chem. 2008, 106, 1188–1194. [Google Scholar] [CrossRef]
- Jayathilakan, K.; Sharma, G.K.; Radhakrishna, K.; Bawa, A.S. Antioxidant Potential of Synthetic and Natural Antioxidants and Its Effect on Warmed-over-Flavour in Different Species of Meat. Food Chem. 2007, 105, 908–916. [Google Scholar] [CrossRef]
- FDA. CFR—Code of Federal Regulations Title 21. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?CFRPart=101 (accessed on 9 May 2020).
- Karre, L.; Lopez, K.; Getty, K.J.K. Natural Antioxidants in Meat and Poultry Products. Meat Sci. 2013, 94, 220–227. [Google Scholar] [CrossRef]
- Pinto, L.; Tapia-Rodríguez, M.R.; Baruzzi, F.; Ayala-Zavala, J.F. Plant Antimicrobials for Food Quality and Safety: Recent Views and Future Challenges. Foods 2023, 12, 2315. [Google Scholar] [CrossRef]
- Burt, S. Essential Oils: Their Antibacterial Properties and Potential Applications in Foods--a Review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Chuang, S.; Sheen, S.; Sommers, C.H.; Zhou, S.; Sheen, L.-Y. Survival Evaluation of Salmonella and Listeria Monocytogenes on Selective and Nonselective Media in Ground Chicken Meat Subjected to High Hydrostatic Pressure and Carvacrol. J. Food Prot. 2020, 83, 37–44. [Google Scholar] [CrossRef]
- Piñon, M.I.; Alarcon-Rojo, A.D.; Renteria, A.L.; Mendez, G.; Janacua-Vidales, H. Reduction of Microorganisms in Marinated Poultry Breast Using Oregano Essential Oil and Power Ultrasound. Acta Aliment. 2015, 44, 527–533. [Google Scholar] [CrossRef]
- Hernández-Ochoa, L.; Aguirre-Prieto, Y.B.; Nevárez-Moorillón, G.V.; Gutierrez-Mendez, N.; Salas-Muñoz, E. Use of Essential Oils and Extracts from Spices in Meat Protection. J. Food Sci. Technol. 2014, 51, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Camo, J.; Beltrán, J.A.; Roncalés, P. Extension of the Display Life of Lamb with an Antioxidant Active Packaging. Meat Sci. 2008, 80, 1086–1091. [Google Scholar] [CrossRef] [PubMed]
- Dufour, V.; Stahl, M.; Baysse, C. The Antibacterial Properties of Isothiocyanates. Microbiology 2015, 161, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.; Barry-Ryan, C.; Bourke, P. Antimicrobial Activity of Plant Essential Oils Using Food Model Media: Efficacy, Synergistic Potential and Interactions with Food Components. Food Microbiol. 2009, 26, 142–150. [Google Scholar] [CrossRef]
- Olaimat, A.N.; Holley, R.A. Inhibition of Listeria Monocytogenes on Cooked Cured Chicken Breasts by Acidified Coating Containing Allyl Isothiocyanate or Deodorized Oriental Mustard Extract. Food Microbiol. 2016, 57, 90–95. [Google Scholar] [CrossRef]
- Moller, A.; Leone, C.; Kataria, J.; Sidhu, G.; Rama, E.N.; Kroft, B.; Thippareddi, H.; Singh, M. Effect of a Carrageenan/Chitosan Coating with Allyl Isothiocyanate on Microbial Load in Chicken Breast. LWT 2022, 161, 113397. [Google Scholar] [CrossRef]
- Nadarajah, D.; Han, J.H.; Holley, R.A. Inactivation of Escherichia Coli O157:H7 in Packaged Ground Beef by Allyl Isothiocyanate. Int. J. Food Microbiol. 2005, 99, 269–279. [Google Scholar] [CrossRef]
- Chacon, P.A.; Muthukumarasamy, P.; Holley, R.A. Elimination of Escherichia Coli O157:H7 from Fermented Dry Sausages at an Organoleptically Acceptable Level of Microencapsulated Allyl Isothiocyanate. Appl. Environ. Microbiol. 2006, 72, 3096–3102. [Google Scholar] [CrossRef]
- Hintz, T.; Matthews, K.K.; Di, R. The Use of Plant Antimicrobial Compounds for Food Preservation. BioMed Res. Int. 2015, 2015, e246264. [Google Scholar] [CrossRef]
- Tajkarimi, M.M.; Ibrahim, S.A.; Cliver, D.O. Antimicrobial Herb and Spice Compounds in Food. Food Control 2010, 21, 1199–1218. [Google Scholar] [CrossRef]
- Lin, C.M.; Preston, J.F.; Wei, C.I. Antibacterial Mechanism of Allyl Isothiocyanate. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Delaquis, P.J.; Mazza, G. Antimicrobial Properties of Isothiocyanates in Food Preservation. Food Technol. 1995, 49, 73–84. [Google Scholar]
- Kyung, K.H.; Fleming, H.P. Antimicrobial Activity of Sulfur Compounds Derived from Cabbage. J. Food Prot. 1997, 60, 67–71. [Google Scholar] [CrossRef]
- Isshiki, K.; Tokuoka, K.; Mori, R.; Chiba, S. Preliminary Examination of Allyl Isothiocyanate Vapor for Food Preservation. Biosci. Biotechnol. Biochem. 1992, 56, 1476–1477. [Google Scholar] [CrossRef]
- Ward, S.M.; Delaquis, P.J.; Holley, R.A.; Mazza, G. Inhibition of Spoilage and Pathogenic Bacteria on Agar and Pre-Cooked Roast Beef by Volatile Horseradish Distillates. Food Res. Int. 1998, 31, 19–26. [Google Scholar] [CrossRef]
- Dias, M.; Soares, N.; Borges, S.; Sousa, M.; Nunes, C.; Oliveira, I.; Medeiros, E. Use of Allyl Isothiocyanate and Carbon Nanotubes in an Antimicrobial Film to Package Shredded, Cooked Chicken Meat. Food Chem. 2013, 141, 3160–3166. [Google Scholar] [CrossRef]
- EFSA Scientific Opinion on the Safety of Allyl Isothiocyanate for the Proposed Uses as a Food Additive. EFSA J. 2010, 8, 1943. [CrossRef]
- CIE (Commission Internationale de L’Eclairage). Colorimetry, Publication CIE 15.2-1986, 2nd ed.; Commission Internationale de L’Eclairage: Viena, Austria, 1986; ISBN 3-900-734-00-3. [Google Scholar]
- Grau, R.; Hamm, R. A Simple Method for the Determination of Water Binding in Muscles. Naturswissenschaften 1953, 40, 29–30. [Google Scholar] [CrossRef]
- Utama, D.T.; Lee, S.G.; Baek, K.H.; Chung, W.S.; Chung, I.A.; Jeon, J.T.; Lee, S.K. High Pressure Processing for Dark-Firm-Dry Beef: Effect on Physical Properties and Oxidative Deterioration during Refrigerated Storage. Asian-Australas. J. Anim. Sci. 2017, 30, 424–431. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A. Krzywicki Revisited: Equations for Spectrophotometric Determination of Myoglobin Redox Forms in Aqueous Meat Extracts. J. Food Sci. 2004, 69, C717–C720. [Google Scholar] [CrossRef]
- Ganhão, R.; Estévez, M.; Morcuende, D. Suitability of the TBA Method for Assessing Lipid Oxidation in a Meat System with Added Phenolic-Rich Materials. Food Chem. 2011, 126, 772–778. [Google Scholar] [CrossRef]
- Dussault, D.; Vu, K.D.; Lacroix, M. In Vitro Evaluation of Antimicrobial Activities of Various Commercial Essential Oils, Oleoresin and Pure Compounds against Food Pathogens and Application in Ham. Meat Sci. 2014, 96, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Semeniuc, C.A.; Pop, C.R.; Rotar, A.M. Antibacterial Activity and Interactions of Plant Essential Oil Combinations against Gram-Positive and Gram-Negative Bacteria. J. Food Drug Anal. 2017, 25, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Jridi, M.; Siala, R.; Fakhfakh, N.; Ayadi, M.A.; Elhatmi, M.; Taktak, M.A.; Nasri, M.; Zouari, N. Effect of Rosemary Leaves and Essential Oil on Turkey Sausage Quality. Acta Aliment. 2015, 44, 534–541. [Google Scholar] [CrossRef]
- Friedrich, L.; Siró, I.; Dalmadi, I.; Horváth, K.; Ágoston, R.; Balla, C. Influence of Various Preservatives on the Quality of Minced Beef under Modified Atmosphere at Chilled Storage. Meat Sci. 2008, 79, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Marshall, M.R.; Wei, C. Antibacterial Activity of Some Essential Oil Components against Five Foodborne Pathogens. J. Agric. Food Chem. 1995, 43, 2839–2845. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Guarda, A.; Rubilar, J.F.; Miltz, J.; Galotto, M.J. The Antimicrobial Activity of Microencapsulated Thymol and Carvacrol. Int. J. Food Microbiol. 2011, 146, 144–150. [Google Scholar] [CrossRef]
- López, P.; Sanchez, C.; Batlle, R.; Nerín, C. Vapor-Phase Activities of Cinnamon, Thyme, and Oregano Essential Oils and Key Constituents against Foodborne Microorganisms. J. Agric. Food Chem. 2007, 55, 4348–4356. [Google Scholar] [CrossRef]
- Lopez-Romero, J.C.; González-Ríos, H.; Borges, A.; Simões, M. Antibacterial Effects and Mode of Action of Selected Essential Oils Components against Escherichia Coli and Staphylococcus aureus. Available online: https://www.hindawi.com/journals/ecam/2015/795435/ (accessed on 6 August 2020).
- Boskovic, M.; Djordjevic, J.; Ivanovic, J.; Janjic, J.; Zdravkovic, N.; Glisic, M.; Glamoclija, N.; Baltic, B.; Djordjevic, V.; Baltic, M. Inhibition of Salmonella by Thyme Essential Oil and Its Effect on Microbiological and Sensory Properties of Minced Pork Meat Packaged under Vacuum and Modified Atmosphere. Int. J. Food Microbiol. 2017, 258, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The Mechanisms of Action of Carvacrol and Its Synergism with Nisin against Listeria Monocytogenes on Sliced Bologna Sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of Broiler Chicken Meat Quality and Factors Affecting Them: A Review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Shirzadegan, K.; Falahpour, P. The Physicochemical Properties and Antioxidative Potential of Raw Thigh Meat from Broilers Fed a Dietary Medicinal Herb Extract Mixture. Open Vet. J. 2014, 4, 69–77. [Google Scholar] [CrossRef]
- Jauhar, S.; Ismail-Fitry, M.R.; Chong, G.H.; Nor-Khaizura, M.A.R.; Ibadullah, W.Z.W. Different Pressures, Low Temperature, and Short-Duration Supercritical Carbon Dioxide Treatments: Microbiological, Physicochemical, Microstructural, and Sensorial Attributes of Chill-Stored Chicken Meat. Appl. Sci. 2020, 10, 6629. [Google Scholar] [CrossRef]
- Bekhit, A.E.D.; Cassidy, L.; Hurst, R.D.; Farouk, M.M. Post-Mortem Metmyoglobin Reduction in Fresh Venison. Meat Sci. 2007, 75, 53–60. [Google Scholar] [CrossRef]
- Csehi, B.; Szerdahelyi, E.; Pásztor-Huszár, K.; Salamon, B.; Tóth, A.; Zeke, I.; Jónás, G.; Friedrich, L. Changes of Protein Profiles in Pork and Beef Meat Caused by High Hydrostatic Pressure Treatment. Acta Aliment. 2016, 45, 565–571. [Google Scholar] [CrossRef]
- Toldrà, M.; Dàvila, E.; Saguer, E.; Fort, N.; Salvador, P.; Parés, D.; Carretero, C. Functional and Quality Characteristics of the Red Blood Cell Fraction from Biopreserved Porcine Blood as Influenced by High Pressure Processing. Meat Sci. 2008, 80, 380–388. [Google Scholar] [CrossRef]
- Arshad, M.S.; Amjad, Z.; Yasin, M.; Saeed, F.; Imran, A.; Sohaib, M.; Anjum, F.M.; Hussain, S. Quality and Stability Evaluation of Chicken Meat Treated with Gamma Irradiation and Turmeric Powder. Int. J. Food Prop. 2019, 22, 154–172. [Google Scholar] [CrossRef]
- Karwowska, M.; Dolatowski, Z.J. Effect of Mustard on Lipid Oxidation in Model Pork Meat Product. Eur. J. Lipid Sci. Technol. 2014, 116, 311–318. [Google Scholar] [CrossRef]
- Bellucci, E.R.B.; Bis-Souza, C.V.; Domínguez, R.; Bermúdez, R.; Barretto, A.C.D.S. Addition of Natural Extracts with Antioxidant Function to Preserve the Quality of Meat Products. Biomolecules 2022, 12, 1506. [Google Scholar] [CrossRef] [PubMed]
- Hussein, K.; Friedrich, L.; Kisko, G.; Ayari, E.; Nemeth, C.; Dalmadi, I. Use of Allyl-Isothiocyanate and Carvacrol to Preserve Fresh Chicken Meat during Chilling Storage. Czech J. Food Sci. 2019, 37, 417–424. [Google Scholar] [CrossRef]
- Ahn, E.S.; Kim, Y.S.; Shin, D.H. Observation of Bactericidal Effect of Allyl Isothiocyanate on Listeria Monocytogenes. Food Sci. Biotechnol. 2001, 10, 31–35. [Google Scholar]
- Meira, N.V.B.; Holley, R.A.; Bordin, K.; de Macedo, R.E.F.; Luciano, F.B. Combination of Essential Oil Compounds and Phenolic Acids against Escherichia Coli O157:H7 in Vitro and in Dry-Fermented Sausage Production. Int. J. Food Microbiol. 2017, 260, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, M.P.J.C.; Pires, A.C.D.S.; Soares, N.d.F.F.; Araújo, E.A. Use of Allyl Isothiocyanate Sachet to Preserve Cottage Cheese. J. Foodserv. 2009, 20, 275–279. [Google Scholar] [CrossRef]
- Guo, M.; Jin, T.Z.; Scullen, O.J.; Sommers, C.H. Effects of Antimicrobial Coatings and Cryogenic Freezing on Survival and Growth of Listeria Innocua on Frozen Ready-to-Eat Shrimp during Thawing. J. Food Sci. 2013, 78, M1195–M1200. [Google Scholar] [CrossRef]
- Jin, T.; Gurtler, J.B. Inactivation of Salmonella on Tomato Stem Scars by Edible Chitosan and Organic Acid Coatings. J. Food Prot. 2012, 75, 1368–1372. [Google Scholar] [CrossRef]
- Luciano, F.B.; Holley, R.A. Enzymatic Inhibition by Allyl Isothiocyanate and Factors Affecting Its Antimicrobial Action against Escherichia Coli O157:H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ahn, E.S.; Shin, D.H. Extension of Shelf Life by Treatment with Allyl Isothiocyanate in Combination with Acetic Acid on Cooked Rice. J. Food Sci. 2002, 67, 274–279. [Google Scholar] [CrossRef]





| Bacterial Strains | Storage Time (h) | Bioactive Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thymol | Eugenol | Limonene | Cuminaldehyde | AITC | β-Citronellol | Geraniol | (−)-α-Bisabolol | BHT | ||
| Thickness of the Zone of Inhibition (mm) ± SD | ||||||||||
| Pseudomonas lundensis | 24 | 3.33 ± 0.78 | 1.38 ± 0.00 | NI | 1.22 ± 0.30 | CI | 1.59 ± 0.27 | 1.70 ± 0.36 | 1.45 ± 0.11 | 0.58 ± 0.14 |
| 48 | 2.95 ± 1.13 | 1.72 ± 0.00 | NI | 1.25 ± 0.33 | CI | 1.58 ± 0.28 | 1.25 ± 0.41 | NI | NI | |
| 72 | 2.50 ± 0.67 | 1.68 ± 0.00 | NI | 0.80 ± 0.01 | 10.80 ± 0.5.28 | 1.40 ± 0.25 | 1.10 ± 0.49 | NI | NI | |
| Escherichia coli | 24 | 7.38 ± 0.62 | 5.57 ± 0.21 | 1.48 ± 0.01 | 4.80 ± 0.25 | 1.63 ± 0.49 | 1.15 ± 0.38 | 2.69 ± 0.44 | NI | NI |
| 48 | 7.21 ± 0.66 | 4.63 ± 0.10 | 1.09 ± 0.40 | 3.74 ± 0.69 | 2.0 ± 0.91 | 1.15 ± 0.21 | 2.67 ± 0.50 | NI | NI | |
| 72 | 6.68 ± 0.53 | 4.70 ± 0.36 | 1.20 ± 0.54 | 3.69 ± 0.41 | 1.72 ± 0.70 | 0.91 ± 0.14 | 2.33 ± 0.42 | NI | NI | |
| Staphylococcus aureus | 24 | 7.15 ± 0.98 | 6.91 ± 0.00 | 0.85 ± 0.10 | 9.42 ± 2.22 | CI | 1.82 ± 0.04 | 2.05 ± 0.05 | 0.47 ± 0.02 | 1.09 ± 0.51 |
| 48 | 5.77 ± 0.51 | 6.67 ± 0.00 | 0.86 ± 0.00 | 7.17 ± 0.10 | CI | 1.73 ± 0.19 | 2.02 ± 0.15 | 0.42 ± 0.06 | 0.84 ± 0.25 | |
| 72 | 5.76 ± 0.29 | 6.49 ± 0.00 | 0.87 ± 0.00 | 6.85 ± 0.68 | CI | 0.99 ± 0.38 | 1.94 ± 0.02 | 0.39 ± 0.09 | 0.80 ± 0.30 | |
| Listeria monocytogenes | 24 | 5.41 ± 0.68 | 2.75 ± 0.00 | NI | 1.64 ± 0.05 | 1.53 ± 0.12 | 1.86 ± 0.10 | 0.82 ± 0.02 | NI | NI |
| 48 | 5.34 ± 0.41 | 2.62 ± 0.00 | NI | 1.03 ± 0.09 | 1.41 ± 0.18 | 1.01 ± 0.16 | NI | NI | NI | |
| 72 | 5.28 ± 0.62 | 2.61 ± 0.00 | NI | 1.07 ± 0.06 | 1.28 ± 0.06 | 1.10 ± 0.31 | NI | NI | NI | |
| Salmonella Typhimurium | 24 | 6.62 ± 0.19 | 3.35 ± 0.00 | 0.00 | 2.52 ± 0.17 | CI | 1.85 ± 0.24 | 0.82 ± 0.16 | NI | 0.51 ± 0.52 |
| 48 | 6.61 ± 0.51 | 3.17 ± 0.00 | 0.00 | 1.92 ± 0.08 | 7.03 ± 4.09 | 1.42 ± 0.12 | 0.77 ± 0.77 | NI | NI | |
| 72 | 5.55 ± 0.70 | 3.37 ± 0.00 | 0.00 | 1.96 ± 0.24 | 5.33 ± 3.75 | 1.25 ± 0.07 | 0.67 ± 0.78 | NI | NI | |
| Bacillus cereus | 24 | 7.30 ± 0.01 | 5.10 ± 0.00 | NI | 6.54 ± 0.14 | 2.48 ± 0.97 | 2.15 ± 0.09 | 2.04 ± 0.39 | 0.44 ± 0.45 | NI |
| 48 | 5.03 ± 0.01 | 5.56 ± 0.00 | NI | 3.08 ± 0.58 | 0.99 ± 0.06 | 2.05 ± 0.16 | 1.11 ± 1.11 | 0.30 ± 0.30 | NI | |
| 72 | 5.77 ± 0.38 | 4.20 ± 0.00 | NI | 3.02 ± 0.53 | 1.24 ± 0.35 | 1.09 ± 0.69 | 1.07 ± 1.07 | 0.30 ± 0.30 | NI | |
| Bacterial Strains | Ethanol | Bioactive Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Thymol | Eucgenol | Limonene | Cuminaldehyde | AITC | β-Citronellol | Geraniol | (−)-α-Bisabolol | BHT | ||
| MIC (μL/mL) | ||||||||||
| Pseudomonas lundensis | NI | 0.25 | 0.125 | 1 | 0.25 | 0.063 | 0.5 | 0.5 | 1 | 0.25 |
| Escherichia coli | NI | 0.063 | 0.125 | 0.5 | 0.25 | 0.008 | 0.25 | 0.063 | 0.5 | 0.5 |
| Staphylococcus aureus | NI | 0.063 | 0.125 | 0.5 | 0.25 | 0.004 | 0.063 | 0.063 | 0.25 | 0.063 |
| Listeria monocytogenes | NI | 0.125 | 0.125 | 0.5 | 0.25 | 0.008 | 0.063 | 0.063 | 0.25 | 0.5 |
| Salmonella Typhimurium | NI | 0.25 | 0.125 | 0.5 | 0.125 | 0.004 | 0.25 | 0.063 | 0.5 | 0.5 |
| Bacillus cereus | NI | 0.125 | 0.125 | 1 | 0.25 | 0.031 | 0.063 | 0.125 | 0.25 | 0.5 |
| Parameters | Storage Time (d) | Treatments | |||
|---|---|---|---|---|---|
| No-BAC | AITC-MIC-1 | AITC-MIC-2 | AITC-MIC-4 | ||
| pH | 0 | 6.02 ± 0.01 cA | 6.03 ± 0.03 cA | 6.05 ± 0.04 cA | 6.04 ± 0.04 dA |
| 3 | 5.92 ± 0.05 bA | 5.99 ± 0.01 cAB | 6.01 ± 0.01 cB | 6.01 ± 0.00 dB | |
| 7 | 5.85 ± 0.01 abA | 5.89 ± 0.01 bB | 5.88 ± 0.01 bB | 5.91 ± 0.01 cC | |
| 10 | 5.83 ± 0.03 aB | 5.77 ± 0.02 aA | 5.78 ± 0.02 aA | 5.83 ± 0.01 bB | |
| 14 | 5.82 ± 0.02 aB | 5.76 ± 0.03 aAB | 5.73 ± 0.02 aA | 5.72 ± 0.03 aA | |
| L* | 0 | 51.15 ± 0.57 aA | 52.40 ± 1.91 aA | 53.26 ± 1.73 aA | 53.83 ± 2.28 aA |
| 3 | 54.13 ± 0.17 cA | 55.39 ± 0.46 bAB | 55.73 ± 0.36 bB | 60.81 ± 1.42 bC | |
| 7 | 53.02 ± 1.61 bcA | 56.32 ± 0.51 bcB | 56.60 ± 0.89 bcB | 62.40 ± 0.84 bC | |
| 10 | 52.98 ± 0.43 bcA | 58.12 ± 0.39 cB | 58.63 ± 0.16 dB | 62.44 ± 0.59 bC | |
| 14 | 52.34 ± 0.56 abA | 55.55 ± 1.36 bB | 58.09 ± 0.72 cdC | 62.82 ± 0.33 bD | |
| a* | 0 | 2.77 ± 0.32 aA | 2.91 ± 0.62 aA | 2.81 ± 0.68 abA | 2.75 ± 0.18 bA |
| 3 | 3.84 ± 1.11 bA | 3.32 ± 0.32 abA | 3.12 ± 0.22 abA | 3.09 ± 0.12 bA | |
| 7 | 3.98 ± 0.65 bC | 3.75 ± 0.35 bBC | 3.20 ± 0.17 bAB | 2.64 ± 0.38 bA | |
| 10 | 4.07 ± 1.20 bA | 3.20 ± 0.49 abA | 3.37 ± 0.20 bA | 2.94 ± 0.25 bA | |
| 14 | 3.25 ± 0.80 abB | 2.83 ± 0.28 aAB | 2.31 ± 0.60 aAB | 1.87 ± 0.45 aA | |
| b* | 0 | 3.95 ± 0.64 aA | 4.22 ± 0.57 aA | 4.17 ± 0.70 abA | 4.01 ± 0.46 aA |
| 3 | 4.18 ± 0.88 aA | 4.70 ± 0.67 aA | 4.65 ± 0.51 bA | 5.32 ± 0.50 bA | |
| 7 | 3.32 ± 0.52 aA | 3.73 ± 0.50 aA | 4.19 ± 0.57 abA | 5.69 ± 0.65 bB | |
| 10 | 3.15 ± 0.90 aA | 3.80 ± 0.57 aA | 3.37 ± 0.20 aA | 5.12 ± 0.38 bB | |
| 14 | 5.74 ± 0.75 bA | 6.09 ± 0.72 bAB | 7.17 ± 0.34 cB | 8.76 ± 0.61 cC | |
| C* | 0 | 4.84 ± 0.58 aA | 5.16 ± 0.56 aA | 5.03 ± 0.94 aA | 4.87 ± 0.40 aA |
| 3 | 5.72 ± 1.17 abA | 5.77 ± 0.56 abA | 5.61 ± 0.38 aA | 6.16 ± 0.37 bA | |
| 7 | 5.23 ± 0.37 abA | 5.30 ± 0.52 aA | 5.29 ± 0.38 aA | 6.29 ± 0.51 bB | |
| 10 | 5.27 ± 0.79 abAB | 4.97 ± 0.75 aAB | 4.76 ± 0.29 aA | 5.91 ± 0.24 bB | |
| 14 | 6.62 ± 0.87 cA | 6.73 ± 0.59 bA | 7.54 ± 0.45 bA | 8.97 ± 0.53 cB | |
| h* | 0 | 0.96 ± 0.09 abA | 0.97 ± 0.13 bA | 0.98 ± 0.05 bA | 0.97 ± 0.06 aA |
| 3 | 0.84 ± 0.14 abA | 0.95 ± 0.09 bAB | 0.98 ± 0.07 bAB | 1.04 ± 0.06 abB | |
| 7 | 0.70 ± 0.14 aA | 0.78 ± 0.06 aAB | 0.91 ± 0.09 bB | 1.13 ± 0.09 bC | |
| 10 | 0.67 ± 0.26 aA | 0.87 ± 0.02 abAB | 0.79 ± 0.00 aA | 1.05 ± 0.07 abB | |
| 14 | 1.06 ± 0.10 bA | 1.13 ± 0.07 cAB | 1.26 ± 0.07 cBC | 1.36 ± 0.06 cC | |
| WHC | 0 | 1.71 ± 0.12 aA | 1.78 ± 0.21 aA | 1.87 ± 0.20 aA | 1.96 ± 0.11 aA |
| 3 | 1.61 ± 0.14 aA | 1.75 ± 0.30 aA | 1.78 ± 0.17 aA | 1.84 ± 0.21 aA | |
| 7 | 1.72 ± 0.26 aA | 1.75 ± 0.09 aA | 2.05 ± 0.07 aA | 2.13 ± 0.16 aA | |
| 10 | 1.63 ± 0.03 aA | 1.72 ± 0.13 aA | 2.12 ± 0.11 aB | 2.21 ± 0.22 aB | |
| 14 | 1.34 ± 0.13 aA | 1.67 ± 0.03 aA | 2.15 ± 0.22 aB | 2.22 ± 0.11 aB | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, K.N.; Friedrich, L.; Dalmadi, I.; Kiskó, G. Evaluation of In Vitro Antimicrobial Activity of Bioactive Compounds and the Effect of Allyl-Isothiocyanate on Chicken Meat Quality under Refrigerated Conditions. Appl. Sci. 2023, 13, 10953. https://doi.org/10.3390/app131910953
Hussein KN, Friedrich L, Dalmadi I, Kiskó G. Evaluation of In Vitro Antimicrobial Activity of Bioactive Compounds and the Effect of Allyl-Isothiocyanate on Chicken Meat Quality under Refrigerated Conditions. Applied Sciences. 2023; 13(19):10953. https://doi.org/10.3390/app131910953
Chicago/Turabian StyleHussein, Khabat Noori, László Friedrich, István Dalmadi, and Gabriella Kiskó. 2023. "Evaluation of In Vitro Antimicrobial Activity of Bioactive Compounds and the Effect of Allyl-Isothiocyanate on Chicken Meat Quality under Refrigerated Conditions" Applied Sciences 13, no. 19: 10953. https://doi.org/10.3390/app131910953
APA StyleHussein, K. N., Friedrich, L., Dalmadi, I., & Kiskó, G. (2023). Evaluation of In Vitro Antimicrobial Activity of Bioactive Compounds and the Effect of Allyl-Isothiocyanate on Chicken Meat Quality under Refrigerated Conditions. Applied Sciences, 13(19), 10953. https://doi.org/10.3390/app131910953






