An In Vitro Study of the Healing Potential of Black Mulberry (Morus nigra L.) Extract in a Liposomal Formulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
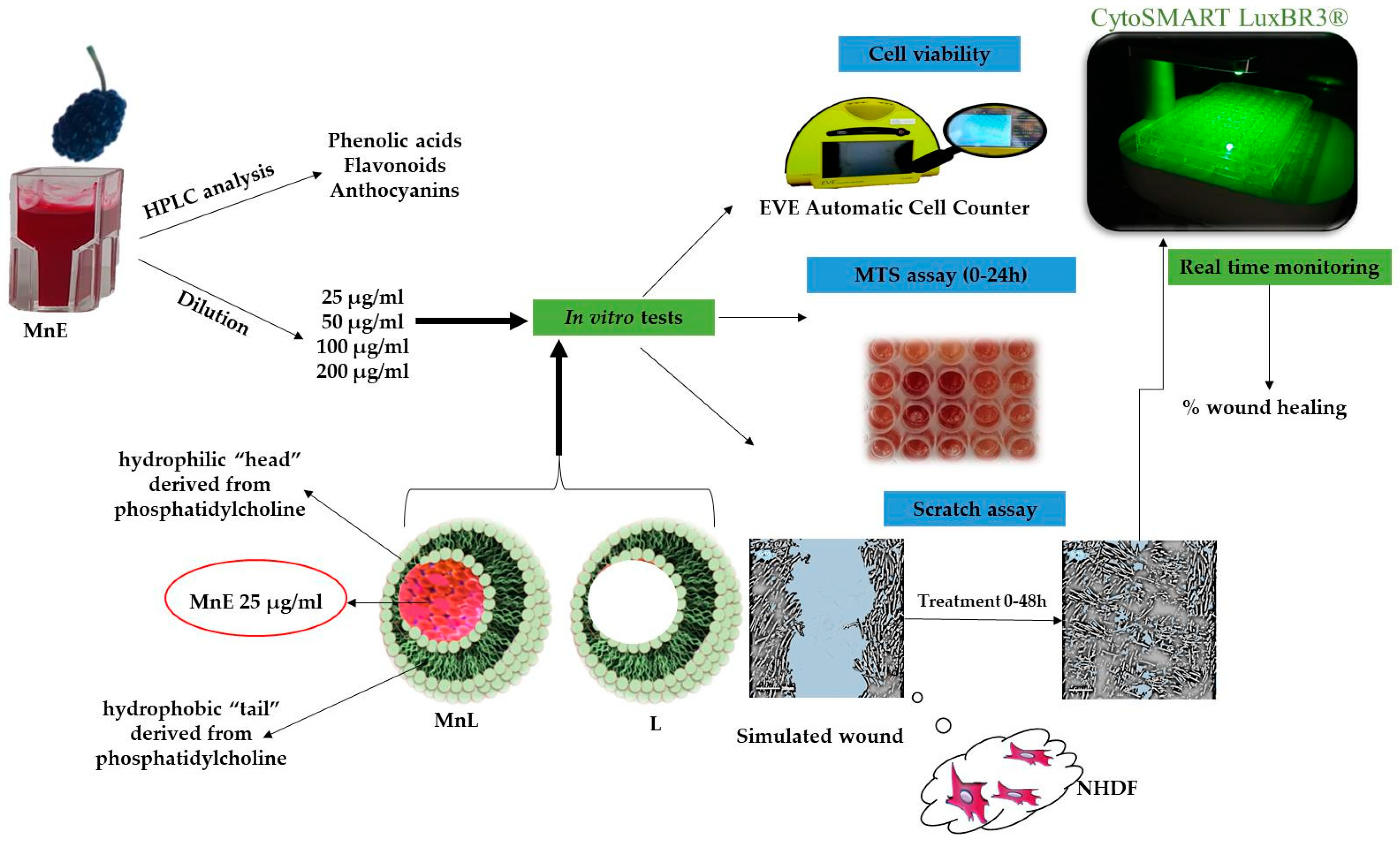
2.2. Preparation of Mulberry Extract
2.3. HPLC-DAD-MS (ESI+) Characterization of the Extract from the Point of View of Anthocyanin Content
2.4. Determination of Total Phenol Content (TPc)
2.5. Determination of Total Flavonoids Content (TFc)
2.6. Ascorbic Acid Determination
2.7. Determination of Antioxidant Capacity
FRAP Assay
2.8. Formulation of Liposomes with Mulberry Extract and Their Characterization
2.8.1. Formulation of Liposomes Using the Lipid Film Hydration Method
2.8.2. Microscopic Characterization of the Formed Liposomes
2.8.3. Characterization of the Size and the Electric Surface Potential of the Liposomes by DLS
2.8.4. Determination of the Entrapment Efficiency (EE%) of the Mulberry Extract in Liposomes
2.9. Cell Culture and Treatment
2.9.1. Cell Viability Assay
2.9.2. Cell Proliferation Assay (MTS)
2.9.3. In Vitro Testing of the Healing Effect of Mulberry Extracts and Its Liposomes Formulated Using the Scratch Method
2.10. Statistical Analyses
3. Results and Discussions
3.1. Phytochemical Characterization of the MnE
3.2. Determination of Total Phenols, Flavonoids, Ascorbic Acid Content and Antioxidant Capacity
3.3. Formulation of Liposomes with Mulberry Extract and Their Characterization
3.3.1. Formulation of Liposomes Using the Lipid Film Hydration Method and Microscopic Characterization of the Formed Liposomes
3.3.2. Characterization of the Size and the Electric Surface Potential of the Liposomes by DLS
3.3.3. Determination of the Entrapment Efficiency (EE%) of the Mulberry Extract in Liposomes
3.4. Cell Culture and Treatment
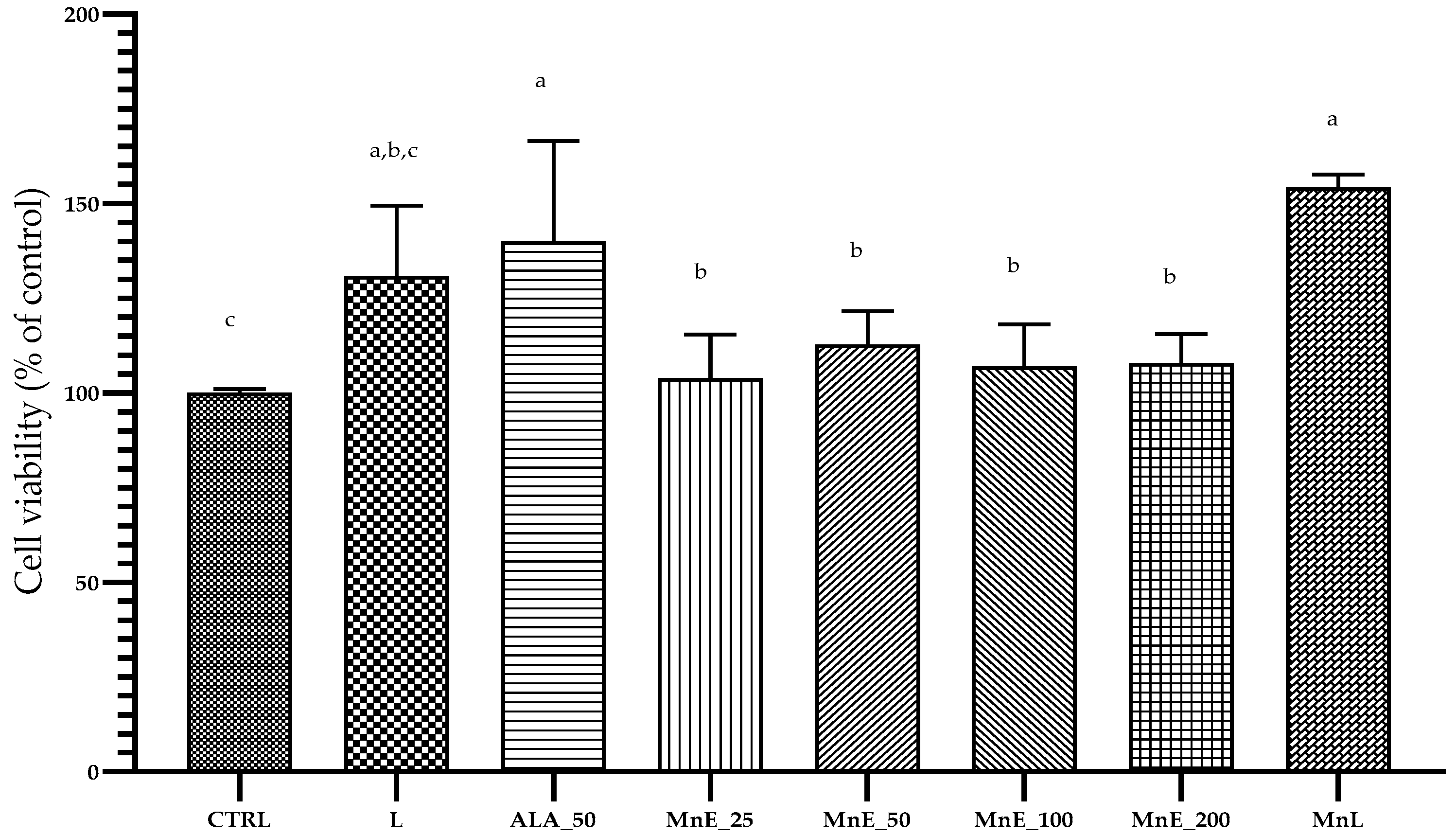
3.4.1. Cell Viability and Proliferation Assay (MTS) of Samples
3.4.2. In Vitro Testing of the Healing Effect of Mulberry Extracts and Its Liposome Using the Scratch Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodrigues, E.L.; Marcelino, G.; Silva, G.T.; Figueiredo, P.S.; Garcez, W.S.; Corsino, J.; Guimarães, R.D.C.A.; Freitas, K.D.C. Nutraceutical and Medicinal Potential of the Morus Species in Metabolic Dysfunctions. Int. J. Mol. Sci. 2019, 20, 301. [Google Scholar] [CrossRef] [Green Version]
- D’Urso, G.; Mes, J.J.; Montoro, P.; Hall, R.D.; De Vos, R.C.H. Identification of Bioactive Phytochemicals in Mulberries. Metabolites 2019, 10, 7. [Google Scholar] [CrossRef] [Green Version]
- Jin, Q.; Yang, J.; Ma, L.; Wen, D.; Chen, F.; Li, J. Identification of polyphenols in mulberry (genus Morus) cultivars by liquid chromatography with time-of-flight mass spectrometer. J. Food Compos. Anal. 2017, 63, 55–64. [Google Scholar] [CrossRef]
- Sánchez-Salcedo, E.M.; Tassotti, M.; Del Rio, D.; Hernández, F.; Martínez, J.J.; Mena, P. (Poly)phenolic fingerprint and chemometric analysis of white (Morus alba L.) and black (Morus nigra L.) mulberry leaves by using a non-targeted UHPLC–MS approach. Food Chem. 2016, 212, 250–255. [Google Scholar] [CrossRef]
- Tomas, M.; Toydemir, G.; Boyacioglu, D.; Hall, R.D.; Beekwilder, J.; Capanoglu, E. Processing black mulberry into jam: Effects on antioxidant potential and in vitro bioaccessibility. J. Sci. Food Agric. 2017, 97, 3106–3113. [Google Scholar] [CrossRef] [PubMed]
- Memete, A.R.; Teusdea, A.C.; Timar, A.V.; Vuscan, A.N.; Mintaș, O.S.; Cavalu, S.; Vicas, S.I. Effects of Different Edible Coatings on the Shelf Life of Fresh Black Mulberry Fruits (Morus nigra L.). Agriculture 2022, 12, 1068. [Google Scholar] [CrossRef]
- Memete, A.R.; Timar, A.V.; Vuscan, A.N.; Venter, A.C.; Vicas, S.I. Phytochemical Composition of Different Botanical Parts of Morus Species, Health Benefits and Application in Food Industry. Plants 2022, 11, 152. [Google Scholar] [CrossRef]
- Pawlowska, A.M.; Oleszek, W.; Braca, A. Quali-quantitative Analyses of Flavonoids of Morus nigra L. and Morus alba L. (Moraceae) Fruits. J. Agric. Food Chem. 2008, 56, 3377–3380. [Google Scholar] [CrossRef]
- Gültekin-Özgüven, M.; Karadağ, A.; Duman, Ş.; Özkal, B.; Özçelik, B. Fortification of dark chocolate with spray dried black mulberry (Morus nigra) waste extract encapsulated in chitosan-coated liposomes and bioaccessability studies. Food Chem. 2016, 201, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Nie, W.-J. Chemical properties in fruits of mulberry species from the Xinjiang province of China. Food Chem. 2015, 174, 460–466. [Google Scholar] [CrossRef]
- Ercisli, S.; Orhan, E. Chemical composition of white (Morus alba), red (Morus rubra) and black (Morus nigra) mulberry fruits. Food Chem. 2007, 103, 1380–1384. [Google Scholar] [CrossRef]
- Humbert, P.G.; Haftek, M.; Creidi, P.; Lapière, C.; Nusgens, B.; Richard, A.; Schmitt, D.; Rougier, A.; Zahouani, H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003, 12, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Maione-Silva, L.; de Castro, E.G.; Nascimento, T.L.; Cintra, E.R.; Moreira, L.C.; Cintra, B.A.S.; Valadares, M.C.; Lima, E.M. Ascorbic acid encapsulated into negatively charged liposomes exhibits increased skin permeation, retention and enhances collagen synthesis by fibroblasts. Sci. Rep. 2019, 9, 522. [Google Scholar] [CrossRef] [Green Version]
- Thulabandu, V.; Chen, D.; Atit, R.P. Dermal fibroblast in cutaneous development and healing. WIREs Dev. Biol. 2018, 7, e307. [Google Scholar] [CrossRef]
- Lynch, M.D.; Watt, F.M. Fibroblast heterogeneity: Implications for human disease. J. Clin. Investig. 2018, 128, 26–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rippa, A.L.; Kalabusheva, E.P.; Vorotelyak, E.A. Regeneration of Dermis: Scarring and Cells Involved. Cells 2019, 8, 607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorrell, J.M.; Caplan, A.I. Fibroblasts—A Diverse Population at the Center of It All. Int. Rev. Cell Mole. Biol. 2009, 276, 161–214. [Google Scholar]
- Salehi, B.; Butnariu, M.; Corneanu, M.; Sarac, I.; Vlaisavljevic, S.; Kitic, D.; Rahavian, A.; Abedi, A.R.; Karkan, M.F.; Bhatt, I.D.; et al. Chronic pelvic pain syndrome: Highlighting medicinal plants toward biomolecules discovery for upcoming drugs formulation. Phytother. Res. 2020, 34, 769–787. [Google Scholar] [CrossRef] [PubMed]
- Teușdea, A.C.; Laslo, V.; Cavalu, S.; Fritea, L.; Dobjanschi, L.; Zdrinca, M.; Zdrinca, M.; Ganea, M.; Pașc, P.; Memete, A.R.; et al. Evaluation of In Vitro Wound-Healing Potential, Antioxidant Capacity, and Antimicrobial Activity of Stellaria media (L.) Vill. Appl. Sci. 2021, 11, 11526. [Google Scholar] [CrossRef]
- Mohammadabadi, M.; Mozafari, M. Enhanced efficacy and bioavailability of thymoquinone using nanoliposomal dosage form. J. Drug Deliv. Sci. Technol. 2018, 47, 445–453. [Google Scholar] [CrossRef]
- Mozafari, M.R. Nanoliposomes: Preparation and Analysis. In Liposomes; Methods in Molecular Biology; Weissig, V., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 605, pp. 29–50. [Google Scholar]
- Zoghi, A.; Khosravi-Darani, K.; Omri, A. Process Variables and Design of Experiments in Liposome and Nanoliposome Research. Mini Rev. Med. Chem. 2018, 18, 324–344. [Google Scholar] [CrossRef]
- Sakla, M.; Breitinger, U.; Breitinger, H.-G.; Mansour, S.; Tammam, S.N. Delivery of trans-membrane proteins by liposomes; the effect of liposome size and formulation technique on the efficiency of protein delivery. Int. J. Pharm. 2021, 606, 120879. [Google Scholar] [CrossRef] [PubMed]
- Miere, F.; Vicas, S.I.; Timar, A.V.; Ganea, M.; Zdrinca, M.; Cavalu, S.; Fritea, L.; Vicas, L.; Muresan, M.; Pallag, A.; et al. Preparation and Characterization of Two Different Liposomal Formulations with Bioactive Natural Extract for Multiple Applications. Processes 2021, 9, 432. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [Green Version]
- Van Tran, V.; Moon, J.-Y.; Lee, Y.-C. Liposomes for delivery of antioxidants in cosmeceuticals: Challenges and development strategies. J. Control. Release 2019, 300, 114–140. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.; Rugina, O.D.; Pintea, A.M.; Sconţa, Z.; Bunea, C.I.; Socaciu, C. Comparative Polyphenolic Content and Antioxidant Activities of Some Wild and Cultivated Blueberries from Romania. Not. Bot. Horti Agrobot. Cluj Napoca 2011, 39, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Lee, J. Variations in Anthocyanin Profiles and Antioxidant Activity of 12 Genotypes of Mulberry (Morus spp.) Fruits and Their Changes during Processing. Antioxidants 2020, 9, 242. [Google Scholar] [CrossRef] [Green Version]
- Kleszken, E.; Purcarea, C.; Pallag, A.; Ranga, F.; Memete, A.R.; Miere, F.; Vicas, S.I. Phytochemical Profile and Antioxidant Capacity of Viscum album L. Subsp. album and Effects on Its Host Trees. Plants 2022, 11, 3021. [Google Scholar] [CrossRef]
- Budău, R.; Memete, A.R.; Timofte, A.I.; Vicas, S.I. Phytochemical screening and antioxidant capacity of two berry cultivars, ‘Ruben’ and ‘Duke’, depending on their harvesting time. Bull. Univ. Agric. Sci. Vet. Med. Cluj Napoca. Food Sci. Technol. 2022, 1, 27. [Google Scholar] [CrossRef]
- Bandici, L.; Teusdea, A.; Pavel Milian, O.; Ramona Memete, A.; Ioana Vicas, S.; Vasile Timar, A.; Emil Bandici, G. The Use of Microwaves in the Process of Reducing the Browning of Apple Slices. In Proceedings of the 2021 16th International Conference on Engineering of Modern Electric Systems (EMES), Oradea, Romania, 10 June 2021; pp. 1–4. [Google Scholar]
- Win, N.N.C.; Soe, T.T.; Kar, A.; Soe, Y.Y.; Lin, M. Effects of Syrup Solution with Different Concentrations of Citric Acid on Quality and Storage Life of Canned Litchi. Open Access Libr. J. 2021, 8, 1–16. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Khorasani, S.; Ghandehari Yazdi, A.P.; Taghavi, E.; Alipoor Amro Abadi, M.; Ghobadi, H.; Zihayat, B.; Rasti, B.; Mozafari, M.R. 3.32-Recent Trends in the Nanoencapsulation Processes for Food and Nutraceutical Applications. In Innovative Food Processing Technologies; Knoerzer, K., Muthukumarappan, K., Eds.; Elsevier: Oxford, UK, 2021; pp. 532–545. [Google Scholar]
- Miere, F.; Fritea, L.; Cavalu, S.; Vicaş, S. Formulation, Characterization, and Advantages of Using Liposomes in Multiple Therapies. Pharmacophore 2020, 11, 1–12. [Google Scholar]
- Gibis, M.; Ruedt, C.; Weiss, J. In vitro release of grape-seed polyphenols encapsulated from uncoated and chitosan-coated liposomes. Food Res. Int. 2016, 88, 105–113. [Google Scholar] [CrossRef]
- Chen, A.; Leith, M.; Tu, R.; Tahim, G.; Sudra, A.; Bhargava, S. Effects of diluents on cell culture viability measured by automated cell counter. PLoS ONE 2017, 12, e0173375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malich, G.; Markovic, B.; Winder, C. The sensitivity and specificity of the MTS tetrazolium assay for detecting the in vitro cytotoxicity of 20 chemicals using human cell lines. Toxicology 1997, 124, 179–192. [Google Scholar] [CrossRef]
- Kabakov, A.E.; Kudryavtsev, V.A.; Gabai, V.L. Determination of Cell Survival or Death. In Molecular Chaperones; Methods in Molecular Biology; Calderwood, S.K., Prince, T.L., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 787, pp. 231–244. [Google Scholar]
- Antonescu, A.; Fritea, L.; Teușdea, A.C.; Vicaș, L.; Vicaș, S.I.; Brihan, I.; Domuța, M.; Zdrinca, M.; Zdrinca, M.; Cavalu, S.; et al. Evaluation of Wound Healing Potential of Novel Hydrogel Based on Ocimum basilicum and Trifolium pratense Extracts. Processes 2021, 9, 2096. [Google Scholar] [CrossRef]
- Thabti, I.; Elfalleh, W.; Hannachi, H.; Ferchichi, A.; Campos, M.D.G. Identification and quantification of phenolic acids and flavonol glycosides in Tunisian Morus species by HPLC-DAD and HPLC–MS. J. Funct. Foods 2012, 4, 367–374. [Google Scholar] [CrossRef]
- Jaiswal, R.; Sovdat, T.; Vivan, F.; Kuhnert, N. Profiling and Characterization by LC-MSn of the Chlorogenic Acids and Hydroxycinnamoylshikimate Esters in Maté (Ilex paraguariensis). J. Agric. Food Chem. 2010, 58, 5471–5484. [Google Scholar] [CrossRef]
- Tocai, A.-C.; Ranga, F.; Teodorescu, A.G.; Pallag, A.; Vlad, A.M.; Bandici, L.; Vicas, S.I. Evaluation of Polyphenolic Composition and Antimicrobial Properties of Sanguisorba officinalis L. and Sanguisorba minor Scop. Plants 2022, 11, 3561. [Google Scholar] [CrossRef]
- Wu, X.; Prior, R.L. Systematic Identification and Characterization of Anthocyanins by HPLC-ESI-MS/MS in Common Foods in the United States: Fruits and Berries. J. Agric. Food Chem. 2005, 53, 2589–2599. [Google Scholar] [CrossRef]
- Singh, R.; Wu, B.; Tang, L.; Liu, Z.; Hu, M. Identification of the Position of Mono-O-glucuronide of Flavones and Flavonols by Analyzing Shift in Online UV Spectrum (λmax) Generated from an Online Diode Array Detector. J. Agric. Food Chem. 2010, 58, 9384–9395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Salcedo, E.M.; Mena, P.; García-Viguera, C.; Hernández, F.; Martínez, J.J. (Poly)phenolic compounds and antioxidant activity of white (Morus alba) and black (Morus nigra) mulberry leaves: Their potential for new products rich in phytochemicals. J. Funct. Foods 2015, 18, 1039–1046. [Google Scholar] [CrossRef]
- Radojković, M.M.; Zeković, Z.P.; Dojčinović, B.P.; Stojanović, Z.S.; Cvetanović, A.D.; Manojlović, D.D. Characterization of Morus species in respect to micro, macro, and toxic elements. Acta Period. Technol. 2014, 45, 229–237. [Google Scholar] [CrossRef]
- Saewan, N.; Jimtaisong, A. Natural products as photoprotection. J. Cosmet. Dermatol. 2015, 14, 47–63. [Google Scholar] [CrossRef]
- Ju, M.-J.; Kwon, J.-H.; Kim, H.-K. Physiological Activities of Mulberry Leaf and Fruit Extracts with Different Extraction Conditions. Korean J. Food Preserv. 2009, 16, 442–448. [Google Scholar]
- Soto, M.L.; Falqué, E.; Domínguez, H. Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics 2015, 2, 259–276. [Google Scholar] [CrossRef] [Green Version]
- Kitagawa, S.; Yoshii, K.; Morita, S.-Y.; Teraoka, R. Efficient Topical Delivery of Chlorogenic Acid by an Oil-in-Water Microemulsion to Protect Skin against UV-Induced Damage. Chem. Pharm. Bull. 2011, 59, 793–796. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.; Long, X.; Zhao, Q.; Zheng, Y.; Song, M.; Ma, S.; Jing, Y.; Wang, S.; He, Y.; Esteban, C.R.; et al. A Single-Cell Transcriptomic Atlas of Human Skin Aging. Dev. Cell 2021, 56, 383–397. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, Y.; Jiang, X.; Chen, T.; Ma, Y.; Wu, S.; Sun, J.; Jiao, R.; Li, X.; Deng, L.; et al. Cyanidin-3-O-glucoside inhibits the UVB-induced ROS/COX-2 pathway in HaCaT cells. J. Photochem. Photobiol. B Biol. 2017, 177, 24–31. [Google Scholar] [CrossRef]
- Phillips, K.M.; Tarrago-Trani, M.T.; McGinty, R.M.; Rasor, A.S.; Haytowitz, D.B.; Pehrsson, P.R. Seasonal variability of the vitamin C content of fresh fruits and vegetables in a local retail market. J. Sci. Food Agric. 2018, 98, 4191–4204. [Google Scholar] [CrossRef]
- Li, Y.; Bao, T.; Chen, W. Comparison of the protective effect of black and white mulberry against ethyl carbamate-induced cytotoxicity and oxidative damage. Food Chem. 2018, 243, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Uzun, H.İ.; Bayir, A. Biochemical Contents of Mulberry (Morus Spp.) Fruits. Planta Med. 2012, 78, PA1. [Google Scholar] [CrossRef]
- Pullar, J.M.; Carr, A.C.; Vissers, M.C.M. The Roles of Vitamin C in Skin Health. Nutrients 2017, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Shindo, Y.; Witt, E.; Han, D.; Epstein, W.; Packer, L. Enzymic and Non-Enzymic Antioxidants in Epidermis and Dermis of Human Skin. J. Investig. Dermatol. 1994, 102, 122–124. [Google Scholar] [CrossRef] [Green Version]
- Popovska, O.; Simonovska, J.; Kavrakovski, Z.; Rafajlovska, V. Preparation of Ketoconazole Liposomes with an Ultrasonic and an Injection Method Using Vegetable Oils. Indian J. Pharm. Educ. Res. 2020, 54, 946–953. [Google Scholar] [CrossRef]
- Asprea, M.; Tatini, F.; Piazzini, V.; Rossi, F.; Bergonzi, M.C.; Bilia, A.R. Stable, Monodisperse, and Highly Cell-Permeating Nanocochleates from Natural Soy Lecithin Liposomes. Pharmaceutics 2019, 11, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenz, S.F.; Sengupta, K. Giant vesicles as cell models. Integr. Biol. 2012, 4, 982–995. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M. Entrapment of rosemary extract by liposomes formulated by Mozafari method: Physicochemical characterization and optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Fritea, L.; Pasca, P.M.; Vlase, L.; Gheldiu, A.-M.; Moldovan, L.; Banica, F.; Dobjanschi, L.; Cavalu, S. Electrochemical Methods for Evaluation of Antioxidant Properties of Propolis Extract Incorporated in Chitosan Nanoparticles. Mater. Plast. 2021, 57, 96–108. [Google Scholar] [CrossRef]
- Cavalu, S.; Kamel, E.; Laslo, V.; Fritea, L.; Costea, T.; Antoniac, I.V.; Vasile, E.; Antoniac, A.; Semenescu, A.; Mohan, A.; et al. Eco-friendly, Facile and Rapid Way for Synthesis of Selenium Nanoparticles Production, structural and morphological characterisation. Rev. Chim. 2018, 68, 2963–2966. [Google Scholar] [CrossRef]
- Miere, F.G.; Ganea, M.; Teodorescu, A.G.; Fritea, L.; Lestyan, M.; Horvath, T.; Hanga-Fărcaș, A.; Vicaș, S.I. The Green Synthesıs of Sılver and Selenıum Nanopartıcles usıng the Plant Stellarıa Medıa (L.) Vıll. Pharmacophore 2022, 13, 88–95. [Google Scholar] [CrossRef]
- Li, Z.; Liu, M.; Wang, H.; Du, S. Increased cutaneous wound healing effect of biodegradable liposomes containing madecassoside: Preparation optimization, in vitro dermal permeation, and in vivo bioevaluation. Int. J. Nanomed. 2016, 11, 2995–3007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, B.W. Drug delivery routes in skin: A novel approach. Adv. Drug Deliv. Rev. 2002, 54, S31–S40. [Google Scholar] [CrossRef]
- Francke, N.M.; Bunjes, H. Influence of drug loading on the physical stability of phospholipid-stabilised colloidal lipid emulsions. Int. J. Pharm. X 2020, 2, 100060. [Google Scholar] [CrossRef]
- Wu, S.; Lin, X. Trials in developing a nanoscale material for extravascular contrast-enhanced ultrasound targeting hepatocellular carcinoma. Peerj 2020, 8, e10403. [Google Scholar] [CrossRef]
- Liu, X.; Han, M.; Xu, J.; Geng, S.; Zhang, Y.; Ye, X.; Gou, J.; Yin, T.; He, H.; Tang, X. Asialoglycoprotein receptor-targeted liposomes loaded with a norcantharimide derivative for hepatocyte-selective targeting. Int. J. Pharm. 2017, 520, 98–110. [Google Scholar] [CrossRef]
- Wichayapreechar, P.; Anuchapreeda, S.; Phongpradist, R.; Rungseevijitprapa, W.; Ampasavate, C. Dermal targeting of Centella asiatica extract using hyaluronic acid surface modified niosomes. J. Liposome Res. 2020, 30, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, M.J. Cell Viability Assays: Introduction. In Mammalian Cell Viability; Methods in Molecular Biology; Stoddart, M.J., Ed.; Humana Press: Totowa, NJ, USA, 2011; Volume 740, pp. 1–6. [Google Scholar]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Im, J.; Hyun, J.; Kim, S.-W.; Bhang, S.H. Enhancing the Angiogenic and Proliferative Capacity of Dermal Fibroblasts with Mulberry (Morus alba. L) Root Extract. Tissue Eng. Regen. Med. 2022, 19, 49–57. [Google Scholar] [CrossRef]
- Hyun, J.; Im, J.; Kim, S.-W.; Kim, H.; Seo, I.; Bhang, S. Morus alba Root Extract Induces the Anagen Phase in the Human Hair Follicle Dermal Papilla Cells. Pharmaceutics 2021, 13, 1155. [Google Scholar] [CrossRef]
- Jafari, S.M. An Overview of Nanoencapsulation Techniques and Their Classification. In Nanoencapsulation Technologies for the Food and Nutraceutical Industries; Jafari, S.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 1–34. [Google Scholar]
- Guo, S.; DiPietro, L.A. Factors Affecting Wound Healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef]
- Junker, J.P.; Caterson, E.; Eriksson, E. The Microenvironment of Wound Healing. J. Craniofacial Surg. 2013, 24, 12–16. [Google Scholar] [CrossRef]
- Ghiulai, R.; Roşca, O.; Antal, D.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Macaşoi, I.; Olariu, T.; Dehelean, C.; Creţu, O.; et al. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef] [PubMed]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, H.N.; Hardman, M.J. Wound healing: Cellular mechanisms and pathological outcomes. Open Biol. 2020, 10, 200223. [Google Scholar] [CrossRef]
- Du, C.; Fikhman, D.A.; Monroe, M.B.B. Shape Memory Polymer Foams with Phenolic Acid-Based Antioxidant Properties. Antioxidants 2022, 11, 1105. [Google Scholar] [CrossRef]
- Sivakumar, S.; Murali, R.; Arathanaikotti, D.; Gopinath, A.; Senthilkumar, C.; Kesavan, S.; Madhan, B. Ferulic acid loaded microspheres reinforced in 3D hybrid scaffold for antimicrobial wound dressing. Int. J. Biol. Macromol. 2021, 177, 463–473. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Özay, Y.; Güzel, S.; Yumrutaş, Ö.; Pehlivanoğlu, B.; Erdoğdu, I.H.; Yildirim, Z.; Türk, B.A.; Darcan, S. Wound Healing Effect of Kaempferol in Diabetic and Nondiabetic Rats. J. Surg. Res. 2019, 233, 284–296. [Google Scholar] [CrossRef] [PubMed]


| Peak No. | Rt (min) | UV λmax (nm) | [M+H]+ (m/z) | Phenolic Compounds | Subclass | MnE (µg/g fw) * |
|---|---|---|---|---|---|---|
| 1. | 3.05 | 270 | 155 | Dihydroxybenzoic acid | Hydroxybenzoic acid | 1143.714 ± 115.38 |
| 2. | 3.84 | 270 | 139 | Hydroxybenzoic acid | Hydroxybenzoic acid | 177.988 ± 16.99 |
| 3. | 9.36 | 280 | 579 | Procyanidin dimer | Flavanol | 672.943 ± 67.94 |
| 4. | 10.91 | 520 | 449 | Cyanidin 3-O-glucoside Cyanidin 3-O-rutinoside | Anthocyanins | 947.464 ± 93.96 |
| 5. | 595 | Anthocyanins | ||||
| 6. | 12.02 | 360, 255 | 773, 303 | Quercetin 3-glucosyl-(1->2)-rhamnoside-7-glucoside | Flavonol | 204.503 ± 19.59 |
| 7. | 12.35 | 322 | 355 | 5-Caffeoylquinic acid (chlorogenic acid) | Hydroxycinnamic acid | 1078.456 ± 109.08 |
| 8. | 12.65 | 322 | 355 | 1-Caffeoylquinic acid | Hydroxycinnamic acid | 587.256 ± 57.76 |
| 9. | 13.19 | 323 | 369 | 3-Feruloylquinic acid | Hydroxycinnamic acid | 202.347 ± 19.93 |
| 10. | 13.73 | 270 | 331 | Vanillic acid-glucoside | Phenolic glucoside | 408.647 ± 40.89 |
| 11. | 14.50 | 323 | 369 | 4-Feruloylquinic acid | Hydroxycinnamic acid | 609.269 ± 60.93 |
| 12. | 15.17 | 323 | 369 | 5-Feruloylquinic acid | Hydroxycinnamic acid | 431.909 ± 43.71 |
| 13. | 15.44 | 360, 255 | 611, 303 | Quercetin 3-O-rutinoside (rutin) | Flavonol | 1654.227 ± 166.02 |
| 14. | 16.11 | 360, 255 | 465, 303 | Quercetin 3-O-glucoside | Flavonol | 3402.965 ± 339.97 |
| 15. | 17.10 | 360, 255 | 449, 303 | Quercetin 3-O-rhamnoside | Flavonol | 459.747 ± 46.07 |
| 16. | 18.16 | 350, 250 | 473, 287 | Kaempferol 3-O-glucuronide | Flavonol | 335.817 ± 32.98 |
| Samples | TPc (mg GAE/100 g dw) | TFc (mg QE/100 g dw) | Ascorbic acid (mg AA/100 g fw) | FRAP (mmol TE/100 g dw) |
|---|---|---|---|---|
| MnE | 1460.37 ± 61.42 | 296.48 ± 79.53 | 43.71 ± 1.481 | 80.23 ± 0.56 |
| Liposomal Formula * | Size up to 500 nm (%) | Zeta Potential (mV) | EE% |
|---|---|---|---|
| L | 87.12 | −2.80 | - |
| MnL | 78.68 | −2.5 | 88.25 |
| Samples | % of Cell Viability |
|---|---|
| CTRL | 95.25 ± 7.31 |
| ALA_50 | 94.77 ± 5.51 |
| MnE_25 | 98.55 ± 5.90 |
| MnE_50 | 97.21 ± 7.11 |
| MnE_100 | 95.48 ± 8.24 |
| MnE_200 | 96.78 ± 7.48 |
| L | 95.15 ± 6.20 |
| MnL | 95.30 ± 6.50 |
| Samples | CTRL | ALA_50 | MnE_25 | MnE_50 | MnE_100 | MnE_200 | L | MnL | |
|---|---|---|---|---|---|---|---|---|---|
| Hours | |||||||||
| 12 | 35.66 ± 0.27 a | 47.09 ± 1.76 a | 50.48 ± 7.37 a | 43.55 ± 15.08 a | 30.90 ± 4.98 a | 46.88 ± 22.83 a | 44.83 ± 10.30 a | 40.39 ± 15.86 a | |
| 24 | 51.40 ± 6.41 a | 64.19 ± 3.37 a | 66.70 ± 15.04 a | 68.33 ± 0.16 a | 46.62 ± 14.57 a,b | 72.68 ± 2.38 a | 73.21 ± 3.05 b | 81.15 ± 14.80 a,c | |
| 36 | 66.61 ± 3.79 a | 74.93 ± 2.27 a,b | 75.87 ± 7.62 a,b | 70.66 ± 0.74 a,b | 64.78 ± 4.59 a,e | 78.62 ± 3.72 b | 94.15 ± 1.89 c,d | 98.78 ± 1.09 d | |
| 48 | 82.37 ± 1.65 a | 92.73 ± 1.68 b,c,d,e | 93.81 ± 2.45 c,d,e,f | 84.81 ± 0.07 a,d | 84.63 ± 1.04 a,d | 89.32 ± 3.29 d,e | 94.20 ± 1.89 e | 99.72 ± 1.07 f | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memete, A.R.; Miere, F.; Laslo, V.; Purcarea, C.; Vicas, L.; Ganea, M.; Antonescu, A.; Vicas, S.I. An In Vitro Study of the Healing Potential of Black Mulberry (Morus nigra L.) Extract in a Liposomal Formulation. Appl. Sci. 2023, 13, 1041. https://doi.org/10.3390/app13021041
Memete AR, Miere F, Laslo V, Purcarea C, Vicas L, Ganea M, Antonescu A, Vicas SI. An In Vitro Study of the Healing Potential of Black Mulberry (Morus nigra L.) Extract in a Liposomal Formulation. Applied Sciences. 2023; 13(2):1041. https://doi.org/10.3390/app13021041
Chicago/Turabian StyleMemete, Adriana Ramona, Florina Miere (Groza), Vasile Laslo, Cornelia Purcarea, Laura Vicas, Mariana Ganea, Angela Antonescu, and Simona Ioana Vicas. 2023. "An In Vitro Study of the Healing Potential of Black Mulberry (Morus nigra L.) Extract in a Liposomal Formulation" Applied Sciences 13, no. 2: 1041. https://doi.org/10.3390/app13021041
APA StyleMemete, A. R., Miere, F., Laslo, V., Purcarea, C., Vicas, L., Ganea, M., Antonescu, A., & Vicas, S. I. (2023). An In Vitro Study of the Healing Potential of Black Mulberry (Morus nigra L.) Extract in a Liposomal Formulation. Applied Sciences, 13(2), 1041. https://doi.org/10.3390/app13021041







