Abstract
Meibomitis is an ocular disease which leads to a dysfunction of the meibomian glands. This ophthalmologic disease may cause severe pain and obvious vision loss. The therapeutic protocol used in the treatment of this pathology consists in local and systemic antibiotic therapy. The results obtained using this approach are scarce and, in many cases, result in adverse events. In this study, we propose an alternative and original approach using TECAR therapy in the treatments of meibomitis disease. The endogenous heat produced by the TECAR device produced beneficial effects from both a histological and anatomical point of view. Different parameters (TBUT, interferometry, tear meniscus height, meibography and OCTA) were evaluated before the TECAR treatments, immediately afterwards, and 15 days after the end of the treatments. The obtained results suggest a new possible use of TECAR therapy on ophthalmological patients, opening an innovative scenario in a non-invasive manner.
1. Introduction
Meibomitis is an ocular disorder that causes inflammation of the eyelid edge in correspondence with the meibomian glands [1].
This condition is accompanied by a dysfunction of the meibomian glands with an unstable tear film, shortened tear film breakup time (TBUT) and superficial punctuate keratopathy [2]. The meibomian glands are responsible for the secretion of some important lipid substances present in tears.
The dysfunction of the meibomian glands implies a pathological unicum with other structures of the eye.
Indeed, meibomitis is strongly related to ocular surface inflammation, such as corneal cellular infiltrates and neovascularization, superficial punctate keratopathy (SPK), and conjunctivitis [3]. Moreover, it is difficult to differentiate SPK caused by dry eye from that caused by meibomitis. Meibomian gland inflammation, “meibomitis,” is associated with ocular surface inflammatory diseases; for these reasons, “these diseases are poorly defined clinically, making effective treatment difficult” [4,5]. In the literature, there is no univocal therapeutic protocol for this condition; usually, meibomitis disease is treated by using local and systemic antibiotic therapy [6,7]. The results of this approach are insufficient to draw any meaningful conclusions on the use of oral antibiotics for chronic blepharitis; on the other hand, some studies suggest that oral antibiotics may improve clinical signs but also cause more adverse events [8]. Other approach strategies have been employed, such as exogenous heating through warm applications on the eyeball—the mechanical toilet of the glandular region—and pulsed light therapy [9,10,11,12]. Nevertheless, new versatile and fast alternatives for accurate therapeutical approaches are needed [13]. In this context, the use of short waves with resistive-capacitive energy transfer (TECAR) in the integrated treatment of pathologies of ophthalmological interest can play an original role [14,15,16]. This therapeutic strategy is based on the ability to produce endogenous heat with consequent beneficial effects from both a histological and anatomical point of view [17].
The produced heat is endogenous and, unlike physical means with the production of exogenous heat (for example, Infrared therapy), it does not induce ab extrinsic heating of the treated structures with the consequent risk of burns and/or tissue damage [18].
The mechanism that leads to the production of deep tissue hyperthermia is constituted by a flow of electric charges generated by the passage of non-ionizing electromagnetic short waves [19]. This flow is generated between two poles (one active—in “capacitive” and/or “resistive” mode—and one passive), which are placed around the area to be treated [20].
To our knowledge, no studies are currently available on TECAR therapy and ophthalmological diseases.
The aim of this study was to evaluate whether the application of TECAR on the eye of patients affected by meibomitis disease can produce positive effects both in terms of subjective symptoms and in terms of anatomical tissue evidence. In addition, the vascular flow of the optic nerve of the subjects recruited for the therapy was evaluated, in order to provide clinicians with valuable information to be translated in terms of the improvement of visual acuity. The reliability of the proposed innovative methodology was evaluated by conducting a statistical analysis using the Anderson–Darling test and the one-way ANOVA.
2. Materials and Methods
2.1. Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of Tedesco’s ophthalmic outpatients clinic (18 November 2021 protocol number 18113), where the patients were enrolled before participating in this study. Informed consent was obtained from all the subjects after an explanation of the nature and possible consequences of the study.
2.2. Clinical Data
In the present study, 15 patients (8 male (72.62 ± 7.25) and 7 female (75.86 ± 6.09)) were enrolled according to the following inclusion criteria:
- All non-responders to administered therapies (i.e., pulsed light);
- Bilateral disease history of at least 3 years;
- All with an equal degree of alteration in both eyes;
- Meibography of a value not less than 1;
- TBUT from 1 to 5 s;
- Lacrimal meniscus height greater than 0.20 mm;
- Interferometry less than 50 (it goes up to 100);
- Jenvis report 30 (it ranges from 1 to 100);
- ANGIO TC SCAN (OCTA) of optic nerve head.
The same patient was enrolled in the group containing the sick subjects undergoing TECAR therapy (Group 1), as well as in the control group (Group 2). One of the patient’s eyes was treated using a TECAR device, and the sham treatment was performed in the fellow eye. For each group, two weekly treatments, for a total of 10 treatments, were carried out. After the end of the ten treatments, the first patient follow-up visit was conducted, representing the T1 session; 15 days after the end of the TECAR treatments, the second follow-up visit was conducted, representing the T2 session. The T0 session is represented by the evaluation of the parameters, detailed in Table 1 and reevaluated during T1 and T2 sessions, before the treatments. In the following sub-sections, the description of the TECAR and placebo treatments is better detailed.

Table 1.
Outcomes used for the evaluation of the effectiveness of the cure.
2.3. TECAR Treatment (Group 1)
All treatments were performed by dedicated equipment using the C400 Tecarterapia (CAPENERGY manufacturer).
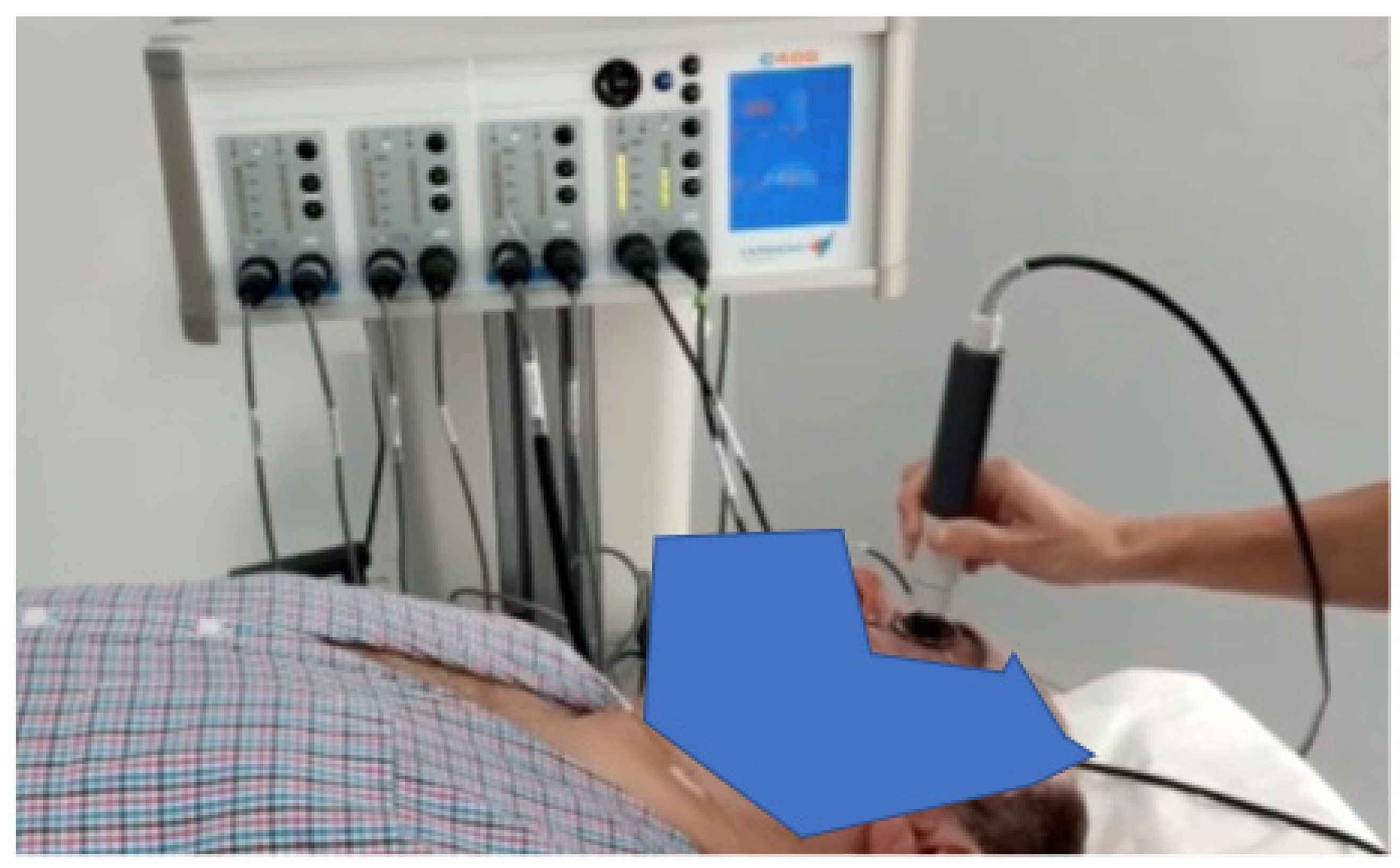
The device can automatically identify the optimal tissue impedance and set the best frequency to be used (from three different frequencies); this last choice depends on the impedance signal. TECAR therapy was conducted by placing the negative electrode under the patient’s cervical dorsal region and the positive electrode in capacitive mode by massaging the eyeball (with a closed eye) and the periorbital area (Figure 1).
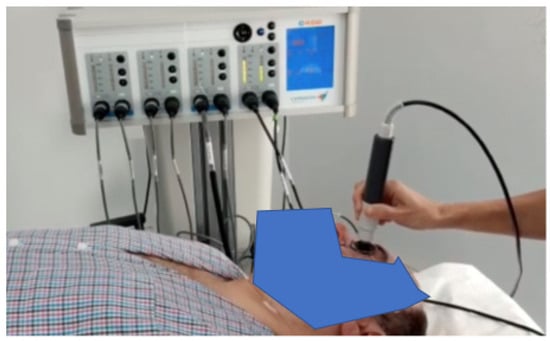
Figure 1.
Experimental set-up used during the treatments.
Each treatment had a duration of 5 min, and the used power, displayed on the device and related to temperature, was maintained at 45% (treatment in athermia) for the first four minutes; in the last minute of treatment, 60% of power was reached in terms of the endogenous heat produced.
After each treatment, the patient was asked if he felt any disturbances or discomfort.
2.4. PLACEBO Treatment (Group 2)
The sham treatment was performed immediately after finishing the session described in Section 2.3. in the eye treated with TECAR.
The TECAR capacitive electrode maintained heat energy in contact with the un-treated eyeball, even if, for the sham treatment, the machine was not started and was kept on “STAND BY” mode for the entire 5 min of the “SHAM” session. In this manner, no electric charge flowed through in the tissues causing heat.
Also in this case, after each treatment, the patient was asked if he felt any disturbances or discomfort.
Different outcomes were used to evaluate the advantages of the proposed methodology, and these are summarized in Table 1.
Meibomian glands were analyzed according to the degree of loss on the Jenvis grading scale: 1 for a loss under 33%, 2 for a loss between 33 and 66% and 3 for a greater loss. The Jenvis grading scale includes the analysis of the following parameters: infrared meibography, TBUT, interferometry and the tear meniscus height.
Infrared meibography appears to be a valuable clinical test in the diagnoses of dry eye and meibomian gland dysfunction [21,22]. We used a four-point scale for the quantification of meibomian gland loss, with scores of 0–3 corresponding to the absence of partial glands, <25% partial glands, 25–75% partial glands and >75% partial glands, respectively.
The TBUT is a key indicator of tear stability and one of the simplest clinical tests for diagnosing tear dysfunction syndrome. Interferometry of the tear film is used to evaluate and study the lipid layer and its formation and distribution in the eye, providing extremely detailed images of the tear. The procedure is non-invasive and takes less than five minutes [23]. TBUT images were acquired by using the OCULUS Keratograph 5 M device (Oculus, Arlington, WA, USA).
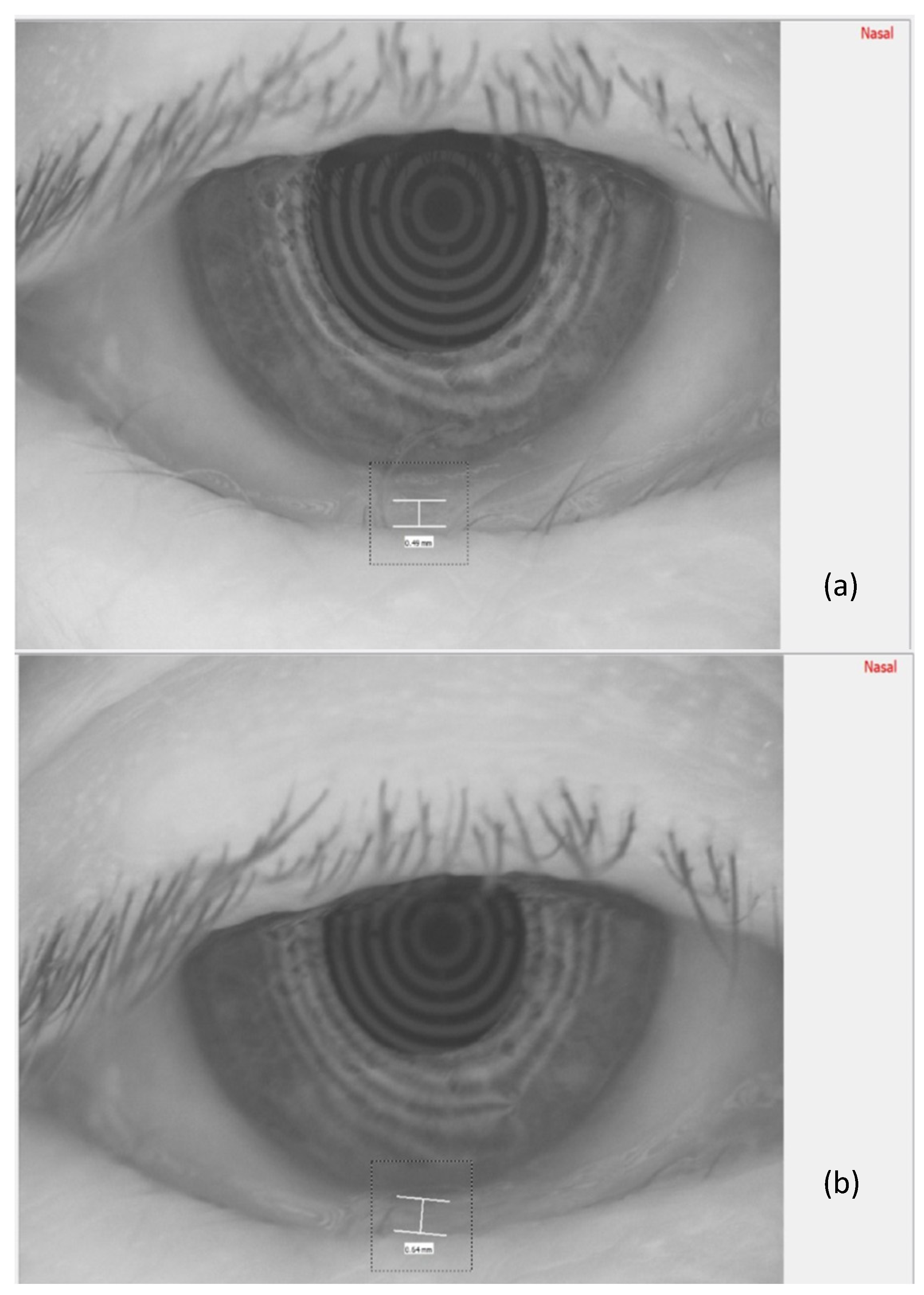
Tear meniscus height was qualitatively analyzed based on its height, assuming a value of 1 as very high (≥0.35 mm); 2 as normal (0.20–0.35 mm); 3 as slightly reduced (0.15–0.20 mm); and 4 as low (≤0.15 mm) [12].
In addition, OCTA was performed to evaluate the radial pericapillary capillary (RPC) vessel density and retinal nerve fiber layer (RNFL). OCTA represents a non-invasive imaging technique which can be used to provide a three-dimensional visualization of the perfused vasculature of the retina and choroid [24,25]. It not only analyzes the intensity of the reflected light, but also the temporal changes of the OCT signal [26]. OCTA also allows the automated measurement of tissue thickness (useful for monitoring the evolution of diseases over time) and cornea, analyzing structural alterations and identifying the depth of lesions.
It represents a hard outcome because from the direct vision of the tissues a numerical score is obtained, which expresses a certain degree of alteration. In this sense, it represents both a qualitative and a quantitative examination. In this study, OCTA images were obtained by using the DRI OCT Triton device (Topcon Corporation, Tokyo, Japan). The built-in software was used to evaluate the RPC densities, the analysis of the optical nerve head (ONH) and the RNFL thickness. In particular, the RPC density analysis included the densities analysis of the whole-body image, inside the optic disc area and the peripapillary area; the ONH analysis included the evaluation of the disc area, of the rim area and the cup-to-disc ratio.
2.5. Statistical Analysis
The statistical analysis was performed using MATLAB® R2017a (MathWorks, Natick, MA, USA).
First, we tested the assumption of normality of distribution for each dataset by using the Anderson–Darling test (MATLAB function adtest). As for sixteen of the twenty tested datasets (five for each of the four variables analyzed), the Anderson–Darling test resulted in a p-value < 0.05, so we decided to use non-parametric tests for the subsequent analyses.
To test if the datasets from the different sessions and with different treatments (with and without TECAR) belonged to the same statistical distribution, a one-way ANOVA with data grouped for session and treatment was performed (MATLAB function anova1). A post hoc, multiple comparison with Tukey’s honestly significant difference test (MATLAB function multcompare) was used.
3. Results
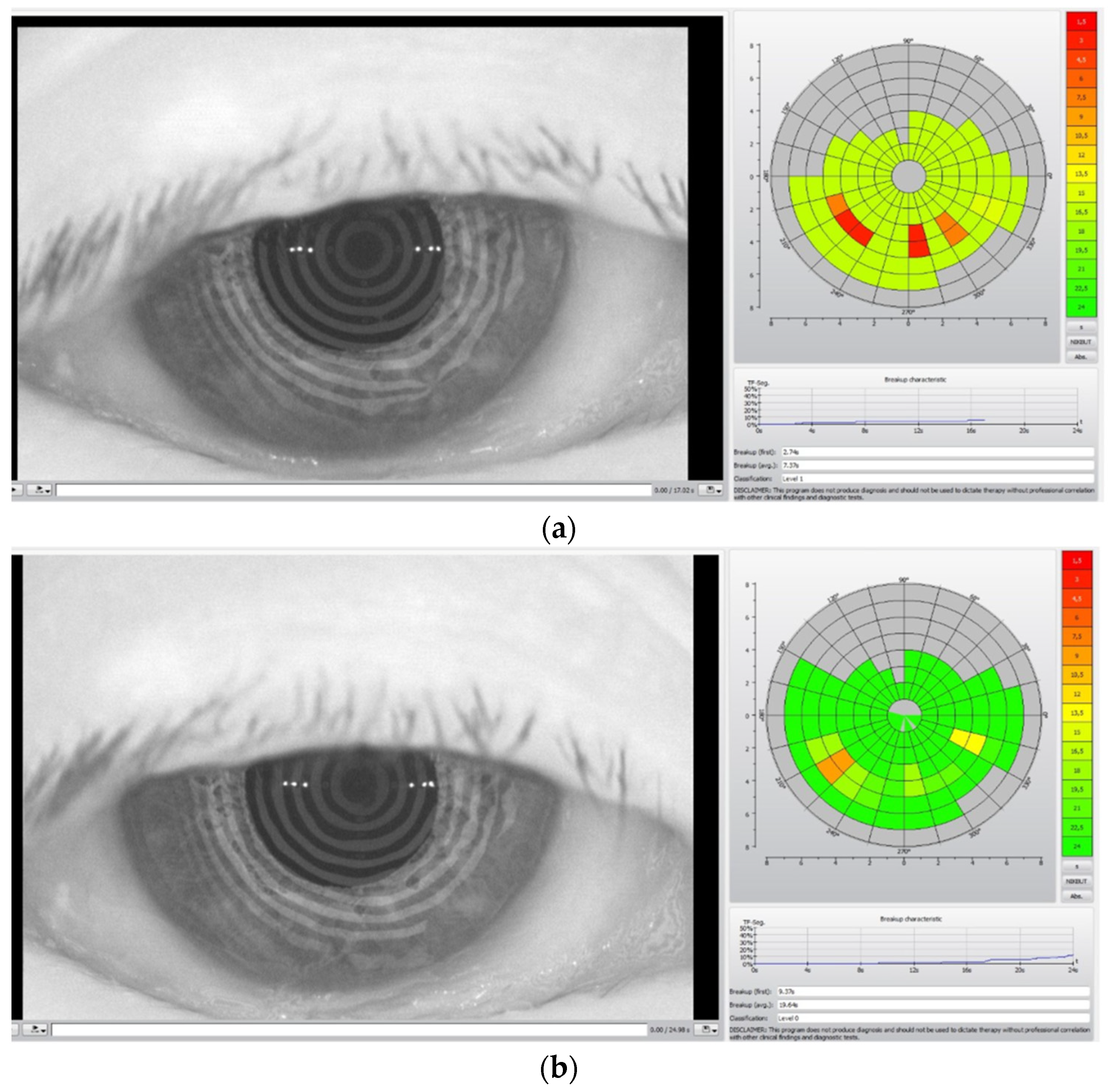
In Figure 2, the TBUT analysis before the TECAR treatment (a) and after the treatment (b) is reported.
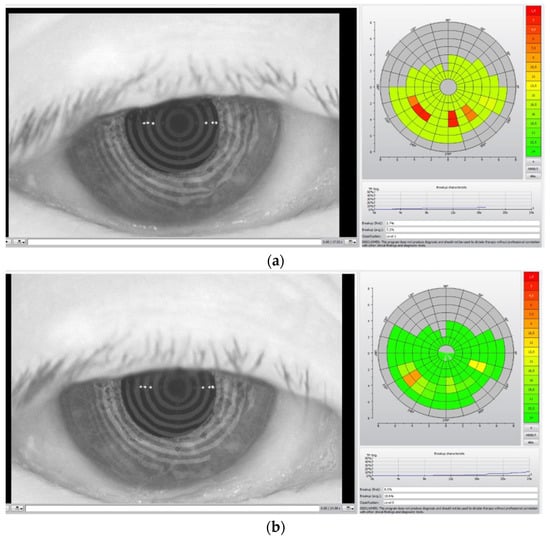
Figure 2.
TBUT analysis before (a) and after (b) TECAR treatments. The acquired images refer to a different subject from Figure 1.
The colored diagrams on the right of the (a) and (b) figures represent the state of the tear film. The colored scale, from green to red, indicates the suffering degree of the tear film, where green denotes no suffering and red indicates high suffering of the tear film.
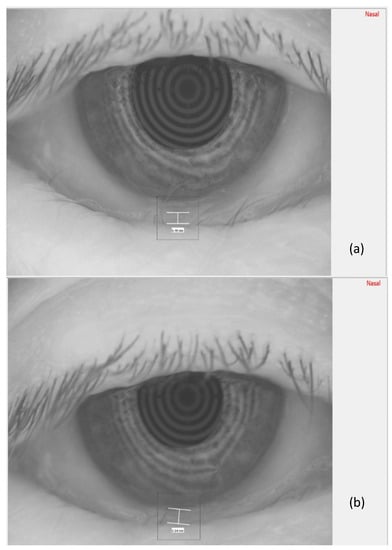
In Figure 3, the measurement of the meniscus height, before TECAR treatment (a) and after it (b), is reported.
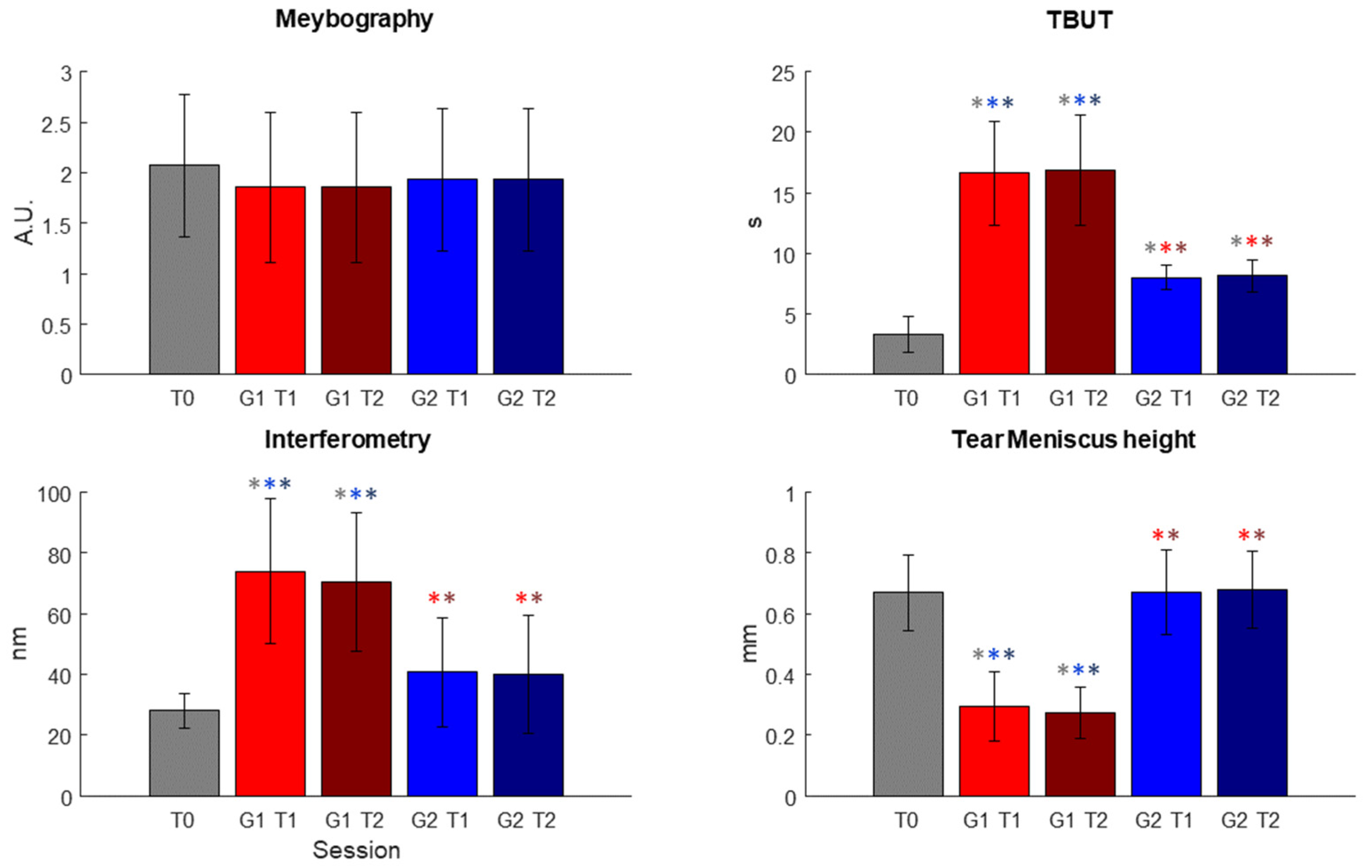
In Figure 4, the results obtained after the statistical analysis are reported. In Figure 4, the outcomes used for the evaluation of the effectiveness of the treatment are plotted for each session.
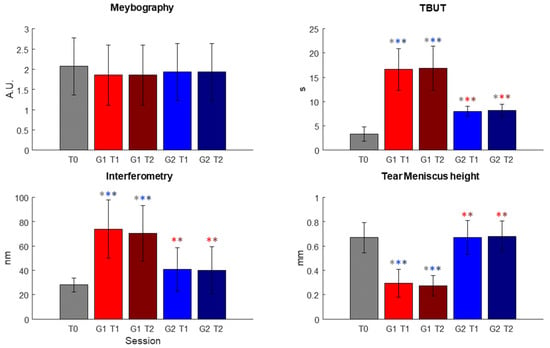
Figure 4.
Results of the statistical analysis conducted on the parameters used for the evaluation of the effectiveness of the treatment conducted in the two different sessions, T1 and T2, respectively. Significant comparisons are highlighted using * of the color of the significantly different dataset (In figure, a.u.: arbitrary unit; s: seconds; nm: nanometer; mm: millimeter; T0, T1 and T2 represent the three different sessions; G(1 or 2)T(1 or 2): Group (1 or 2) at session (1 or 2)).

Table 2.
Comparisons of T1 and T2 datasets with T0 session for both Group 1 and 2. First row: mean ± SD; second row: p-value.

Table 3.
Comparisons of T1 and T2 datasets for Group 1 and 2 between each other.
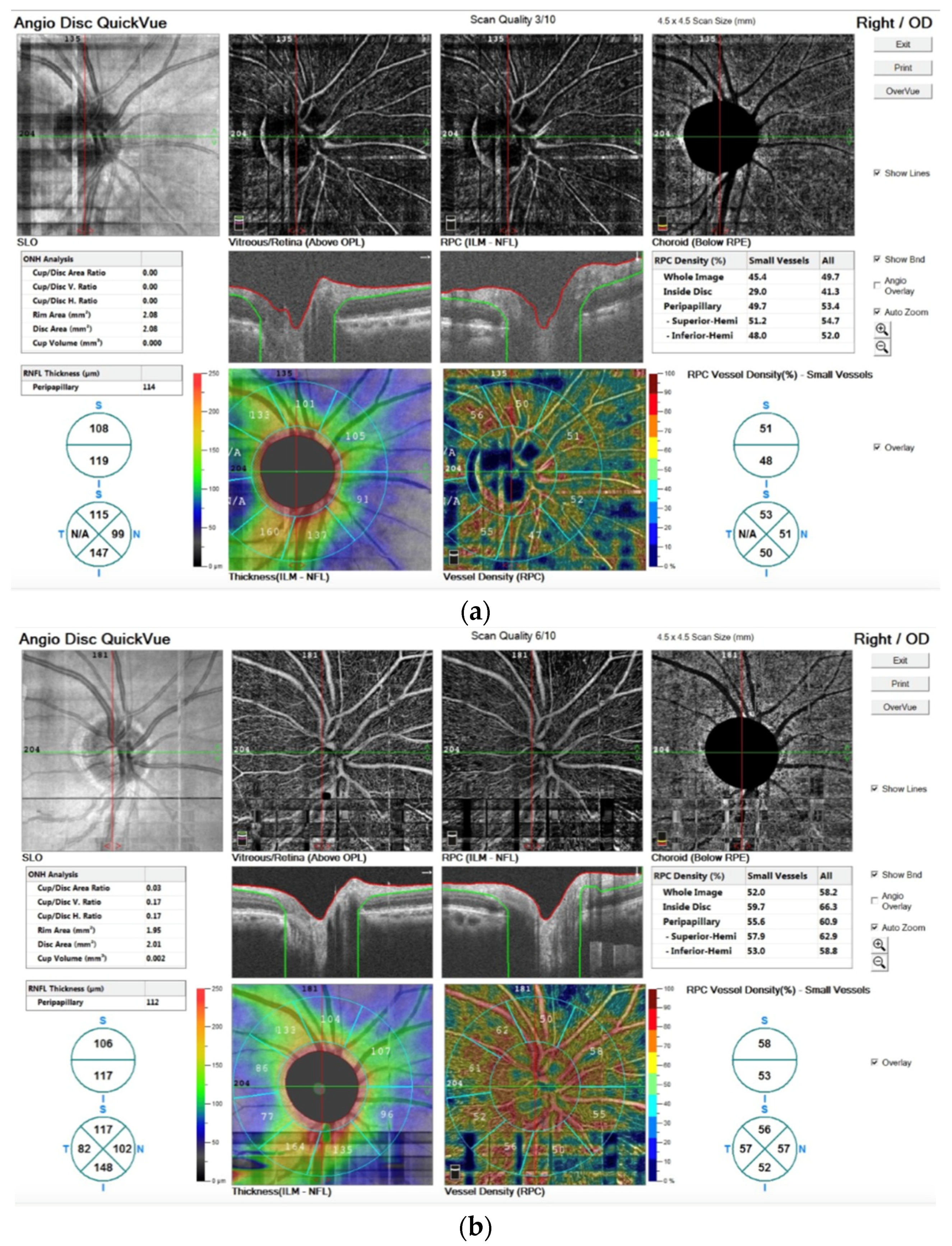
In addition, in Figure 5 we report the OCTA image before (a) and after the TECAR treatments (b).
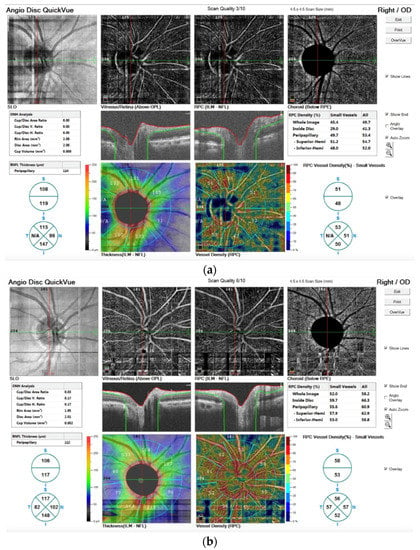
Figure 5.
OCTA images stored before (a) and after the TECAR treatments (b). In both images, in the upper line, the images are related to the angiographic image of the 4.5 mm × 4.5 mm rectangle scan of the ONH; in the medium line, the images refer to the segmentation of volumetric data that leads to identification of retinal capillary plexuses; in the bottom line, the vessel density is shown by the color maps. The different numbers of the outer ring represent the capillary density of the peripapillary area.
The OCTA technique permits the visualization of structure and blood flow within the vitreous, retina and choroid, separately. It is also possible to examine the distinct capillary networks of the retina using this technique. Figure 5a visualizes the vascular networks (or plexuses) in the retina, showing a pathological alteration. After TECAR therapy, Figure 5b, we can observe an improvement of the pathological alteration, resulting in a major vascularization and in an improvement of vessel density. In particular, we observed no significant difference in RNFL thickness before and after the TECAR treatments. On the other hand, the RPC densities analysis showed large differences between the two groups. After the TECAR treatments, the whole-body image density increased by approximately 8%, the inside optic disc area density increased by approximately 23% and the peripapillary area density increased by approximately 5%. No significant difference was found related to the ONH analysis.
After the end of the treatments, the patients did not show any symptoms of discomfort.
4. Discussion
In this study, the action of eye stimulation with TECAR was investigated.
This therapeutic methodology represents the most advanced step of thermotherapy in physical medicine and rehabilitation, using heat as a therapeutic means in terms of interaction between heat and tissues. The current passage through tissues induces an increase in tissue temperature, causing vasodilatation; reducing muscle spasms; and increasing pain threshold, cell activity and the elasticity of the connective tissue [27,28]. The biological effects are attributable not only to the phenomenon of active hyperemia, but also to bio-stimulation and cellular communication processes inherent in the cellular component of the treated tissue [29]. The positive effects of the therapy could be attributed to the ability of the TECAR therapy technique to stimulate deep tissue through endogenous heat, evoking anti-inflammatory and regenerative effects [30]. The electromagnetic field probably affects the kinetics of ionic binding in cellular macromolecules, by modulating the release of cytokines, driving the immune response toward an anti-inflammatory/reparative profile [31,32]. Currently, TECAR therapy seems to be one of the most beneficial therapies used in recovery [33]; it also has several contraindications that the operator must consider before starting the treatment. Absolute contraindications of TECAR therapy are patients with pacemakers, hemorrhagic gastrointestinal ulcers, infusion pumps and electric cable implants, deep-vein thrombosis, uncontrolled ischemic heart disease and localized cancerous areas/tumors [34].
Meibomitis is a condition that involves multiple structures of the eye causing a series of symptoms all related to the same cause. This ophthalmologic disease may cause severe pain and obvious vision loss, which seriously affect the normal life of patients [35].
After the TECAR treatments, an improvement was demonstrated both in terms of subjective symptoms and in terms of anatomical tissue evidence.
The statistical analysis highlighted no statistical differences between each of the datasets for meibography, as shown in Table 2 and Table 3, but it is possible to observe a decrease in the scale values.
On the other hand, for TBUT, both T1 and T2 sessions for Group 1 and Group 2 presented significant different distribution with respect to the T0 dataset; in particular, after the T1 session, a marked improvement could already be seen. For interferometry and tear meniscus height, the Kruskal–Wallis test showed a significant difference only for Group 1 datasets (see Table 2). For all these three variables, however, the test highlighted statistical differences in all comparisons between Group 1 datasets and Group 2 datasets (see Table 3).
The second target of the present study was to evaluate the blood flow in the ophthalmic nerve. For the objective assessment of disease progression, qualitative and quantitative analyses were performed using the OCTA technique. After the TECAR treatments, the OCTA images (Figure 5b) showed an improvement in blood flow and density, probably due to an improvement of the oxygenation of vessels. The optic nerve showed revascularization with a greater blood supply guaranteed by the process of active hyperemia, with consequent better trophism of the optic nerve. The anti-inflammatory effect was also important at the level of the most superficial part of the eye without highlighting side effects in terms of burns and damage to the eye itself.
In this study, no side effect was observed in terms of direct damage of the external structures of the eye or in the lens of the eye; in fact, the lens represents the most thermosensitive part of the eye.
Meibomite is a complex process that affects all the noble structures aimed at guaranteeing the normal hydration of the eye.
Recent evidence from various research works has shown that intense pulsed light modifies the mechanism of meibomian gland dysfunction, which helps to relieve the symptoms of dry eye disease [36,37,38]. The working principle of the intense pulsed light is based on selective photo thermolysis, in which thermally mediated radiation damage is limited to chosen epidermal and dermal pigmented targets at the cellular or tissue structural levels [39]. The physical principle of this technique is different compared to TECAR therapy, particularly regarding the interaction with matter. In our opinion, based on the literature, the combination of TECAR and intense pulsed light techniques may be ideal for treating eye disorders to accelerate healing and will lead to a better quality of life.
5. Conclusions
It was possible to demonstrate that TECAR therapy treatments can induce improvements in ocular disease; in particular, patients affected by meibomitis disorder showed positive effects in the symptoms associated with the disease. It is important to underline that the same patients were refractories to previous local pharmacologic therapies. In addition, the optical nerve flow also benefited from the TECAR treatments.
The present study could represent an opportunity to investigate, in future studies, a rational use of TECAR therapy in the treatment of degenerative conditions of the retina and the posterior chamber of the eye.
Positive action on the trophism of the nervous side, on the biostimulation of the nerve (neurotrophic action) and on the microcirculation could represent a rational treatment mechanism for conditions affecting the most delicate part of the macula.
Finally, in this study, the increase in temperature inside the ocular globe did not show negative effects on highly heat-sensitive structures such as the lens.
The overall results, which must be confirmed by the study in a larger population, suggest a new possible use of TECAR therapy on ophthalmological patients, opening an innovative scenario in a non-invasive manner.
Author Contributions
Conceptualization, A.T., A.M. and G.A.; methodology, A.T., G.R.T. and A.M.; software, S.G.; validation, A.T., G.R.T. and A.M.; formal analysis, G.A. and S.G.; investigation, A.T. and A.M.; resources, G.R.T.; data curation, A.T. and G.A.; writing—original draft preparation, A.T. and G.A.; writing—review and editing, A.T., S.G. and G.A.; supervision, A.M. and G.A.; project administration, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board of Tedesco’s ophthalmic outpatients clinic (18/11/2021 protocol number 18113).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kuchay, R.H.; Hassan, A.; Mir, Y. Ocular findings and genomics of X-linked recessive disorders: A review. Indian J. Ophthalmol. 2022, 70, 2386. [Google Scholar] [CrossRef]
- Terry, M.A. Dry Eye in the Elderly. Drugs Aging 2001, 18, 101–107. [Google Scholar] [CrossRef]
- Suzuki, T. Inflamed Obstructive Meibomian Gland Dysfunction Causes Ocular Surface Inflammation. Investig. Opthalmology Vis. Sci. 2018, 59, DES94–DES101. [Google Scholar] [CrossRef]
- Suzuki, T.; Teramukai, S.; Kinoshita, S. Meibomian Glands and Ocular Surface Inflammation. Ocul. Surf. 2015, 13, 133–149. [Google Scholar] [CrossRef]
- Suzuki, T. Meibomitis-related keratoconjunctivitis: Implications and clinical significance of meibomian gland inflammation. Cornea 2012, 31 (Suppl. S1), S41–S44. [Google Scholar] [CrossRef]
- Al-Hity, A.; Lockington, D. Oral azithromycin as the systemic treatment of choice in the treatment of meibomian gland disease. Clin. Exp. Ophthalmol. 2016, 44, 199–201. [Google Scholar] [CrossRef]
- Opitz, D.L.; Tyler, K.F. Efficacy of azithromycin 1% ophthalmic solution for treatment of ocular surface disease from posterior blepharitis. Clin. Exp. Optom. 2011, 94, 200–206. [Google Scholar] [CrossRef]
- Halim, M.S.; Onghanseng, N.; Hassan, M.; Besalti, Z.; Ng, S.M.; Nguyen, Q.D. Oral antibiotics for chronic blepharitis. Cochrane Database Syst. Rev. 2021, 6, CD013697. [Google Scholar]
- Wong, C.W.; Busoy, J.M.F.; Cheung, N.; Barathi, V.A.; Storm, G.; Wong, T.T. Endogenous or Exogenous Retinal Pigment Epithelial Cells: A Comparison of Two Experimental Animal Models of Proliferative Vitreoretinopathy. Transl. Vis. Sci. Technol. 2020, 9, 46. [Google Scholar] [CrossRef]
- Magliocca, K.R.; Vivas, E.X.; Griffith, C.C. Idiopathic, Infectious and Reactive Lesions of the Ear and Temporal Bone. Head Neck Pathol. 2018, 12, 328–349. [Google Scholar] [CrossRef]
- Ribeiro, B.B.; Marta, A.; Ramalhão, J.P.; Marques, J.H.; Barbosa, I. Pulsed Light Therapy in the Management of Dry Eye Disease: Current Perspectives. Clin. Ophthalmol. 2022, 16, 3883–3893. [Google Scholar] [CrossRef]
- Suwal, A.; Hao, J.-L.; Zhou, D.-D.; Liu, X.-F.; Suwal, R.; Lu, C.-W. Use of Intense Pulsed Light to Mitigate Meibomian Gland Dysfunction for Dry Eye Disease. Int. J. Med Sci. 2020, 17, 1385–1392. [Google Scholar] [CrossRef]
- Lee, L.; Garrett, Q.; Flanagan, J.; Vaddavalli, P.K.; Papas, E.B. Treatment Practices and Outcomes of Meibomian Gland Dysfunction at a Tertiary Center in Southern India. Eye Contact Lens Sci. Clin. Pract. 2018, 44 (Suppl. S1), S138–S143. [Google Scholar] [CrossRef]
- Ballester, F.F.; Vélez, M.S.; Alonso, M.A. Aplicación de la Transferencia Eléctrica Capacitiva en Oftalmología (T.E.R.). Rev. D’Or Oftalmol. 2001, 73, 43–49. [Google Scholar]
- Úbeda, A.; De Bernardo, S.; Bazan, E.; Trillo, M.A.; Martinez, M.A.; Leal, J. Assay of biocompatibility for RF generated by a System for clinical applications of hypertermia. In Proceedings of the Third World Congress for Electricity and Magnetism in Biology and Medicine, Lake Buena Vista, FL, USA, June 2000. [Google Scholar]
- Cole, A.J.; Eaglestone, M.A. The benefits of deep heat: Ultrasound and electromagnatic diathermy. PhYsic. Sportsmed. 1994, 22, 77–88. [Google Scholar] [CrossRef]
- Rowland, L.A.; Bal, N.C.; Periasamy, M. The role of skeletal-muscle-based thermogenic mechanisms in vertebrate endothermy. Biol. Rev. 2015, 90, 1279–1297. [Google Scholar] [CrossRef]
- Tramontana, A.; Page, J.C. Does the physiatrist rep-licate the functions of the neurologist or orthopedist? Ann. Phys. Rehabil. Med. 2016, 59, 349. [Google Scholar] [CrossRef]
- Tramontana, A.; Miangolarra Page, J.C. High power multichannel diathermy treatment of a calcific De Quervain’s Tendinopathy in a tennis player: Echographic evidence of the result. Med. Dello Sport 2019, 72, 663–666. [Google Scholar] [CrossRef]
- Lubkowska, A.; Pluta, W. Infrared Thermography as a Non-Invasive Tool in Musculoskeletal Disease Rehabilitation—The Control Variables in Applicability—A Systematic Review. Appl. Sci. 2022, 12, 4302. [Google Scholar] [CrossRef]
- Arita, R. Meibography: A Japanese Perspective. Investig. Opthalmology Vis. Sci. 2018, 59, DES48–DES55. [Google Scholar] [CrossRef]
- Swiderska, K.; Read, M.L.; Blackie, C.A.; Maldonado-Codina, C.; Morgan, P.B. Latest developments in meibography: A review. Ocul. Surf. 2022, 25, 119–128. [Google Scholar] [CrossRef]
- Martínez-Hergueta, M.C.; del Barrio, J.L.A.; Canto-Cerdan, M.; Amesty, M.A. Efficacy and safety of intense pulsed light direct eyelid application. Sci. Rep. 2022, 12, 15592. [Google Scholar] [CrossRef]
- Spaide, R.F.; Klancnik, J.M., Jr.; Cooney, M.J. Retinal Vascular Layers Imaged by Fluorescein Angiography and Optical Coherence Tomography Angiography. JAMA Ophthalmol 2015, 133, 45–50. [Google Scholar] [CrossRef]
- Matsunaga, D.; Yi, J.; Puliafito, C.A.; Kashani, A.H. OCT Angiography in Healthy Human Subjects. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 510–515. [Google Scholar] [CrossRef]
- Rocholz, R.; Corvi, F.; Weichsel, J.; Schmidt, S.; Staurenghi, G. OCT Angiography (OCTA) in Retinal Diagnostics. In High Resolution Imaging in Microscopy and Ophthalmology: New Frontiers in Biomedical Optics; Bille, J.F., Ed.; Springer: Cham, Germany, 2019; Chapter 6. [Google Scholar] [CrossRef]
- Talia, F. Marconiterapia nell’ascesso polmonare [Marconitherapy in lung abscess]. Arch. Radiol. 1946, 19, 7–12. [Google Scholar]
- Aguilar, A. Radarterapia [Radar therapy]. Sem. Med. 1955, 107, 541–544. [Google Scholar] [PubMed]
- Barassi, G.; Mariani, C.; Supplizi, M.; Prosperi, L.; Di Simone, E.; Marinucci, C.; Pellegrino, R.; Guglielmi, V.; Younes, A.; Di Iorio, A. Capacitive and Resistive Electric Transfer Therapy: A Comparison of Operating Methods in Non-specific Chronic Low Back Pain; Advances in Experimental Medicine and Biology. In Integrative Clinical Research; Pokorski, M., Ed.; Springer: Cham, Germany, 2022; Volume 1375. [Google Scholar] [CrossRef]
- Szabo, D.A.; Neagu, N.; Teodorescu, S.; Predescu, C.; Sopa, I.S.; Panait, L. TECAR Therapy Associated with High-Intensity Laser Therapy (Hilt) and Manual Therapy in the Treatment of Muscle Disorders: A Literature Review on the Theorised Effects Supporting Their Use. J. Clin. Med. 2022, 11, 6149. [Google Scholar] [CrossRef]
- Salmeri, F.M.; Denaro, L.; Ruello, E.; Acri, G.; Gurgone, S.; Sansotta, C.; Testagrossa, B. Irradiation with Polychromatic Incoherent Low Energy Radiation of Human Peripheral Blood Mononuclear Cells In Vitro: Effects on Cytokine Production. Int. J. Environ. Res. Public Health 2020, 17, 1233. [Google Scholar] [CrossRef]
- Ongaro, A.; Varani, K.; Masieri, F.; Pellati, A.; Massari, L.; Cadossi, R.; Vincenzi, F.; Borea, P.; Fini, M.; Caruso, A.; et al. Electromagnetic fields (EMFs) and adenosine receptors modulate prostaglandin E2 and cytokine release in human osteoarthritic synovial fibroblasts. J. Cell. Physiol. 2011, 227, 2461–2469. [Google Scholar] [CrossRef]
- Kim, J.-H.; Park, J.-H.; Yoon, H.-B.; Lee, J.-H.; Jeon, H.-S. Immediate Effects of High-frequency Diathermy on Muscle Architecture and Flexibility in Subjects With Gastrocnemius Tightness. Phys. Ther. Korea 2020, 27, 133–139. [Google Scholar] [CrossRef]
- Shamrock, A.G.; Varacallo, M. Achilles Tendon Rupture; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Bertone, C.; Mollicone, A.; Russo, S.; Sasso, P.; Fasciani, R.; Riccardi, C.; Lizzano, M.; ALI working group. The role of hypochlorous acid in the management of eye infections: A case series. Drugs Context 2022, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xue, A.L.; Wang, M.T.M.; Ormonde, S.E.; Craig, J.P. Randomised double-masked placebo-controlled trial of the cumulative treatment efficacy profile of intense pulsed light therapy for meibomian gland dysfunction. Ocul. Surf. 2020, 18, 286–297. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.A.; Taher, I.M.E.; Ghoneim, D.F.; Safwat, A.E.M. Effect of intense pulsed light therapy on tear proteins and lipids in meibomian gland dysfunction. J. Ophthalmic Vis. Res. 2019, 14, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Fukuoka, S.; Morishige, N. Therapeutic efficacy of intense pulsed light in patients with refractory meibomian gland dysfunction. Ocul. Surf. 2019, 17, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Raulin, C.; Grema, H. IPL technology: A review. Lasers Surg. Med. 2003, 32, 78–87. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).