Inspiring Real-Time Evaluation and Optimization of Human–Robot Interaction with Psychological Findings from Human–Human Interaction
Abstract
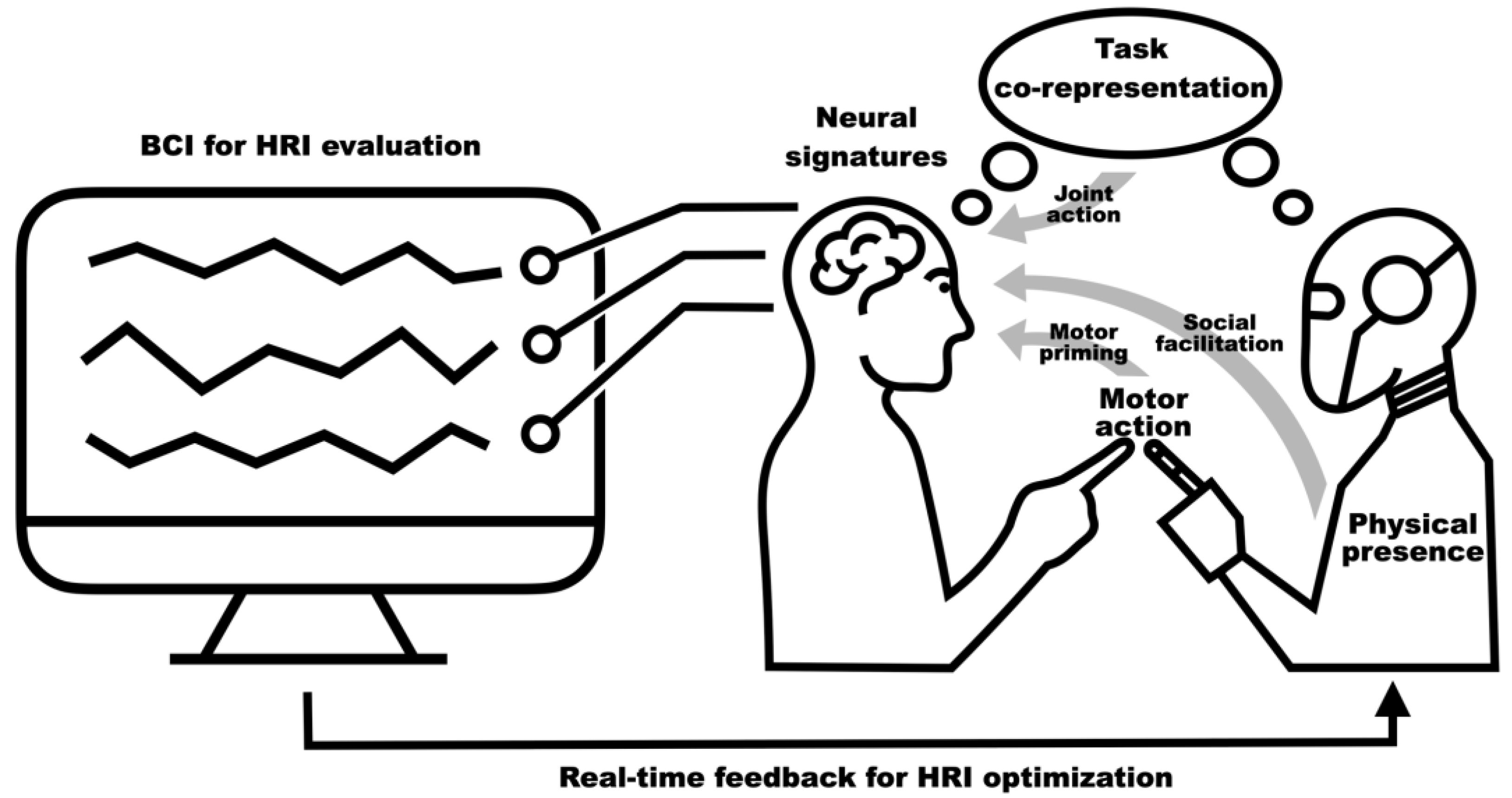
1. Introduction
2. The Effect of Physical Presence in Social Interaction
2.1. Social Facilitation
2.2. The Implication of Social Facilitation for HRI
3. The Effect of Motor Actions in Social Interaction
3.1. Motor Priming
3.2. The Implication of Motor Priming for HRI
4. The Effect of Task Co-Representation in Social Interaction
4.1. Joint Action
4.2. The Implication of Joint Action for HRI
5. Real-Time Evaluation of HRI with BCI
5.1. EEG Signatures of the Three Psychological Effects in HRI
5.2. Developing BCI Systems for HRI Evaluation and Optimization
5.3. Application of BCI in Different Fields of HRI
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Toh, L.P.E.; Causo, A.; Tzuo, P.-W.; Chen, I.M.; Yeo, S.H. A review on the use of robots in education and young children. J. Educ. Technol. Soc. 2016, 19, 148–163. [Google Scholar]
- Li, B.; Li, G.; Sun, Y.; Jiang, G.; Kong, J.; Jiang, D. A review of rehabilitation robot. In Proceedings of the 2017 32nd Youth Academic Annual Conference of Chinese Association of Automation (YAC), Hefei, China, 19–21 May 2017; pp. 907–911. [Google Scholar]
- Abdi, J.; Al-Hindawi, A.; Ng, T.; Vizcaychipi, M.P. Scoping review on the use of socially assistive robot technology in elderly care. BMJ Open 2018, 8, e018815. [Google Scholar] [CrossRef]
- Licklider, J.C.R. Man-Computer Symbiosis. Ire. T. Hum. Fact. Electf. 1960, 1, 4–11. [Google Scholar] [CrossRef]
- Henschel, A.; Hortensius, R.; Cross, E.S. Social Cognition in the Age of Human–Robot Interaction. Trends Neurosci. 2020, 43, 373–384. [Google Scholar] [CrossRef]
- Bethel, C.L.; Murphy, R.R. Review of Human Studies Methods in HRI and Recommendations. Int. J. Soc. Robot. 2010, 2, 347–359. [Google Scholar] [CrossRef]
- Wiese, E.; Metta, G.; Wykowska, A. Robots As Intentional Agents: Using Neuroscientific Methods to Make Robots Appear More Social. Front. Psychol. 2017, 8, 1663. [Google Scholar] [CrossRef]
- Kompatsiari, K.; Pérez-Osorio, J.; Tommaso, D.D.; Metta, G.; Wykowska, A. Neuroscientifically-Grounded Research for Improved Human-Robot Interaction. In Proceedings of the 2018 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), Madrid, Spain, 1–5 October 2018; pp. 3403–3408. [Google Scholar]
- Krämer, N.C.; von der Pütten, A.; Eimler, S. Human-Agent and Human-Robot Interaction. In Human-Computer Interaction: The Agency Perspective; Zacarias, M., de Oliveira, J.V., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 215–240. [Google Scholar]
- Zajonc, R.B. Social Facilitation. Science 1965, 149, 269–274. [Google Scholar] [CrossRef]
- Triplett, N. The Dynamogenic Factors in Pacemaking and Competition. Am. J. Psychol. 1898, 9, 507–533. [Google Scholar] [CrossRef]
- Meumann, E. Haus-und Schularbeit Experimente an Kindern der Volksschule; Kessinger Publishing: Whitefish, MT, USA, 1904. [Google Scholar]
- Dashiell, J.F. An experimental analysis of some group effects. J. Abnorm. Soc. Psychol. 1930, 25, 190–199. [Google Scholar] [CrossRef]
- Moore, H.T. Laboratory Tests of Anger, Fear and Sex Interest. Am. J. Psychol. 1917, 28, 390–395. [Google Scholar] [CrossRef]
- Ekdahl, A.G. Effects of attitude on free word association time. Genet. Psychol. Monogr. 1929, 5, 253–338. [Google Scholar]
- Burri, C. The influence of an audience upon recall. J. Educ. Psychol. 1931, 22, 683–690. [Google Scholar] [CrossRef]
- Bond, C.F. Social facilitation: A self-presentational view. J. Personal. Soc. Psychol. 1982, 42, 1042–1050. [Google Scholar] [CrossRef]
- Baron, R.S. Distraction-Conflict Theory: Progress and Problems. Adv. Exp. Soc. Psychol. 1986, 19, 1–40. [Google Scholar] [CrossRef]
- Strauss, B. Social facilitation in motor tasks: A review of research and theory. Psychol. Sp. Exerc. 2002, 3, 237–256. [Google Scholar] [CrossRef]
- Liu, N.; Yu, R.; Yang, L.; Lin, X. Gender composition mediates social facilitation effect in co-action condition. Sci. Rep. 2017, 7, 15073. [Google Scholar] [CrossRef]
- Dube, S.K.; Tatz, S.J. Audience effects in tennis performance. Percept. Mot. Ski. 1991, 73, 844–846. [Google Scholar] [CrossRef]
- Oviatt, D.P. Social Facilitation and Motor Performance: A Meta-Analysis. Master’s Thesis, University of Maryland, College Park, MD, USA, 2005. [Google Scholar]
- Schermerhorn, P.; Scheutz, M.; Crowell, C.R. Robot social presence and gender: Do females view robots differently than males? In Proceedings of the Proceedings of the 3rd ACM/IEEE International Conference on Human Robot Interaction, Amsterdam, The Netherlands, 12–15 March 2008; pp. 263–270. [Google Scholar]
- Riether, N.; Hegel, F.; Wrede, B.; Horstmann, G. Social facilitation with social robots? In Proceedings of the 2012 7th ACM/IEEE International Conference on Human-Robot Interaction (HRI), Boston, MA, USA, 5–8 March 2012; pp. 41–47. [Google Scholar]
- Irfan, B.; Kennedy, J.; Lemaignan, S.; Papadopoulos, F.; Senft, E.; Belpaeme, T. Social Psychology and Human-Robot Interaction: An Uneasy Marriage. In Proceedings of the Companion of the 2018 ACM/IEEE International Conference on Human-Robot Interaction, Chicago, IL, USA, 5–8 March 2018; pp. 13–20. [Google Scholar]
- Buccino, G.; Binkofski, F.; Fink, G.R.; Fadiga, L.; Fogassi, L.; Gallese, V.; Seitz, R.J.; Zilles, K.; Rizzolatti, G.; Freund, H.J. Action observation activates premotor and parietal areas in a somatotopic manner: An fMRI study: Cortical activation during action observation. Eur. J. Neurosci. 2001, 13, 400–404. [Google Scholar] [CrossRef]
- Buccino, G.; Binkofski, F.; Riggio, L. The mirror neuron system and action recognition. Brain Lang 2004, 89, 370–376. [Google Scholar] [CrossRef]
- Fadiga, L.; Craighero, L.; Olivier, E. Human motor cortex excitability during the perception of others’ action. Curr. Opin. Neurobiol. 2005, 15, 213–218. [Google Scholar] [CrossRef]
- Wearden, J.H.; Edwards, H.; Fakhri, M.; Percival, A. Why “sounds are judged longer than lights”: Application of a model of the internal clock in humans. Q. J. Exp. Psychol. Sect. B 1998, 51, 97–120. [Google Scholar]
- Brass, M.; Bekkering, H.; Wohlschläger, A.; Prinz, W. Compatibility between Observed and Executed Finger Movements: Comparing Symbolic, Spatial, and Imitative Cues. Brain Cogn. 2000, 44, 124–143. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Stoykov, M.E. Editorial: Motor Priming for Motor Recovery: Neural Mechanisms and Clinical Perspectives. Front. Neurol. 2017, 8, 448. [Google Scholar] [CrossRef] [PubMed]
- Castiello, U.; Lusher, D.; Mari, M.; Edwards, M.; Humphreys, G.W. Observing a human and a robotic hand grasping an object: Differential motor priming effects. In Common Mechanisms in Perception and Action, Attention and Performance XIX; Prinz, W., Hommel, B., Eds.; Oxford University Press: New York, NY, USA, 2002; pp. 315–333. [Google Scholar]
- Tai, Y.F.; Scherfler, C.; Brooks, D.J.; Sawamoto, N.; Castiello, U. The Human Premotor Cortex Is Mirror Only for Biological Actions. Curr. Biol. 2004, 14, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Kilner, J.M.; Paulignan, Y.; Blakemore, S.J. An Interference Effect of Observed Biological Movement on Action. Curr. Biol. 2003, 13, 522–525. [Google Scholar] [CrossRef] [PubMed]
- Pierno, A.C.; Mari, M.; Lusher, D.; Castiello, U. Robotic movement elicits visuomotor priming in children with autism. Neuropsychologia 2008, 46, 448–454. [Google Scholar] [CrossRef]
- Eizicovits, D.; Edan, Y.; Tabak, I.; Levy-Tzedek, S. Robotic gaming prototype for upper limb exercise: Effects of age and embodiment on user preferences and movement. Restor. Neurol. Neurosci. 2018, 36, 261–274. [Google Scholar] [CrossRef]
- Kashi, S.; Levy-Tzedek, S. Smooth leader or sharp follower? Playing the mirror game with a robot. Restor. Neurol. Neurosci. 2018, 36, 147–159. [Google Scholar] [CrossRef]
- Vannucci, F.; Sciutti, A.; Lehman, H.; Sandini, G.; Nagai, Y.; Rea, F. Cultural differences in speed adaptation in human-robot interaction tasks. Paladyn J. Behav. Robot. 2019, 10, 256–266. [Google Scholar] [CrossRef]
- Press, C.; Bird, G.; Flach, R.; Heyes, C. Robotic movement elicits automatic imitation. Cogn. Brain Res. 2005, 25, 632–640. [Google Scholar] [CrossRef]
- Chaminade, T.; Cheng, G. Social cognitive neuroscience and humanoid robotics. J. Physiol. 2009, 103, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Gazzola, V.; Rizzolatti, G.; Wicker, B.; Keysers, C. The anthropomorphic brain: The mirror neuron system responds to human and robotic actions. Neuroimage 2007, 35, 1674–1684. [Google Scholar] [CrossRef]
- Langer, A.; Levy-Tzedek, S. Priming and Timing in Human-Robot Interactions. In Modelling Human Motion: From Human Perception to Robot Design; Noceti, N., Sciutti, A., Rea, F., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 335–350. [Google Scholar]
- Curioni, A.; Knoblich, G.; Sebanz, N. Joint Action in Humans: A Model for Human-Robot Interactions. In Humanoid Robotics: A Reference; Goswami, A., Vadakkepat, P., Eds.; Springer: Dordrecht, The Netherlands, 2016; pp. 1–19. [Google Scholar]
- Simon, J.R.; Small, A.M. Processing auditory information: Interference from an irrelevant cue. J. Appl. Psychol. 1969, 53, 433–435. [Google Scholar] [CrossRef]
- Eriksen, B.A.; Eriksen, C.W. Effects of noise letters upon the identification of a target letter in a nonsearch task. Percept. Psychophys. 1974, 16, 143–149. [Google Scholar] [CrossRef]
- Sebanz, N.; Knoblich, G.; Prinz, W. Representing others’ actions: Just like one’s own? Cognition 2003, 88, B11–B21. [Google Scholar] [CrossRef] [PubMed]
- Atmaca, S.; Sebanz, N.; Knoblich, G. The joint flanker effect: Sharing tasks with real and imagined co-actors. Exp. Brain Res. 2011, 211, 371–385. [Google Scholar] [CrossRef] [PubMed]
- Ciardo, F.; Lugli, L.; Nicoletti, R.; Rubichi, S.; Iani, C. Action-space coding in social contexts. Sci. Rep. 2016, 6, 22673. [Google Scholar] [CrossRef]
- Ciardo, F.; Wykowska, A. Response Coordination Emerges in Cooperative but Not Competitive Joint Task. Front. Psychol. 2018, 9, 1919. [Google Scholar] [CrossRef]
- Kourtis, D.; Sebanz, N.; Knoblich, G. Predictive representation of other people’s actions in joint action planning: An EEG study. Soc. Neurosci. 2013, 8, 31–42. [Google Scholar] [CrossRef]
- Kourtis, D.; Knoblich, G.; Woźniak, M.; Sebanz, N. Attention allocation and task representation during joint action planning. J. Cogn. Neurosci. 2014, 26, 2275–2286. [Google Scholar] [CrossRef]
- Huang, C.-M.; Cakmak, M.; Mutlu, B. Adaptive Coordination Strategies for Human-Robot Handovers. Robot. Sci. Syst. 2015, 11, 1–11. [Google Scholar]
- Hsieh, Y.W.; Wu, C.Y.; Wang, W.E.; Lin, K.C.; Chang, K.C.; Chen, C.C.; Liu, C.T. Bilateral robotic priming before task-oriented approach in subacute stroke rehabilitation: A pilot randomized controlled trial. Clin. Rehabil. 2017, 31, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, Y.; Chen, X.; Gao, S. Interface, interaction, and intelligence in generalized brain–computer interfaces. Trends Cogn. Sci. 2021, 25, 671–684. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.; Mühl, C.; Lotte, F.; Hachet, M. Review of the Use of Electroencephalography as an Evaluation Method for Human-Computer Interaction. In Proceedings of the PhyCS-International Conference on Physiological Computing Systems, Lisbonne, Portugal, 7–9 January 2014; pp. 214–223. [Google Scholar]
- Rashid, M.; Sulaiman, N.; PP Abdul Majeed, A.; Musa, R.M.; Ab Nasir, A.F.; Bari, B.S.; Khatun, S. Current Status, Challenges, and Possible Solutions of EEG-Based Brain-Computer Interface: A Comprehensive Review. Front. Neurorobotics 2020, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Cano, S.; Soto, J.; Acosta, L.; Peñeñory, V.M.; Moreira, F. Using Brain-Computer Interface to evaluate the User eXperience in interactive systems. Comput. Methods Biomech. Biomed. Eng. Imaging Vis. 2022, 1–9. [Google Scholar] [CrossRef]
- Klimesch, W. Alpha-band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 2012, 16, 606–617. [Google Scholar] [CrossRef]
- Orekhova, E.V.; Stroganova, T.A.; Posikera, I.N. Alpha activity as an index of cortical inhibition during sustained internally controlled attention in infants. Clin. Neurophysiol. 2001, 112, 740–749. [Google Scholar] [CrossRef]
- Liu, N.; Yu, R. Influence of social presence on eye movements in visual search tasks. Ergonomics 2017, 60, 1667–1681. [Google Scholar] [CrossRef]
- Klimesch, W. EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Res. Rev. 1999, 29, 169–195. [Google Scholar] [CrossRef]
- Baek, H.; Min, B.-K. Blue light aids in coping with the post-lunch dip: An EEG study. Ergonomics 2015, 58, 803–810. [Google Scholar] [CrossRef]
- Barry, R.J.; Clarke, A.R.; McCarthy, R.; Selikowitz, M.; Rushby, J.A.; Ploskova, E. EEG differences in children as a function of resting-state arousal level. Clin. Neurophysiol. 2004, 115, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Tran, Y.; Craig, A.; McIsaac, P. Extraversion–introversion and 8–13 Hz waves in frontal cortical regions. Pers. Indiv. Differ. 2001, 30, 205–215. [Google Scholar] [CrossRef]
- Lazzaro, I.; Gordon, E.; Li, W.; Lim, C.L.; Plahn, M.; Whitmont, S.; Clarke, S.; Barry, R.J.; Dosen, A.; Meares, R. Simultaneous EEG and EDA measures in adolescent attention deficit hyperactivity disorder. Int. J. Psychophysiol. 1999, 34, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Mann, C.A.; Lubar, J.F.; Zimmerman, A.W.; Miller, C.A.; Muenchen, R.A. Quantitative analysis of EEG in boys with attention-deficit-hyperactivity disorder: Controlled study with clinical implications. Pediatr. Neurol. 1992, 8, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Ullsperger, M.; Danielmeier, C.; Jocham, G. Neurophysiology of performance monitoring and adaptive behavior. Physiol. Rev. 2014, 94, 35–79. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, A.; Dieterich, R.; Riesel, A. Error-related brain activity in the age of RDoC: A review of the literature. Int. J. Psychophysiol. 2015, 98, 276–299. [Google Scholar] [CrossRef] [PubMed]
- Gehring, W.J.; Goss, B.; Coles, M.G.H.; Meyer, D.E.; Donchin, E. The Error-Related Negativity. Perspect. Psychol. Sci. 2018, 13, 200–204. [Google Scholar] [CrossRef]
- Alanis, J.C.G.; Baker, T.E.; Peper, M.; Chavanon, M.-L. Social context effects on error-related brain activity are dependent on interpersonal and achievement-related traits. Sci. Rep. 2019, 9, 1728. [Google Scholar] [CrossRef]
- Kim, E.Y.; Iwaki, N.; Uno, H.; Fujita, T. Error-Related Negativity in Children: Effect of an Observer. Dev. Neuropsychol. 2005, 28, 871–883. [Google Scholar] [CrossRef]
- Hajcak, G.; Moser, J.S.; Yeung, N.; Simons, R.F. On the ERN and the significance of errors. Psychophysiology 2005, 42, 151–160. [Google Scholar] [CrossRef]
- Riesel, A.; Weinberg, A.; Endrass, T.; Kathmann, N.; Hajcak, G. Punishment has a lasting impact on error-related brain activity. Psychophysiology 2012, 49, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Van Meel, C.S.; Van Heijningen, C.A. The effect of interpersonal competition on monitoring internal and external error feedback. Psychophysiology 2010, 47, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Decety, J.; Grèzes, J.; Costes, N.; Perani, D.; Jeannerod, M.; Procyk, E.; Grassi, F.; Fazio, F. Brain activity during observation of actions. Influence of action content and subject’s strategy. Brain 1997, 120, 1763–1777. [Google Scholar] [CrossRef] [PubMed]
- Iacoboni, M.; Woods, R.P.; Brass, M.; Bekkering, H.; Mazziotta, J.C.; Rizzolatti, G. Cortical mechanisms of human imitation. Science 1999, 286, 2526–2528. [Google Scholar] [CrossRef] [PubMed]
- Rizzolatti, G.; Fadiga, L.; Matelli, M.; Bettinardi, V.; Paulesu, E.; Perani, D.; Fazio, F. Localization of grasp representations in humans by PET: 1. Observation versus execution. Exp. Brain Res. 1996, 111, 246–252. [Google Scholar] [CrossRef]
- Muthukumaraswamy, S.D.; Johnson, B.W.; McNair, N.A. Mu rhythm modulation during observation of an object-directed grasp. Cogn. Brain Res. 2004, 19, 195–201. [Google Scholar] [CrossRef]
- Oberman, L.M.; Hubbard, E.M.; McCleery, J.P.; Altschuler, E.L.; Ramachandran, V.S.; Pineda, J.A. EEG evidence for mirror neuron dysfunction in autism spectrum disorders. Cogn. Brain Res. 2005, 24, 190–198. [Google Scholar] [CrossRef]
- Pineda, J.A. The functional significance of mu rhythms: Translating seeing and hearing into doing. Brain Res. Rev. 2005, 50, 57–68. [Google Scholar] [CrossRef]
- Silas, J.; Levy, J.P.; Holmes, A. Sensitivity of mu rhythm modulation to the relevance of an observed movement but not to goal congruency. Int. J. Psychophysiol. 2012, 85, 168–173. [Google Scholar] [CrossRef]
- Sebanz, N.; Bekkering, H.; Knoblich, G. Joint action: Bodies and minds moving together. Trends Cogn. Sci. 2006, 10, 70–76. [Google Scholar] [CrossRef]
- Sebanz, N.; Knoblich, G.; Prinz, W.; Wascher, E. Twin Peaks: An ERP Study of Action Planning and Control in Coacting Individuals. J. Cogn. Neurosci. 2006, 18, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Ruissen, M.I.; Bruijn, E.R.A.d. Is it me or is it you? Behavioral and electrophysiological effects of oxytocin administration on self-other integration during joint task performance. Cortex 2015, 70, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Peterburs, J.; Liepelt, R.; Voegler, R.; Ocklenburg, S.; Straube, T. It’s not me, it’s you-Differential neural processing of social and non-social nogo cues in joint action. Soc. Neurosci. 2017, 14, 114–124. [Google Scholar] [CrossRef]
- Han, Y.; Ziebell, P.; Riccio, A.; Halder, S. Two sides of the same coin: Adaptation of BCIs to internal states with user-centered design and electrophysiological features. Brain Comput. Interfaces 2022, 9, 102–114. [Google Scholar] [CrossRef]
- Singh, H.; Daly, I. Translational Algorithms: The Heart of a Brain Computer Interface. In Brain-Computer Interfaces: Current Trends and Applications; Hassanien, A.E., Azar, A.T., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 97–121. [Google Scholar]
- Nam, C.S.; Nijholt, A.; Lotte, F. Brain–Computer Interfaces Handbook; CRC Press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Clerc, M.; Bougrain, L.; Lotte, F. Brain-Computer Interfaces 1: Methods and Perspectives; John and Wiley and Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Kübler, A.; Holz, E.M.; Riccio, A.; Zickler, C.; Kaufmann, T.; Kleih, S.C.; Staiger-Sälzer, P.; Desideri, L.; Hoogerwerf, E.-J.; Mattia, D. The User-Centered Design as Novel Perspective for Evaluating the Usability of BCI-Controlled Applications. PLoS ONE 2014, 9, e112392. [Google Scholar] [CrossRef]
- Lenz, C.; Nair, S.; Rickert, M.; Knoll, A.; Rosel, W.; Gast, J.; Bannat, A.; Wallhoff, F. Joint-action for humans and industrial robots for assembly tasks. In Proceedings of the RO-MAN 2008-The 17th IEEE International Symposium on Robot and Human Interactive Communication, Munich, Germany, 1–3 August 2008; pp. 130–135. [Google Scholar]
- Nikolaidis, S.; Lasota, P.; Rossano, G.; Martinez, C.; Fuhlbrigge, T.; Shah, J. Human-robot collaboration in manufacturing: Quantitative evaluation of predictable, convergent joint action. In Proceedings of the IEEE ISR, Seoul, Republic of Korea, 24–26 October 2013; pp. 1–6. [Google Scholar]
- Hentout, A.; Aouache, M.; Maoudj, A.; Akli, I. Human–robot interaction in industrial collaborative robotics: A literature review of the decade 2008–2017. Adv. Robot. 2019, 33, 764–799. [Google Scholar] [CrossRef]

| Psychological Effects | Related Factor | Cognitive Processes Involved (And Their Potential EEG Signatures *) | References |
|---|---|---|---|
| Social facilitation | Physical presence | Arousal level (alpha and beta power) | [61,62,63,64,65,66] |
| Performance monitoring (error-related negativity, ERN) | [71,72,73] | ||
| Motor priming | Motor action | Sensorimotor mirroring (event-related mu suppression) | [80,81] |
| Joint action | Task co-representation | Response conflict (N2 component) | [84,85] |
| Stimulus classification, response discrimination (P3 component) | [50,82,83,85] | ||
| Action planning (contingent negative variation, CNV; movement-related potential, MRP) | [50,51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, H.; Wang, F.; Zhang, D. Inspiring Real-Time Evaluation and Optimization of Human–Robot Interaction with Psychological Findings from Human–Human Interaction. Appl. Sci. 2023, 13, 676. https://doi.org/10.3390/app13020676
Liu H, Wang F, Zhang D. Inspiring Real-Time Evaluation and Optimization of Human–Robot Interaction with Psychological Findings from Human–Human Interaction. Applied Sciences. 2023; 13(2):676. https://doi.org/10.3390/app13020676
Chicago/Turabian StyleLiu, Huashuo, Fei Wang, and Dan Zhang. 2023. "Inspiring Real-Time Evaluation and Optimization of Human–Robot Interaction with Psychological Findings from Human–Human Interaction" Applied Sciences 13, no. 2: 676. https://doi.org/10.3390/app13020676
APA StyleLiu, H., Wang, F., & Zhang, D. (2023). Inspiring Real-Time Evaluation and Optimization of Human–Robot Interaction with Psychological Findings from Human–Human Interaction. Applied Sciences, 13(2), 676. https://doi.org/10.3390/app13020676







