A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes the Growth of 3D Spheroid Cultures of Gingival Fibroblasts
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Morphometric Analysis
2.4. Cell Viability
2.5. Cell Behavior
2.6. Statistical Analysis
3. Results
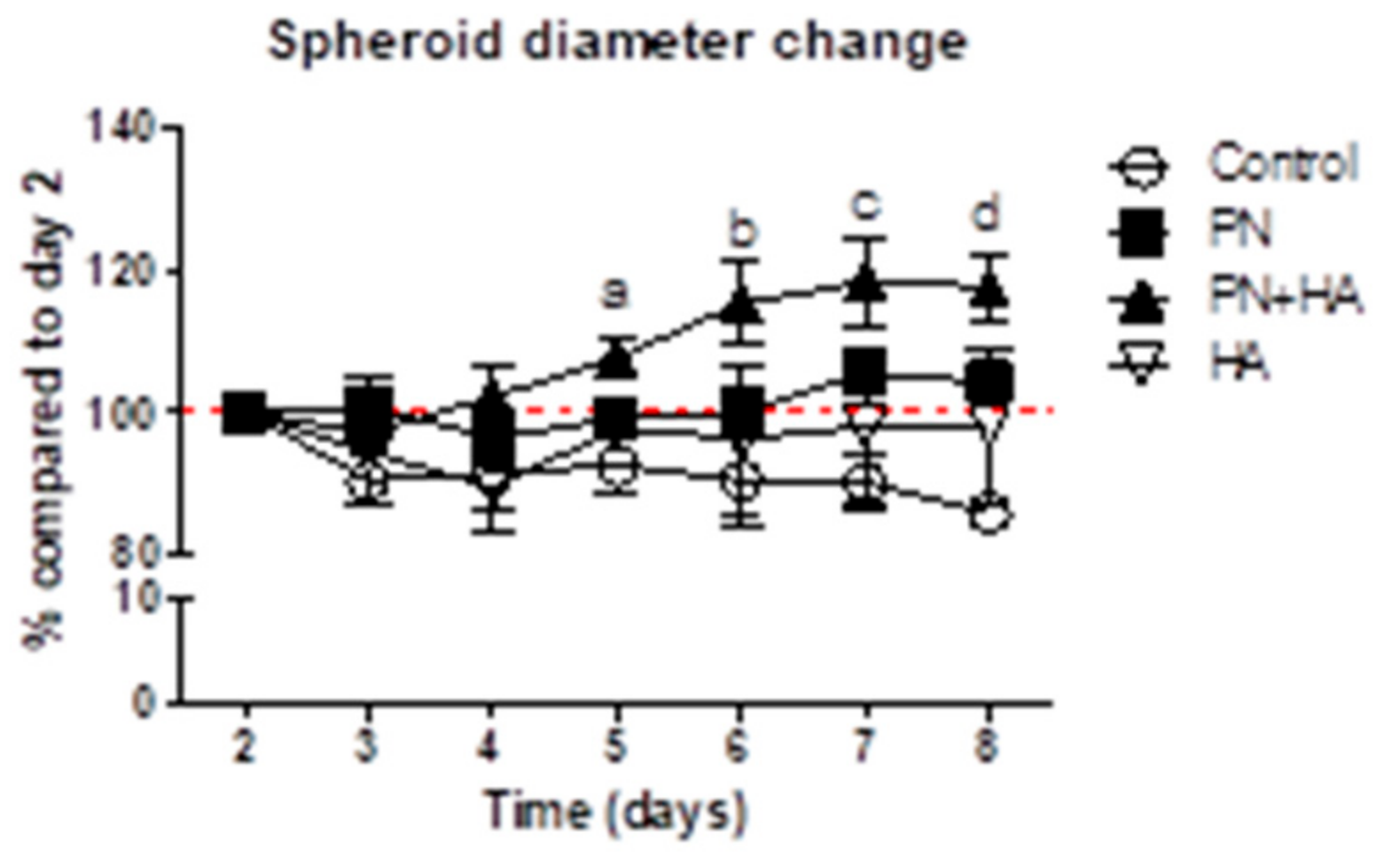
3.1. PN + HA Promotes the Dimensional Growth of Spheroids
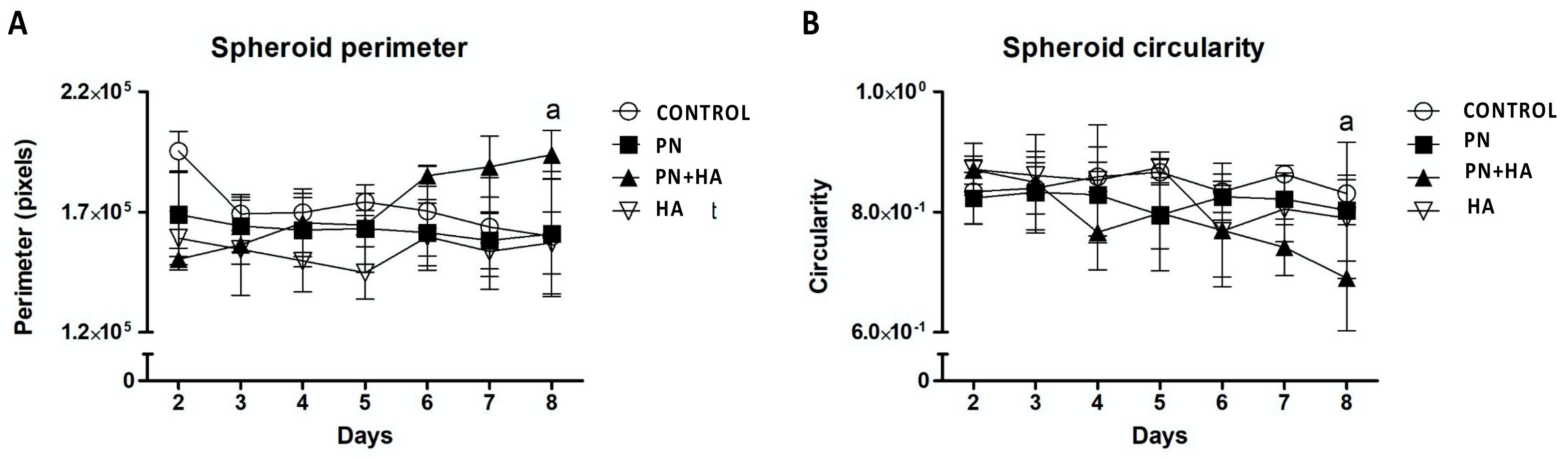
3.2. PN + HA Induces Morphological Changes in Spheroids
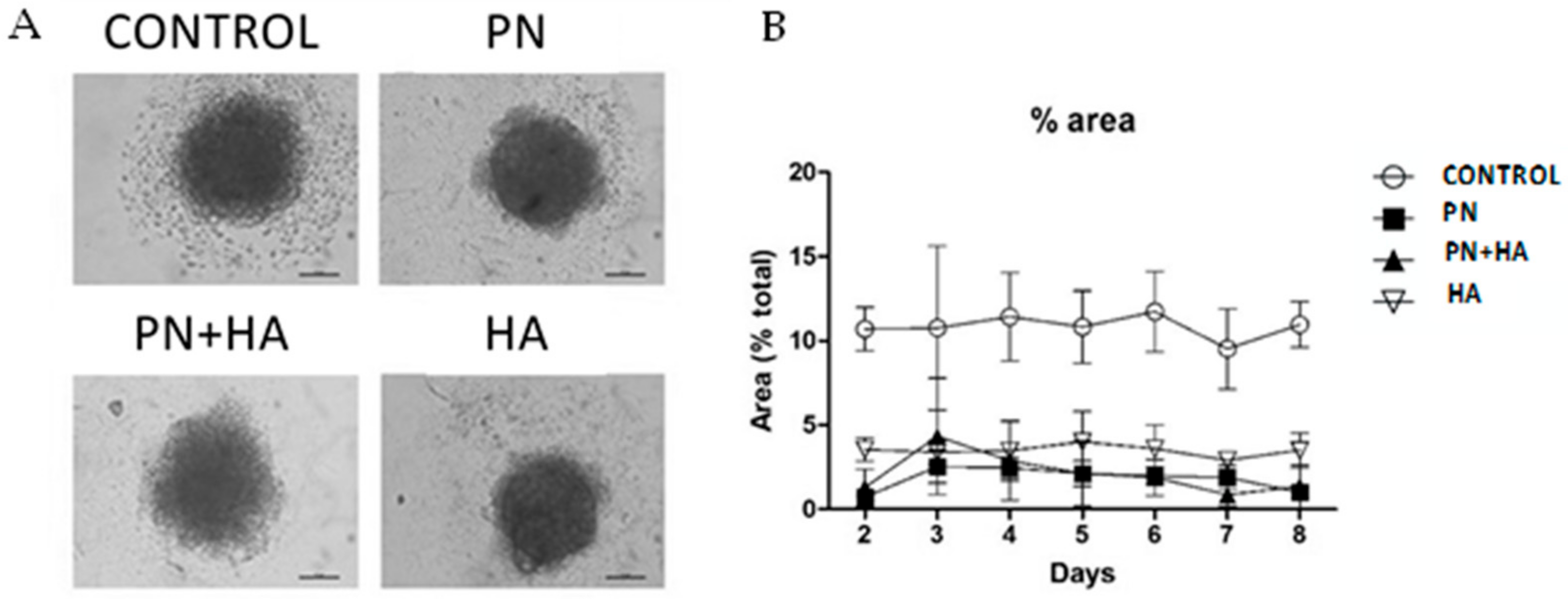
3.3. PN Preserves the Integrity of Cell Spheroids
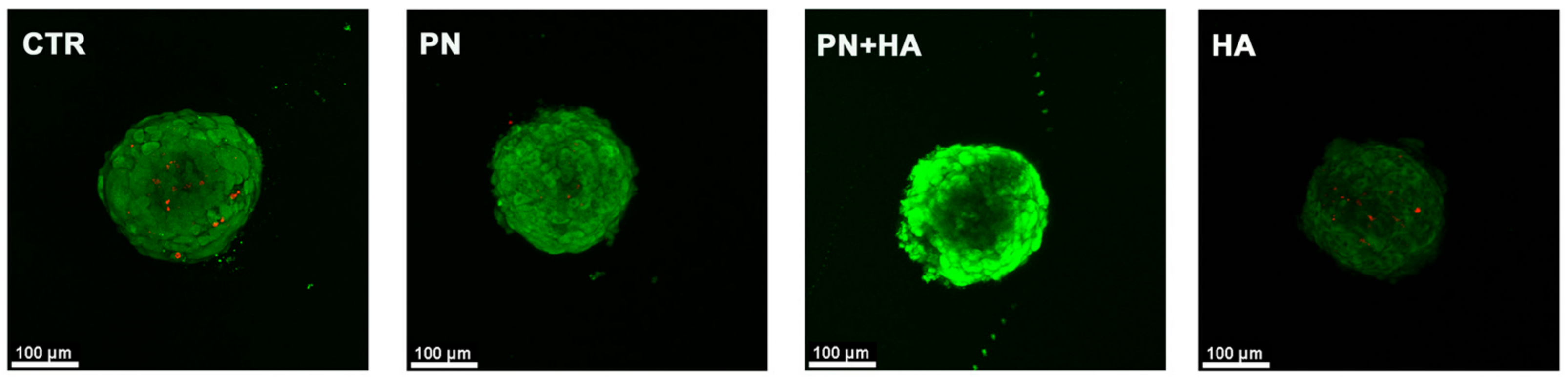
3.4. PN Increases Cell Viability within Spheroids
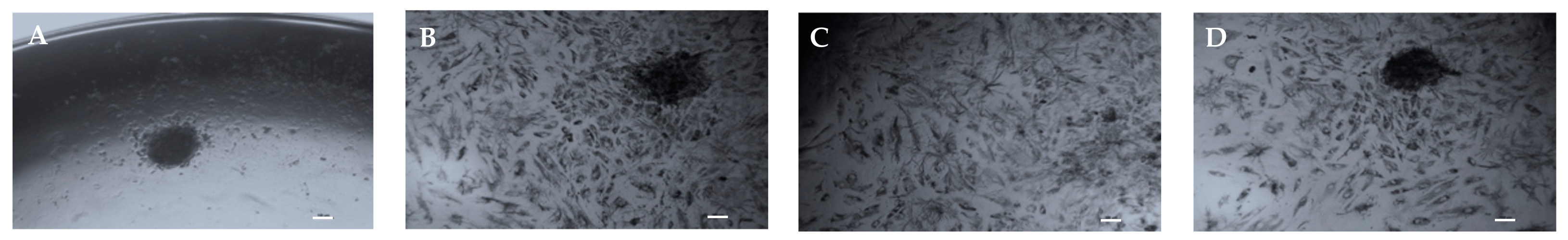
3.5. PN Increases Cell Proliferation and Migration from Spheroids
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Paternoster, J.L.; Vranckx, J.J. State of the Art of Clinical Applications of Tissue Engineering in 2021. Tissue Eng. Part B Rev. 2022, 28, 592–612. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guo, L.; Li, X.; Liu, S.; Du, J.; Xu, J.; Hu, J.; Liu, Y. Challenges and Tissue Engineering Strategies of Periodontal Guided Tissue Regeneration. Tissue Eng. Part C Methods 2022, 28, 405–419. [Google Scholar] [CrossRef] [PubMed]
- Colangelo, M.T.; Govoni, P.; Belletti, S.; Squadrito, F.; Guizzardi, S.; Galli, C. Polynucleotide Biogel Enhances Tissue Repair, Matrix Deposition and Organization. J. Biol. Regul. Homeost. Agents 2021, 35, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; Bartoletti, E.; Maioli, L.; Massirone, A.; Pia Palmieri, I.; Papagni, M.; Priori, M.; Trocchi, G. Consensus Report on the Use of PN-HPTTM (Polynucleotides Highly Purified Technology) in Aesthetic Medicine. J. Cosmet. Dermatol. 2021, 20, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Cavallini, M.; de Luca, C.; Prussia, G.; Raichi, M. PN-HPT® (Polynucleotides Highly Purified Technology) in Facial Middle Third Rejuvenation. Exploring the Potential. J. Cosmet. Dermatol. 2022, 21, 615–624. [Google Scholar] [CrossRef]
- Gennero, L.; Denysenko, T.; Calisti, G.F.; Vercelli, A.; Vercelli, C.M.; Amedeo, S.; Mioletti, S.; Parino, E.; Montanaro, M.; Melcarne, A.; et al. Protective Effects of Polydeoxyribonucleotides on Cartilage Degradation in Experimental Cultures. Cell Biochem. Funct. 2013, 31, 214–227. [Google Scholar] [CrossRef]
- Giarratana, L.S.; Marelli, B.M.; Crapanzano, C.; de Martinis, S.E.; Gala, L.; Ferraro, M.; Marelli, N.; Albisetti, W. A Randomized Double-Blind Clinical Trial on the Treatment of Knee Osteoarthritis: The Efficacy of Polynucleotides Compared to Standard Hyaluronian Viscosupplementation. Knee 2014, 21, 661–668. [Google Scholar] [CrossRef]
- Colangelo, M.T.; Galli, C.; Guizzardi, S. The Effects of Polydeoxyribonucleotide on Wound Healing and Tissue Regeneration: A Systematic Review of the Literature. Regen. Med. 2020, 15, 1801–1821. [Google Scholar] [CrossRef]
- Scognamiglio, C.; Soloperto, A.; Ruocco, G.; Cidonio, G. Bioprinting Stem Cells: Building Physiological Tissues One Cell at a Time. Am. J. Physiol.-Cell Physiol. 2020, 319, C465–C480. [Google Scholar] [CrossRef]
- Pia Palmieri, I.; Raichi, M. Biorevitalization of Postmenopausal Labia Majora, the Polynucleotide/Hyaluronic Acid Option. Obstet. Gynecol. Rep. 2019, 3, 1–5. [Google Scholar] [CrossRef]
- Guizzardi, S.; Uggeri, J.; Belletti, S.; Cattarini, G. Hyaluronate Increases Polynucleotides Effect on Human Cultured Fibroblasts. J. Cosmet. Dermatol. Sci. Appl. 2013, 3, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Dallari, D.; Sabbioni, G.; del Piccolo, N.; Carubbi, C.; Veronesi, F.; Torricelli, P.; Fini, M. Efficacy of Intra-Articular Polynucleotides Associated With Hyaluronic Acid Versus Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis. Clin. J. Sport Med. 2020, 30, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Lei, N.; Chang, R.; Yang, C.; Li, Q.; Zuo, N.; Gu, Y. Efficacy of Intra-Articular Polynucleotides Associated with Hyaluronic Acid vs. Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis: A Systematic Review and Meta-Analysis of Randomized Clinical Trial. Medicine 2020, 99, e20689. [Google Scholar] [CrossRef] [PubMed]
- Pilloni, A.; Rojas, M.A.; Dh, C.T.; Carere, M.; de Filippis, A.; Marsala Dh, R.L.; Marini, L. Clinical Effects of the Adjunctive Use of a Polynucleotides and Hyaluronic Acid Based Gel in the Subgingival Re-Instrumentation of Residual Periodontal Pockets: A Randomized, Split-Mouth Clinical Trial. J. Periodontol. 2022, 1–10. [Google Scholar] [CrossRef]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [Green Version]
- Schindler, M.; Nur-E-Kamal, A.; Ahmed, I.; Kamal, J.; Liu, H.-Y.; Amor, N.; Ponery, A.S.; Crockett, D.P.; Grafe, T.H.; Chung, H.Y.; et al. Living in Three Dimensions: 3D Nanostructured Environments for Cell Culture and Regenerative Medicine. Cell Biochem. Biophys. 2006, 45, 215–227. [Google Scholar] [CrossRef]
- Wolff, A.; Frank, M.; Staehlke, S.; Peters, K. A Comparative Study on the Adipogenic Differentiation of Mesenchymal Stem/Stromal Cells in 2D and 3D Culture. Cells 2022, 11, 1313. [Google Scholar] [CrossRef]
- Jubelin, C.; Muñoz-Garcia, J.; Griscom, L.; Cochonneau, D.; Ollivier, E.; Heymann, M.-F.; Vallette, F.M.; Oliver, L.; Heymann, D. Three-Dimensional in Vitro Culture Models in Oncology Research. Cell Biosci. 2022, 12, 155. [Google Scholar] [CrossRef]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards Organoid Culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef]
- Squadrito, F.; Bitto, A.; Irrera, N.; Pizzino, G.; Pallio, G.; Minutoli, L.; Altavilla, D. Pharmacological Activity and Clinical Use of PDRN. Front. Pharmacol. 2017, 8, 224. [Google Scholar] [CrossRef]
- Bigelow, C.E.; Mitra, S.; Knuechel, R.; Foster, T.H. ALA- and ALA-Hexylester-Induced Protoporphyrin IX Fluorescence and Distribution in Multicell Tumour Spheroids. Br. J. Cancer 2001, 85, 727–734. [Google Scholar] [CrossRef] [Green Version]
- Stagni, C.; Rocchi, M.; Mazzotta, A.; del Piccolo, N.; Rani, N.; Govoni, M.; Vivarelli, L.; Veronesi, F.; Fini, M.; Dallari, D. Randomised, Double-Blind Comparison of a Fixed Co-Formulation of Intra-Articular Polynucleotides and Hyaluronic Acid versus Hyaluronic Acid Alone in the Treatment of Knee Osteoarthritis: Two-Year Follow-Up. BMC Musculoskelet. Disord. 2021, 22, 773. [Google Scholar] [CrossRef]
- de Caridi, G.; Massara, M.; Acri, I.; Zavettieri, S.; Grande, R.; Butrico, L.; de Franciscis, S.; Serra, R. Trophic Effects of Polynucleotides and Hyaluronic Acid in the Healing of Venous Ulcers of the Lower Limbs: A Clinical Study. Int. Wound J. 2016, 13, 754–758. [Google Scholar] [CrossRef]
- Segreto, F.; Carotti, S.; Marangi, G.F.; Francesconi, M.; Scaramuzzino, L.; Gratteri, M.; Caldaria, E.; Morini, S.; Persichetti, P. The Use of Acellular Porcine Dermis, Hyaluronic Acid and Polynucleotides in the Treatment of Cutaneous Ulcers: Single Blind Randomised Clinical Trial. Int. Wound J. 2020, 17, 1702–1708. [Google Scholar] [CrossRef]
- Maioli, L. Polynucleotides Highly Purified Technology and Nucleotides for the Acceleration and Regulation of Normal Wound Healing. Aesthetic Med. 2020, 6, 48–52. [Google Scholar]
- Colangelo, M.T.; Belletti, S.; Govoni, P.; Guizzardi, S.; Galli, C. A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes Wound Healing in a Primary Gingival Fibroblast Model. Appl. Sci. 2021, 11, 4405. [Google Scholar] [CrossRef]
- Al Shboul, S.; DeLuca, V.J.; Al Dweiri, Y.; Saleh, T. Can 3D Bioprinting Solve the Mystery of Senescence in Cancer Therapy? Ageing Res. Rev. 2022, 81, 101732. [Google Scholar] [CrossRef]
- AlHowaish, N.A.; AlSudani, D.I.; AlMuraikhi, N.A. Evaluation of a Hyaluronic Acid Hydrogel (Restylane Lyft) as a Scaffold for Dental Pulp Regeneration in a Regenerative Endodontic Organotype Model. Odontology 2022, 110, 726–734. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colangelo, M.T.; Vicedomini, M.L.; Belletti, S.; Govoni, P.; Guizzardi, S.; Galli, C. A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes the Growth of 3D Spheroid Cultures of Gingival Fibroblasts. Appl. Sci. 2023, 13, 743. https://doi.org/10.3390/app13020743
Colangelo MT, Vicedomini ML, Belletti S, Govoni P, Guizzardi S, Galli C. A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes the Growth of 3D Spheroid Cultures of Gingival Fibroblasts. Applied Sciences. 2023; 13(2):743. https://doi.org/10.3390/app13020743
Chicago/Turabian StyleColangelo, Maria Teresa, Maria Luisa Vicedomini, Silvana Belletti, Paolo Govoni, Stefano Guizzardi, and Carlo Galli. 2023. "A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes the Growth of 3D Spheroid Cultures of Gingival Fibroblasts" Applied Sciences 13, no. 2: 743. https://doi.org/10.3390/app13020743
APA StyleColangelo, M. T., Vicedomini, M. L., Belletti, S., Govoni, P., Guizzardi, S., & Galli, C. (2023). A Biomimetic Polynucleotides–Hyaluronic Acid Hydrogel Promotes the Growth of 3D Spheroid Cultures of Gingival Fibroblasts. Applied Sciences, 13(2), 743. https://doi.org/10.3390/app13020743







