Mitigation of Non-Steroidal Anti-Inflammatory and Antiretroviral Drugs as Environmental Pollutants by Adsorption Using Nanomaterials as Viable Solution—A Critical Review
Abstract
1. Introduction
2. Wastewater Treatment Methods
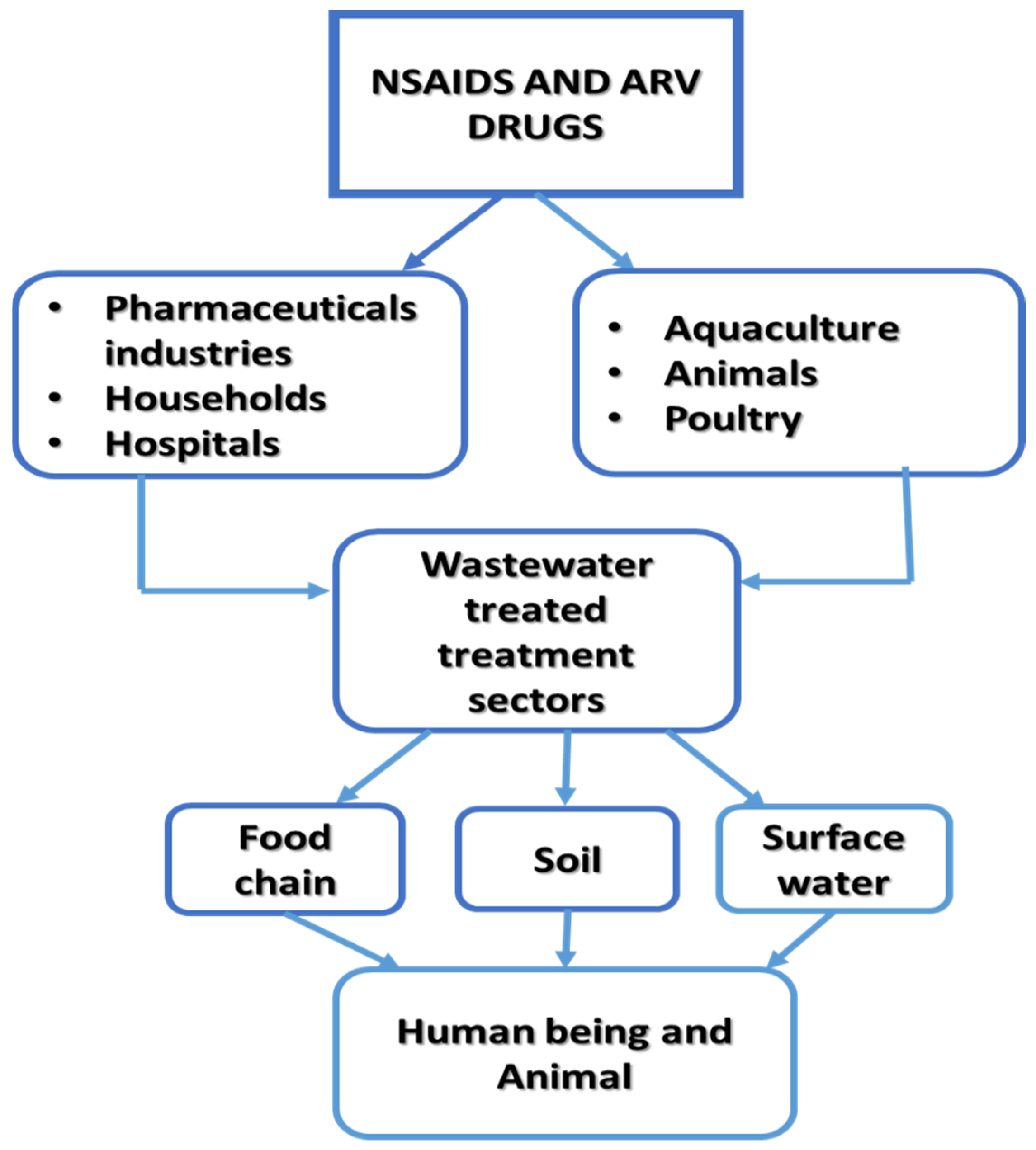
3. Pharmaceuticals Pollutants
3.1. Occurrence, Fate and Removal Technologies of Pharmaceuticals in Water
3.1.1. Non-Steroidal Anti-Inflammatory Drugs
3.1.2. Antiretroviral Drugs
3.2. Detection, Sample Preparation and Analysis of Nsaids and ARV Drugs
4. Adsorption
Factors Affecting Adsorption
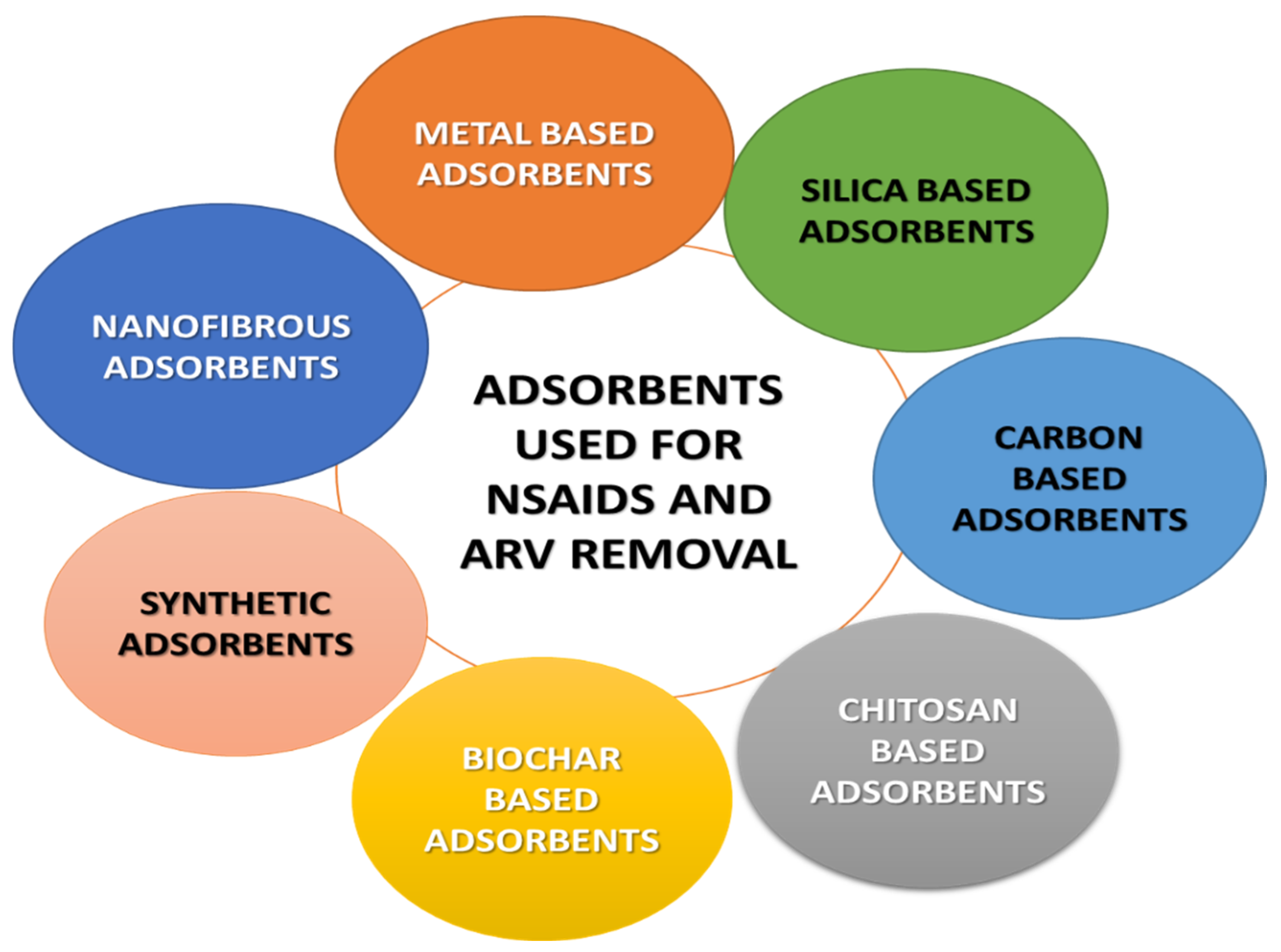
5. Mitigation of Pharmaceutical Pollutants by Nanomaterials
5.1. Classification of Nano-Sorbents
5.1.1. Carbon-Based Nanomaterial
Biochar-Based Adsorbents
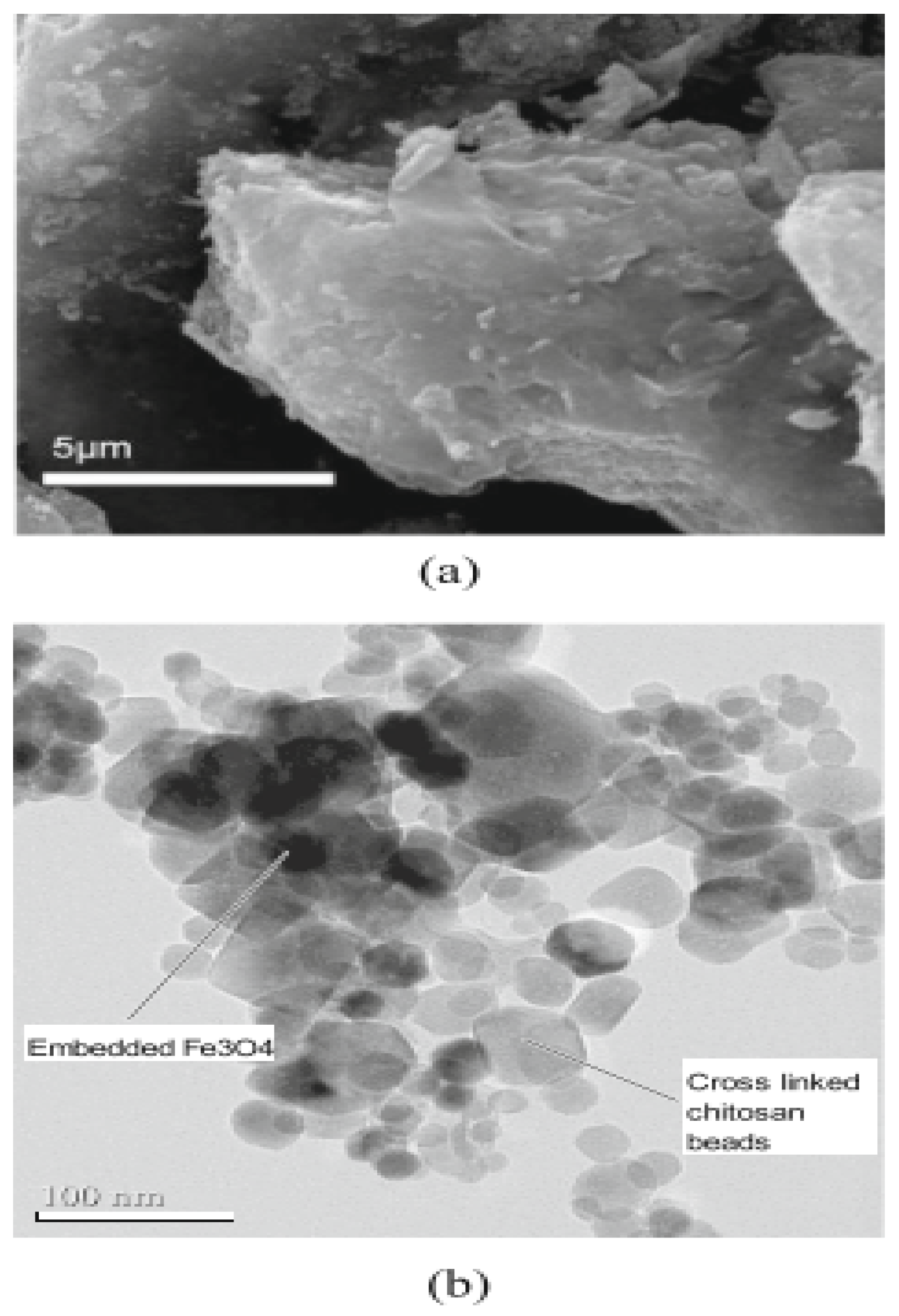
5.1.2. Chitosan-Based Nanoparticles
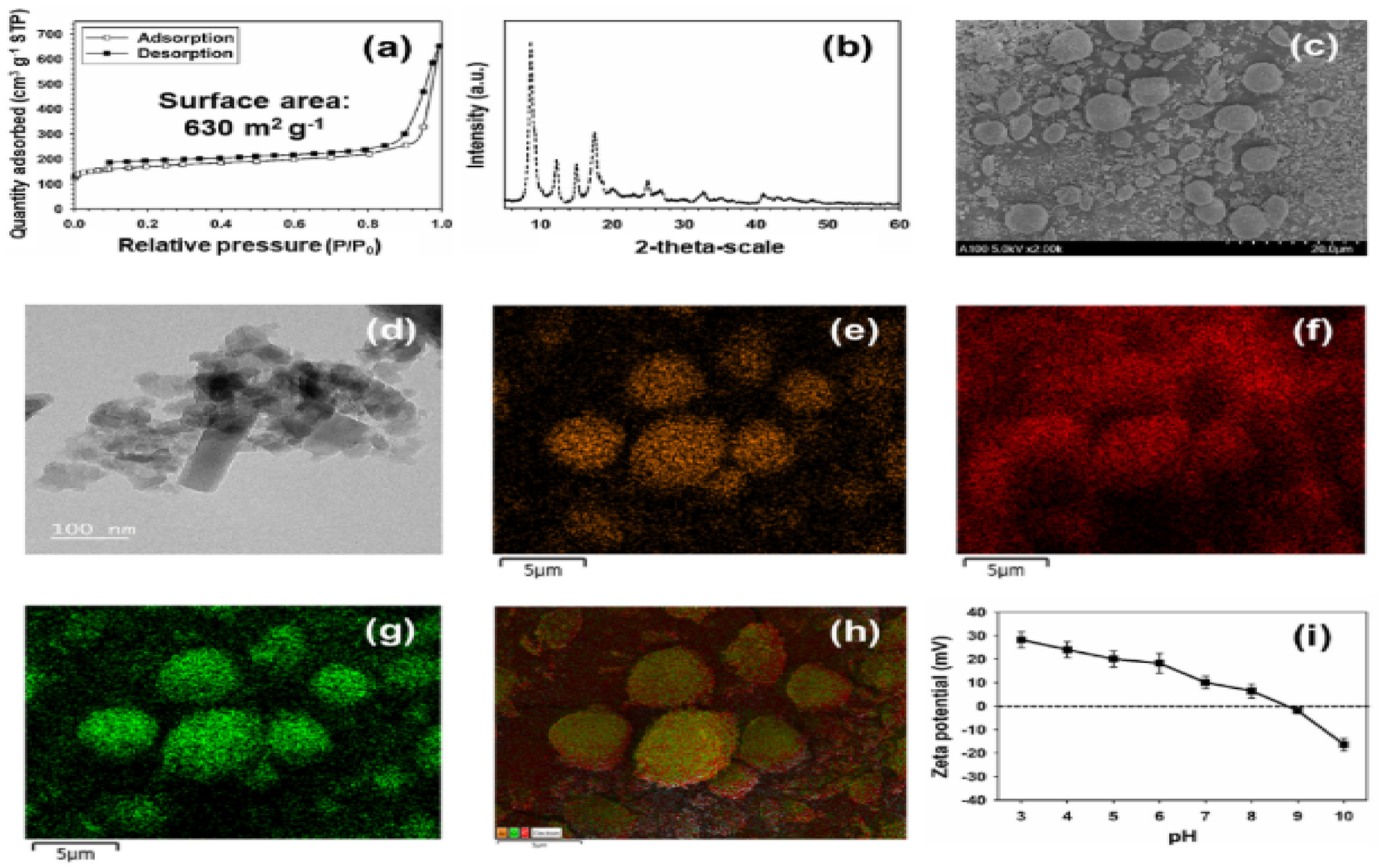
5.1.3. Metal-Based Nanoparticles (MNPs)
5.1.4. Silica-Based Nano-Sorbents
5.1.5. Electrospun-Based Adsorbents
5.2. Nanomembranes
5.3. Nanostructured Catalytic Membranes
5.4. Characteristics of Nanosorbents
5.5. Interaction of Pharmaceuticals and Chemical Nature of Adsorbents
6. Removal of Pharmaceutical Pollutants in Wastewater by Nanosorbents
6.1. Removal of Non-Steroidal Anti-Inflammatory Drugs
6.2. Removal of Antiretroviral Drugs in Water
6.3. Mitigation Using Poly (Vinyl Alcohol) (PVA)-Based Nanofibers
7. Limitations of Nanomaterials
8. Future Prospects
9. Conclusions
Funding
Conflicts of Interest
References
- Shen, R.; Andrews, S.A. Demonstration of 20 pharmaceuticals and personal care products (PPCPs) as nitrosamine precursors during chloramine disinfection. Water Res. 2011, 45, 944–952. [Google Scholar] [CrossRef] [PubMed]
- Carmalin Sophia, A.; Lima, E.C.; Allaudeen, N.; Rajan, S. Application of graphene based materials for adsorption of pharmaceutical traces from water and wastewater—A review. Desalin. Water Treat. 2016, 57, 27573–27586. [Google Scholar] [CrossRef]
- Sigonya, S.; Chibuzor, S.; Phumlani, O.; Mdluli, S.; Hendrica, T. Method optimisation and application based on solid phase extraction of non steroidal anti–inflammatory drugs, antiretroviral drugs, and a lipid regulator from coastal areas of Durban, South Africa. SN Appl. Sci. 2022, 4, 231. [Google Scholar] [CrossRef]
- Awwad, N.S.; El-Zahhar, A.A.; Fouda, A.M.; Ibrahium, H.A. Removal of heavy metal ions from ground and surface water samples using carbons derived from date pits. J. Environ. Chem. Eng. 2013, 1, 416–423. [Google Scholar] [CrossRef]
- Arora, R. Adsorption of heavy metals-a review. Mater. Today Proc. 2019, 18, 4745–4750. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Foster, J.E. Plasma-based water purification: Challenges and prospects for the future. Phys. Plasmas 2017, 24, 055501. [Google Scholar] [CrossRef]
- Shafiq, I.; Hussain, M.; Shehzad, N.; Maafa, I.M.; Akhter, P.; Amjad, U.E.S.; Shafique, S.; Razzaq, A.; Yang, W.; Tahir, M.; et al. The effect of crystal facets and induced porosity on the performance of monoclinic BiVO4 for the enhanced visible-light driven photocatalytic abatement of methylene blue. J. Environ. Chem. Eng. 2019, 7, 103265. [Google Scholar] [CrossRef]
- Comerton, A.M.; Andrews, R.C.; Bagley, D.M.; Hao, C. The rejection of endocrine disrupting and pharmaceutically active compounds by NF and RO membranes as a function of compound and water matrix properties. J. Memb. Sci. 2008, 313, 323–335. [Google Scholar] [CrossRef]
- Fuerhacker, M.; Dürauer, A.; Jungbauer, A. Adsorption isotherms of 17β-estradiol on granular activated carbon (GAC). Chemosphere 2001, 44, 1573–1579. [Google Scholar] [CrossRef]
- Kaya, Y.; Ersan, G.; Vergili, I.; Gönder, Z.B.; Yilmaz, G.; Dizge, N.; Aydiner, C. The treatment of pharmaceutical wastewater using in a submerged membrane bioreactor under different sludge retention times. J. Memb. Sci. 2013, 442, 72–82. [Google Scholar] [CrossRef]
- Stasinakis, A.S. Use of selected advanced oxidation processes ({AOPs}) for wastewater treatment—A mini review. Glob. NEST J. 2008, 2008, 376–385. [Google Scholar]
- Westerhoff, P.; Yoon, Y.; Snyder, S.; Wert, E. Fate of endocrine-disruptor, pharmaceutical, and personal care product chemicals during simulated drinking water treatment processes. Environ. Sci. Technol. 2005, 39, 6649–6663. [Google Scholar] [CrossRef]
- Oller, I.; Malato, S.; Sánchez-Pérez, J.A. Combination of Advanced Oxidation Processes and biological treatments for wastewater decontamination—A review. Sci. Total Environ. 2011, 409, 4141–4166. [Google Scholar] [CrossRef] [PubMed]
- Radjenović, J.; Petrović, M.; Barceló, D. Fate and distribution of pharmaceuticals in wastewater and sewage sludge of the conventional activated sludge (CAS) and advanced membrane bioreactor (MBR) treatment. Water Res. 2009, 43, 831–841. [Google Scholar] [CrossRef] [PubMed]
- Yoon, Y.; Ryu, J.; Oh, J.; Choi, B.G.; Snyder, S.A. Occurrence of endocrine disrupting compounds, pharmaceuticals, and personal care products in the Han River (Seoul, South Korea). Sci. Total Environ. 2010, 408, 636–643. [Google Scholar] [CrossRef]
- Mohanty, K.; Das, D.; Biswas, M.N. Preparation and characterization of activated carbons from Sterculia alata nutshell by chemical activation with zinc chloride to remove phenol from wastewater. Adsorption 2006, 12, 119–132. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Haider, W. Heterogeneous photocatalysis and its potential applications in water and wastewater treatment: A review. Nanotechnology 2018, 29, 342001. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Juang, R.S. Efficient removal of cationic dyes from water by a combined adsorption-photocatalysis process using platinum-doped titanate nanomaterials. J. Taiwan Inst. Chem. Eng. 2019, 99, 166–179. [Google Scholar] [CrossRef]
- Gerbersdorf, S.U.; Cimatoribus, C.; Class, H.; Engesser, K.H.; Helbich, S.; Hollert, H.; Lange, C.; Kranert, M.; Metzger, J.; Nowak, W.; et al. Anthropogenic Trace Compounds (ATCs) in aquatic habitats—Research needs on sources, fate, detection and toxicity to ensure timely elimination strategies and risk management. Environ. Int. 2015, 79, 85–105. [Google Scholar] [CrossRef] [PubMed]
- Ngumba, E.; Gachanja, A.; Tuhkanen, T. Occurrence of selected antibiotics and antiretroviral drugs in Nairobi River Basin, Kenya. Sci. Total Environ. 2016, 539, 206–213. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajjadi, M.; Iravani, S.; Varma, R.S. Starch, cellulose, pectin, gum, alginate, chitin and chitosan derived (nano)materials for sustainable water treatment: A review. Carbohydr. Polym. 2021, 251, 116986. [Google Scholar] [CrossRef]
- Konig-Péter, A.; Kocsis, B.; Kilár, F.; Pernyeszi, T. Bio-adsorption characteristics of Pseudomonas aeruginosa PAO1. J. Serbian Chem. Soc. 2014, 79, 495–508. [Google Scholar] [CrossRef]
- Shon, H.K.; Vigneswaran, S.; Snyder, S.A. Effluent organic matter (EfOM) in wastewater: Constituents, effects, and treatment. Crit. Rev. Environ. Sci. Technol. 2006, 36, 327–374. [Google Scholar] [CrossRef]
- Wong, J.K.H.; Tan, H.K.; Lau, S.Y.; Yap, P.S.; Danquah, M.K. Potential and challenges of enzyme incorporated nanotechnology in dye wastewater treatment: A review. J. Environ. Chem. Eng. 2019, 7, 103261. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Collado, N.; Rodriguez-Mozaz, S.; Gros, M.; Rubirola, A.; Barceló, D.; Comas, J.; Rodriguez-Roda, I.; Buttiglieri, G. Pharmaceuticals occurrence in a WWTP with significant industrial contribution and its input into the river system. Environ. Pollut. 2014, 185, 202–212. [Google Scholar] [CrossRef]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U.; Mohan, D. Pharmaceuticals of emerging concern in aquatic systems: Chemistry, occurrence, effects, and removal methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef]
- De Andrade, J.R.; Oliveira, M.F.; Da Silva, M.G.C.; Vieira, M.G.A. Adsorption of Pharmaceuticals from Water and Wastewater Using Nonconventional Low-Cost Materials: A Review. Ind. Eng. Chem. Res. 2018, 57, 3103–3127. [Google Scholar] [CrossRef]
- Brutzkus, J.C.; Shahrokhi, M.; Varacallo, M. Naproxen; StatPearls Publishing: Treuasure Island, FL, USA, 2018; pp. 1–5. [Google Scholar]
- Dahane, S.; Gil García, M.D.; Martínez Bueno, M.J.; Uclés Moreno, A.; Martínez Galera, M.; Derdour, A. Determination of drugs in river and wastewaters using solid-phase extraction by packed multi-walled carbon nanotubes and liquid chromatography-quadrupole-linear ion trap-mass spectrometry. J. Chromatogr. A 2013, 1297, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.; Bosch, B.; Brune, K.; Patrignani, P.; Young, C. Advances in NSAID development: Evolution of diclofenac products using pharmaceutical technology. Drugs 2015, 75, 859–877. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.J. Adsorption of non-steroidal anti-inflammatory drugs from aqueous solution using activated carbons: Review. J. Environ. Manage. 2017, 190, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Ngubane, N.P.; Naicker, D.; Ncube, S.; Chimuka, L.; Madikizela, L.M. Determination of naproxen, diclofenac and ibuprofen in Umgeni estuary and seawater: A case of northern Durban in KwaZulu–Natal Province of South Africa. Reg. Stud. Mar. Sci. 2019, 29, 100675. [Google Scholar] [CrossRef]
- Álvarez-Torrellas, S.; Rodríguez, A.; Ovejero, G.; García, J. Comparative adsorption performance of ibuprofen and tetracycline from aqueous solution by carbonaceous materials. Chem. Eng. J. 2016, 283, 936–947. [Google Scholar] [CrossRef]
- Weigel, S.; Kuhlmann, J.; Huhnerfuss, H. Drugs and personal care products as ubiquitous pollutants: Occurrence and distribution of clofibric acid, caffeine and DEET in the North Sea. Sci. Total Environ. 2002, 295, 131–141. [Google Scholar] [CrossRef]
- Baccar, R.; Sarrà, M.; Bouzid, J.; Feki, M.; Blánquez, P. Removal of pharmaceutical compounds by activated carbon prepared from agricultural by-product. Chem. Eng. J. 2012, 211–212, 310–317. [Google Scholar] [CrossRef]
- Jedynak, K.; Szczepanik, B.; Rȩdzia, N.; Slomkiewicz, P.; Kolbus, A.; Rogala, P. Ordered mesoporous carbons for adsorption of paracetamol and non-steroidal anti-inflammatory drugs: Ibuprofen and naproxen from aqueous solutions. Water 2019, 11, 1099. [Google Scholar] [CrossRef]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Li, Y.; Han, S.; Ma, J. Adsorptive removal of antibiotics from aqueous solution using carbon materials. Chemosphere 2016, 153, 365–385. [Google Scholar] [CrossRef]
- Rakić, V.; Rac, V.; Krmar, M.; Otman, O.; Auroux, A. The adsorption of pharmaceutically active compounds from aqueous solutions onto activated carbons. J. Hazard. Mater. 2015, 282, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Bhadra, B.N.; Seo, P.W.; Jhung, S.H. Adsorption of diclofenac sodium from water using oxidized activated carbon. Chem. Eng. J. 2016, 301, 27–34. [Google Scholar] [CrossRef]
- Larous, S.; Meniai, A.H. Adsorption of Diclofenac from aqueous solution using activated carbon prepared from olive stones. Int. J. Hydrogen Energy 2016, 41, 10380–10390. [Google Scholar] [CrossRef]
- Mestre, A.S.; Pires, J.; Nogueira, J.M.F.; Carvalho, A.P. Activated carbons for the adsorption of ibuprofen. Carbon N. Y. 2007, 45, 1979–1988. [Google Scholar] [CrossRef]
- Guedidi, H.; Reinert, L.; Lévêque, J.M.; Soneda, Y.; Bellakhal, N.; Duclaux, L. The effects of the surface oxidation of activated carbon, the solution pH and the temperature on adsorption of ibuprofen. Carbon N. Y. 2013, 54, 432–443. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, L.; Yang, S.; Schott, J.A.; Liu, X.; Mahurin, S.M.; Huang, C.; Zhang, Y.; Fulvio, P.F.; Chisholm, M.F.; et al. Solid-state synthesis of ordered mesoporous carbon catalysts via a mechanochemical assembly through coordination cross-linking. Nat. Commun. 2017, 8, 15020. [Google Scholar] [CrossRef]
- Wood, T.P.; Duvenage, C.S.J.; Rohwer, E. The occurrence of anti-retroviral compounds used for HIV treatment in South African surface water. Environ. Pollut. 2015, 199, 235–243. [Google Scholar] [CrossRef]
- Lofrano, G.; Carotenuto, M.; Libralato, G.; Domingos, R.F.; Markus, A.; Dini, L.; Gautam, R.K.; Baldantoni, D.; Rossi, M.; Sharma, S.K.; et al. Polymer functionalized nanocomposites for metals removal from water and wastewater: An overview. Water Res. 2016, 92, 22–37. [Google Scholar] [CrossRef]
- Peng, X.; Wang, C.; Zhang, K.; Wang, Z.; Huang, Q.; Yu, Y.; Ou, W. Profile and behavior of antiviral drugs in aquatic environments of the Pearl River Delta, China. Sci. Total Environ. 2014, 466–467, 755–761. [Google Scholar] [CrossRef]
- Ncube, S.; Madikizela, L.M.; Chimuka, L.; Nindi, M.M. Environmental fate and ecotoxicological effects of antiretrovirals: A current global status and future perspectives. Water Res. 2018, 145, 231–247. [Google Scholar] [CrossRef]
- Mosekiemang, T.T.; Stander, M.A.; de Villiers, A.; Villiers, D.; Mosekiemang, T.T.; Stander, M.A. Simultaneous quantification of commonly prescribed antiretroviral drugs and their selected metabolites in aqueous environmental samples by direct injection and solid phase extraction liquid chromatography—Tandem mass spectrometry. Chemosphere 2019, 220, 983–992. [Google Scholar] [CrossRef] [PubMed]
- Sanderson, H.; Johnson, D.J.; Reitsma, T.; Brain, R.A.; Wilson, C.J.; Solomon, K.R. Ranking and prioritization of environmental risks of pharmaceuticals in surface waters. Regul. Toxicol. Pharmacol. 2004, 39, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Van Langenhove, H.; Abira, M.A.; Vergeynst, L.; Demeestere, K.; K’oreje, K.O.; Kandie, F.J.; Okoth, M. Occurrence, fate and removal of pharmaceuticals, personal care products and pesticides in wastewater stabilization ponds and receiving rivers in the Nzoia Basin, Kenya. Sci. Total Environ. 2018, 637–638, 336–348. [Google Scholar] [CrossRef]
- Kebede, T.G.; Seroto, M.B.; Chokwe, R.C.; Dube, S.; Nindi, M.M. Adsorption of antiretroviral (ARVs) and related drugs from environmental wastewaters using nanofibers. J. Environ. Chem. Eng. 2020, 8, 104049. [Google Scholar] [CrossRef]
- Afkhami, A.; Saber-Tehrani, M.; Bagheri, H. Simultaneous removal of heavy-metal ions in wastewater samples using nano-alumina modified with 2,4-dinitrophenylhydrazine. J. Hazard. Mater. 2010, 181, 836–844. [Google Scholar] [CrossRef]
- Hua, M.; Zhang, S.; Pan, B.; Zhang, W.; Lv, L.; Zhang, Q. Heavy metal removal from water/wastewater by nanosized metal oxides: A review. J. Hazard. Mater. 2012, 211–212, 317–331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Shen, Z.; Dai, C.; Zhou, X. Removal of selected pharmaceuticals from aqueous solution using magnetic chitosan: Sorption behavior and mechanism. Environ. Sci. Pollut. Res. 2014, 21, 12780–12789. [Google Scholar] [CrossRef]
- Kebede, T.G.; Dube, S.; Nindi, M.M. Biopolymer electrospun nanofibres for the adsorption of pharmaceuticals from water systems. J. Environ. Chem. Eng. 2019, 7, 103330. [Google Scholar] [CrossRef]
- Ren, X.; Chen, C.; Nagatsu, M.; Wang, X. Carbon nanotubes as adsorbents in environmental pollution management: A review. Chem. Eng. J. 2011, 170, 395–410. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Mehmood, S.; Tang, H.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Mitigation of environmentally-related hazardous pollutants from water matrices using nanostructured materials—A review. Chemosphere 2020, 253, 126770. [Google Scholar] [CrossRef]
- Bagbi, Y.; Pandey, A.; Solanki, P.R. Electrospun Nanofibrous Filtration Membranes for Heavy Metals and Dye Removal. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 275–288. [Google Scholar] [CrossRef]
- Cui, J.; Li, F.; Wang, Y.; Zhang, Q.; Ma, W.; Huang, C. Electrospun nanofiber membranes for wastewater treatment applications. Sep. Purif. Technol. 2020, 250, 117116. [Google Scholar] [CrossRef]
- Karnik, B.S.; Davies, S.H.; Baumann, M.J.; Masten, S.J. The effects of combined ozonation and filtration on disinfection by-product formation. Water Res. 2005, 39, 2839–2850. [Google Scholar] [CrossRef] [PubMed]
- Mlunguza, N.Y.; Ncube, S.; Nokwethemba Mahlambi, P.; Chimuka, L.; Madikizela, L.M. Adsorbents and removal strategies of non-steroidal anti-inflammatory drugs from contaminated water bodies. J. Environ. Chem. Eng. 2019, 7, 103142. [Google Scholar] [CrossRef]
- Ngumba, E.; Kosunen, P.; Gachanja, A.; Tuhkanen, T. A multiresidue analytical method for trace level determination of antibiotics and antiretroviral drugs in wastewater and surface water using SPE-LC-MS/MS and matrix-matched standards. Anal. Methods 2016, 8, 6720–6729. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multi-residue determination of 115 veterinary drugs and pharmaceutical residues in milk powder, butter, fish tissue and eggs using liquid chromatography-tandem mass spectrometry. Anal. Chim. Acta 2015, 880, 103–121. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, H.; Wu, B.; Ma, C.; Ye, Y. Sensitive determination of carbamates in fruit and vegetables by a combination of solid-phase extraction and dispersive liquid-liquid microextraction prior to HPLC. Microchim. Acta 2012, 176, 419–427. [Google Scholar] [CrossRef]
- Petrie, B.; Youdan, J.; Barden, R.; Kasprzyk-Hordern, B. Multi-residue analysis of 90 emerging contaminants in liquid and solid environmental matrices by ultra-high-performance liquid chromatography tandem mass spectrometry. J. Chromatogr. A 2016, 1431, 64–78. [Google Scholar] [CrossRef]
- Abafe, O.A.; Späth, J.; Fick, J.; Jansson, S.; Buckley, C.; Stark, A.; Pietruschka, B.; Martincigh, B.S. LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere 2018, 200, 660–670. [Google Scholar] [CrossRef]
- Afroze, S.; Sen, T.K. A Review on Heavy Metal Ions and Dye Adsorption from Water by Agricultural Solid Waste Adsorbents. Water Air Soil Pollut. 2018, 229, 225. [Google Scholar] [CrossRef]
- Abdullah, M.O.; Tan, I.A.W.; Lim, L.S. Automobile adsorption air-conditioning system using oil palm biomass-based activated carbon: A review. Renew. Sustain. Energy Rev. 2011, 15, 2061–2072. [Google Scholar] [CrossRef]
- Ezugbe, E.O.; Rathilal, S. Membrane technologies in wastewater treatment: A review. Membranes 2020, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Bazan-Wozniak, A.; Pietrzak, R. Adsorption of organic and inorganic pollutants on activated bio-carbons prepared by chemical activation of residues of supercritical extraction of raw plants. Chem. Eng. J. 2020, 393, 124785. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Das, S.; Mondal, M.K. Exhaustive studies on toxic Cr(VI) removal mechanism from aqueous solution using activated carbon of Aloe vera waste leaves. J. Mol. Liq. 2020, 307, 112956. [Google Scholar] [CrossRef]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Hussain, M.; Fino, D.; Russo, N. N2O decomposition by mesoporous silica supported Rh catalysts. J. Hazard. Mater. 2012, 211–212, 255–265. [Google Scholar] [CrossRef]
- Montes, D.; Tocuyo, E.; Gonzaĺez, E.; Rodriǵuez, D.; Solano, R.; Atencio, R.; Ramos, M.A.; Moronta, A. Reactive H2S chemisorption on mesoporous silica molecular sieve-supported CuO or ZnO. Microporous Mesoporous Mater. 2013, 168, 111–120. [Google Scholar] [CrossRef]
- Ali, Z.; Ahmad, R. Nanotechnology for Water Treatment. Environ. Nanotechnol. 2020, 3, 143–163. [Google Scholar] [CrossRef]
- Jaria, G.; Calisto, V.; Gil, M.V.; Otero, M.; Esteves, V.I. Removal of fluoxetine from water by adsorbent materials produced from paper mill sludge. J. Colloid Interface Sci. 2015, 448, 32–40. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Rachna Water purification by using Adsorbents: A Review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Krstić, V. Role of zeolite adsorbent in water treatment. In Handbook of Nanomaterials for Wastewater Treatment: Fundamentals and Scale Up Issues; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Iftekhar, S.; Ramasamy, D.L.; Srivastava, V.; Asif, M.B.; Sillanpää, M. Understanding the factors affecting the adsorption of Lanthanum using different adsorbents: A critical review. Chemosphere 2018, 204, 413–430. [Google Scholar] [CrossRef] [PubMed]
- Iftekhar, S.; Srivastava, V.; Sillanpää, M. Enrichment of lanthanides in aqueous system by cellulose based silica nanocomposite. Chem. Eng. J. 2017, 320, 151–159. [Google Scholar] [CrossRef]
- Akhtar, J.; Amin, N.A.S.; Shahzad, K. A review on removal of pharmaceuticals from water by adsorption. Desalin. Water Treat. 2016, 57, 12842–12860. [Google Scholar] [CrossRef]
- Oba, S.N.; Ighalo, J.O.; Aniagor, C.O.; Adaobi, C. Science of the Total Environment Removal of ibuprofen from aqueous media by adsorption: A comprehensive review. Sci. Total Environ. 2021, 780, 146608. [Google Scholar] [CrossRef]
- Bernal, V.; Giraldo, L. Physicochemical Properties of Activated Carbon: Their Effect on the Adsorption of Pharmaceutical Compounds and Adsorbate—Adsorbent Interactions. C 2018, 4, 62. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Anil, A.G.; Kapoor, D.; Khasnabis, S.; Shekar, S.; Pavithra, N.; Samuel, J.; Subramanian, S.; Singh, J.; et al. Adsorption and detoxification of pharmaceutical compounds from wastewater using nanomaterials: A review on mechanism, kinetics, valorization and circular economy. J. Environ. Manage. 2021, 300, 113569. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.H.; Huang, H.; Schwab, K. Effects of solution chemistry on the adsorption of ibuprofen and triclosan onto carbon nanotubes. Langmuir 2011, 27, 12960–12967. [Google Scholar] [CrossRef]
- Hasan, Z.; Khan, N.A.; Jhung, S.H. Adsorptive removal of diclofenac sodium from water with Zr-based metal–organic frameworks. Chem. Eng. J. 2016, 284, 1406–1413. [Google Scholar] [CrossRef]
- Rafati, L.; Ehrampoush, M.H.; Rafati, A.A.; Mokhtari, M.; Mahvi, A.H. Modeling of adsorption kinetic and equilibrium isotherms of naproxen onto functionalized nano-clay composite adsorbent. J. Mol. Liq. 2016, 224, 832–841. [Google Scholar] [CrossRef]
- Lotfi, R.; Hayati, B.; Rahimi, S.; Shekarchi, A.A.; Mahmoodi, N.M.; Bagheri, A. Synthesis and characterization of PAMAM/SiO2 nanohybrid as a new promising adsorbent for pharmaceuticals. Microchem. J. 2019, 146, 1150–1159. [Google Scholar] [CrossRef]
- Mondal, S.; Patel, S.; Majumder, S.K. Naproxen Removal Capacity Enhancement by Transforming the Activated Carbon into a Blended Composite Material. Water. Air. Soil Pollut. 2020, 231, 37. [Google Scholar] [CrossRef]
- Cai, Z.; Dwivedi, A.D.; Lee, W.N.; Zhao, X.; Liu, W.; Sillanpää, M.; Zhao, D.; Huang, C.H.; Fu, J. Application of nanotechnologies for removing pharmaceutically active compounds from water: Development and future trends. Environ. Sci. Nano 2018, 5, 27–47. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Y.; Zhang, L.; Huang, H.; Hu, J.; Shah, S.M.; Su, X. Adsorption and removal of tetracycline antibiotics from aqueous solution by graphene oxide. J. Colloid Interface Sci. 2012, 368, 540–546. [Google Scholar] [CrossRef]
- Chen, H.; Gao, B.; Li, H. Removal of sulfamethoxazole and ciprofloxacin from aqueous solutions by graphene oxide. J. Hazard. Mater. 2015, 282, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Liu, X.; Cao, Z.; Zhan, Y.; Shi, X.; Yang, Y.; Zhou, J.; Xu, J. Adsorption behavior and mechanism of chloramphenicols, sulfonamides, and non-antibiotic pharmaceuticals on multi-walled carbon nanotubes. J. Hazard. Mater. 2016, 310, 235–245. [Google Scholar] [CrossRef]
- Feng, X.; Qiu, B.; Sun, D. Enhanced naproxen adsorption by a novel β-cyclodextrin immobilized the three-dimensional macrostructure of reduced graphene oxide and multiwall carbon nanotubes. Sep. Purif. Technol. 2022, 290, 120837. [Google Scholar] [CrossRef]
- Yi, Y.; Huang, Z.; Lu, B.; Xian, J.; Tsang, E.P.; Cheng, W.; Fang, J.; Fang, Z. Magnetic biochar for environmental remediation: A review. Bioresour. Technol. 2020, 298, 122468. [Google Scholar] [CrossRef]
- Ai, T.; Jiang, X.; Zhong, Z.; Li, D.; Dai, S. Methanol-modified ultra-fine magnetic orange peel powder biochar as an effective adsorbent for removal of ibuprofen and sulfamethoxazole from water. Adsorpt. Sci. Technol. 2020, 38, 304–321. [Google Scholar] [CrossRef]
- Shan, D.; Deng, S.; Zhao, T.; Wang, B.; Wang, Y.; Huang, J.; Yu, G.; Winglee, J.; Wiesner, M.R. Preparation of ultrafine magnetic biochar and activated carbon for pharmaceutical adsorption and subsequent degradation by ball milling. J. Hazard. Mater. 2016, 305, 156–163. [Google Scholar] [CrossRef]
- Liyanage, A.S.; Canaday, S.; Pittman, C.U.; Mlsna, T. Rapid remediation of pharmaceuticals from wastewater using magnetic Fe3O4/Douglas fir biochar adsorbents. Chemosphere 2020, 258, 127336. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Ngo, H.H.; Guo, W.; Huo, J.; Du, Q.; Zhang, Y.; Li, C.; Yang, F. Sorptive removal of ibuprofen from water by natural porous biochar derived from recyclable plane tree leaf waste. J. Water Process Eng. 2022, 46, 102627. [Google Scholar] [CrossRef]
- Divya, K.; Jisha, M.S. Chitosan nanoparticles preparation and applications. Environ. Chem. Lett. 2018, 16, 101–112. [Google Scholar] [CrossRef]
- Liu, F.F.; Zhao, J.; Wang, S.; Du, P.; Xing, B. Effects of solution chemistry on adsorption of selected pharmaceuticals and personal care products (PPCPs) by graphenes and carbon nanotubes. Environ. Sci. Technol. 2014, 48, 13197–13206. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Wang, R.; Zhang, S.; Li, G.; Zhang, Y. Treatment of wastewater containing oil using phosphorylated silica nanotubes (PSNTs)/polyvinylidene fluoride (PVDF) composite membrane. Desalination 2014, 332, 109–116. [Google Scholar] [CrossRef]
- Natarajan, R.; Saikia, K.; Ponnusamy, S.K.; Rathankumar, A.K.; Rajendran, D.S.; Venkataraman, S.; Tannani, D.B.; Arvind, V.; Somanna, T.; Banerjee, K.; et al. Understanding the factors affecting adsorption of pharmaceuticals on different adsorbents—A critical literature update. Chemosphere 2022, 287, 131958. [Google Scholar] [CrossRef]
- Bindhu, M.R.; Umadevi, M. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy Silver and gold nanoparticles for sensor and antibacterial applications. Spectroscopy 2014, 128, 37–45. [Google Scholar] [CrossRef]
- Yu, Y.; Yu, L.; Koh, K.Y.; Wang, C.; Chen, J.P. Rare-earth metal based adsorbents for effective removal of arsenic from water: A critical review. Crit. Rev. Environ. Sci. Technol. 2018, 48, 1127–1164. [Google Scholar] [CrossRef]
- Jun, B.M.; Heo, J.; Park, C.M.; Yoon, Y. Comprehensive evaluation of the removal mechanism of carbamazepine and ibuprofen by metal organic framework. Chemosphere 2019, 235, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; He, S.; Shi, G.; Ma, Y.; Ruan, C.; Jin, X.; Chen, Q.; Liu, X.; Dai, H.; Chen, X.; et al. In-situ immobilization of ZIF-67 on wood aerogel for effective removal of tetracycline from water. Chem. Eng. J. 2021, 423, 130184. [Google Scholar] [CrossRef]
- Zeb, S.; Ali, N.; Ali, Z.; Bilal, M.; Adalat, B.; Hussain, S.; Gul, S.; Ali, F.; Ahmad, R.; Khan, S.; et al. Silica-based nanomaterials as designer adsorbents to mitigate emerging organic contaminants from water matrices. J. Water Process Eng. 2020, 38, 101675. [Google Scholar] [CrossRef]
- Hartmann, M. Ordered mesoporous materials for bioadsorption and biocatalysis. Chem. Mater. 2005, 17, 4577–4593. [Google Scholar] [CrossRef]
- Bui, T.X.; Choi, H. Adsorptive removal of selected pharmaceuticals by mesoporous silica SBA-15. J. Hazard. Mater. 2009, 168, 602–608. [Google Scholar] [CrossRef]
- Natarajan, R.; Banerjee, K.; Kumar, P.S.; Somanna, T.; Tannani, D.; Arvind, V.; Raj, R.I.; Vo, D.V.N.; Saikia, K.; Vaidyanathan, V.K. Performance study on adsorptive removal of acetaminophen from wastewater using silica microspheres: Kinetic and isotherm studies. Chemosphere 2021, 272, 129896. [Google Scholar] [CrossRef]
- Lokhande, R.; Gedam, B.; Shah, Y.; Tandon, M.; Bansod, P.Y. Rationale for near total thyroidectomy in patients with nodular goitre. Int. J. Biomed. Adv. Res. 2015, 6, 427–430. [Google Scholar] [CrossRef]
- Saeed, K.; Haider, S.; Oh, T.J.; Park, S.Y. Preparation of amidoxime-modified polyacrylonitrile (PAN-oxime) nanofibers and their applications to metal ions adsorption. J. Memb. Sci. 2008, 322, 400–405. [Google Scholar] [CrossRef]
- Zhu, F.; Zheng, Y.M.; Zhang, B.G.; Dai, Y.R. A critical review on the electrospun nanofibrous membranes for the adsorption of heavy metals in water treatment. J. Hazard. Mater. 2021, 401, 123608. [Google Scholar] [CrossRef] [PubMed]
- Shabafrooz, V.; Mozafari, M.; Vashaee, D.; Tayebi, L. Electrospun nanofibers: From filtration membranes to highly specialized tissue engineering scaffolds. J. Nanosci. Nanotechnol. 2014, 14, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Haghi, A.K.; Akbari, M. Trends in electrospinning of natural nanofibers. Phys. Status Solidi Appl. Mater. Sci. 2007, 204, 1830–1834. [Google Scholar] [CrossRef]
- Camiré, A.; Espinasse, J.; Chabot, B.; Lajeunesse, A. Development of electrospun lignin nanofibers for the adsorption of pharmaceutical contaminants in wastewater. Environ. Sci. Pollut. Res. 2020, 27, 3560–3573. [Google Scholar] [CrossRef]
- Mozzaquatro, S.; Val, A.; Yin, G.; Wang, J.; Selene, M.; De Souza, A.G.U.; Hotza, D. An overview on nanostructured TiO2—Containing fibers for photocatalytic degradation of organic pollutants in wastewater treatment. J. Water Process Eng. 2021, 40, 101827. [Google Scholar] [CrossRef]
- Li, L.; Iqbal, J.; Zhu, Y.; Wang, F.; Zhang, F.; Chen, W.; Wu, T.; Du, Y. Chitosan/Al2O3-HA nanocomposite beads for efficient removal of estradiol and chrysoidin from aqueous solution. Int. J. Biol. Macromol. 2020, 145, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Huang, W.; Zhu, H.; Xiong, J.; Bei, H.; Wei, X.; Wang, S. Modified TiO2 particles for heterogeneous photocatalysis under solar irradiation. Mater. Lett. 2020, 279, 128472. [Google Scholar] [CrossRef]
- Khanna, P.K.; Singh, N.; Charan, S. Synthesis of nano-particles of anatase-TiO2 and preparation of its optically transparent film in PVA. Mater. Lett. 2007, 61, 4725–4730. [Google Scholar] [CrossRef]
- Sze, S.; Chiang, K.; Gordon, A. The stability of polymeric membranes in a TiO2 photocatalysis process. J. Membr. Sci. 2006, 275, 202–211. [Google Scholar] [CrossRef]
- Luo, M.; Tang, W.; Zhao, J.; Pu, C. Hydrophilic modification of poly (ether sulfone) used TiO2 nanoparticles by a sol—Gel process. J. Mater. Process. Technol. 2006, 172, 431–436. [Google Scholar] [CrossRef]
- Song, M.; Cao, H.; Zhu, Y.; Wang, Y.; Zhao, S.; Huang, C. Electrochemical and photocatalytic properties of electrospun C/TiO2 nanofibers. Chem. Phys. Lett. 2020, 747, 137355. [Google Scholar] [CrossRef]
- Kolbasov, A.; Sinha-ray, S.; Yarin, A.L.; Pourdeyhimi, B. Heavy metal adsorption on solution-blown biopolymer nano fi ber membranes. J. Memb. Sci. 2017, 530, 250–263. [Google Scholar] [CrossRef]
- An, S.; Joshi, B.N.; Lee, M.W.; Kim, N.Y.; Yoon, S.S. Applied Surface Science Electrospun graphene-ZnO nanofiber mats for photocatalysis applications. Appl. Surf. Sci. 2014, 294, 24–28. [Google Scholar] [CrossRef]
- Li, J.; Ma, L.Y.; Li, L.S.; Xu, L. Photodegradation kinetics, transformation, and toxicity prediction of ketoprofen, carprofen, and diclofenac acid in aqueous solutions. Environ. Toxicol. Chem. 2017, 36, 3232–3239. [Google Scholar] [CrossRef]
- Filep, T.; Szabó, L.; Kondor, A.C.; Jakab, G.; Szalai, Z. Evaluation of the effect of the intrinsic chemical properties of pharmaceutically active compounds (PhACs) on sorption behaviour in soils and goethite. Ecotoxicol. Environ. Saf. 2021, 215, 112120. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, M.; Duan, C.; Sun, J.; Xu, Y. Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr. Polym. 2019, 206, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Carmosini, N.; Lee, L.S. Ciprofloxacin sorption by dissolved organic carbon from reference and bio-waste materials. Chemosphere 2009, 77, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Almeida, B.; Oehmen, A.; Marques, R.; Brito, D.; Carvalho, G.; Barreto Crespo, M.T. Modelling the biodegradation of non-steroidal anti-inflammatory drugs (NSAIDs) by activated sludge and a pure culture. Bioresour. Technol. 2013, 133, 31–37. [Google Scholar] [CrossRef]
- Feng, Z.; Odelius, K.; Rajarao, G.K.; Hakkarainen, M. Microwave carbonized cellulose for trace pharmaceutical adsorption. Chem. Eng. J. 2018, 346, 557–566. [Google Scholar] [CrossRef]
- Lung, I.; Soran, M.L.; Stegarescu, A.; Opris, O.; Gutoiu, S.; Leostean, C.; Lazar, M.D.; Kacso, I.; Silipas, T.D.; Porav, A.S. Evaluation of CNT-COOH/MnO2/Fe3O4 nanocomposite for ibuprofen and paracetamol removal from aqueous solutions. J. Hazard. Mater. 2021, 403, 123528. [Google Scholar] [CrossRef]
- Vicente-Martínez, Y.; Caravaca, M.; Soto-Meca, A. Determination of very low concentration of bisphenol A in toys and baby pacifiers using dispersive liquid–liquid microextraction by in situ ionic liquid formation and high-performance liquid chromatography. Pharmaceuticals 2020, 13, 301. [Google Scholar] [CrossRef]
- Chandrashekar Kollarahithlu, S.; Balakrishnan, R.M. Adsorption of pharmaceuticals pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the aqueous phase using amine functionalized superparamagnetic silica nanocomposite. J. Clean. Prod. 2021, 294, 126155. [Google Scholar] [CrossRef]
- Leone, V.O.; Pereira, M.C.; Aquino, S.F.; Oliveira, L.C.A.; Correa, S.; Ramalho, T.C.; Gurgel, L.V.A.; Silva, A.C. Adsorption of diclofenac on a magnetic adsorbent based on maghemite: Experimental and theoretical studies. New J. Chem. 2018, 42, 437–449. [Google Scholar] [CrossRef]
- Nadolny, B.; Heineck, R.G.; Bazani, H.A.G.; Hemmer, J.V.; Biavatti, M.L.; Radetski, C.M.; Almerindo, G.I. Use of brewing industry waste to produce carbon-based adsorbents: Paracetamol adsorption study. J. Environ. Sci. Health Part A 2020, 55, 947–956. [Google Scholar] [CrossRef]
- Chen, C.; Wang, P.; Lim, T.T.; Liu, L.; Liu, S.; Xu, R. A facile synthesis of monodispersed hierarchical layered double hydroxide on silica spheres for efficient removal of pharmaceuticals from water. J. Mater. Chem. A 2013, 1, 3877–3880. [Google Scholar] [CrossRef]
- Alnajjar, M.; Hethnawi, A.; Nafie, G.; Hassan, A.; Vitale, G.; Nassar, N.N. Silica-alumina composite as an effective adsorbent for the removal of metformin from water. J. Environ. Chem. Eng. 2019, 7, 102994. [Google Scholar] [CrossRef]
- Crini, G.; Badot, P.M. Application of chitosan, a natural aminopolysaccharide, for dye removal from aqueous solutions by adsorption processes using batch studies: A review of recent literature. Prog. Polym. Sci. 2008, 33, 399–447. [Google Scholar] [CrossRef]
- Adeola, A.O.; de Lange, J.; Forbes, P.B.C. Adsorption of antiretroviral drugs, efavirenz and nevirapine from aqueous solution by graphene wool: Kinetic, equilibrium, thermodynamic and computational studies. Appl. Surf. Sci. Adv. 2021, 6, 100157. [Google Scholar] [CrossRef]
- de Souza dos Santos, G.E.; Ide, A.H.; Duarte, J.L.S.; McKay, G.; Silva, A.O.S.; Meili, L. Adsorption of anti-inflammatory drug diclofenac by MgAl/layered double hydroxide supported on Syagrus coronata biochar. Powder Technol. 2020, 364, 229–240. [Google Scholar] [CrossRef]
- Hossein Beyki, M.; Mohammadirad, M.; Shemirani, F.; Saboury, A.A. Magnetic cellulose ionomer/layered double hydroxide: An efficient anion exchange platform with enhanced diclofenac adsorption property. Carbohydr. Polym. 2017, 157, 438–446. [Google Scholar] [CrossRef]
- Tran, T.V.; Nguyen, D.T.C.; Le, H.T.N.; Vo, D.V.N.; Nanda, S.; Nguyen, T.D. Optimization, equilibrium, adsorption behavior and role of surface functional groups on graphene oxide-based nanocomposite towards diclofenac drug. J. Environ. Sci. 2020, 93, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Guerra, A.C.S.; de Andrade, M.B.; Tonial dos Santos, T.R.; Bergamasco, R. Adsorption of sodium diclofenac in aqueous medium using graphene oxide nanosheets. Environ. Technol. 2021, 42, 2599–2609. [Google Scholar] [CrossRef]
- Khalil, A.M.E.; Memon, F.A.; Tabish, T.A.; Salmon, D.; Zhang, S.; Butler, D. Nanostructured porous graphene for efficient removal of emerging contaminants (pharmaceuticals) from water. Chem. Eng. J. 2020, 398, 125440. [Google Scholar] [CrossRef]
- Hiew, B.Y.Z.; Lee, L.Y.; Lai, K.C.; Gan, S.; Thangalazhy-Gopakumar, S.; Pan, G.T.; Yang, T.C.K. Adsorptive decontamination of diclofenac by three-dimensional graphene-based adsorbent: Response surface methodology, adsorption equilibrium, kinetic and thermodynamic studies. Environ. Res. 2019, 168, 241–253. [Google Scholar] [CrossRef]
- Li, L.; Zhao, N.; Wei, W.; Sun, Y. A review of research progress on CO2 capture, storage, and utilization in Chinese Academy of Sciences. Fuel 2013, 108, 112–130. [Google Scholar] [CrossRef]
- Munoz, M.; Nieto-Sandoval, J.; Álvarez-Torrellas, S.; Sanz-Santos, E.; Calderón, B.; de Pedro, Z.M.; Larriba, M.; Fullana, A.; García, J.; Casas, J.A. Carbon-encapsulated iron nanoparticles as reusable adsorbents for micropollutants removal from water. Sep. Purif. Technol. 2021, 257, 117974. [Google Scholar] [CrossRef]
- Shahnaz, T.; Vishnu Priyan, V.; Pandian, S.; Narayanasamy, S. Use of Nanocellulose extracted from grass for adsorption abatement of Ciprofloxacin and Diclofenac removal with phyto, and fish toxicity studies. Environ. Pollut. 2021, 268, 115494. [Google Scholar] [CrossRef] [PubMed]
- Ighalo, J.O.; Ajala, O.J.; Umenweke, G.; Ogunniyi, S.; Adeyanju, C.A.; Igwegbe, C.A.; Adeniyi, A.G. Mitigation of clofibric acid pollution by adsorption: A review of recent developments. J. Environ. Chem. Eng. 2020, 8, 104264. [Google Scholar] [CrossRef]
- Lawal, I.A.; Lawal, M.M.; Akpotu, S.O.; Azeez, M.A.; Ndungu, P.; Moodley, B. Theoretical and experimental adsorption studies of sulfamethoxazole and ketoprofen on synthesized ionic liquids modified CNTs. Ecotoxicol. Environ. Saf. 2018, 161, 542–552. [Google Scholar] [CrossRef]
- Hoppen, M.I.; Carvalho, K.Q.; Ferreira, R.C.; Passig, F.H.; Pereira, I.C.; Rizzo-Domingues, R.C.P.; Lenzi, M.K.; Bottini, R.C.R. Adsorption and desorption of acetylsalicylic acid onto activated carbon of babassu coconut mesocarp. J. Environ. Chem. Eng. 2019, 7, 102862. [Google Scholar] [CrossRef]
- Rafati, L.; Ehrampoush, M.H.; Rafati, A.A.; Mokhtari, M.; Mahvi, A.H. Removal of ibuprofen from aqueous solution by functionalized strong nano-clay composite adsorbent: Kinetic and equilibrium isotherm studies. Int. J. Environ. Sci. Technol. 2018, 15, 513–524. [Google Scholar] [CrossRef]
- Moacǎ, E.A.; Mihali, C.V.; Macaşoi, I.G.; Racoviceanu, R.; Şoica, C.; Dehelean, C.A.; Pǎcurariu, C.; Florescu, S. Fe3O4@C matrix with tailorable adsorption capacities for paracetamol and acetylsalicylic acid: Synthesis, characterization, and kinetic modeling. Molecules 2019, 24, 1727. [Google Scholar] [CrossRef]
- ALOthman, Z.A.; Badjah, A.Y.; Alharbi, O.M.L.; Ali, I. Synthesis of chitosan composite iron nanoparticles for removal of diclofenac sodium drug residue in water. Int. J. Biol. Macromol. 2020, 159, 870–876. [Google Scholar] [CrossRef]
- Ameri, A.; Shakibaie, M.; Pournamdari, M.; Ameri, A.; Foroutanfar, A.; Doostmohammadi, M.; Forootanfar, H. Degradation of diclofenac sodium using UV/biogenic selenium nanoparticles/H2O2: Optimization of process parameters. J. Photochem. Photobiol. A Chem. 2020, 392, 112382. [Google Scholar] [CrossRef]
- Ye, X.; Li, Y.; Lin, H.; Chen, Y.; Liu, M. Lignin-Based Magnetic Nanoparticle Adsorbent for Diclofenac Sodium Removal: Adsorption Behavior and Mechanisms. J. Polym. Environ. 2021, 29, 3401–3411. [Google Scholar] [CrossRef]
- Teo, H.T.; Siah, W.R.; Yuliati, L. Enhanced adsorption of acetylsalicylic acid over hydrothermally synthesized iron oxide-mesoporous silica MCM-41 composites. J. Taiwan Inst. Chem. Eng. 2016, 65, 591–598. [Google Scholar] [CrossRef]
- Kamarudin, N.H.N.; Jalil, A.A.; Triwahyono, S.; Sazegar, M.R.; Hamdan, S.; Baba, S.; Ahmad, A. Elucidation of acid strength effect on ibuprofen adsorption and release by aluminated mesoporous silica nanoparticles. RSC Adv. 2015, 5, 30023–30031. [Google Scholar] [CrossRef]
- Kumar, S.; Krishnakumar, B.; Sobral, A.J.F.N.; Koh, J. Bio-based (chitosan/PVA/ZnO) nanocomposites film: Thermally stable and photoluminescence material for removal of organic dye. Carbohydr. Polym. 2019, 205, 559–564. [Google Scholar] [CrossRef]
- Tian, H.; Yuan, L.; Wang, J.; Wu, H.; Wang, H.; Xiang, A.; Ashok, B.; Rajulu, A.V. Electrospinning of polyvinyl alcohol into crosslinked nanofibers: An approach to fabricate functional adsorbent for heavy metals. J. Hazard. Mater. 2019, 378, 120751. [Google Scholar] [CrossRef]
- Subramanian, S.; Seeram, R. New directions in nanofiltration applications—Are nanofibers the right materials as membranes in desalination? Desalination 2013, 308, 198–208. [Google Scholar] [CrossRef]



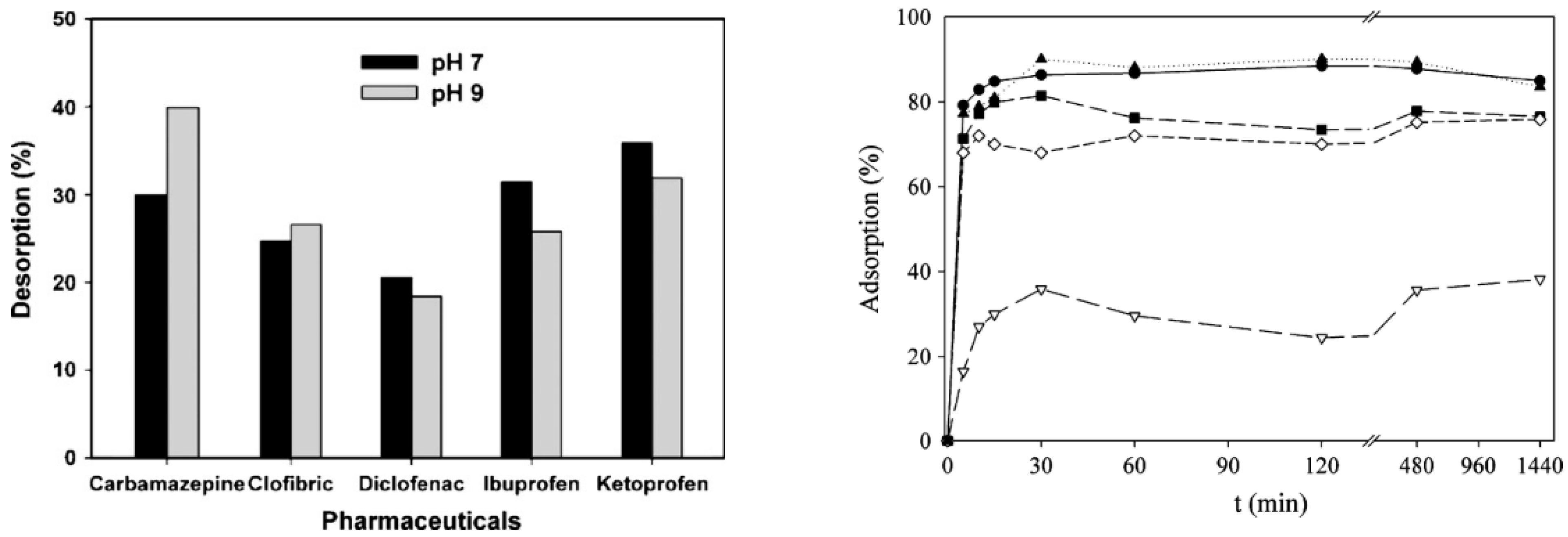
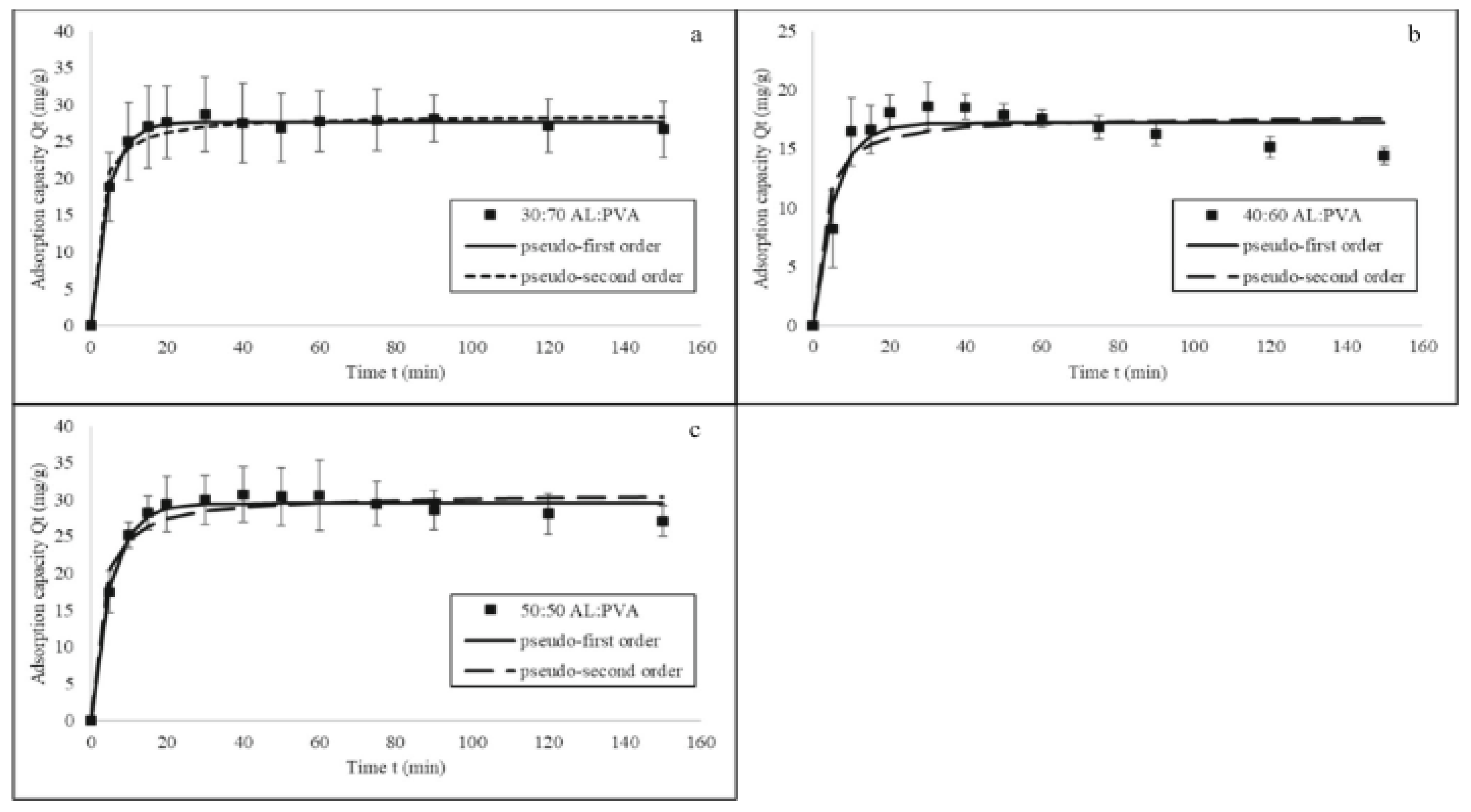


| Name of Pharmaceuticals | Chemical Formula | Molecular Weight (g/mol) | Water Solubility (mg/L) | pKa | Kd | logKow |
|---|---|---|---|---|---|---|
| NSAIDs | ||||||
| Diclofenac | C14H10Cl2NNaO2 | 318.1 | 4.8 | 4.15 | 0.2 | 4.51 |
| Ibuprofen | C13H18O2 | 206.29 | 21 | 4.91 | 3.7 × 10−7 | 3.97 |
| Naproxen | C14H14O3 | 230.26 | 29.9 | 4.15 | - | 3.18 |
| Ketoprofen | C16H14O3 | 254.28 | 5 | 4.45 | 15 | 3.12 |
| ARVs | ||||||
| Efavirenz | C14H9ClF3NO2 | 315.6 | 0.093 | 10.2/12.52 | - | 4.7 |
| Emtricitabine | C8H10FN3O3S | 247.25 | 1.12 × 106 | 2.65 | - | −0.43 |
| Nevaripine | C15H14N4O | 266.29 | 0.705 | 2.80 | - | 3.89 |
| Adsorbent | Adsorbate | Preparation Method | Particle Size (nm) | Surface Area (m2/g) | pH | Initial Conc. (mg/L) | Temp (K) | Adsorbent Dosage (mg) | %Removal | Adsorption Capacity (mg/g) | Adsorption Order | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biochar-based adsorbents | ||||||||||||
| MgAl Layered double hydroxide | Diclofenac | Co-precipitation | 10.21–0.589 | 212.89 | 5.6 | 200 | 333 | - | 82 | 168 | Psuedo Second order PSO | [147] |
| CaAl-LDH | Diclofenac | Precipitation | 50 | 30.6 | 9 | 0.5–200 | 298 | 10 | 87.1 | 268 | PSO | [148] |
| Graphene oxide nanocomposite | Diclofenac | Acid functionalization of graphene | 14.5 | 239.5 | 4 | 10 | 298 | 450 | 86.1 | 18.4–32.4 | PSO | [149] |
| Graphene oxide nanosorbent | Diclofenac | Acid functionalization of graphene | 2000 | - | 6.2 | 250 | 298 | 10 | 74 | 128.74 | PSO | [150] |
| Nanostructured porous graphene | Diclofenac | Chemical oxidative thermal treatment | 1.5 | 670 | 7.5 | 100 | 298 | 250 | 99 | 13.08 | PSO | [151] |
| 3D reduced graphene oxide aerogel | Diclofenac | Acid functionalization of graphene | 2–4 | 132.19 | 6 | 250 | 298 | 250 | 32.5 | 596.71 | PSO | [152] |
| Magnetic Fe3O4 Douglas fir biochar | Ibuprofen | Magnetization method | 1.32 | 322 | 8 | 100 | 308 | 2500 | 91 | 149.9–4.5 | PSO | [103] |
| Cellulosic sisal nanoparticles | Ibuprofen | Chemical oxidative polymerization | 1000 | High | 5 | 30 | 313 | 150 | 83 | 19.45 | PSO | |
| Carbon-based nanomaterial | ||||||||||||
| Al2O3 CNTs | Diclofenac | Sol-gel | 60–100 | 237 | 7 | 50 | 298 | 1.78 | 64.2 | 0.1065 | PSO | [153] |
| Granular 450 CNTs | Diclofenac | Heating filtration method | 1.1 | 288 | 6 | 30 | 298 | 15 | 92.95 | 369.5 | PSO | [102] |
| Carbon encapsulated iron nanoparticle | Diclofenac | Magnetic stirring and solvent extraction | 40 | 169 | 10 | 100 | 298 | 300 | 95 | 24 | PSO | [154] |
| Grass nanocellulose | Diclofenac | Cyprus rotundas extracted by hydrogen peroxide | 40.50 | high | 8 | 250 | 298 | 10 | 89 | 192.37 | PSO | [155] |
| Carbon nanosphere | Diclofenac | Microwave carbonization of cellulose | 7 | - | 5.98 | 10 | 298 | 1 | 59 | 27.3 | PSO | [137] |
| SWCNT | Ibuprofen | Chemical vapour deposition method | 5 | 1020 | 7 | 2 | 298 | 0.1 | - | 231.5 | PSO | [89] |
| O-MWCNT | 5 ± 15 | |||||||||||
| MWCNT | 5 ± 15 | |||||||||||
| Ordered mesoporous AC | Ibuprofen | Soft templating method | 2–50 | 670 | 6 | 100 | 298 | 10 | 21 | 120.1 | PSO | [39] |
| Ordered mesoporous AC | Ibuprofen | Impregnation method | 2–50 | 670 | 9 | - | 303 | 35 | - | 9.74 | PSO | [156] |
| 1-benzyl-3-hexadecyl imidazolium | ketoprofen | Solvent extraction ultrasonification and precipitation | 1 | 170.86 | 5 | 50 | 298 | 0.02 | 85 | 50 | PSO | [157] |
| Surface modification AC | Naproxen | Chemical activation | 26.66 | - | 4.4 | 70 | 298 | 20 | 61.99 | 159.8 | PSO | [93] |
| Brewed industry AC | Paracetamol | ASTM E 1756-01 method | 10µm | 364 | 5 | 200 | 298 | 750 | 98.3 | 29.45 | Pseudo First Order PFO | [142] |
| AC babbassu activated mesocarp | Acetylsalicylic acid | Tobasa Agro industry supply | 2 ± 0.05 | 951 | 5 | 200 | 298.15 | 100 | 84.66 | 89.87 | PSO | [158] |
| Chitosan-based adsorbents | ||||||||||||
| Genipin-crosslinked chitosan/graphene oxide SO3H composite | Ketoprofen | Covalent attachment | 80–90 | - | 6.6 | 15 | 298 | 50 | 88.5 | 113.27–138.4 | PSO | [159] |
| Metal-based adsorbents | ||||||||||||
| Fe3O4@C Method | Acetylsalicylic acid | Solution combustion method | 30 | high | 3 | 100 | 298 | - | 80–98 | 234.02 | PSO | [160] |
| UiO66 18% SO3H UiO66 NH2 UiO66 | Diclofenac | Zirconium inorganic building brick | 3–4 | 1082 | 5.4 | 20 | 298 | 1 | 59 | 189 | PSO | [90] |
| 902 | 263 | |||||||||||
| 910 | 106 | |||||||||||
| Composite iron nanoparticle | Diclofenac | Epichlorohydrin as cross-linker and chitosan | 15.8–90.4 | - | 5 | 0.002 | 298 | 0.4 | 85 | - | PFO | [161] |
| Biogenic selenium nanocomposite | Diclofenac | Produced from S. griseobunnues | 73.8 | - | 5.5 | 32 | 298 | 5 | 97.93 | - | PFO | [162] |
| Lignin-based magnetic nanoparticles | Diclofenac | Magnetic agitation and condensation | 0.02 | 739.2 | 5 | 10 | 298 | 5 | - | 106.4 | PSO | [163] |
| Silica-based adsorbent | ||||||||||||
| Iron oxide mesoporous silica MCM 41 Composite | Acetylsalicylic acid | Impregnation method | 100 | 721 | 6 | 500 | 300 | 200 | - | 1.98–3.7 | PSO | [164] |
| Polyamidoamine silica | Diclofenac | Soxhlet extraction | 32 | 7.2 | 9 | 1 | 298 | 1 | 93 | 134 | PSO | [92] |
| Ibuprofen | 98 | 124 | ||||||||||
| Ketoprofen | 94 | 112 | ||||||||||
| Mesoporous silica nanoparticles | Ibuprofen | Wet impregnation method | 60–80 | 1107 | 7 | 8 | 298 | 6.38 | 79 | - | PSO | [165] |
| Nanofibrous adsorbents | ||||||||||||
| MW/PVA blends nanofibers | Didanosine | Electrospun Mondia Whitei root extract | 0.022 | 1754 ± 0.62 | 7 | 0.5 | 303 | 40 | 72.5 | 109.8 | PSO | [55] |
| Sulfamethoxan | 0.003 | 146.4 | 110.17 | |||||||||
| Lidocain | 0.005 | 65.4 | 182.85 | |||||||||
| Nevirapne | 0.008 | 189.1 | 263.4 | |||||||||
| Prednisolone | 0.001 | 74.0 | 156.6 | |||||||||
| Isoniazid | 0.002 | 154.2 | 131.6 | |||||||||
| Dexamethosane | 0.004 | 81.9 | 144.8 | |||||||||
| Rifampin | 0.005 | 75.1 | 131.8 | |||||||||
| Ritonavir | 0.002 | 94.57 | 79.35 | |||||||||
| Efavirenz | 0.001 | 72.8 | 184.9 | |||||||||
| Fluconazole | 0.009 | 137.7 | 202.6 | |||||||||
| Stavodine | 0.007 | 150.2 | 188.33 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sigonya, S.; Mokhothu, T.H.; Mokhena, T.C.; Makhanya, T.R. Mitigation of Non-Steroidal Anti-Inflammatory and Antiretroviral Drugs as Environmental Pollutants by Adsorption Using Nanomaterials as Viable Solution—A Critical Review. Appl. Sci. 2023, 13, 772. https://doi.org/10.3390/app13020772
Sigonya S, Mokhothu TH, Mokhena TC, Makhanya TR. Mitigation of Non-Steroidal Anti-Inflammatory and Antiretroviral Drugs as Environmental Pollutants by Adsorption Using Nanomaterials as Viable Solution—A Critical Review. Applied Sciences. 2023; 13(2):772. https://doi.org/10.3390/app13020772
Chicago/Turabian StyleSigonya, Sisonke, Thabang Hendrica Mokhothu, Teboho Clement Mokhena, and Talent Raymond Makhanya. 2023. "Mitigation of Non-Steroidal Anti-Inflammatory and Antiretroviral Drugs as Environmental Pollutants by Adsorption Using Nanomaterials as Viable Solution—A Critical Review" Applied Sciences 13, no. 2: 772. https://doi.org/10.3390/app13020772
APA StyleSigonya, S., Mokhothu, T. H., Mokhena, T. C., & Makhanya, T. R. (2023). Mitigation of Non-Steroidal Anti-Inflammatory and Antiretroviral Drugs as Environmental Pollutants by Adsorption Using Nanomaterials as Viable Solution—A Critical Review. Applied Sciences, 13(2), 772. https://doi.org/10.3390/app13020772









