The Use of Coagulation–Flocculation for Industrial Colored Wastewater Treatment—(I) The Application of Hybrid Materials
Abstract
:Featured Application
Abstract
1. Introduction
2. Principal Textile Colored WW Characteristics and Composition
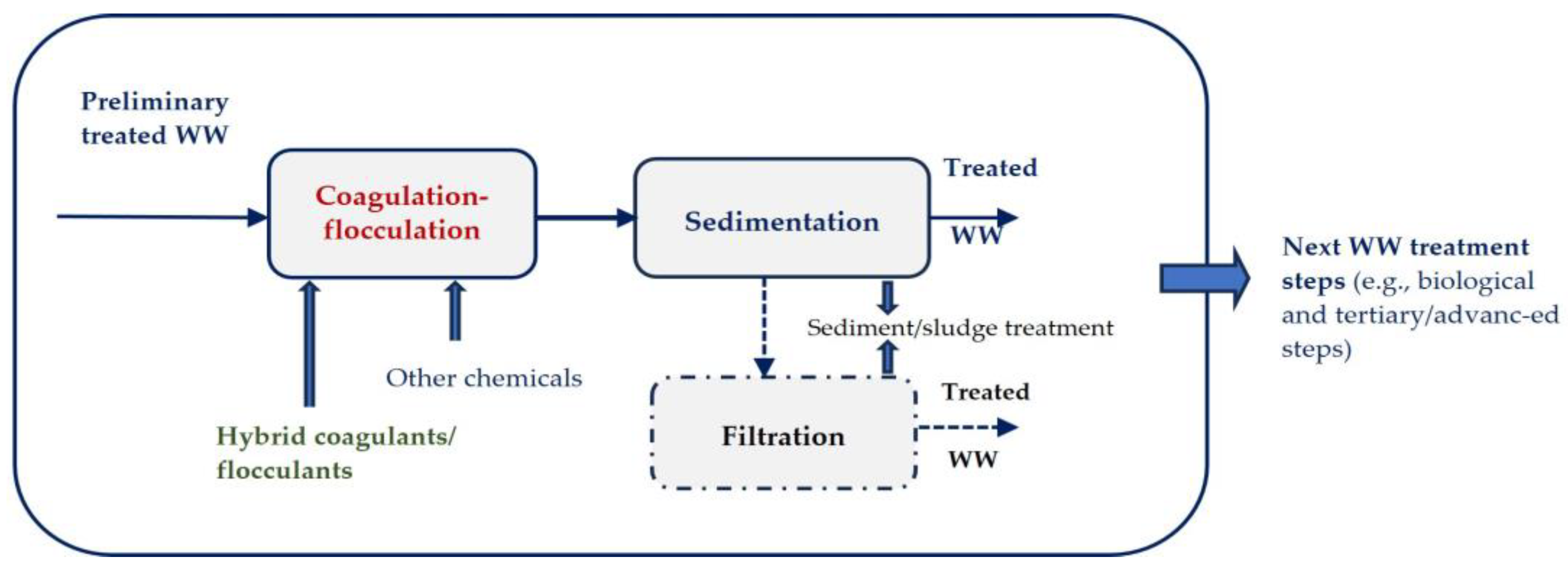
3. Textile WW Treatment Processes for Dye(s) and Color Removal—Chemical Coagulation–Flocculation Technologies and Their Performance
3.1. Textile WW Treatment Processes for Dye(s) and Color Removal
3.2. Coagulation–Flocculation Technologies and Its Performances in Colored Textile WWs Treatment
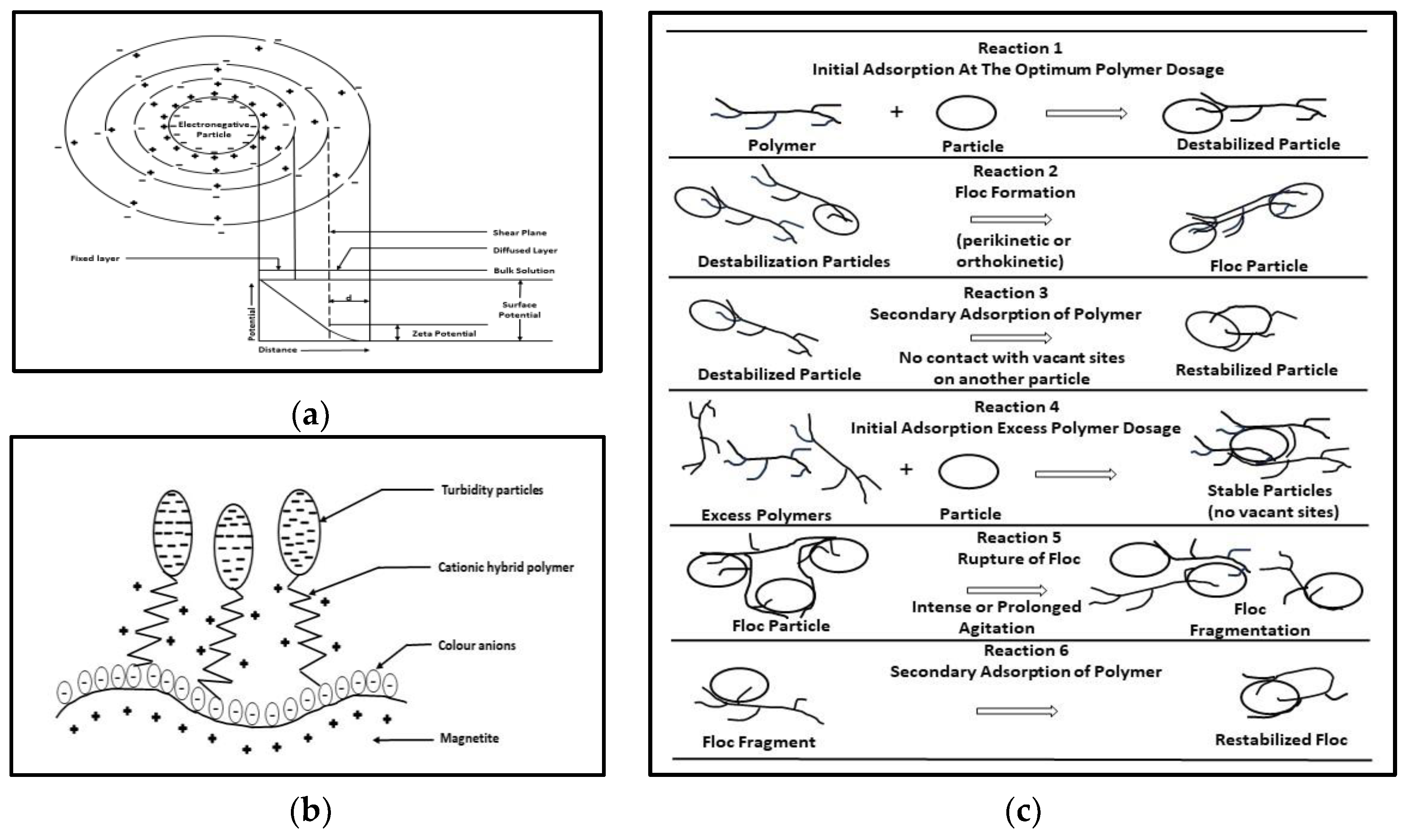
- –
- Attraction forces: these decrease the surface charge and enhance the aggregation of solids in distinct, separable aggregates or flocs (coagulation process).
- –
- The simple electrostatic adsorption of counterions: effectively neutralizes the solid surface charge and decreases the surface potential (dependent on ionic species or large, complex molecules, and ordinary adsorption) (coagulation–flocculation process).
- –
- Precipitation: hydrated metal hydroxides (precipitates) are formed that can adsorb on the solid surface with other existing colloids and neutralize the surface charge (pH-sensitive, with the characteristic value of the isoelectric point of metal hydroxide) (coagulation–flocculation process).
- –
- Enmeshment in an agglomerate precipitate and adsorption: is when organic polymers are used (cationic, anionic, and nonionic ones). The existing ions in the WW interact with the polymeric chains, forming solid aggregates (flocculation process).
4. Hybrid Materials and Their Performance in Colored WW Treatments Based on Coagulation–Flocculation Processes
4.1. Hybrid Materials Used as Coagulation–Flocculation Agents in Colored WW Treatment
- –
- Inorganic–inorganic hybrid materials (e.g., PASiC, PFC–Na–bentonite, PFC–magnetic nanoparticles, clinoptilolite–Al2(SO4)3, oxo titanium sulphate–Al2(SO4)3, FeCl3–PAC, iron–aluminum polymer hybrid),
- –
- Inorganic–organic hybrid materials (e.g., Al(OH)3–PAM, Al(OH)3–P(AM–co–AA), CaCl2–PAM, MgCl2–PAM, PFC–PAM, PAC–PAM, Al2(SO4)3–PDMDAAC, FeCl3–PDMDAAC, PFC–PDMDAAC, PAC–starch–graft–PAM, and PAC–EPI–DMA, etc.),
- –
- Inorganic–natural polymer hybrid materials (e.g., Al2(SO4)3–chitosan, PAC–chitosan, poly (aluminum ferric silicate)–chitosan, chitosan–PAC–Na2SiO3, rectorite–amylose, red mud–hydrochloric pickle liquor of bauxite, etc.),
- –
- Inorganic–biopolymer hybrid materials (e.g., pullulan–PAC, microbial flocculant GA1–PAC, MBF (Aspergillus niger)—zeolite);
- –
- Organic–organic hybrid materials (e.g., poly (acrylamide–co–acrylic acid),
- –
- Organic–natural polymer hybrid materials (e.g., sodium alginate grafted PAM, chitosan–g–N,N–dimethylacrylamide, PAM–g–carboxymethyl starch, CMC–starch, starch–g–PAM, chitosan–g-N–vinyl formamide, starch–g-PAM–co-sodium xanthan);
- –
- Natural polymer–natural polymer hybrid materials (e.g., cationic starch–chitosan cross-linking co-polymer).
- –
- Physical blending (at ambient temperature) (e.g., for structurally hybridized materials such as PDMDAAC, PAM, PFC–PDMDAAC, PAC–PDMDAAC, PFC–PDMDAAC, FeCl3–PDMDAAC), PAC–chitosan, PAC–EPI–DMA, MgCl2–PAM, PFC–PDMDAAC, etc.);
- –
- Elevated temperature blending (e.g., for structurally hybridized materials such as PFS–PDMDAAC, PFS–PAM, CaCl2–PAM, FeCl3–PAM, etc.);
- –
- Hydroxylation–pre-polymerization (e.g., for chemically bound-hybridized materials such as PASiC, PAFC, PAFSiC, PMAS, PFSiS, PASiC, poly aluminum silicate, PAC, etc.);
- –
- Co-polymerization (e.g., for chemically bound-hybridized materials as Al(OH)3–PAM, Al(OH)3–P(AM–co–AA), PGS–PAM (polygorskite–polyacrylamide), etc.);
- –
- Chemical grafting/cross-linking (e.g., for chemically bound hybridized materials such as PAM–g–CMS (polyacrylamide grafted carboxymethyl starch), chitosan–g–N,N–dimethylacrylamide, CMC–starch, SAG–g–PAM (sodium alginate grafted polyacrylamide), starch–g–PAM–co sodium xanthate).
4.2. Influencing Factors of Hybrid Materials’ Performance in Coagulation–Flocculation Processes
4.2.1. The Effect of pH on Textile WW Treatment via Coagulation–Flocculation
4.2.2. Effect of Hybrid Material Dose on Textile WW Treatment Using Coagulation–Flocculation
4.2.3. Effect of Stirring Speed and Time on Textile WW Treatment Using Coagulation–Flocculation
4.2.4. Effect of Temperature on Textile WW Treatment Using Coagulation–Flocculation
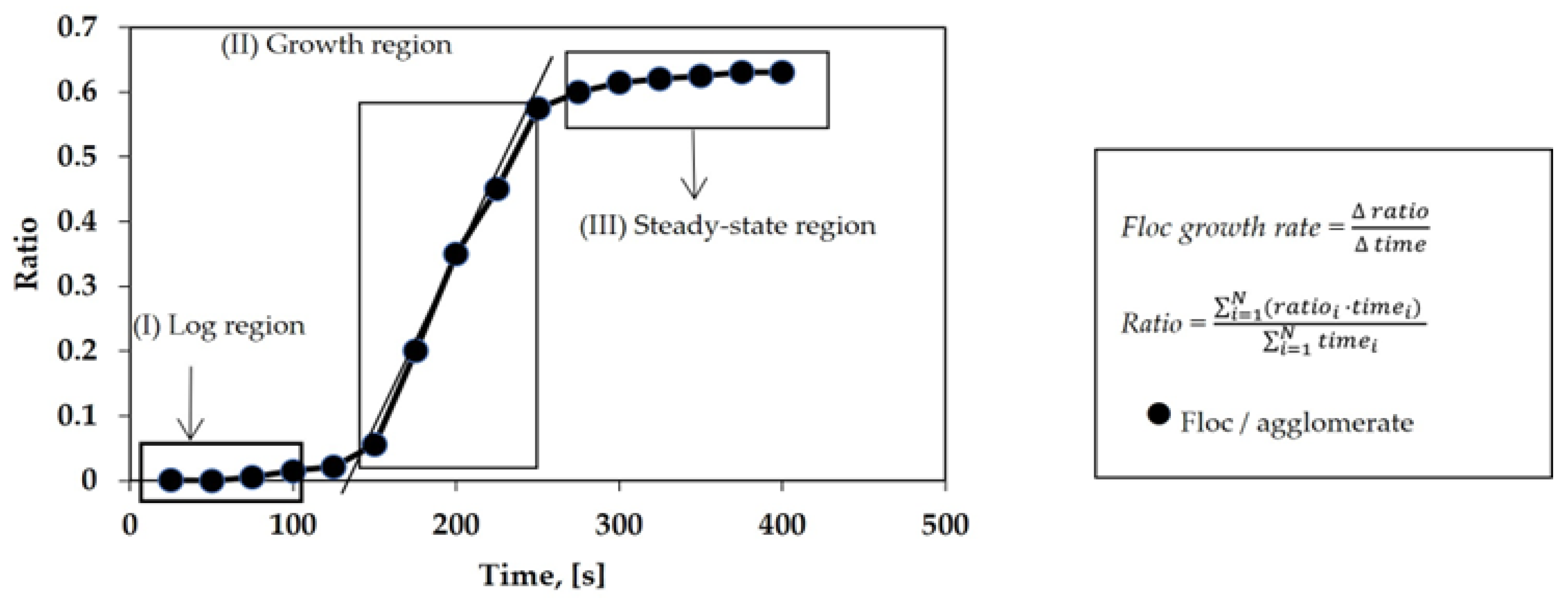
4.2.5. Process Kinetics of Coagulation–Flocculation
4.3. Performance of Different Hybrid Materials Used in Colored Textile WW Treatment
5. Future Perspectives on the Use of Hybrid Materials in Coagulation–Flocculation Treatment of Colored Textile WWs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
List of Abbreviations
| BOD5 | biochemical oxygen demand (after five days) |
| CMC | carboxymethyl cellulose |
| CMS | carboxymethyl starch |
| COD-Cr | chemical oxygen demand (analyzed using potassium dichromate method) |
| DMA | dimethylamine |
| DO | dissolved oxygen |
| DSC | differential scanning calorimetric analysis |
| EDX | energy-dispersive X-ray diffraction. |
| EPI | epichlorohydrin |
| MBF (Aspergillus niger) | Aspergillus niger sp.-based microbial biomass fiber |
| MW | molecular weight |
| P(AM-co-AA) | poly (acrylamide-co-acrylic acid) |
| PAC | poly aluminum chloride |
| PAFC | poly (aluminum ferric chloride) |
| PAFSiC | poly (aluminum-ferric-silicate-chloride) |
| PAM | poly acrylamide |
| PAS | poly (aluminum sulphate) |
| PASiS | poly (aluminum silicate sulphate) hybrid material |
| PASiC | poly (aluminum silicate chloride) hybrid material |
| PDMDAAC | poly (dimethyl diallyl ammonium chloride) |
| PFC | poly (ferric chloride) |
| PFSiS | poly (ferric silicate sulphate) |
| PGS | polygorskite |
| PMAS | poly (magnesium-aluminum sulphate) |
| SAG | sodium alginate |
| SBP | soybean peroxidase |
| SEM | scanning electron microscopy |
| SMA | poly (styrene-co-maleic anhydride) |
| TEM | transmission electron microscopy |
| TGA | thermal gravimetric analysis |
| TOC | total organic carbon |
| UV254 | UV absorbance at 254 nm per cm (cm−1) |
| WW | wastewater |
References
- Somma, S.; Reverchon, E.; Baldino, L. Water purification of classical and emerging organic pollutants: An extensive review. ChemEng. 2021, 5, 47. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef] [PubMed]
- Samsami, S.; Mohamadizaniani, M.; Sarrafzadeh, M.-H.; Rene, E.R.; Firoozbahr, M. Recent advances in the treatment of dye-containing wastewater from textile industries: Overview and perspectives. Proc. Safety Environ. Prot. 2020, 143, 136–163. [Google Scholar] [CrossRef]
- Yaseen, D.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- De Gisi, S.; Lofrano, G.; Grassi, M.; Notarnicola, M. Characteristics and adsorption capacities of low-cost sorbents for wastewater treatment: A review. Sustain. Mater. Technol. 2016, 3, 10–40. [Google Scholar] [CrossRef]
- Water Use in Europe. Available online: http://www.eea.europa.eu/signals/signals-2018-content-list/infographic/water-use-in-europe/view (accessed on 16 March 2020).
- Sanchez, W.; Egea, E. Health and environmental risks associated with emerging pollutants and novel processes. Environ. Sci. Pollut. Res. 2018, 25, 6085–6086. [Google Scholar] [CrossRef]
- Ali, J.; Ali, M.; Khan, I.; Khan, A.; Rafique, Z.; Waseem, H. Advances in Biodegradation and Bioremediation of Emerging Contaminants in the Environment (Chapter 7). In Biological Approaches to Controlling Pollutants, Advances in Pollution Research; Kumar, S., Hashmi, M.Z., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 121–138. ISBN 978-0-12-824316-9. [Google Scholar] [CrossRef]
- Zaharia, C.; Suteu, D. Textile Organic Dyes—Characteristics, Polluting effects and Separation/Elimination Procedures from Industrial Effluents—A Critical Overview. In Organic Pollutants—Ten Years after the Stockholm Convention—Environmental and Analytical Update; Puzyn, T., Mostrag-Szlichtyng, A., Eds.; InTech: Rijeka, Croatia, 2012; Chapter 3; pp. 55–86. ISBN 978-953-307-917-2. [Google Scholar] [CrossRef]
- EPA. Profile of the Textile Industry (Guide Manual); Environmental Protection Agency: Washington, DC, USA, 1997.
- Crini, G.; Lichtfouse, E. Advantages and disadvantages of techniques used for wastewater treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Chauhan, P.S.; Kant, R.; Rai, A.; Gupta, A.; Bhattacharya, S. facile synthesis of ZnO/GO nanoflowers over Si substrate for improved photocatalytic decolorization of MB dye and industrial wastewater under solar irradiation. Mater. Sci. Semicond. Process 2019, 89, 6–17. [Google Scholar] [CrossRef]
- Saravanan, A.; Kumar, P.S.; Yaashikaa, P.R.; Kanmani, S.; Varthine, R.H.; Muthu, C.M.M.; Yuvaraj, D. Modelling on the removal of dye from industrial wastewater using surface improved Enteromorpha intestinalis. Int. J. Environ. Res. 2019, 13, 349–366. [Google Scholar] [CrossRef]
- Behera, M.; Nayak, J.; Banerjee; Chakrabortty; Tripathy, S.K. A review on the treatment of textile industry waste effluents towards the development of efficient mitigation strategy: An integrated system design approach. J. Environ. Chem. Eng. 2021, 9, 105277. [Google Scholar] [CrossRef]
- Sekomo, C.B.; Rousseau, D.P.L.; Saleh, S.A.; Lens, P.N.L. Heavy metal removal in duckweed and algae ponds as a polishing step for textile wastewater treatment. Ecol. Eng. 2012, 44, 102–110. [Google Scholar] [CrossRef]
- Sharma, K.P.; Sharma, S.; Sharma, S.; Singh, P.K.; Kumar, S.; Grover, R.; Sharma, P.K. A comparative study on characterization of textile wastewaters (untreated and treated) toxicity by chemical and biological tests. Chemosphere 2007, 69, 48–54. [Google Scholar] [CrossRef]
- Shah, M.P.; Patel, K.A.; Nair, S.S.; Darji, A. Optimization of environmental parameters on microbial degradation of reactive black dye. J. Bioremed. Biodegrad. 2013, 4, 10–15. [Google Scholar] [CrossRef]
- Kehinde, F.O.; Aziz, H.A. Textile waste water and the advanced oxidative treatment process, an overview. Int. J. Innovat. Res. Sci. Eng. Technol. 2014, 3, 15310–15317. [Google Scholar] [CrossRef]
- Bhuvaneswari, A.; Asha, B.; Selvakumar, D. Start up and enhancement granulation in an anaerobic baffled reactor for the treatment of textile wastewater. Int. J. Civ. Eng. 2016, 9, 645–652. [Google Scholar]
- Punzi, M.; Nilsson, F.; Anbalagan, A.; Svensson, N.M.; Jonsson, K.; Mattiasson, B.; Jonstrup, M. Combined anaerobic-ozonation process for treatment of textile wastewater: Removal of acute toxicity and mutagenicity. J. Hazard. Mater. 2015, 292, 52–60. [Google Scholar] [CrossRef]
- Manekar, P.; Patkar, G.; Aswale, P.; Mahure, M.; Nandy, T. Detoxifying of high strength textile effluent through chemical and bio-oxidation processes. Bioresour. Technol. 2014, 157, 44–51. [Google Scholar] [CrossRef]
- Singh, D.; Singh, V.; Agnihotri, A.K. Study of textile effluent in and around Ludhiana district in Punjab. India. Int. J. Environ. Sci. 2013, 3, 1271–1278. [Google Scholar]
- Paul, S.A.; Chavan, S.K.; Khambe, S.D. Studies on characterization of textile industrial waste water in Solapur city. Int. J. Chem. Sci. 2012, 10, 635–642. [Google Scholar]
- Imtiazuddin, S.M.; Mumtaz, M.; Mallick, K.A. Pollutants of wastewater characteristics in textile industries. J. Basic Appl. Sci. 2012, 8, 554–556. [Google Scholar] [CrossRef]
- Syafalni, S.; Abustan, I.; Dahlan, I.; Wah, C.K.; Umar, G. Treatment of dye wastewater using granular activated carbon and zeolite filter. Mod. Appl. Sci. 2012, 6, 37–51. [Google Scholar]
- Lim, S.L.; Chu, W.L.; Phang, S.M. Use of chlorella vulgaris for bioremediation of textile wastewater. Biores. Technol. 2010, 101, 7314–7322. [Google Scholar] [CrossRef]
- Verma, A.K.; Dash, R.R.; Bhunia, P. A review on chemical coagulation/flocculation technologies dor removal of colour from textile wastewaters. J. Environ. Manag. 2012, 93, 154–168. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Islam, A. A review on textile wastewater characterization in Bangladesh. Resour. Environ. 2015, 5, 15–44. [Google Scholar] [CrossRef]
- Nopkhuntod, S.; Dararat, S.; Yimrattanabovorn, J. Removal of reactive dyes from wastewater by shale. Songklanakarin J. Sci. Technol. 2012, 34, 117–123. [Google Scholar]
- Sun, Y.; Zheng, H.; Tan, M.; Wang, Y.; Tang, X.; Feng, L.; Xiang, X. Synthesis and characterization of composite flocculant PAFS-CPAM for the treatment of textile dye wastewater. J. Appl. Polym. Sci. 2014, 131, 1–8. [Google Scholar] [CrossRef]
- Liu, H.I.; Chang, F.Q.; Wang, D.S. Interaction of ozone and organic matter in coagulation with inorganic polymer flocculant-PACl: Role of organic components. Desalination 2009, 249, 556–601. [Google Scholar] [CrossRef]
- Bes-Pia, A.; Cuartas-Uribe, B.; Mendoza-Roca, J.A.; Alcaina-Mirand, M.I. Study of the behavior of different NF membranes for the reclamation of a secondary textile effluent in rinsing processes. J. Hazard Mater. 2010, 178, 341–348. [Google Scholar] [CrossRef]
- Aouni, A.; Fersi, C.; Cuartas-Uribe, B.; Bes-Pia, A.; Alcaina-Mirand, M.I.; Dhahbi, M. Reactive dyes rejection and textile effluent treatment study using ultrafiltration and nanofiltration processes. Desalination 2012, 221, 259–267. [Google Scholar] [CrossRef]
- Buscio, V.; Marin, M.J.; Crespi, M.; Gutierrez-Bouzan, C. Reuse of textile wastewater after homogenization-decantation treatment coupled to PVDF ultrafiltration membranes. Chem. Eng. J. 2015, 265, 122–128. [Google Scholar] [CrossRef]
- Tomei, M.C.; Pascual, J.S.; Angelucci, D.M. Analysing performance of real textile wastewater bio-decolourization under different reaction environments. J. Clean. Prod. 2016, 129, 468–477. [Google Scholar] [CrossRef]
- Al-Shiwaik, N.M.; Abid, B.A.; Brbooti, M.M.M. Color removal from industrial textile wastewater using chemical adsorption. Eng. Tech. J. Part B 2013, 31, 471–489. [Google Scholar] [CrossRef]
- Hussein, F.H. Chemical properties of treated textile dyeing wastewater. Asian J. Chem. 2013, 25, 9393–9400. [Google Scholar] [CrossRef]
- Abid, M.F.; Zablouk, M.A.; Abid-Alamer, A.M. Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iran J. Environ. Health Sci. Eng. 2012, 9, 17. [Google Scholar] [CrossRef] [PubMed]
- Almazan-Sanchez, P.T.; Linares-Hernandez, I.; Solache-Rios, M.J.; Marinez-Miranda, V. Textile wastewater treatment using iron-modified clay and copper-modified carbon in batch and column systems. Water Air Soil Pollut. 2016, 227, 100. [Google Scholar] [CrossRef]
- Uysal, Y.; Aktas, D.; Caglar, Y. Determination of colour removal efficiency of Lemna minor L. from industrial effluents. J. Environ. Prot. Ecol. 2014, 15, 1718–1726. [Google Scholar]
- Un, U.T.; Aytac, E. Electrocoagulation in a packed bed reactor-complete treatment of color and COD from real textile wastewater. J. Environ. Manag. 2013, 123, 113–119. [Google Scholar]
- Verma, A.K.; Bhunia, P.; Dash, R.R. Carbonaceous organics removal kinetics in an upflow anaerobic blanket (UASB) reactor treating physico-chemically pre-treated textile wastewater. Desal. Water Treat. 2015, 54, 1577–1588. [Google Scholar] [CrossRef]
- Mountassir, Y.; Benyaich, A.; Bercot, P.; Rezrazi, M. Potential use of clay in electrocoagulation process of textile wastewater: Treatment performance and flocs characterization. J. Environ. Chem. Eng. 2015, 3, 2900–2908. [Google Scholar] [CrossRef]
- Marquez, M.; Costa, C. Biomass concentration in pact process. Chem. Water Res. 1996, 30, 2079–2085. [Google Scholar] [CrossRef]
- Avlonitis, S.A.; Poulios, I.; Sotiriou, D.; Pappas, M.; Moutesidis, K. Simulated cotton dye effluents treatment and reuse by nanofiltration. Desalination 2008, 297, 259–267. [Google Scholar] [CrossRef]
- Bali, U.; Karagozoglu, B. Performance comparison of Fenton process, ferric coagulation and H2O2/pyridine/Cu(II) system for decolorization of Remazol Turquoise Blue H-133. Dyes Pigments 2007, 74, 73–80. [Google Scholar] [CrossRef]
- Mbuligwe, S.E. Comparative treatment of dye-rich wastewater in engineered wetland systems (EWSs) vegetated with different plants. Water Res. 2005, 39, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.F.; Liao, C.H.; Chen, M.C. Pre-oxidation and coagulation of textile wastewater by the Fenton process. Chemosphere 2002, 46, 923–928. [Google Scholar] [CrossRef]
- Basibuyuk, M.; Forster, C.F. An examination of the treatability of a simulated textile wastewater containing Maxilon Red BL-N. Proc. Biochem. 1997, 32, 523–527. [Google Scholar] [CrossRef]
- Mohan, S.V.; Rao, N.C.; Prasad, K.K.; Karthikeyan, J. Treatment of simulated Reactive Yellow 22 (Azo) dye effluents using Spirogyra species. Waste Manag. 2002, 22, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Dhaouefi, Z.; Toledo-Cervantes, A.; Garcia, D.; Bedoui, A.; Ghedira, K.; Chekir-Ghedira, I.; Mufloz, R. Assessing textile wastewater treatment in an anoxic-aerobic photobioreactor and the potential of the treated water for irrigation. Algal Res. 2018, 29, 170–178. [Google Scholar] [CrossRef]
- Neamtu, M.; Zaharia, C.; Catrinescu, C.; Yediler, Y.; Macoveanu, M.; Kettrup, A. Fe-exchanged Y zeolite as catalyst for wet peroxide oxidation of reactive azo dye Procion Marine H-EXL. Appl. Catal. B Environ. 2004, 48, 287–294. [Google Scholar] [CrossRef]
- Robinson, T.; McMullan, G.; Marchant, R.; Nigam, P. Remediation of dyes in textile effluents: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 2001, 77, 247–255. [Google Scholar] [CrossRef]
- Mezohegyi, G.; van der Zee, F.P.; Font, J.; Fortuny, A.; Fabregat, A. Towards advanced aqueous dye removal processes: A short review on the versatile role of activated carbon. J. Environ. Manag. 2012, 102, 148–164. [Google Scholar] [CrossRef]
- Hao, O.J.; Kim, H.; Chiang, P.-C. Decolorization of wastewater. Crit. Rev. Environ. Sci. Technol. 2000, 30, 449–505. [Google Scholar] [CrossRef]
- Pandey, A.; Singh, P.; Iyengar, L. Bacterial decolorization and degradation of azo dyes. Int. Biodeterior. Biodegrad. 2007, 59, 73–84. [Google Scholar] [CrossRef]
- Ramos, M.D.N.; Pereira Lima, J.P.; de Aquino, S.F.; Aguiar, A. A critical analysis of the alternative treatments applied to effluents from Brazilian textile industries. J. Water Proc. Eng. 2021, 43, 102273. [Google Scholar] [CrossRef]
- Macoveanu, M.; Teodosiu, C.; Duca, G. Advanced Treatment of Wastewaters with Non-Biodegradable Organic Compounds; Technical University Ed.; Gheorghe Asachi: Iasi, Romania, 1997. (In Romanian) [Google Scholar]
- Zaharia, C. Innovative Wastewater Treatment Technologies: Opportunities, Perspectives and Challenges; Ecozone Ed.: Iasi, Romania, 2023; ISBN 978-606-8625-39-3. [Google Scholar]
- Musteret, C.P.; Fighir, D.; Gavrilescu, D.; Zaharia, C.; Teodosiu, C. Waters and Wastewaters Treatment: Practice Applications; Politehnium: Iasi, Romania, 2014; ISBN 978-973-621-442-4. (In Romanian) [Google Scholar]
- Cooper, P. Colour in Dyehouse Effluent; Publishing House of the Society of Dyers and Colourists: Nottingham, UK, 1995. [Google Scholar]
- Zaharia, C.; Suteu, D.; Muresan, A. Options and solutions for textile effluent decolorization using some specific physico-chemical treatment steps. Environ. Eng. Manag. J. 2012, 11, 493–509. [Google Scholar] [CrossRef]
- Zaharia, C.; Surpateanu, M. Study of flocculation with Prodefloc CRC 301 polyelectrolyte applied into a chemical wastewater treatment. Ovidius Univ. Annals Chem. 2006, 17, 50–53. [Google Scholar]
- Zaharia, C.; Diaconescu, R.; Surpateanu, M. Optimization study of a wastewater chemical treatment with PONILIT GT-2 anionic polyelectrolyte. Environ. Eng. Manag. J. 2006, 5, 1141–1152. [Google Scholar] [CrossRef]
- Zaharia, C.; Diaconescu, R.; Surpateanu, M. Study of flocculation with Ponilit GT-2 anionic polyelectrolyte applied into a chemical wastewater treatment. Open Chem. 2007, 5, 239–256. [Google Scholar] [CrossRef]
- Khan, M.S.; Knapp, J.; Clemett, A.; Chadwick, M.; Mahmood, M.; Sharif, M.I. Managing and monitoring effluent treatment plants. In Managing Industrial Pollution from Small and Medium Scale Industries in Bangladesh; Booklet Series SEI, BCAS; University of Leeds: Leeds, UK, 2006. [Google Scholar]
- Crini, G.; Badot, P.M. Traitement et Épuration des Eaux Industrielles Polluées; PUFC: Besançon, France, 2010. [Google Scholar]
- Anjaneyulu, Y.; Sreedhara Chary, N.; Samuel Suman Raj, D. Decolourization of industrial effluents: Available methods and emerging technologies—A review. Rev. Environ. Sci. Bio/Technol. 2005, 4, 245–273. [Google Scholar] [CrossRef]
- Berefield, L.D.; Judkins, J.F.; Weand, B.L. Process Chemistry for Water and Wastewater Treatment; Prentice-Hall: Saddle River, NJ, USA, 1982. [Google Scholar]
- Abujazar, M.S.; Karaagac, S.U.; Amr, S.S.A.; Alazaiza, M.Y.D.; Bashir, M.J.K. Recent advancement in the application of hybrid coagulants in coagulation-flocculation of wastewater: A review. J. Clean. Prod. 2022, 345, 131133. [Google Scholar] [CrossRef]
- Katheresan, V.; Kansedo, J.; Lau, S.Y. Efficiency of various recent wastewater dye removal methods: A review. J. Environ. Chem. Eng. 2018, 6, 4676–4697. [Google Scholar] [CrossRef]
- Vajnhandl, S.; Valh, J.V. The status of water reuse in European textile sector. J. Environ. Manag. 2014, 141, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Bilinska, L.; Cmurek, M.; Ledakowicz, S. Textile wastewater treatment by AOPs for brine reuse. Process Saf. Environ. Prot. 2017, 109, 420–428. [Google Scholar] [CrossRef]
- Stefan, M.I. Advanced Oxidation Processes for Water Treatment: Fundamentals and Applications; IWA Publishing: London, UK, 2017. [Google Scholar]
- Oturan, M.A.; Aaron, J.J. Advanced oxidation processes in water/wastewater treatment: Principles and applications: A review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2577–2641. [Google Scholar] [CrossRef]
- Fernandes, A.; Makos, P.; Boczkaj, G. Treatment of bitumen post oxidative effluents by sulphate radicals based advanced oxidation processes (S-AOPs) under alkaline pH conditions. J. Clean Prod. 2018, 195, 374–384. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, M.I.; Van Tran, T.T.; Juang, R.S. Enhanced removal of various dyes from aqueous solutions by UV and simulated solar photocatalysts over TiO2/ZnO/rGO composites. Sep. Purif. Technol. 2020, 232, 115962. [Google Scholar] [CrossRef]
- Babu, S.G.; Ashokkumar, M.; Neppolian, B. The role of ultrasound on advanced oxidation processes. Top. Curr. Chem. 2016, 374, 75. [Google Scholar] [CrossRef] [PubMed]
- Butani, S.A.; Mane, S.J. Coagulation/flocculation process for cationic, anionic dye removal using water treatment residuals—A review. Int. J. Technol. Manag. 2017, 6, 121–125. [Google Scholar]
- Collivignarelli, M.C.; Abba, A.; Miino, M.C.; Damiani, S. Treatments for color removal from wastewater: State of art. J. Environ. Managn. 2019, 236, 727–745. [Google Scholar] [CrossRef]
- Patel, H.; Vashi, R.T. Comparison of naturally prepared coagulants for removal of COD and color from textile wastewater. Global NEST J. 2013, 15, 522–528. [Google Scholar]
- Chellam, S.; Sari, M.A. Aluminium electrocoagulation as pretreatment during microfiltration of surface water containing NOM: A review of fouling, NOM, DBP and virus content. J. Hazard. Mater. 2016, 304, 490–501. [Google Scholar] [CrossRef]
- He, C.C.; Hu, C.Y.; Lo, S.L. Evaluation of sono-electrocoagulation for the removal of reactive Blue 19 passive film removed by ultrasound. Sep. Purif. Technol. 2016, 165, 107–113. [Google Scholar] [CrossRef]
- Hamad, H.; Bassyouni, D.; El-Ashroukhy, E.S.; Amin, N.; El-Latif, M.A. Comparative performance of anode oxidation and electrocoagulation as clean processes for electrocatalytic degradation of diazo dye Acid Brown 14 in aqueous medium. J. Hazard. Mater. 2017, 335, 178–187. [Google Scholar]
- Rosales, E.; Pazos, M.; Longo, M.A.; Sanroman, M.A. Electro-Fenton decoloration of dyes in a continuous reactor: A promising technology in colored wastewater treatment. Chem. Eng. J. 2009, 155, 62–67. [Google Scholar] [CrossRef]
- Dos Santos, A.J.; Garcia-Segura, S.; Dosta, S.; Cano, I.G.; Martinez-Huitie, C.A.; Brillas, E. A ceramic electrode of ZnO2-V2O5 for the generation of oxidant species in anodic oxidation. Assessment of the treatment of Acid Blue 29 dye in sulphate and chloride media. Sep. Purif. Technol. 2019, 228, 115747. [Google Scholar] [CrossRef]
- Martinez-Huite, C.A.; Panizza, M. Electrochemical oxidation of organic pollutants for wastewater treatment. Curr. Opin. Electrochem. 2018, 11, 62–71. [Google Scholar] [CrossRef]
- Rambabu, K.; Bharach, C.; Monash, P.; Veiu, S.; Banat, F.; Naushat, M.; Arthanameswaran, G.; Show, P.I. Effective treatment of dye polluted wastewater using nanoporous CaCl2 modified polyethersulphone membrane. Process Saf. Environ. Prot. 2019, 124, 266–278. [Google Scholar] [CrossRef]
- Gunawan, F.M.; Mangindzan, D.; Khoiruddin, K.; Wenten, I.C. Nanofiltration membrane cross-linked by m-phenylenediamine for dye removal from textile wastewater. Polym. Adv. Technol. 2019, 30, 360–367. [Google Scholar] [CrossRef]
- Gao, J.; Thang, Z.; Wang, K.Y.; Cheng, T.S. Fabrication of loose inner-selective polyethersulphine (PES) hollow fibers by one-step spinning process for nanofiltration (NF) of textile dyes. J. Memb. Sci. 2017, 541, 413–424. [Google Scholar] [CrossRef]
- Wiesmann, U.; Choi, I.S.; Dombrowski, E.M. Fundamentals of Biological Wastewater Treatment; Wiley-VCH Verlag GmbH&Co. KgaA: Weinheim, Germany, 2017. [Google Scholar]
- Ødegaard, H. Particle separation in wastewater treatment. In Proceedings of the 7th European Sewage and Refuse Symposium, Munich, Germany, 19–22 May 1987; pp. 351–400. [Google Scholar]
- Walldal, C. Electrokinetic study of silica particles flocculated by two cationic polyelectrolytes: Sequential and simultaneous addition. Colloids Surf. A Physicochem. Eng. Asp. 2001, 194, 111–121. [Google Scholar] [CrossRef]
- Yen, T.F. Chemical Processes for Environmental Engineering; Imperial College Press: Danvers, MA, USA, 2007; ISBN 978-1-86094-759-9. [Google Scholar]
- Zaharia, C. Wastewater Chemical Treatment; Performantica: Iasi, Romania, 2006. (In Romanian) [Google Scholar]
- Haller, E.J. Simplified Wastewater Treatment Plant Operations; Technomic Publishing Co., Inc.: Lancaster, PA, USA, 1995; pp. 107–119. [Google Scholar]
- Qian, F.; Sun, X.; Liu, Y. Removal characteristics of organics in bio-treated textile wastewater reclamation by a stepwise coagulation and intermediate GAC/O3 oxidation process. Chem. Eng. J. 2013, 214, 112–118. [Google Scholar] [CrossRef]
- Wang, J.; Guo, M.; Luo, Y.; Shao, D.; Ge, S.; Cai, L.; Xia, C.; Lan, S.S. Production of magnetic sodium alginate polyelectrolyte nanospheres for lead ions removal from wastewater. J. Environ. Manag. 2021, 289, 112506. [Google Scholar] [CrossRef] [PubMed]
- Ukiwe, L.N.; Alinnor, J.I. Assessment of polyacrylamide and aluminium sulphate coagulants in turbidity removal in wastewater. Terr. Aquat. Environ. Toxicol. 2012, 6, 132–135. [Google Scholar]
- Kurniawan, S.B.; Imron, M.F.; Chik, C.E.N.C.E.; Owodunni, A.A.; Ahmad, A.; Alnawajha, M.M.; Rahim, N.F.M.; Said, N.S.M.; Sheikh Abdullah, S.R.; Kasan, N.A.; et al. What compound inside biocoagulants/bioflocculants is contributing the most to the coagulation and flocculation processes? Sci. Total Environ. 2022, 806, 150902. [Google Scholar] [CrossRef] [PubMed]
- Morosanu, I.; Paduraru, C.; Bucatariu, F.; Fighir, D.; Mihai, M.; Teodosiu, C. Shaping polyelectrolyte composites for heavy metals adsorption from wastewater: Experimental assessment and equilibrium studies. J. Environ. Manag. 2022, 321, 115999. [Google Scholar] [CrossRef]
- Rigoletto, M.; Calza, P.; Gaggero, E.; Laurenti, E. Hybrid materials for the removal of emerging pollutants in water: Classification. Sysnthesis, and properties. Chem. Eng. J. Adv. 2022, 10, 100252. [Google Scholar] [CrossRef]
- Sher, F.; Malik, A.; Liu, H. Industrial polymer effluent treatment by chemical coagulation and flocculation. J. Environ. Chem. Eng. 2013, 1, 684–689. [Google Scholar] [CrossRef]
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, B.T. Development, characterization and the application of hybrid materials in coagulation/flocculation in wastewater: A review. Chem. Eng. J. 2012, 203, 370–386. [Google Scholar] [CrossRef]
- Ayalew, Z.M.; Guo, X.; Zhang, X. Synthesis and application of polyethyleneimine (PEI)-based composite/nanocomposite material for heavy metals removal from wastewater: A critical review. J. Hazard. Mater. Adv. 2022, 8, 100158. [Google Scholar] [CrossRef]
- El-Taweel, R.M.; Mohamed, N.; Alrefaey, K.A.; Husien, S.; Abdel-Aziz, A.B.; Salim, A.I.; Mostafa, N.G.; Said, L.A.; Ahim, I.S.; Radwan, A.G. A review of coagulation explaining its definition, mechanism, coagulant types, and optimization models; RSM, and ANN. Curr. Res. Green Sustain. Chem. 2023, 6, 100358. [Google Scholar] [CrossRef]
- Shi, C.; Wang, Q.; Li, D.; Zeng, B.; Liu, Q.; Cui, Y.; Wang, J.; Wang, X. Inorganic composite coagulant for wool scouring wastewater treatment: Performance, kinetics and coagulation mechanism. Sep. Purif. Technol. 2023, 313, 123482. [Google Scholar] [CrossRef]
- Qadeer, H.A.; Mahomoodally, M.F.; Nadeem, F.; Khanam, A. Wastewater treatment and dyes removal using electrocoagulation aided by natural biosorbents—A review. Int. J. Chem. Biochem. Sci. 2018, 14, 77–87. [Google Scholar]
- Saravanan, A.; Thamarai, P.; Kumar, P.S.; Rangasamy, G. Recent advances in polymer composite, extraction, and their application for wastewater treatment: A review. Chemosphere 2022, 308, 136368. [Google Scholar] [CrossRef]
- Owodunni, A.A.; Ismail, S. Revolutionary technique for sustainable plant-based green coagulants in industrial wastewater treatment—A review. J. Water Proc. Eng. 2021, 42, 102096. [Google Scholar] [CrossRef]
- Bediako, J.K.; El Ouardi, Y.; Masima Mouele, E.S.; Mensah, B.; Repo, E. Polyelectrolyte and polyelectrolyte complex-incorporated adsorbents in water and wastewater remediation—A review of recent advances. Chemosphere 2023, 325, 138418. [Google Scholar] [CrossRef] [PubMed]
- Elgarahy, A.M.; Maged, A.; Eloffy, M.G.; Zahran, M.; Kharbish, S.; Elwakeel, K.Z.; Bhatnagar, A. Geopolymers as sustainable eco-friendly materials: Classification, synthesis routes, and applications in wastewater treatment. Sep. Purif. Technol. 2023, 324, 124631. [Google Scholar] [CrossRef]
- Nageswara, R.L.; Feroz, S.; Karunya, S.; Motilal, L.; Saidireddy, P.; Suman, G. Synthesis, characterization and application of polymer composite materials in wastewater treatment. Mater. Today Proc. 2022, 59, 1726–1734. [Google Scholar] [CrossRef]
- Dutt, M.A.; Hanif, M.A.; Nadeem, F.; Bhatti, H.N. A review of advances in engineered composite materials popular for wastewater treatment. J. Env. Chem. Eng. 2020, 8, 104073. [Google Scholar] [CrossRef]
- Jaspal, D.; Malviya, A. Composites for wastewater purification: A review. Chemosphere 2020, 246, 125788. [Google Scholar] [CrossRef]
- Lin, S.H.; Chen, M.L. Purification of textile wastewater effluents by a combined Fenton process and ion exchange. Desalination 1997, 109, 121–130. [Google Scholar] [CrossRef]
- Venkat Mohan, S.; Srimurli, M.; Sailaja, P.; Karthikeyan, J. A study of acid dye colour removal using adsorption and coagulation. Environ. Eng. Policy 1999, 1, 149–154. [Google Scholar] [CrossRef]
- Sanghi, R.; Bhattacharya, B.; Singh, V. Use of Cassia javahikai seed gumand gum polyacrylamide as coagulant aid for the decolorization of textile dye solutions. Biores. Technol. 2006, 97, 1259–1264. [Google Scholar] [CrossRef] [PubMed]
- Chitanu, G.C.; Carpov, A.; Asaftei, T. Romanian Invention Patent (OSIM) No. 106745; ‘P. Poni’ Institute of Macromolecular Chemistry of Iasi: Iasi, Romania, 1987. [Google Scholar]
- Zaharia, C.; Macoveanu, M. Separation of some heavy metal ions from wastewaters using polyelectrolytes. Sci. Ann. 2000, VIII, 199–206. [Google Scholar]
- Zaharia, C.; Surpăţeanu, M.; Creţescu, I.; Macoveanu, M.; Braunstein, H. Electrocoagulation/electroflotation—Methods applied for wastewater treatment. Environ. Eng. Manag. J. 2005, 4, 463–472. [Google Scholar] [CrossRef]
- Daneshvar, N.; Sorkhabi, H.A.; Tizpar, A. Decolorization of Orange II by electrocoagulation method. Separ. Purific. Technol. 2003, 31, 153–162. [Google Scholar] [CrossRef]
- Ramesh Babu, B.; Parande, A.K.; Raghu, S.; Prem Kumar, T. Textile technology. Cotton Textile processing: Waste Generation and Effluent Treatment. J. Cotton Sci. 2007, 11, 141–153125. [Google Scholar]
- Wei, Y.; Ding, A.; Chen, Y. Removal of refractory dyes by a novel chlorine-free coagulant of polyferric-silicate-acetate (PFSA): Characterization and performance evaluation. J. Environ. Chem. Eng. 2022, 10, 108524. [Google Scholar] [CrossRef]
- Bazrafshan, E.; Alipour, M.R.; Mahvi, A.H. Textile wastewater treatment by application of combined chemical coagulation, electrocoagulation, and adsorption processes. Desalin. Water Treat. 2015, 108, 9203–9215. [Google Scholar] [CrossRef]
- Huang, X.; Wan, Y.; Shi, B.; Shi, J.; Chen, H.; Liang, H. Characterization and application of poly-ferric-titanium-silicate-sulfate in disperse and reactive dye wastewaters treatment. Chemosphere 2020, 249, 126129. [Google Scholar] [CrossRef]
- Wei, Y.; Cheng, X.; Ding, A.; Xu, J. Magnesium silicate polymer as a coagulant for reactive dye removal from wastewater: Considering the intrinsic pH in magnesium silicate polymer and coagulation behavior. ACS Omega 2020, 5, 26094–26100. [Google Scholar] [CrossRef]
- Pang, F.M.; Kumar, P.; Teng, T.T.; Mohd Omar, A.K.; Wasewar, K.L. Removal of lead, zinc and iron by coagulation-flocculation. J. Taiwan Inst. Chem. Eng. 2011, 42, 809–815. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, B.; Yue, Q.; Wang, Y. Effect of viscosity, basicity and organic content of composite flocculant on the decolorization performance and mechanism for reactive dyeing wastewater. J. Environ. Sci. 2011, 23, 1626–1633. [Google Scholar] [CrossRef] [PubMed]
- Yeap, K.L.; Teng, T.T.; Poh, B.T.; Morad, N.; Lee, K.E. Preparation and characterization of coagulation/flocculation behavior of a novel inorganic–organic hybrid polymer for reactive and disperse dyes removal. Chem. Eng. J. 2014, 243, 305–314, ISSN 1385-8947. [Google Scholar] [CrossRef]
- Lee, K.E.; Morad, N.; Teng, T.T.; Poh, T. An application study of inorganic-organic composite polymer in flocculating reactive dye wastewater under different conditions. Int. Conf. Biol. Environ. Chem. 2011, 24, 139–143. [Google Scholar]
- Su, C.X.; Teng, T.T.; Morad, N.; Rafatullah, M. Optimisation of the coagulation-flocculation of reactive dye wastewater using novel inorganic-organic hybrid polymer. Iran. J. Energy Environ. 2016, 7, 31–38. [Google Scholar] [CrossRef]
- Lee, K.E.; Teng, T.T.; Morad, N.; Poh, B.T.; Mahalingam, M. Flocculation activity of novel ferric chloride-polyacrylamide (FeCl3-PAM) hybrid polymer. Desalination 2011, 266, 108–113. [Google Scholar] [CrossRef]
- Lee, K.E.; Goh, T.L.; Simon, N. Textile industrial wastewater treatment by polyacrylamide aided magnesium chloride hybrid coagulant. Nat. Environ. Pollut. Technol. 2017, 16, 399–407. [Google Scholar]
- Al-Ani, Y.; Li, Y. Degradation of C.I. Reactive Blue 19 using combined iron scrap process and coagulation/flocculation by a novel Al(OH)3-polyacrylamide hybrid polymer. J. Taiwan Inst. Chem. Eng. 2012, 43, 942–947. [Google Scholar] [CrossRef]
- Lee, K.E.; Hanafiah, M.M.; Halim, A.A.; Mahmud, M.H. Primary treatment of dye wastewater using aloe vera-aided aluminium and magnesium hybrid coagulants. Procedia Environ. Sci. 2015, 30, 56–61. [Google Scholar] [CrossRef]
- Wu, J.; Xue, Y.L.; Yang, G.; Sun, B.Y.; Zhang, L. Preparation and performance of polysilicate aluminum ferric-chitosan. In Proceedings of the 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Abd-Elhakeem, M.A.; Alkhulaqi, T.A. Simple, rapid, and efficient water purification by chitosan coated magnetite nanoparticles. J. Environ. Nanotechnol. 2014, 3, 17–20. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, S.; Sun, W.; Zhu, S.; Zheng, H. Flocculation activity and Evaluation of chitosan-based flocculant CMCTS-g-P(AM-CA) for heavy metal removal. Separ. Purific. Technol. 2020, 1016, 116737. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, H.; Yang, X. Preparation and performance of a novel starch-based inorganic/organic composite coagulant for textile wastewater treatment. Separ. Purific. Technol. 2019, 210, 93–99. [Google Scholar] [CrossRef]
- Dotto, J.; Fagundes-Klen, M.R.; Veit, M.T.; Palacio, S.M.; Bergamasco, R. Performance of different coagulants in the coagulation/flocculation process of textile wastewater. J. Clean. Prod. 2019, 208, 656–665. [Google Scholar] [CrossRef]
- Wang, S.; Kong, F.; Fatehi, P.; Hou, Q. Cationic High Molecular Weight Lignin Polymer: A Flocculant for the Removal of Anionic Azo-Dyes from Simulated Wastewater. Molecules 2018, 23, 2005. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Yue, Q.; Miao, J. Evaluation of polyaluminium ferric chloride (PAPC) as a composite coagulant for water and wastewater treatment. Water Sci. Technol. 2003, 47, 127–132. [Google Scholar]
- Gao, B.Y.; Wang, Y.; Yue, Q.Y. The chemical species distribution of aluminium in composite flocculants prepared from polyaluminium chloride (PAC) and polydimethyldiallylammonium chloride (PDMDAAC). Acta Hydrochim. Hydrobiol. 2005, 33, 365–371. [Google Scholar] [CrossRef]
- Liu, Z.M.; Sang, Y.M.; Tong, Z.G.; Wang, Q.H.; Sun, T.C. Decolourization performance and mechanism of leachate secondary effluent using polyaluminium (III)-magnesium (II) sulphate. Water Environ. J. 2012, 26, 85–93. [Google Scholar] [CrossRef]
- Zhao, H.; Peng, J.; Xue, A.; Ni, J. Distribution and transformation of Al species in organic silicate aluminium hybrid coagulants. Compos. Sci. Technol. 2009, 69, 1629–1634. [Google Scholar] [CrossRef]
- Dong, Y.; Deng, A.; Guo, H.; Tang, X. Preparation and flocculation performance of a polyacrylamide/montmorillonite hybrid flocculant. Huanjing Kexue Xuebao/Acta Sci. Circumstantiae 2009, 29, 2385–2392. [Google Scholar]
- Nanko, M. Definitions and categories of hybrid materials. Azojomo 2009, 6, 1–8. [Google Scholar]
- Zaharia, C. Coagulation-flocculation processes in water and wastewater treatment. (II) Fine particles and its removal using electrolytes and polyelectrolytes. In Current Topics, Concepts and Research Priorities in Environmental Chemistry; Zaharia, C., Ed.; ‘Alexandru Ioan Cuza’ University Publishing House: Iasi, Romania, 2014; Volume III, pp. 165–194. [Google Scholar]
- Moussas, P.A.; Zouboulis, A.I. A new inorganic-organic composite coagulant, consisting of polyferric sulphate (PFS) and polyacrylamide (PAM). Water Res. 2009, 43, 3511–3524. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hao, B.; Yue, Q.; Wei, J.; Li, Q. The characterization and flocculation efficiency of composite flocculant iron salts-polydimethyldiallylammonium chloride. Chem. Eng. J. 2008, 142, 175–181. [Google Scholar] [CrossRef]
- Sen, G.; Kumar, R.; Ghosh, S.; Pal, S. A novel polymeric flocculant based on polyacrylamide grafted carboxymethyl starch. Carbohyd. Polym. 2009, 77, 822–831. [Google Scholar] [CrossRef]
- Lee, K.E.; Khan, I.; Morad, N.; Teng, T.T.; Poh, B.T. Thermal behaviour and morphological properties of novel magnesium salt-polyacrylamide composite polymers. Polym. Compos. 2011, 32, 1515–1522. [Google Scholar] [CrossRef]
- Sun, T.; Sun, C.H.; Zhu, G.I.; Miao, X.J.; Wu, C.C.; Lv, S.B.; Li, W.J. Preparation and coagulation performance of poly-ferric-aluminium-silicate-sulphate from fly ash. Desalination 2011, 268, 270–275. [Google Scholar] [CrossRef]
- Gao, B.Y.; Yang, Y.; Yue, Q.Y.; Wei, J.C.; Li, Q. Color removal from simulated dye water and actual wastewater using a composite coagulant prepared by polyferric chloride and polydimethyldiallylammonium chloride. Sep. Purif. Technol. 2007, 54, 157–163. [Google Scholar] [CrossRef]
- Zhao, y.; Zhang, L.Y.; Ni, F.; Xi, B.; Xia, X.; Peng, X.; Luan, Z. Evaluation of a novel composite inorganic coagulant prepared by red mud for phosphate removal. Desalination 2011, 273, 414–420. [Google Scholar] [CrossRef]
- Huang, P.; Xia, D.; Kazlauciunas, A.; Thornton, P.; Lin, L.; Menzel, R. Dye -mediated interactions in chitosan-based polyelectrolyte organoclay hybrids for enhanced adsorption of industrial dyes. ACS Appl. Mater. Interfaces 2019, 11, 11961–11966. [Google Scholar] [CrossRef]
- Jagaba, A.; Birniwa, A.H.; Usman, A.; Mu’azu, N.; Yaro, N.; Usman, A.K.; Mu’azu, N.D.; Yaro, N.S.A.; Soja, U.B.; Abioye, K.J.; et al. Trend and current practices of coagulation-based hybrid systems for pulp and paper mill effluent treatment: Mechanisms, optimization techniques and performance evaluation. J. Clean. Prod. 2023, 429, 139543. [Google Scholar] [CrossRef]
- Nonfodji, O.M.; Fatombi, J.K.; Ahoyo, T.A.; Osseni, S.A.; Aminou, T. Performance of Mringa oleifera seeds protein and Moringa oleifera seeds protein-polyaluminum chloride composite coagulant in removing organic matter and antibiotic resistant bacteria from hospital wastewater. J. Water Process Eng. 2020, 33, 101103. [Google Scholar] [CrossRef]
- De Souza, M.T.F.; Ambrosio, E.; de Almeida, C.A.; de SouzaFreitas, T.K.F.; Santos, I.B.; de Cinque Almeida, V.; Garcia, J.C. The use of natural coagulant (Opuntia ficus-indica) in the removal for organic materials of textile effluents. Environ. Monit. Assess. 2014, 186, 5261–5271. [Google Scholar] [CrossRef] [PubMed]
- Calza, P.; Zacchigna, D.; Laurenti, E. Degradation of Orange dyes and carbamazepine by soybean peroxidase immobilized on silica monoliths and titanium dioxide. Environ. Sci. Pollut. Res. 2016, 23, 23742–23748. [Google Scholar] [CrossRef]
- Chen, I.; Yan, K.; Zhang, J. Integration of graphene-hemin hybrid materials in an electroenzymatic system for degradation of diclofenac. Electrochim. Acta 2016, 190, 980–987. [Google Scholar] [CrossRef]
- Trache, D.; Tarchoun, A.F.; Derradjii, M.; Hamidon, T.S.; Masruchin, N.; Brosse, M.; Hussin, H. Nanocellulose: From fundamentals to advanced applications. Front. Chem. 2020, 8, 392. [Google Scholar] [CrossRef] [PubMed]
- Daniel-da-Silva, A.I.; Soares, S.F.; Fernandes, T.; Sacramento, M.; Trindade, T.; Daniel-da-Silva, A.L. Trimethyl chitosan/siloxane-hybrid coated Fe3O4 nanoparticles for the uptake of sulfamethoxazole from water. Molecules 2019, 24, 1958. [Google Scholar] [CrossRef]

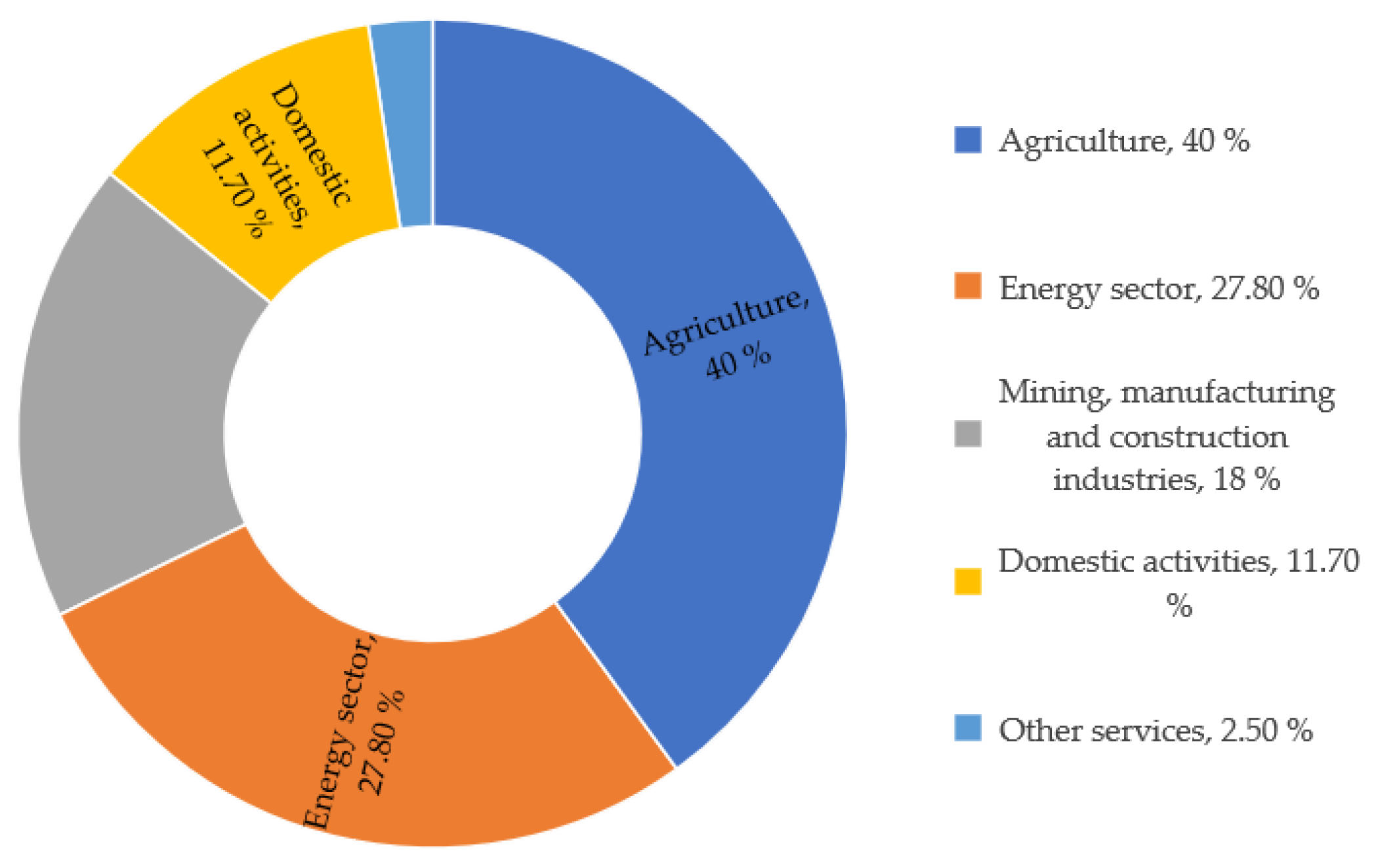
| Textile Process | Water Consumption (×103 m3/kg of Textile Product) | Textile Operation or Process | Water Consumption (×103 m3/kg of Textile Product) |
|---|---|---|---|
| Wool finishing | 110.90–657.20 | Raw wool washing | 4.20–77.60 |
| Fabric finishing | 10.80–276.90 | Fiber finishing | 3.30–557.10 |
| Carpet finishing | 8.30–162.60 | Yarn finishing | 33.40–930.70 |
| Cloth finishing | 5.80–392.80 |
| Process | Singering/De-Sizing | Scouring | Bleaching | Mercerizing | Dyeing/Printing/ Finishing |
|---|---|---|---|---|---|
| Indicators * | |||||
| pH | - | 10–13 | 8.5–9.6 | 5.5–9.5 | 5–10 |
| Color (ADMI), [color units] | - | 650–700 | 153–190 | - | 1450–4750 |
| Total suspended solids (TSSs), [mg/L] | 16,000–32,000 | 7600–17,400 | 2300–14,400 | 600–1900 | 500–14,100 |
| Total dissolved salts (TDSs), [mg/L] | - | - | 4800–19,500 | 4300–4600 | 50–500 |
| COD-Cr, [mgO2/L] | 4600–5900 | 8000–8600 | 6700–13,500 | 1600–1700 | 1100–4600 |
| BOD5, [mgO2/L] | 1700–5200 | 100–2900 | 100–1700 | 50–150 | 10–1800 |
| Water usage, [L/kg cotton] | 3–9 | 26–43 | 3–124 | 232–308 | 8–300 |
| Continent | Asia | Europe | Arabia | America | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Country [Reference] | India [20,21,22,23,24] | Pakistan [25] | Malaysia [26,27] | Bangladesh [29] | Thailand [30] | China [31,32] | Turkey [41,42] | Spain [33,34,35] | Italy [36] | Austria [4] | Romania [10] | Greece [4] | Iraq [37,38,39] | Mexico [40] |
| Textile WW Source | Raw WWs of a Textile Factory (Tirupur/ Sumukh) | Raw WWs from 7 Mills/ Treatment Steps | Effluent Garment Factory/Pen Fabric Mill | Raw WWs from a Mill | Real Effluents from Dyebath | Raw Dye WW/Jinyang Industry/ Biostep | Real Effluent Factory/ Bursa/ Eskisehir | Raw WW of Rinsing Baths/ColorTex Industry | Real Effluents from Dyebath | Mixed WWs from Poly-Ester Fibers Processing | Real WWs from Cotton Fabrics Factory | Real WWs of Epilektos SA Factory | WWs of Al-Hilla Factory/ 14 Mills | Rinsing Step of a Denim Factory |
| Quality Indicator | ||||||||||||||
| pH | 4.8–10 | 7.5–11 | 5.5–10 | 3.9–14 | 5.59 | 7.4–8.3 | 7.8–9.1 | 6.9–7.8 | 8.5–9.5 | 6.36–6.67 | 6.6–8.3 | 8–8.2 | 5–8.5 | 6.84–7 |
| Turbidity, [NTU] | 240–290 | - | 63–74 | - | 417–423 | 40–137 | - | 8–15 | - | - | 205–280 | - | - | 100–105 |
| Color [ADMI/ Pt-Co] * | 420–2500 | - | 680–750 | - | 420–3000 | 310–325 | 1400–3000 | 390–540 | 60–660 | - | 150–390 | - | 85 | 330–600 |
| CODCr [mg O2/L] | 381–4400 | 125–705 | 231–990 | 41–2430 | 750–2600 | 61–1350 | 200–1953 | 200–806 | 972–1075 | 1380–6033 | 665–950 | 150 | 80–90 | 344–500 |
| BOD5 [mg O2/L] | 130–1750 | 115–653 | - | 10–876 | 25–530 | 6–10 | - | - | 8.8–100 | 177–720 | 250–320 | 80 | 50–60 | 85–120 |
| TSS [mg/L] | 8300–101,580 | 200–1619 | 39–11,689 | 25–3950 | 90–140 | - | 115–245 | 46–112 | - | 75–220 | 350–500 | - | 312–400 | 180–3000 |
| TDS [mg/L] | 2070–4800 | 2469–7295 | 14,000–107,500 | 90–5980 | 4800–5700 | - | - | 1456–1568 | - | - | 2000–2500 | - | 400–1350 | 2000–2100 |
| Chlorides [mg/L] | 980–4320 | 950–2750 | 1500 | 600 | - | - | - | 270–365 | 950–2750 | - | 80–500 | - | 542–550 | 300–330 |
| Dissolved phosphates [mg/L] | 72–87 | - | 5–10 | - | - | - | - | - | 3.1–3.3 | - | 5–15 | - | 0.64 | 50–287 |
| Sulphates [mg/L] | 2050–2250 | 600–1000 | 250 | 100 | - | - | - | 124–176 | 300–450 | - | 100–800 | - | 410–580 | 200–230 |
| Total N [mg/L] | - | - | - | - | - | - | - | - | - | 7.53–5.2 | 20 | - | - | - |
| NO3-N [mg/L] | 3.6–627 | - | - | - | - | - | - | - | 3.7–3.9 | 0.26–1.1 | 3.2–3.5 | - | - | 1.9 |
| NH4-N [mg/L] | 1.7 | - | 2.1–3.8 | - | - | - | - | - | 36–44 | 0.76–3.7 | 2.5 | - | - | - |
| Carbonate (CO32−) [mg/L] | 110–120 | - | - | - | - | - | - | 96–98 | - | - | - | - | - | 0.35 |
| HCO3− [mg/L] | 555–1464 | - | - | - | - | - | - | 800–1000 | - | - | - | - | - | 101.5 |
| Chemical Constituents of Textile Colored WW | Concentration, [mg/L] | Dye Type | Reported Treatment Method | Testing Country | Reference |
|---|---|---|---|---|---|
| Starch, Acetic acid, Sucrose, NaOH, H2SO4, Na2CO3, NaCl, Sodium lauryl sulphate, Dyes mixture: Reactive Black 5+ Congo Red + Disperse Blue 3 | 1000, 200, 600, 500, 300, 500, 3000, and 100 200 | Reactive Acid Disperse | Coagulation–flocculation | India | [28,43] |
| Starch, Ammonium sulphate, Disodium phosphate, Reactive Violet 4 | 2.78, 5.56, 5.56 80 | Reactive | Electrocoagulation | Marocco | [44] |
| NaCl, NaOH, Na2CO3, Reactive dye | 40,000, 1500, 2000, 600 | Reactive | Adsorption by shale column | Thailand | [30] |
| Meat extract, Urea, K2HPO4, NaCl, CaCl2.H2O, MgSO4.7H2O, Acid Orange 7 | 110, 30, 28, 7, 4, 2, 20 | Acid | Adsorption by PAC and activated sludge | Spain | [45] |
| NaCl, Dyes mixture: Everzol Black + Everzol Blue + Everzol Red | 500, 60 (mixture) | Reactive | Ultrafiltration/nanofiltration | Spain | [34] |
| Na2CO3, NaOH, NaCl, Reactive Black 5 | 40, 20, 600, 130 | Reactive | Nanofiltration | Austria | [46] |
| Polyvinyl alcohol, Remazol Turquoise Blue G-133, Irgapadol MP, Reactive Black 5, Disperse Yellow 211, Vat Yellow 46 | 100, 50, 2000, 30, 30, 30 | Reactive, Vat Disperse | Advanced oxidation–Fenton process | Turkey | [47] |
| Acetic acid, NaCl, Na2CO3, NaOH, Polyether based co-polymer micro-dispersion, Acryl co-polymer-phosphorus mixture, Acryl phenol polyglycol ether, Procion Blue HERS, Procion Crimson HEXL, Procion Yellow HE4R, Procion Navy HEXL, Procion Yellow HEXL | 790, 41,000, 13,000, 510, 1200, 850, 500, 6.83, 40.6, 15, 86.3, 33.3 | Reactive | Advanced oxidation (AO) | Australia | [48] |
| Polyvinyl alcohol, Reactive Blue R94H | 125, 20 | Reactive | AO–Fenton process | Taiwan | [49] |
| Starch, NaCl, Remazol Red | 465, 10,000, 10 | Reactive | Anaerobic biofilm reactor | India | [21] |
| Starch, Lab Lemco, Ammonium sulphate, MgSO4.7H2O, CaCl2, FeSO4.7H2O, NiSO4.7H2O, MgCl2. H2O, ZnSO4.7H2O, Boric acid, CoCl2.7H2O, CuSO4.5H2O, Maxilon Red | 1280, 400, 353, 108, 40, 0.75, 0.50, 0.50, 0.50, 0.10, 0.05, 0.005, 25–50 | Reactive | Suspended biofilter using activated sludge | UK | [50] |
| D-glucose, NaCl, FeCl3.H2O, ZnSO4.7H2O, MgSO4.7H2O, Boric acid, CuSO4.5H2O, MgCl2.2H2O, Ammonium molybdate, MnCl2. 2H2O, Al2(SO4)3. 6H2O, CaCl2.2H2O, CoCl2.6H2O, Thiamine-HCl hydrogen, Reactive Yellow 22 | 100, 50, 7100, 1, 5000, 1, 1, 1, 1, 80, 80, 550, 10,000, 2000–250, 500, 1000 | Reactive | Biological treatment by algae | India | [51] |
| Cotton Blanc KRS, Biavin BPA, Meropan DA, Na3PO4, NaOH, Na2SO4, Acetic acid, Ammonium sulphate, Disperse Blue 1, Disperse Orange 3 | 330, 330, 170, 330, 1000, 900, 170, 600, 12, 20 | Disperse | Anoxic-aerobic photo-bioreactor | Spain | [52] |
| Slipper, Mollan, Na2CO3, NaOH, NaCl, Acetic acid, Na2S2O3, Procion Marine HEXL | 1000, 125, 10,000, 1320, 63,000, 500, 2000, 20 | Reactive | Catalytic wet hydrogen peroxide oxidation (FeY11.5 or Y5) | Germany | [53] |
| Operating Characteristics | Coagulation by Double Layer Compression | Coagulation by Charge Neutralization and Adsorption | Co-Precipitation with Coagulation and Adsorption | Flocculation by Interparticle Bridging |
|---|---|---|---|---|
| Destabilization chemicals (coagulant/flocculant) | Non-hydrolyzing counter ions | Hydrolyzing salts (Fe3+, Al3+ salts); superficial active counter ions, soluble polynuclear compounds | Metal ions and anions, Hybrid materials | Polymers/co-polymer, Polyelectrolytes, Hybrid materials |
| Electrostatic effects | Predominant | Important | Important | Sub-ordinated |
| Chemical and adsorption effects | Usually do not take place | Important | Important | Predominant |
| Zeta potential for aggregation | Almost zero | Almost zero | Often, different to zero | Frequent, different of zero |
| Physical properties of formed aggregates | Dense and resistant coagulation | Dense, filtrable, easily dehydrated coagulation | Easily filtrable and settable, tridimensional | Easily breakable, voluminous, tridimensional, easy filtrable flocs |
| Addition of agent | Without effect | Re-stabilization because of charge exchange | Re-stabilization because of charge and ionic exchange | Re-stabilization because of complete surface covering |
| Surface covering degree for destabilization | Not observable | 0 < ϕ < 1 | 0 < ϕ < 1 | ϕ = 0.5 |
| Critical content of destabilization/ re-stabilization agent | Independent of disperse phase concentration. | Stoichiometric towards superficial concentration of disperse phase | Dependent on disperse phase concentration and charge | Stoichiometric |
| Treated WW | Pollutant | Efficiency as Coagulant-Flocculant, [%] | |||
|---|---|---|---|---|---|
| Conventional Coagulants-Flocculants | I—Structurally Hybridized Materials (Composites) | II—Chemically Bound Hybridized Materials | III—Functionally Hybridized Materials | ||
| Effluent from antibiotics’ production company [155,160,161,163] | Antibiotic (amoxicillin, diclofenac, tetracyclin) | - Sodium alginate: 20–39% diclofenac | - chitosan-based magnetic hybrid materials: 80–88% antibiotics | - 3D alginate-based MOP, biopolymer-based hybrid material: 80% diclofenac in 60–90 min | - Chitosan-Fe(II)/Ni(II) nano hybrid material: 93% antibiotic, 85% diclofenac |
| Textile effluent [72,108,116,125,126,127,130,142,156,157,158,159,162] from: - fabric laundry - fabric dyeing mill | Organics as dyes (COD), fine solids (T), suspended solids (SS) | - alum: 20% T, 42% BOD, 43.2–65% COD, 74% color, - FC: 71% dye, 98% SS - FS: 90% dye - PAC: 80% direct dyes | - PAC-extract Hibiscus Rosa-Sinensis leaf: 77.8% COD, 99.4% SS, 78.4% color | - PAM-Alum hybrid material: 68.2% COD, 61.4% SS | - Opuntia ficus-indica-Al(III) hybrid material: 64.77–87.19% COD, 91.26–93.62% T |
| Hospital WW [160] | Organics expressed by COD, fine particles (T) | - PAC: 74–82% COD, 60–78% T | - clay–silica–biopolymer hybrid material: 50–68% COD, 75–84% T | - biopolymer-based hybrid materials: 80% T, 60–80% COD | - PAC-M. oleifera seed hybrid material: 50% COD, 73.80% T |
| Effluent from palm oil mill (POME) [72,159] | Organics (COD, BOD, color), suspended solids (SS) | - alum: 15% T, 46% BOD, 41.8–55% COD, 45% color | - Alum-Cassia obtusifolia seed gum hybrid material: 48.22% COD, 81.58% SS | - Alum–silica monolites-titanium dioxide hybrid material: 70–90% in 60 min | - Alum-PFC-PAM hybrid material: 70–90% COD, 70–95% SS |
| Grey water [103,151,152,153,154] | Organics (COD, BOD, color), antibiotics | - alum: 35% T, 48% BOD, 43.2–63% COD, 71% color, | - laccase immobilized on Fe3O4/SiO2–DTPA hybrid nanocomposite: 99% diclofenac | - soybean peroxidase (SBP)-oxide nano particles-poly(styrene-co-maleic anhydride (SMA): 90% 2,4 dichlorophenol | - Alum-Cassava peels starch: 56.89% COD, 77.48% T, 77.34% SS |
| Property Type | Specific Property | Analysis Technique | Registered Data | Observations |
|---|---|---|---|---|
| Chemical | Chemical structure | FT-IR | - infrared spectrum of absorption, emission, photoconductivity, or Raman scattering, e.g., new peak in PFS–PAM spectra (blending at 50 °C) [152] | - bands associated with –OH vibrations of water or bridging OHs and with –O bond vibrations; - peaks’ intensity variation with molar ratio of constituents |
| Chemical species distribution | - monitoring of hydrolysis–polymerization process for finding changes of Fe(III) and Al(III) active species by - Ferron complexation timed spectro-photometry. - NMP spectroscopy | - difference of complex reaction rate between Ferron and chemical species (Al and Fe) (after reference time = 3 h) E.g., PFC–PDMDAAC, 7% organic excess decreases Fea and Fec but increases Feb [152] | - content/proportion of all kinds of Fe and Al species (Al a,b,c and Fe a,b,c) | |
| Physical | Molecular weight (MW)/intrinsic viscosity (η) | - Mark–Houwink eq.; - static light scattering analysis. - ultrafiltration followed by Al–Ferron timed complex colorimetry | - use of eq. η = f (MW) - selection of size for aggregating actions. - bridging ability efficiency, e.g., CMS-PAM, higher PAM% increases η [153] | - medium and high MWs requested for bridging with multiple interactions with/inter particles |
| Conductivity | - ionic content in aqueous solution | - increase/decrease of conductivity with variation of distinct species molar ratios in aqueous solutions - variation of Fe/Si ratio dependent on degree of hydrolysis | - increase in OH/Fe resulted in slight decrease in conductivity related to the degree of polymerization, e.g., PFSiS [151] | |
| Zeta potential | - electric surface potential measurement | - critical values for destabilization of dispersions, or neutralization, or various composition of materials [152] | - zeta potential value at different critical pH values for stabilization/destabilization of colloids in aqueous systems | |
| Thermal | - Differential scanning calorimetric analysis (DSC). - Thermal gravimetric analysis (TGA) | - DSC: finding of temperature or heat flow during phase transformations and transitions in solids - TGA: thermal decomposition of materials with temperature elevation | - critical temperature for each decomposition step; - weight loss (%) to determine the thermal stability of material. - DT – mass change region, e.g., stability of MgCl2–PAM decreases with increasing MgCl2 content [154] | - thermal decomposition dependent on temperature, heating rate. - hybrid materials with a higher positive value of activation energy (Flyn and Wall’s model) have better thermal stability |
| Morphological | - visual analysis, or - (SEM) scanning electron microscopy; - (TEM) transmission electron microscopy | - SEM: morphology and microstructure of solid hybrid sample. - TEM: molecular structure of solid hybrid in liquid form | - microstructure varies with the composition, functional groups, and reaction time [134,135] | - short chain-like and less branchy inorganic–inorganic hybrid material is a less favorable structure; a multi-branched structure with larger size / fractal dimension is desired |
| Structural | - XRD—energy-dispersive X-ray diffraction. - EDX—X-ray spectroscopy | - identification of the presence of organic material (usually amorphous for inorganics) | - identification of crystalline and amorphous phases, e.g., crystal-line phase prominent when increases (Al + Fe)/Si ratio in XRD [155] - atomic distribution of hybrid materials | - limited studies on the atomic distribution of hybrid materials. - introduction of hygroscopic component increases oxygen content |
| Characteristics | Orthokinetic Flocculation | Perikinetic Coagulation |
|---|---|---|
| Interparticle processes involved [96] | Hydrodynamic fluid motion or agitation in laminar (a) or turbulent (b) regimes (a), or (b) | Brownian interparticle contact |
| Maxwell–Boltzman distribution associated with sedimentation [95,96] | , , ln N/N0 = −4afGt/π, tF = 2/k1N0, or tA = −ln(1 − α)k12N0, ϕ = πd3N/6 | or N = N0/[1 + (4αkTN0/3μ)t], or t1/2 = 3μ/(4αkTN0) = 1.6·1011/(αN0) |
| where d—particle diameter (m); D—Brownian diffusion coefficient (D = kT/(3πμd)); G0—initial velocity gradient; G—velocity gradient; k—Boltzmann constant (J/K); k1, k12—appropiate rate constants; n (N)—number of particles (flocs)/volume unity (m−3); P—real dissipated power (m2 kg/s3, or W); T—absolute temperature (K); tF—flocculation time; tA—adsorption time; V—volume occupied of water (m3); α—fraction of efficient collisions to agglomeration; μ—absolute viscosity; ϕ—volume fraction of colloidal particles; η—dynamic viscosity (Kg/m·s). | ||
| Maxwell–Boltzman distribution associated with filtration [95,150] | For packed-bed filtration: where f—porosity; (1 − f)—the volume of filter media per volume unit of filter bed; d—bed depth, η—a single collector efficiency, reflecting the rate at which particle contacts occur between suspended particles and filter bed; N—number of agglomerated particles/flocs; α—fraction of efficient collisions to agglomeration. | |
| Overall rate for diminishing of particles [95,150]: | , usually G = 10/s, ϕ = 10−4, α = 10−1, and t = 103 s. | |
| Type of Hybrid Materials | Hybrid Materials | Dosage | Wastewater Type | Wastewater Characteristics | Experiments Conditions | Removal Efficiency (%) | Reference |
|---|---|---|---|---|---|---|---|
| Inorganic–inorganic hybrid polymer | PFSA (Polyferric-silicate-acetate) | 16 mg/L | Simulated dye wastewater (Congo Red) | 0.1 g/L, pH 7.50 ± 0.10; temperature, 20 ± 2 °C | Rapid mixing 300 rpm for 2 min, slow mixing 60 rpm for 10 min, settled for 20 min | Dye: 93.3% | [125] |
| Inorganic–inorganic hybrid polymer | PAC (Polyaluminum chloride) | 30 mg/L | Real textile wastewater | Color: 91.7 ± 11.4 Pt/Co, BOD5: 278.54 ± 65.23 mg O2/L, COD: 1346.17 ± 123.36 mg O2/L, TSS: 178.28 ± 23.82 mg/L | Rapid mixing at 150 rpm for 4 min, slow mixing at 40 rpm for 20 min, settle for 1 h | Dye: 44.5% COD: 40% BOD5: 34% TSS: 23.7% | [126] |
| Inorganic–inorganic hybrid polymer | PFTS (Poly-ferric-titanium-silicate-sulfate) | 0.4 mmol/L | Synthetic dye wastewater (Disperse Blue 56) | 0.1 g/L pH 8–9 | Rapid stirring at 200 rpm for 1.5 min, slow stirring at 40 rmp for 15 min for floc formation; rapid stirring at 200 rmp for 5 min for breakage; slow stirring at 40 rmp for 15 min for recovery | Dye: 95.5% Residual turbidity: 7.0 FTU | [127] |
| Inorganic–inorganic hybrid polymer | PFTS (Poly-ferric-titanium-silicate-sulfate) | 0.4 mmol/L | Synthetic dye wastewater (Reactive Yellow) | 0.1 g/L pH 8.0–9.0 | Rapid stirring at 200 rmp for 1.5 min, slow stirring at 40 rmp for 15 min for floc formation; rapid stirring at 200 rmp for 5 min for breakage; slow stirring at 40 rmp for 15 min for recovery | Dye: 49.5% Residual turbidity: 6.4 FTI | [127] |
| Inorganic–inorganic hybrid polymer | MgSiPC (Magnesium silicate) | 62.0–78.0 mg/L | Reactive dye simulated wastewater (Reactive Yellow2) | 100 mg/L pH 12.08/12.0 | Rapid stirring at 300 rpm for 2 min, slow stirring at 60 rpm for 10 min, settle for 20 min. | Dye (RY2): 90–93% | [128] |
| Inorganic–inorganic hybrid polymer | PAC (Polyaluminium chloride) | 1000 mg/L | Aqueous mixed solutions | 7 mg Pb(II) /L, 5 mg Zn(II)/L, pH 8.7–9.2 | Mixed at 60 to 65 rpm for 3 min; settled for 30 min | Pb (II): 92% Zn (II): 98% | [129] |
| Inorganic–organic hybrid material | PAC-EPI-DMA (Polyaluminum chloride-epichloro-hydrin dimethylamine) | 10.8 mg/L | Synthetic dying solution (Reactive Brilliant Red K-2BP) | 100 mg/L pH 8.45 | Rapid mixing at 120 rpm for 3 min, slow mixing at 40 rpm for 12 min, settling time 20 min | Color: 90% | [130] |
| Inorganic–organic hybrid polymer | PACl–PAMIPCl (Polyaluminum chloride–poly(3-acryl-amido-isopropanol chloride) | 50 mg/L | Synthetic dying solution (Reactive Cibacron Blue F3GA) | pH 6.5–6.9, COD 70–80 mg O2/L, color 1050–1100 Pt/Co | Rapid mixing for 3 min at 120 rpm, slow agitation for 12 min at 40 rpm, settling time 30 min pH 7.5 | COD: 92% Color: 95% | [131] |
| Inorganic–organic hybrid polymer | PACl–PAMIPCl (Polyaluminum chloride–poly(3-acrylamido-isopropanol chloride) | 20 mg/L | Synthetic dying solution (Disperse Terasil Yellow W-4G) | pH 7.0–7.4, COD 140–150 mg O2/L, color 4550–4700 Pt/Co | Rapid mixing for 3 min at 120 rpm, slow agitation for 12 min at 40 rpm, settling time 30 min, pH 3 | COD: 93% Color: 96% | [131] |
| Inorganic–organic hybrid polymer | MCPAM (Magnesium chloride-polyacrylamide) | 1200 mg/L | Simulated reactive dye WW (Cibacron Red FN-R) | 200–500 mg/L, pH 12; temperature, 20 °C | Agitation speed of 100 rpm for 5 min, settling time 30 min | Dye: 99% | [132] |
| Inorganic–organic hybrid polymer | MgCl2-PEO (Magnesium chloride-polyethylene oxide) | 1020 mg/L | Synthetic aqueous dye solution (Cibacron Blue F3GA) | 173 mg/L, pH 11.13 | Mixing speed 150 rpm for 6 min, settling time 30 min | COD: 92.09% Color: 99.76% | [133] |
| Inorganic–organic hybrid polymer | FeCl3–PAM (ferric chloride–polyacrylamide) | 500 mg/L | Synthetic dye wastewater (Terasil Red R) | 200 mg/L, pH 5.58–5.95, COD 278–412 mg O2/L, color 3860–4320 Pt/Co | Rapid mixing 200 rpm for 3 min, slow mixing 100 rpm for 6 min, settling for 30 min, pH 5 | COD: 89% Color: 99% | [134] |
| Inorganic–organic hybrid material | MgCl2-PAM (magnesium chloride-polyacrylamide) | 1000 mg/L | Real textile WW | T = 44.2–46.5 °C, pH 11; turbidity, 24.9–26.2 NTU; conductivity, 1919–1967 µS/cm; TDS, 962–987 mg/L; color, 810–850 Pt/Co; COD, 762–784 mg O2/L | Mixing speed 100 rpm for 5 min | COD: 26.4% Color: 82.8% | [135] |
| Inorganic–organic hybrid polymer | Al(OH)3-PAM (aluminium hydroxide-polyacrylamide) | 700 mg/L | Dye wastewater (Reactive Blue 19) | 1000 mg dye/L pH 5–6 | Rapid mixing of 500 rpm for 1 min, slow mixing 200 rpm for 10 min, settling time 60 min | COD: 82% Dye: 90% | [136] |
| Inorganic–natural hybrid material | ALAV (Aluminium sulphate-Aloe vera) | 3000 mg/L | Dye wastewater (Methylene Blue) | 10 mg MB /L, pH 6 | Mixing speed 100 rpm, settling time 30 min | Dye: 50–55% | [137] |
| Inorganic–natural hybrid material | MGAV (Magnesium sulphate -Aloe vera) | 3000 mg/L | Dye wastewater (Methylene Blue) | 10 mg MB /L, pH 12.5 | Mixing speed 100 rpm, settling time 30 min | Dye: 60–70% | [137] |
| Inorganic–natural polymer hybrid materials | PSAF–CTS (Polysilicate Aluminum Ferric-Chitosan) | 18.0 mg/L | Wastewater containing heavy metals ((CrO4)2−, Ni2+) | pH 9 | Rapid stirring at 150 rpm for 5 min, slow stirring at 80 rpm, settle for 30 min. | Cr6+: 100% Ni2+: 82.2% Turbidity: 99.5% | [138] |
| Inorganic–natural polymer hybrid materials | CMNP (Chitosan-Coated Magnetite Nanoparticles) | 1.5 g/50 mL sample | Aqueous solution containing Pb2+ and Cu2+ ions | 0.1 mmol/L, pH 4 | Stirring at 100 rpm for 60 min | Lead (Pb2+): 98% Coopper (Cu2+): 98% | [139] |
| Inorganic–natural polymer hybrid materials | CMCTS-g-P(AM-CA) (Carboxymethyl chitosan—acrylamide—ammonium dithiocarbamate) | 50 mg/L | Simulated heavy metal-containing wastewater | 25 mg Pb2+/L 25 mg Cd2+/L pH 5–6 | Rapid mixing 300–400 rpm for 3–5 min, slow mixing 50–70 rpm for 10–15 min, settling time 15 min | Pb2+: 95.24% Cd2+: 95.72% | [140] |
| Inorganic–natural polymer composite material | PAFC-Starch-g-p(AM-DMDAAC) (polyaluminium ferric chloride-starch graft co-polymer with acrylamide and dimethyl diallyl ammonium chloride) | 0.2 mg/mL dye | Synthetic textile wastewater (Brilliant Blue KN-R) | 100 mg/L, pH 3.54, conductivity, 23.7 mS/cm; temperature, 80 °C | Mixing at 120 rpm for 1 min, 80 rpm for 5 min, 30 rpm for 15 min, and settling for 30 min | Dye: 81.22% | [141] |
| Natural–inorganic composite material | Extract of Moringa oleifera Lam seeds (5 g) in 100 mL of 1M NaCl (1600 mg/L) and KCl solution—1000 mg/L Al2(SO4)3 | pH 5–6 and 820 mg/L AS; pH 2 and 2064 mg/L MO-KCl/2774 mg/L MO-NaCl | Real wastewater from industrial laundry containing reactive dyes, RP-HE8B and OP-HER | pH 10.9, color 4500 mg Pt-Co/L, COD, 5820 mg O2/L; turbidity, 66.8 NTU | Mixing for few min and settling for 30 min | Color: 82.2% COD: 83.04% RP-HE7B dye: 78.4% OP-HER dye: 89.7% | [142] |
| Natural–organic polymer composite material | Lignin-METAC (lignin-[2-(methacryloyloxy) ethyl] trimethyl ammonium chloride) | 120 mg/L | Simulated dye solutions (Reactive Black 5) | 100 mg/L pH 2–8 | Temperature 30 °C, mixing of 150 rpm for 10 min, centrifuged at 1500 rpm for 10 min | RB5: 98% COD: 95% | [143] |
| Natural–organic polymer composite material | Lignin-METAC (lignin-[2-(methacryloyloxy) ethyl] trimethyl ammonium chloride) | 105 mg/L | Simulated dye solutions (Reactive Orange 16) | 100 mg/L pH 2–8 | Temperature 30 °C, mixing of 150 rpm for 10 min, centrifuged at 1500 rpm for 10 min | RO16: 94% COD: 95% | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaharia, C.; Musteret, C.-P.; Afrasinei, M.-A. The Use of Coagulation–Flocculation for Industrial Colored Wastewater Treatment—(I) The Application of Hybrid Materials. Appl. Sci. 2024, 14, 2184. https://doi.org/10.3390/app14052184
Zaharia C, Musteret C-P, Afrasinei M-A. The Use of Coagulation–Flocculation for Industrial Colored Wastewater Treatment—(I) The Application of Hybrid Materials. Applied Sciences. 2024; 14(5):2184. https://doi.org/10.3390/app14052184
Chicago/Turabian StyleZaharia, Carmen, Corina-Petronela Musteret, and Marius-Alexandru Afrasinei. 2024. "The Use of Coagulation–Flocculation for Industrial Colored Wastewater Treatment—(I) The Application of Hybrid Materials" Applied Sciences 14, no. 5: 2184. https://doi.org/10.3390/app14052184
APA StyleZaharia, C., Musteret, C.-P., & Afrasinei, M.-A. (2024). The Use of Coagulation–Flocculation for Industrial Colored Wastewater Treatment—(I) The Application of Hybrid Materials. Applied Sciences, 14(5), 2184. https://doi.org/10.3390/app14052184