Efficiency of Chemical Toilet Sewage (CTS) Co-Fermentation with Typical Energy Crops
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Outline
2.2. Materials
2.3. Anaerobic Reactors
2.4. Analytical Methods
2.5. Calculation Methods
- —portion digested, %;
- —VS in the feedstock, g/dm3;
- —VS in the digestate, g/dm3;
- —feedstock density, g/cm3;
- —digestate density, g/cm3;
- —feedstock volume, cm3/day;
- —digestate volume, cm3/day.
- —specific biogas production per VS in (by volume), cm3/gVS; —daily biogas/CH4 yield (by volume), cm3/day;
- —daily volume of feedstock in cm3.
2.6. Statistical Methods
3. Results
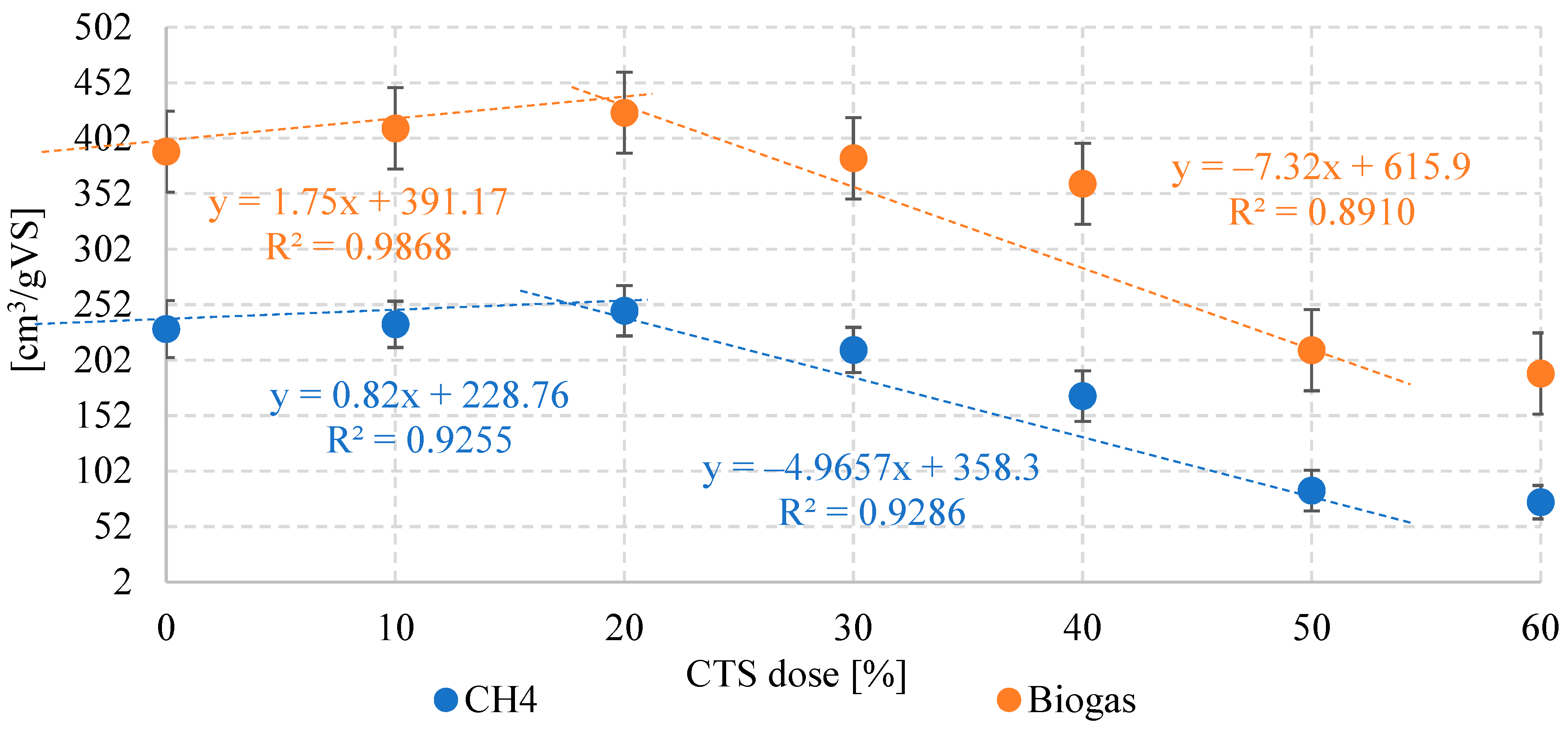
3.1. Biogas and CH4 Production
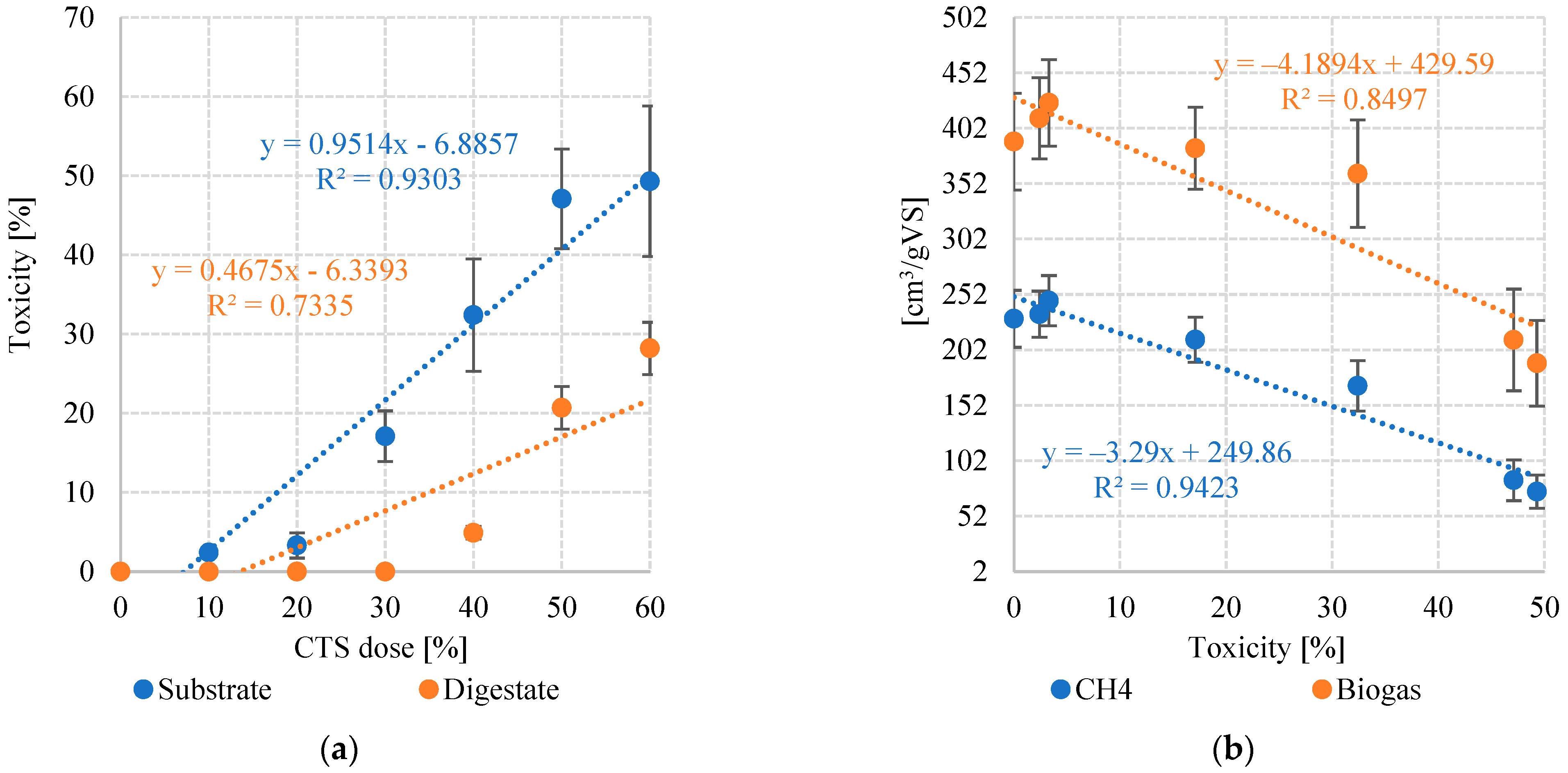
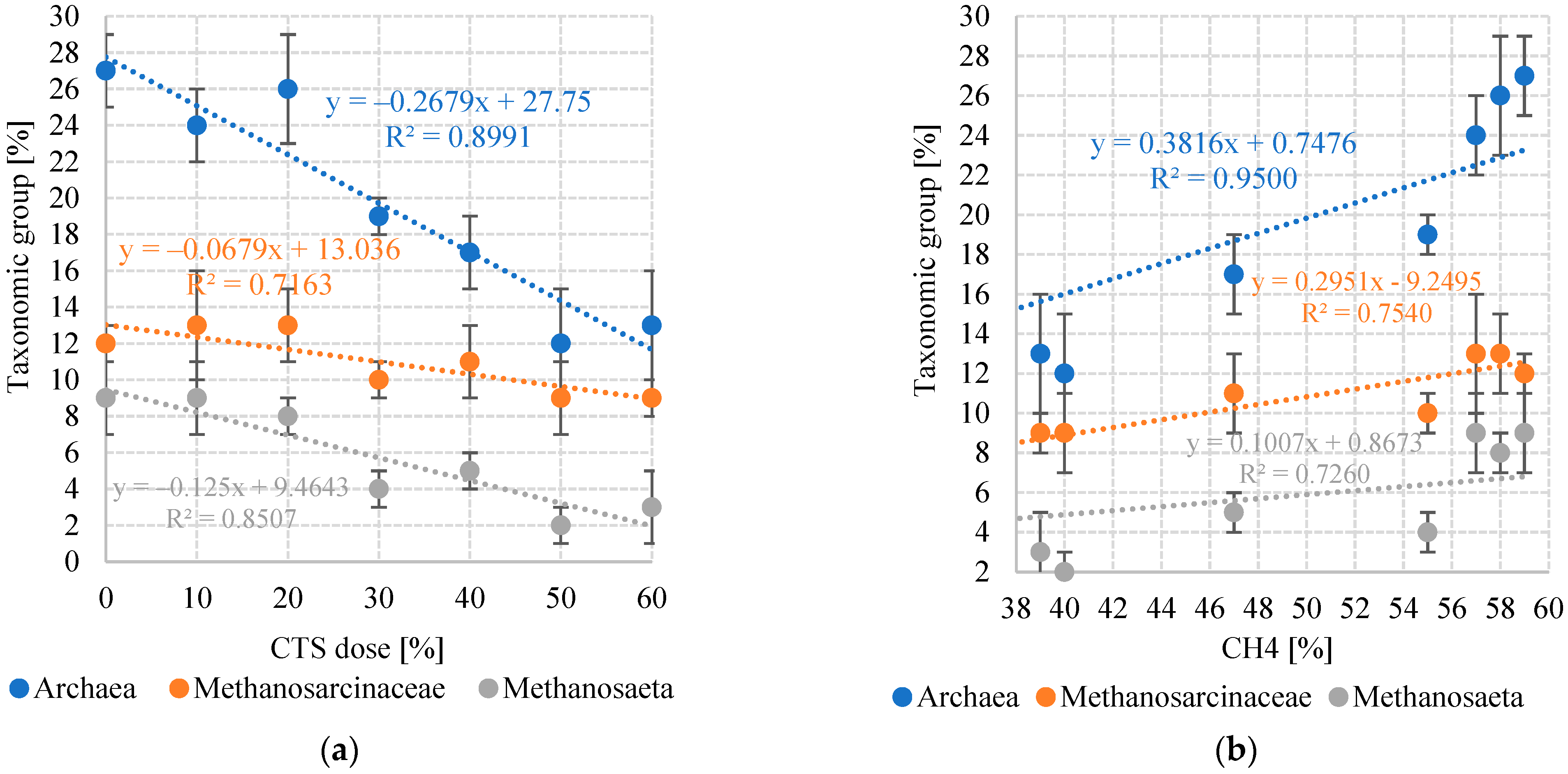
3.2. Toxicity and Bacterial Community
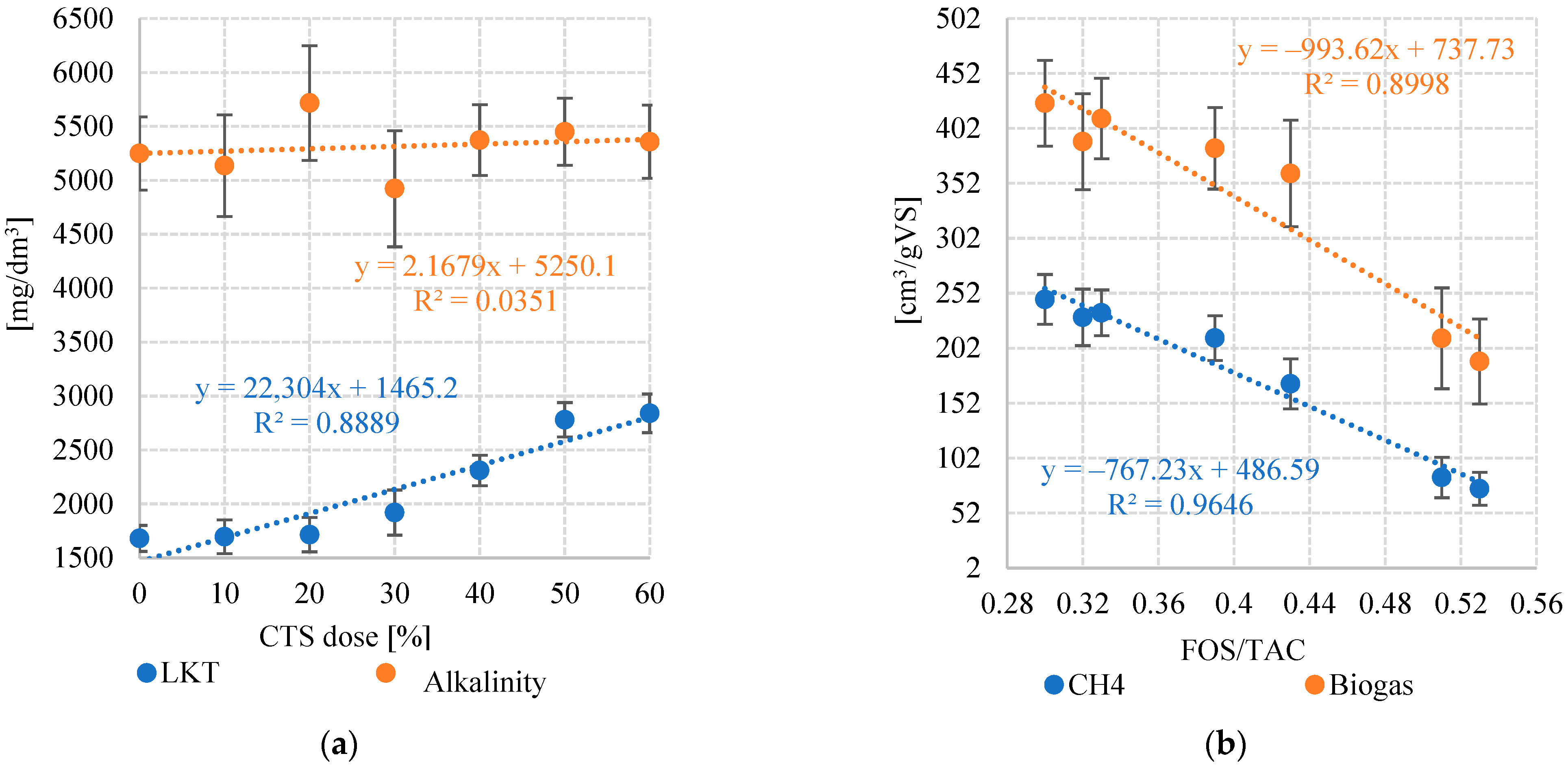
3.3. FOS/TAC and pH
3.4. Portion Digested (Digestion Coefficient) and Digestate Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Št’Astná, M.; Vaishar, A.; Brychta, J.; Tuzová, K.; Zloch, J.; Stodolová, V. Cultural Tourism as a Driver of Rural Development. Case Study: Southern Moravia. Sustainability 2020, 12, 9064. [Google Scholar] [CrossRef]
- Sultan, M.T.; Sharmin, F.; Badulescu, A.; Stiubea, E.; Xue, K. Travelers’ Responsible Environmental Behavior towards Sustainable Coastal Tourism: An Empirical Investigation on Social Media User-Generated Content. Sustainability 2020, 13, 56. [Google Scholar] [CrossRef]
- Siuda, W.; Grabowska, K.; Kaliński, T.; Kiersztyn, B.; Chróst, R.J. Trophic State, Eutrophication, and the Threats for Water Quality of the Great Mazurian Lake System. Pol. River Basins Lakes—Part I 2020, 86, 231–260. [Google Scholar] [CrossRef]
- Furgała-Selezniow, G.; Jankun-Woźnicka, M.; Woźnicki, P.; Cai, X.; Erdei, T.; Boromisza, Z. Trends in Lakeshore Zone Development: A Comparison of Polish and Hungarian Lakes over 30-Year Period. Int. J. Environ. Res. Public Health 2022, 19, 2141. [Google Scholar] [CrossRef] [PubMed]
- Swidy´nska, N.; Swidy´nska, S.; Witkowska, D.; Abrowska, M.; -Fernández, P. Indicators of the Tourist Attractiveness of Urban–Rural Communes and Sustainability of Peripheral Areas. Sustainability 2021, 13, 6968. [Google Scholar] [CrossRef]
- Kaźmierczak, R.; Szczepańska, A.; Grunwald, G.; Kowalczyk, C.; Tanajewski, D. Conceptual Framework of a Global Yacht Positioning System in Poland. Appl. Geomat. 2022, 14, 79–91. [Google Scholar] [CrossRef]
- Klasios, N.; Pozas, A.; Sha, J. The Great Bear Sea—a Toilet Bowl for Marine Vessels: Quantifying Waste and the Ecological, Social, Cultural and Economic Risks of Marine Dumping; The University of British Columbia: Vancouver, BC, Canada, 2020. [Google Scholar] [CrossRef]
- Alamanos, A.; Mcgrath, T.; Linnane, S. Treatment of Domestic WasteWater and Opportunities from Nature-Based Solutions for Small Communities: A Preliminary Assessment. Background Report January 2022. Available online: https://www.researchgate.net/publication/363728547_Treatment_of_Domestic_WasteWater_and_Opportunities_from_Nature-based_Solutions_for_small_communities_a_preliminary_assessment?channel=doi&linkId=632b55ac071ea12e364efdea&showFulltext=true (accessed on 3 January 2023).
- Bedynski, W.; Godz, J.; Łukowski, W. Urban Social Movements in a Small Town: The Case of “Aktywne Giżycko”. Przegląd Kryt. 2021, 3, 79–98. [Google Scholar] [CrossRef]
- Wang, S.; Ji, C.; He, H.; Zhang, Z.; Zhang, L. Tourists’ Waste Reduction Behavioral Intentions at Tourist Destinations: An Integrative Research Framework. Sustain. Prod. Consum. 2021, 25, 540–550. [Google Scholar] [CrossRef]
- Chatema, T.M. Effect of Ageing on the Physicochemical Properties of Fresh Faeces. Ph.D. Thesis, University of KwaZulu-Natal, Durban, South Africa, 2021. [Google Scholar]
- Byrnes, T.A.; Dunn, R.J.K. Boating- and Shipping-Related Environmental Impacts and Example Management Measures: A Review. J. Mar. Sci. Eng. 2020, 8, 908. [Google Scholar] [CrossRef]
- Vítěz, T.; Vítězová, M.; Nováčková, M.; Kushkevych, I. Activated Sludge Respiration Activity Inhibition Caused by Mobile Toilet Chemicals. Process 2020, 8, 598. [Google Scholar] [CrossRef]
- Tawfik, A. Valorization of Anaerobic Digestate from Microalgal Treatment. In Anaerobic Digestate Management; IWA Publishing: London, UK, 2022; p. 111. [Google Scholar] [CrossRef]
- OECD. Guideline for Testing of Chemicals 201. Alga, Growth Inhibition Test; OECD: Paris, France, 1984. [Google Scholar]
- Schinteie, R.; Tran-Dinh, N.; Vergara, T.J.; Midgley, D.J. An Estimation of Chemical Compound Concentrations Used in Onshore Gas Production, a Review of Their Degradation, and Associated Policy Frameworks in South Australia; CSIRO Energy: North Ryde, NSW, Australia, 2019. [Google Scholar]
- Omran, B.A.; Abdel-Salam, M.O. The Catastrophic Battle of Biofouling in Oil and Gas Facilities: Impacts, History, Involved Microorganisms, Biocides and Polymer Coatings to Combat Biofouling; Springer: Berlin/Heidelberg, Germany, 2020; pp. 47–99. [Google Scholar] [CrossRef]
- Anger, C.B.; Rupp, D.; Lo, P.; Takruri, H. Preservation of Dispersed Systems. In Pharmaceutical Dosage Forms 2020; CRC Press: Boca Raton, FL, USA, 2020; pp. 377–436. [Google Scholar] [CrossRef]
- Kircher, M.; Bayer, T. How to Realize an Urban Circular Bioeconomy. In Handbook Waste Biorefinery; Springer: Berlin/Heidelberg, Germany, 2022; pp. 895–913. [Google Scholar] [CrossRef]
- Kazimierowicz, J.; Dzienis, L.; Dębowski, M.; Zieliński, M. Optimisation of Methane Fermentation as a Valorisation Method for Food Waste Products. Biomass Bioenergy 2021, 144, 105913. [Google Scholar] [CrossRef]
- Markowski, M.; Białobrzewski, I.; Zieliński, M.; Dębowski, M.; Krzemieniewski, M. Optimizing Low-Temperature Biogas Production from Biomass by Anaerobic Digestion. Renew. Energy 2014, 69, 219–225. [Google Scholar] [CrossRef]
- Schumüller, K.; Weichgrebe, D.; Köster, S. Biogas Potential of Organic Waste Onboard Cruise Ships—a yet Untapped Energy Source. Biomass Convers. Biorefinery 2021, 12, 5647–5662. [Google Scholar] [CrossRef]
- PN-EN ISO 5815-1:2019-12; Water Quality—Determination of Biochemical Oxygen Demand after n Days (BODn). Polish Committee for Standardization: Warsaw, Poland, 2019. Available online: https://sklep.pkn.pl/pn-en-iso-5815-1-2019-12e.html (accessed on 10 December 2022).
- Wolska, L.; Mędrzycka, K. Assessing the Ecotoxicity of the Bottom Sediments from the Sea Ports of Gdansk and Gdynia. Environ. Pollut. Control 2009, 31, 49–52. [Google Scholar]
- Nielsen, P.H.; Lemmer, H.; Daims, H. Identification and Quantification of Microorganisms in Activated Sludge and Biofilms by FISH: Introduction. In FISH Handbook for Biological Wastewater Treatment; Balint, G., Antala, B., Carty, C., Mabieme, J.-M.A., Amar, I.B., Kaplanova, A., Eds.; IWA Publishing: London, UK, 2009; Volume 7, pp. 1–8. [Google Scholar] [CrossRef]
- Rajeshwari, K.V.; Balakrishnan, M.; Kansal, A.; Lata, K.; Kishore, V.V.N. State-of-the-Art of Anaerobic Digestion Technology for Industrial Wastewater Treatment. Renew. Sustain. Energy Rev. 2000, 4, 135–156. [Google Scholar] [CrossRef]
- Stahl, D.A.; Amann, R.I. Development and Application of Nucleic Acid Probes. In Nucleic Acid Techniques in Bacterial Systematic; Stackbrandt, E., Goodfellow, M., Eds.; John Wiley & Sons: New York, NY, USA, 1991; pp. 205–248. [Google Scholar]
- Raskin, L.; Stromley, J.M.; Rittmann, B.E.; Stahl, D.A. Group-Specific 16S RRNA Hybridization Probes to Describe Natural Communities of Methanogens. Appl. Environ. Microbiol. 1994, 60, 1232–1240. [Google Scholar] [CrossRef] [Green Version]
- Litti, Y.V.; Serdyukov, D.V.; Kanunnikov, O.V.; Akselrod, V.A.; Loiko, N.G. Antimicrobial Properties of a Biocide Based on Quaternary Ammonium Compounds plus Polyhexamethylene Guanidine and Possible Methods for Its Deactivation. Appl. Biochem. Microbiol. 2021, 57, 962–972. [Google Scholar] [CrossRef]
- Wasielewski, S.; Morandi, C.G.; Mouarkech, K.; Minke, R.; Steinmetz, H. Impacts of Blackwater Co-Digestion on Biogas Production in the Municipal Wastewater Treatment Sector Using Pilot-Scale UASB and CSTR Reactors. Desalin. Water Treat 2017, 91, 121–128. [Google Scholar] [CrossRef] [Green Version]
- Wendland, C. Anaerobic Digestion of Blackwater and Kitchen Refuse, Technischen Universität Hamburg-Harburg zur Erlangung des Akademischen Grades; Technische Universität Hamburg: Hamburg, Germany, 2009. [Google Scholar]
- Reysset, M. Comprehensive Overview of Biogas for Sanitation Options—Training of Trainers. Gates Open Res. 2021, 5, 1–64. [Google Scholar] [CrossRef]
- Litti, Y.V.; Serdyukov, D.V.; Kanunnikov, O.V.; Axelrod, V.A.; Loiko, N.G. Treatment of Preserved Fecal Sludge Using Anaerobic Digestion. Ecol. Ind. Russ. 2020, 24, 33–37. [Google Scholar] [CrossRef]
- Krause, A.; Häfner, F.; Augustin, F.; Udert, K.M. Qualitative Risk Analysis for Contents of Dry Toilets Used to Produce Novel Recycling Fertilizers. Circ. Econ. Sustain. 2021, 1, 1107–1146. [Google Scholar] [CrossRef] [PubMed]
- Loiko, N.; Kanunnikov, O.; Serdyukov, D.; Axelrod, V.; Tereshkin, E.; Vishnyakova, A.; Litti, Y. Didecyldimethylammonium Chloride- and Polyhexamethylene Guanidine-Resistant Bacteria Isolated from Fecal Sludge and Their Potential Use in Biological Products for the Detoxification of Biocide-Contaminated Wastewater Prior to Conventional Biological Treatment. Biology 2022, 11, 1332. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Duan, X.; Chen, J.; Fang, K.; Feng, L.; Yan, Y.; Zhou, Q. Enhancing Anaerobic Digestion of Waste Activated Sludge by Pretreatment: Effect of Volatile to Total Solids. Environ. Technol. 2016, 37, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Świątczak, P.; Cydzik-Kwiatkowska, A. Performance and Microbial Characteristics of Biomass in a Full-Scale Aerobic Granular Sludge Wastewater Treatment Plant. Environ. Sci. Pollut. Res. 2018, 25, 1655–1669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alcántara-Hernández, R.J.; Taş, N.; Carlos-Pinedo, S.; Durán-Moreno, A.; Falcón, L.I. Microbial Dynamics in Anaerobic Digestion Reactors for Treating Organic Urban Residues during the Start-up Process. Lett. Appl. Microbiol. 2017, 64, 438–445. [Google Scholar] [CrossRef]
- McHugh, S.; Carton, M.; Mahony, T.; O’Flaherty, V. Methanogenic Population Structure in a Variety of Anaerobic Bioreactors. FEMS Microbiol. Lett. 2003, 219, 297–304. [Google Scholar] [CrossRef] [Green Version]
- Zieliński, M.; Zielińska, M.; Cydzik-Kwiatkowska, A.; Rusanowska, P.; Dębowski, M. Effect of Static Magnetic Field on Microbial Community during Anaerobic Digestion. Bioresour. Technol. 2021, 323, 124600. [Google Scholar] [CrossRef]
- Mata-Alvarez, J.; Macé, S.; Llabrés, P. Anaerobic Digestion of Organic Solid Wastes. An Overview of Research Achievements and Perspectives. Bioresour. Technol. 2000, 74, 3–16. [Google Scholar] [CrossRef]
- Dębowski, M.; Kazimierowicz, J.; Zieliński, M.; Bartkowska, I. Co-Fermentation of Microalgae Biomass and Miscanthus × Giganteus Silage—Assessment of the Substrate, Biogas Production and Digestate Characteristics. Appl. Sci. 2022, 12, 7291. [Google Scholar] [CrossRef]
- Kazimierowicz, J. Organic Waste Used in Agricultural Biogas Plants. J. Ecol. Eng. 2014, 15, 88–92. [Google Scholar] [CrossRef]
- Abbas, Y.; Yun, S.; Mehmood, A.; Shah, F.A.; Wang, K.; Eldin, E.T.; Al-Qahtani, W.H.; Ali, S.; Bocchetta, P. Co-Digestion of Cow Manure and Food Waste for Biogas Enhancement and Nutrients Revival in Bio Circular Economy. Chemosphere 2022, 311, 137018. [Google Scholar] [CrossRef] [PubMed]
- Rwigema, A. Safety and Nutrient Values (NPK) of Bio-Effluent from Human Excreta Used to Feed Biogas Plants in Rwanda. Rwanda J. Eng. Sci. Technol. Environ. 2019, 2, 1–12. [Google Scholar] [CrossRef]
- Nakarmi, A.; Karki, A.B. Biogas as Renewable Source of Energy in Nepal: Theory and Development; Alternative Energy Promotion Centre: Kathmandu, Nepal; Biogas Sector Partnership–Nepal: Kathmandu, Nepal, 2005. [Google Scholar]
- Warnars, L. Bioslurry: A Supreme Fertiliser Positive Effects of Bioslurry on Crops. In Proceedings of the Practitioners’ Track, IFOAM Organic World Congress 2014, ‘Building Organic Bridges’, Istanbul, Turkey, 13–15 October 2014. [Google Scholar]
- Yerizam, M.; Norfhairna, N.; Margaretty, E.; Hajar, I.; Meilianti, M.; Supraptiah, E.; Silviyati, I.; Salsabila, J. Liquid Organic Fertilizer Production from Kersen Leaves (Muntingia calabura L.) and Eggshells with Addition of Spoiled Rice Local Microorganism (Mol) Bioactivator. In Proceedings of the 5th FIRST T1 T2 2021 International Conference (FIRST-T1-T2 2021), Paris, France, 26–28 October 2022; Volume 9, pp. 426–430. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Banerjee, S.; Banerjee, S.; Singh, V.; Das, D. Sustainable Approach for the Treatment of Poultry Manure and Starchy Wastewater by Integrating Dark Fermentation and Microalgal Cultivation. J. Mater. Cycles Waste Manag. 2021, 23, 790–803. [Google Scholar] [CrossRef]

| Parameter | Unit | Value | |
|---|---|---|---|
| Maize Silage | Anaerobic Sludge | ||
| Dry matter (TS) | % | 30.2 ± 0.8 | 9.8 ± 0.2 |
| Organic dry matter (VS) | % TS | 89.0 ± 0.6 | 68.5 ± 2.5 |
| Mineral dry matter (MS) | % TS | 11.0 ± 1.3 | 31.5 ± 2.5 |
| Total nitrogen (TN) | mg/g TS | 11.1 ± 0.9 | 33.1 ± 3.3 |
| Total phosphorus (TP) | mg/g TS | 2.4 ± 0.3 | 1.7 ± 0.2 |
| Total carbon (TC) | mg/g TS | 460 ± 13 | 309 ± 28 |
| Total organic carbon (TOC) | mg/g TS | 441 ± 15 | 199 ± 34 |
| C:N ratio | - | 39.6 ± 1.6 | 9.3 ± 0.1 |
| pH | - | 7.73 ± 0.08 | 7.21 ± 0.31 |
| Protein | % TS | 9.2 ± 0.6 | 20.7 ± 2.8 |
| Lipids | % TS | 2.2 ± 0.5 | 3.1 ± 0.5 |
| Sugars | % TS | 60.4 ± 1.0 | 1.6 ± 0.4 |
| Parameter | Unit | Value |
|---|---|---|
| pH | pH | 8.05 ± 0.74 |
| BOD5 | mg O2/dm3 | 4645 ± 330 |
| COD | mg O2/dm3 | 13860 ± 920 |
| TOC | mg O2/dm3 | 10350 ± 270 |
| TN | mg Ntotal/dm3 | 2240 ± 230 |
| Organic nitrogen | mg N/dm3 | 2150 ± 240 |
| Ammoniacal nitrogen | mg N-NH4/dm3 | 90 ± 50 |
| Nitric nitrogen | mg N-NO3/dm3 | 2.20 ± 0.93 |
| Nitrite nitrogen | mg N-NO2/dm3 | 0.12 ± 0.02 |
| TP | mg Ptotal/dm3 | 202 ± 61 |
| Orthophosphates | mg P-PO4/dm3 | 129 ± 33 |
| Dry residue | mg/dm3 | 12.5 ± 2.2 |
| Chlorides | mgCl−/dm3 | 574 ± 62 |
| Sulfates | mgSO4/dm3 | 0.39 ± 0.12 |
| Lipids | mg/dm3 | 184 ± 36 |
| Variant | Reactor Volume (dm3) | OLR (gVS/dm3·Day) | VS Load (gVS/Day) | Maize Silage Weight (g/Day) | Maize Silage Volume (at 70% Water Content) (cm3) | Maize Silage Volume (at 90% Water Content) (cm3) | Water Volume (cm3) | CTS Volume (cm3) | HRT (doba) | Temperature (°C) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 2.2 | 4.4 | 16.5 | 33 | 100 | 67.0 | 0.0 | 20 | 38 |
| 2 | 60.3 | 6.7 | ||||||||
| 3 | 53.6 | 13.4 | ||||||||
| 4 | 46.9 | 20.1 | ||||||||
| 5 | 40.2 | 26.8 | ||||||||
| 6 | 33.5 | 33.5 | ||||||||
| 7 | 26.8 | 40.2 |
| Parameter | Indicator | Variant | ||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | ||
| Biogas | cm3/gVS | 390 ± 44 | 411 ± 37 | 425 ± 39 | 384 ± 37 | 361 ± 48 | 211 ± 46 | 190 ± 39 |
| cm3/gTS | 347 ± 39 | 366 ± 33 | 378 ± 35 | 342 ± 33 | 321 ± 43 | 188 ± 41 | 169 ± 34 | |
| cm3/gFM | 105 ± 12 | 110 ± 10 | 114 ± 11 | 103 ± 10 | 97 ± 13 | 57 ± 12 | 51 ± 10 | |
| cm3/day | 1716 ± 192 | 1808 ± 161 | 1870 ± 172 | 1690 ± 163 | 1588 ± 213 | 928 ± 202 | 836 ± 170 | |
| Methane | %CH4 | 59 ± 3.1 | 57 ± 2.6 | 58 ± 1.4 | 55 ± 2.7 | 47 ± 3.9 | 40 ± 4.1 | 39 ± 3.4 |
| cm3CH4/gVS | 230 ± 26 | 234 ± 21 | 247 ± 23 | 211 ± 20 | 170 ± 23 | 84.4 ± 18 | 74.1 ± 15 | |
| cm3CH4/gTS | 205 ± 23 | 209 ± 19 | 219 ± 20 | 188 ± 18 | 151 ± 20 | 75.1 ± 16 | 65.9 ± 13 | |
| cm3CH4/gFM | 61.8 ± 6.9 | 63.0 ± 5.6 | 66.3 ± 6.1 | 56.8 ± 5.5 | 45.6 ± 6.1 | 22.7 ± 4.9 | 19.9 ± 4.1 | |
| cm3CH4/day | 1012 ± 113 | 1031 ± 92 | 1085 ± 100 | 929 ± 90 | 747 ± 100 | 371 ± 81 | 326 ± 66 | |
| Taxonomic Group | Variant | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Bacteria (EUB338) | 69 ± 4 | 70 ± 6 | 71 ± 3 | 69 ± 4 | 68 ± 5 | 69 ± 2 | 70 ± 4 |
| Archaea (ARC915) | 27 ± 2 | 24 ± 2 | 26 ± 3 | 19 ± 1 | 17 ± 2 | 12 ± 3 | 13 ± 3 |
| Methanosarcinaceae (MSMX860) | 12 ± 1 | 13 ± 3 | 13 ± 2 | 10 ± 1 | 11 ± 2 | 9 ± 2 | 9 ± 1 |
| Methanosaeta (MX825) | 9 ± 2 | 9 ± 2 | 8 ± 1 | 4 ± 1 | 5 ± 1 | 2 ± 1 | 3 ± 2 |
| Indicator | VFA | Total Alkalinity | FOS/TAC | pH | |
|---|---|---|---|---|---|
| Unit | mgCH3COOH/dm3 | mgCaCO3/dm3 | - | - | |
| Variant | 1 | 1680 ± 121 | 5250 ± 340 | 0.32 ± 0.02 | 7.21 ± 0.07 |
| 2 | 1695 ± 156 | 5136 ± 470 | 0.33 ± 0.04 | 7.34 ± 0.02 | |
| 3 | 1715 ± 160 | 5717 ± 533 | 0.30 ± 0.01 | 7.28 ± 0.04 | |
| 4 | 1920 ± 210 | 4923 ± 538 | 0.39 ± 0.02 | 6.89 ± 0.11 | |
| 5 | 2310 ± 141 | 5372 ± 328 | 0.43 ± 0.04 | 6.42 ± 0.19 | |
| 6 | 2780 ± 159 | 5450 ± 312 | 0.51 ± 0.03 | 6.21 ± 0.13 | |
| 7 | 2840 ± 180 | 5358 ± 340 | 0.53 ± 0.02 | 6.11 ± 0.14 | |
| Variant | PARAMETER | |||||||
|---|---|---|---|---|---|---|---|---|
| Phosphorus (mgP/gTS) | Nitrogen (mgN/gTS) | Calcium (mgCa/gTS) | Magnesium (mgMg/gTS) | Potassium (mgK/gTS) | TS (%) | VS (% TS) | (%) | |
| 1 | 2.46 ± 0.90 | 8.42 ± 1.71 | 29.43 ± 3.12 | 1.66 ± 0.34 | 3.8 ± 0.9 | 12.4 ± 1.1 | 68.2 ± 0.9 | 68.5 ± 1.0 |
| 2 | 2.62 ± 1.12 | 9.76 ± 1.32 | 31.47 ± 5.53 | 1.39 ± 0.42 | 4.1 ± 0.6 | 11.7 ± 0.9 | 69.7 ± 1.4 | 69.7 ± 1.1 |
| 3 | 2.37 ± 0.73 | 8.63 ± 2.63 | 32.61 ± 5.92 | 1.47 ± 0.77 | 3.6 ± 1.3 | 13.9 ± 1.8 | 68.9 ± 1.7 | 64.4 ± 1.8 |
| 4 | 2.50 ± 0.46 | 8.11 ± 1.47 | 33.65 ± 3.27 | 1.19 ± 0.31 | 3.5 ± 0.9 | 11.2 ± 0.6 | 70.3 ± 0.8 | 70.7 ± 0.5 |
| 5 | 2.19 ± 0.38 | 10.73 ± 1.81 | 32.79 ± 4.32 | 1.82 ± 0.72 | 4.4 ± 1.1 | 12.7 ± 1.1 | 71.1 ± 2.3 | 66.4 ± 1.7 |
| 6 | 2.13 ± 0.61 | 9.91 ± 2.27 | 29.62 ± 3.85 | 1.50 ± 0.53 | 4.0 ± 1.0 | 10.9 ± 1.2 | 72.6 ± 1.4 | 70.6 ± 1.3 |
| 7 | 2.43 ± 0.21 | 11.62 ± 0.69 | 30.25 ± 5.23 | 1.71 ± 0.21 | 3.8 ± 0.5 | 11.4 ± 0.8 | 71.4 ± 2.5 | 69.7 ± 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dębowski, M.; Kazimierowicz, J.; Zieliński, M. Efficiency of Chemical Toilet Sewage (CTS) Co-Fermentation with Typical Energy Crops. Appl. Sci. 2023, 13, 818. https://doi.org/10.3390/app13020818
Dębowski M, Kazimierowicz J, Zieliński M. Efficiency of Chemical Toilet Sewage (CTS) Co-Fermentation with Typical Energy Crops. Applied Sciences. 2023; 13(2):818. https://doi.org/10.3390/app13020818
Chicago/Turabian StyleDębowski, Marcin, Joanna Kazimierowicz, and Marcin Zieliński. 2023. "Efficiency of Chemical Toilet Sewage (CTS) Co-Fermentation with Typical Energy Crops" Applied Sciences 13, no. 2: 818. https://doi.org/10.3390/app13020818








