Rules of Heliogeomagnetics Diversely Coordinating Biological Rhythms and Promoting Human Health
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
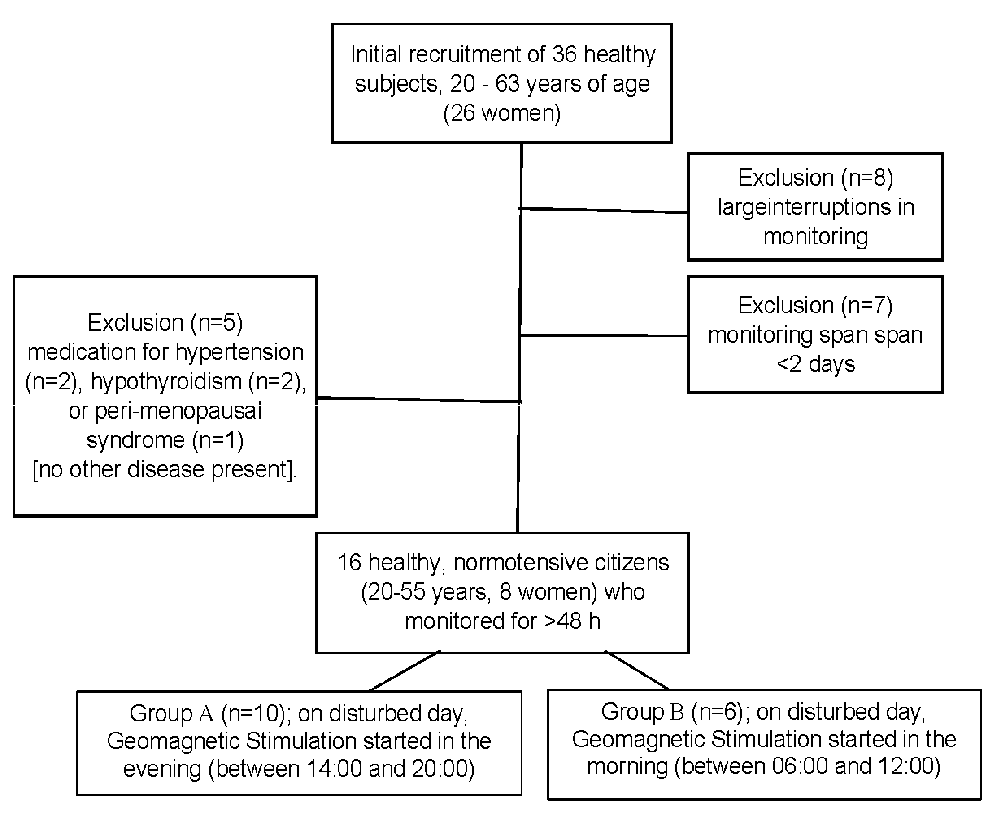
2.1. Subjects
2.2. Ambulatory BP Monitoring
2.3. Geomagnetic Monitoring
2.4. Circadian Parameters of BP and HR
2.5. Data Analysis
3. Results
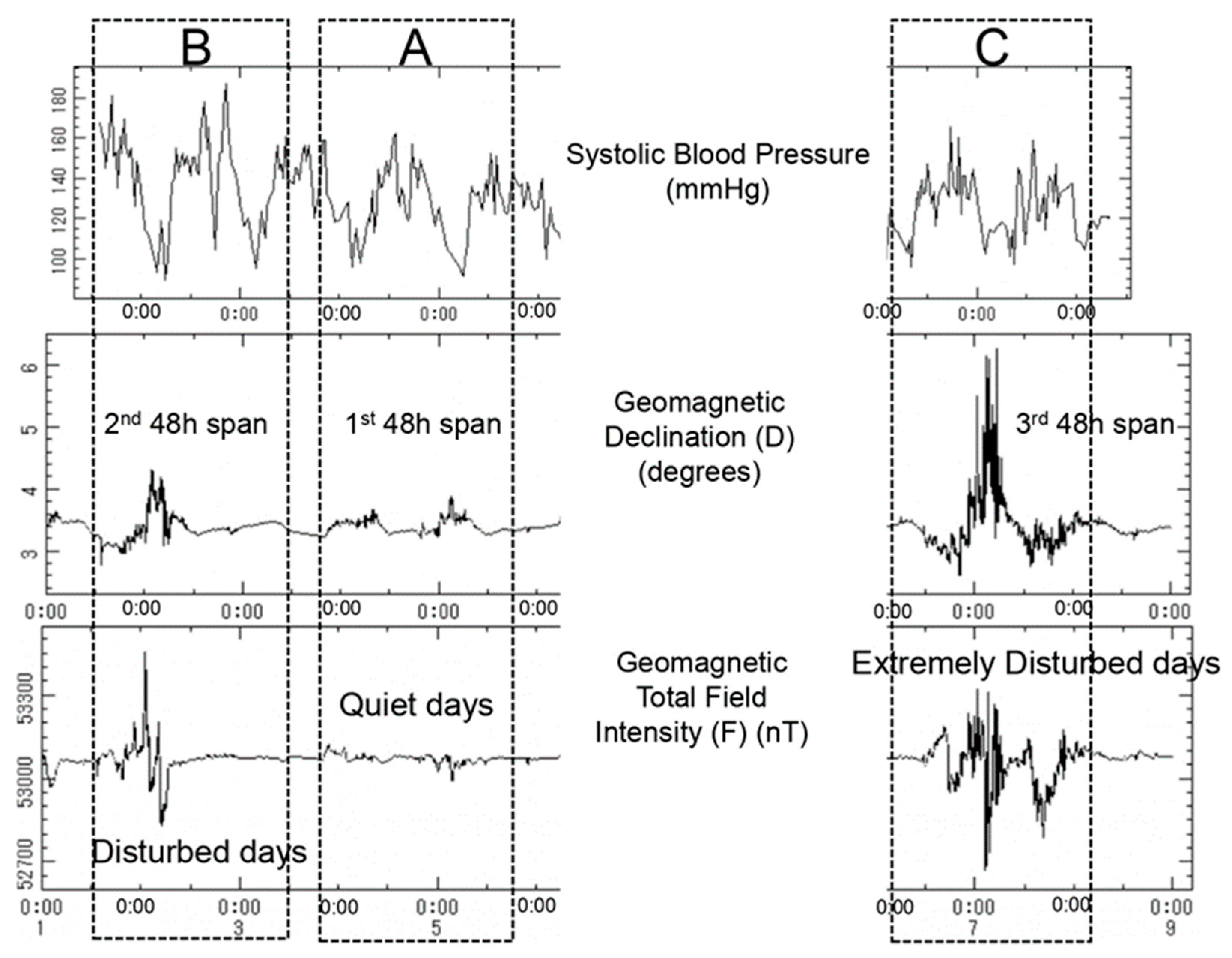
3.1. Changes of Biological Characteristics of BP and HR Associated with Geomagnetic Disturbances
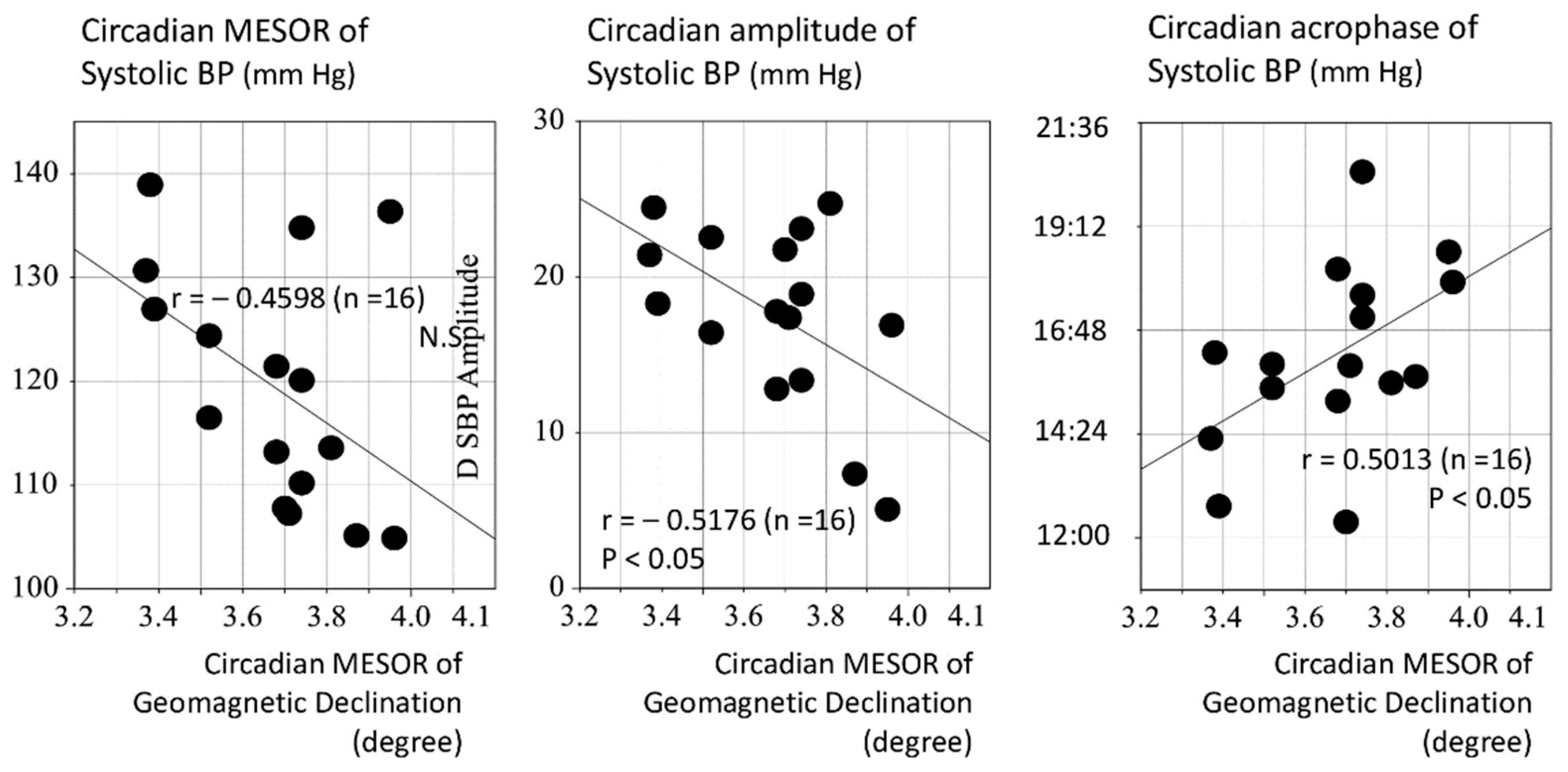
3.2. Assessment of the Effect of Magnetic Fluctuations on the Circadian Period
3.3. Biphasic (Hormetic) Response of SBP to Geomagnetic Stimuli
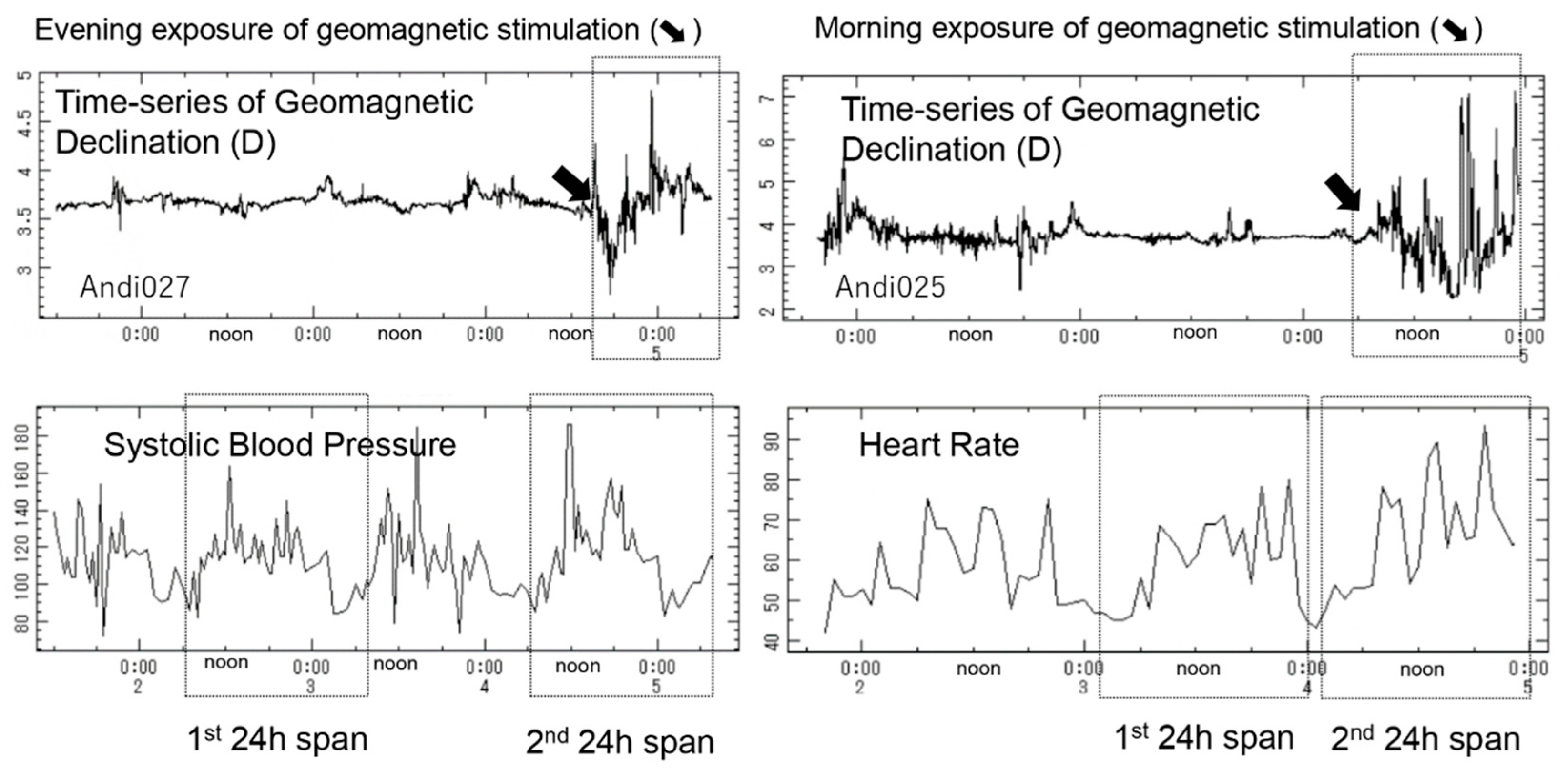
3.4. Circadian-Phase-Dependent Effect of Geomagnetic Stimulation on Circadian Amplitude of SBP and HR
4. Discussion
4.1. Bell-Shaped, Typical of Biphasic, Response of Circadian BP Rhythm to Geomagnetic Stimuli
4.2. Circadian-Phase-Dependent Effect of Geomagnetic Stimulation
4.3. Role of Circasemidian Component Associated with Geomagnetic Fluctuations
4.4. Rules of Procedure in Exploring Heliogeomagnetic Effects on Human Physiology
- (1)
- The atmosphere and the magnetic field provide protection on Earth [111]. Geomagnetic fluctuations in the magnetosphere significantly affect humans not only on Earth but also in space.
- (2)
- (3)
- Effects of geomagnetic disturbances on human physiology are nonlinear and display hormetic responses [72], perhaps understood as part of a broader bell-shaped dose-response curve [85]. Windowed responses appear only in a certain range of doses, which may differ among individuals and change depending on circumstances. They account for the lack of response outside, contrasting with a strong response inside these ‘windows’ [27,28,29,33,55,71,85,87]. Extremely high as well as extremely low geomagnetic activity seem to suppress BP or HRV variability and have adverse health effects [55].
- (4)
- Decreases in HRV linked to geomagnetic storms, occurring more frequently when solar activity is high, reportedly increase cardiovascular risk in susceptible individuals. BP variability, on the other hand, is larger during solar minima and ascending solar cycle phases than during solar maxima, but storms during solar minima are more intense than those during solar maxima, perhaps accounting for changes in BP behavior along the course of the solar cycle [33,42].
- (5)
- Effects of magnetic fluctuations on the activity of the brain’s DMN are modulated by light and/or the circadian clock. Transcranial magnetic stimulation seemed to create a shift in the relationship between the medial prefrontal cortex and the dorsolateral prefrontal cortex, two nodes in the DMN [18,19,20,115].
- (6)
- Geomagnetic stimulation at night improved sleep quality and induced slow-wave deep sleep not only on Earth but also in space. Geomagnetic disturbances also affect psychophysical processes. Their effects depend on individuals’ sensitivity, health status, and capacity for self-regulation [18,19,20,46].
- (7)
- Magnetic stimulation affect the period and amplitude of the endogenous circadian oscillation. These effects are circadian-phase-dependent, as they vary as a function of the time of day when geomagnetic activity occurs [116,117]. Increases in the circadian amplitude of HR and HRV suggest that the circadian system can be amplified in association with geomagnetic disturbances. The circadian amplitude of HR was also found to correlate statistically significantly with the 24 h, 12 h, and 8 h amplitudes of the geomagnetic declination index [19,20,118].
- (8)
- Changes in the time-varying magnetic field above 80 nT over three hours significantly reduced melatonin concentrations in the body. Reduced concentrations of melatonin may play a role in the development of myocardial ischemia, as melatonin was found to improve myocardial microcirculation under laboratory conditions [119,120,121,122].
- (9)
4.5. Limitations and Expectations for Future Investigations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Halberg, F.; Visscher, M.B. Regular diurnal physiological variation in eosinophil levels in five stocks of mice. Proc. Soc. Exp. Biol. N. Y. 1950, 75, 846–847. [Google Scholar] [CrossRef]
- Halberg, F. Quo vadis basic and clinical chronobiology: Promise for health maintenance. Am. J. Anat. 1983, 168, 543–594. [Google Scholar] [CrossRef]
- Ruben, M.D.; Wu, G.; Smith, D.F.; Schmidt, R.E.; Francey, L.J.; Lee, Y.Y.; Anafi, R.C.; Hogenesch, J.B. A database of tissue-specific rhythmically expressed human genes has potential applications in circadian medicine. Sci. Transl. Med. 2018, 10, eaat8806. [Google Scholar] [CrossRef]
- Dyar, K.A.; Lutter, D.; Artati, A.; Ceglia, N.J.; Liu, Y.; Armenta, D.; Jastroch, M.; Schneider, S.; de Mateo, S.; Cervantes, M.; et al. Atlas of circadian metabolism reveals system-wide coordination and communication between clocks. Cell 2018, 174, 1571–1585.e11. [Google Scholar] [CrossRef] [PubMed]
- Molina-Serrano, D.; Kyriakou, D.; Kirmizis, A. Histone modifications as an intersection between diet and longevity. Front. Genet. 2019, 10, 192. [Google Scholar] [CrossRef]
- Gubin, D.; Weinert, D.; Cornelissen Guillaume, G. Chronotheranostics and chronotherapy—Frontiers for personalized medicine. J. Chronomedicine (Tyumen Med. J.) 2020, 22, 3–23. [Google Scholar] [CrossRef]
- Hastings, M.H.; Reddy, A.B.; Maywood, E.S. A clockwork web: Circadian timing in brain and periphery, in health and disease. Nat. Rev. Neurosci. 2003, 4, 649–661. [Google Scholar] [CrossRef] [PubMed]
- Davis, S.; Mirick, D.K. Circadian disruption, shift work and the risk of cancer: A summary of the evidence and studies in Seattle. Cancer Causes Control 2006, 17, 539–545. [Google Scholar] [CrossRef]
- Masri, S.; Kinouchi, K.; Sassone-Corsi, P. Circadian clocks, epigenetics, and cancer. Curr. Opin. Oncol. 2015, 27, 50–56. [Google Scholar] [CrossRef]
- Morris, C.J.; Purvis, T.E.; Hu, K.; Scheer, F.A. Circadian misalignment increases cardiovascular disease risk factors in humans. Proc. Natl. Acad. Sci. USA 2016, 113, E1402–E1411. [Google Scholar] [CrossRef]
- Cornelissen, G.; Otsuka, K. Chronobiology of aging: A mini-review. Gerontology 2017, 63, 118–128. [Google Scholar] [CrossRef]
- Cornelissen, G. Metabolic Syndrome, Adiponectin, Sleep, and the Circadian System. EBioMedicine 2018, 33, 20–21. [Google Scholar] [CrossRef] [PubMed]
- Chellappa, S.L.; Vujovic, N.; Williams, J.S.; Scheer, F.A.J.L. Impact of circadian disruption on cardiovascular function and disease. Trends Endocrinol. Metab. 2019, 30, 767–779. [Google Scholar] [CrossRef] [PubMed]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrauwen, P.; la Fleur, S.E.; Kalsbeek, A. Circadian clocks and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef]
- Xie, Y.; Tang, Q.; Chen, G.; Xie, M.; Yu, S.; Zhao, J.; Chen, L. New insights into the circadian rhythm and its related diseases. Front. Physiol. 2019, 10, 682. [Google Scholar] [CrossRef]
- Maury, E. Off the clock: From circadian disruption to metabolic disease. Int. J. Mol. Sci. 2019, 20, 1597. [Google Scholar] [CrossRef] [PubMed]
- Kervezee, L.; Kosmadopoulos, A.; Boivin, D.B. Metabolic and cardiovascular consequences of shift work: The role of circadian disruption and sleep disturbances. Eur. J. Neurosci. 2020, 51, 396–412. [Google Scholar] [CrossRef]
- Otsuka, K.; Cornelissen, G.; Kubo, Y.; Shibata, K.; Mizuno, K.; Ohshima, H.; Furukawa, S.; Mukai, C. Anti-aging effects of long-term space missions, estimated by heart rate variability. Sci. Rep. 2019, 9, 8995. [Google Scholar] [CrossRef]
- Otsuka, K.; Cornelissen, G.; Furukawa, S.; Kubo, Y.; Shibata, K.; Mizuno, K.; Ohshima, H.; Furukawa, S.; Mukai, C. Astronauts’ well-being and possibly anti-aging improved during long-duration spaceflight. Sci. Rep. 2021, 11, 14907. [Google Scholar] [CrossRef]
- Otsuka, K.; Cornelissen, G.; Furukawa, S.; Shibata, K.; Kubo, Y.; Mizuno, K.; Aiba, T.; Ohshima, H.; Mukai, C. Unconscious mind activates central cardiovascular network and promotes adaptation to microgravity possibly anti-aging during 1-year-long spaceflight. Sci. Rep. 2022, 12, 11862. [Google Scholar] [CrossRef]
- Bigger, J.T., Jr.; Fleiss, J.L.; Steinman, R.C.; Rolnitzky, L.M.; Kleiger, R.E.; Rottman, J.N. Frequency domain measures of heart period variability and mortality after myocardial infarction. Circulation 1992, 85, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Berntson, G.G.; Bigger, J.T., Jr.; Eckberg, D.L.; Grossman, P.; Kaufmann, P.G.; Malik, M.; Nagaraja, H.N.; Porges, S.W.; Saul, J.P.; Stone, P.H.; et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology 1997, 34, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Togo, F.; Kiyono, K.; Struzik, Z.R.; Yamamoto, Y. Unique very low-frequency heart rate variability during deep sleep in humans. IEEE Trans. Biomed. Eng. 2006, 53, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Brämer, D.; Günther, A.; Rupprecht, S.; Nowack, S.; Adam, J.; Meyer, F.; Schwab, M.; Surber, R.; Witte, O.W.; Hoyer, H.; et al. Very Low Frequency Heart Rate Variability Predicts the Development of Post-Stroke Infections. Transl. Stroke Res. 2019, 10, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xiang, L.; Tong, G. Predictive values of heart rate variability, deceleration and acceleration capacity of heart rate in post-infarction patients with LVEF >/=35. Ann. Nonin. Electrocardiol. 2020, 25, e12771. [Google Scholar] [CrossRef]
- Hayano, J.; Ueda, N.; Kisohara, M.; Yuda, E.; Carney, R.M.; Blumenthal, J.A. Survival Predictors of Heart Rate Variability After Myocardial Infarction With and Without Low Left Ventricular Ejection Fraction. Front. Neurosci. 2021, 15, 610955. [Google Scholar] [CrossRef]
- Halberg, F.E.; Cornelissen, G.; Otsuka, K.; Schwartzkopff, O.; Halberg, J.; Bakken, E.E. Chronomics. Biomed. Pharmacother. 2001, 55 (Suppl. S1), s153–s190. [Google Scholar] [CrossRef]
- Cornelissen, G.; Halberg, F.; Breus, T.; Syutkina, E.V.; Baevsky, R.; Weydahl, A.; Watanabe, Y.; Otsuka, K.; Siegelova, J.; Fiser, B.; et al. Non-photic solar associations of heart rate variability and myocardial infarction. J. Atmos. Solar-Terr. Phys. 2002, 64, 707–720. [Google Scholar] [CrossRef]
- Halberg, F.; Cornelissen, G.; Sothern, R.B.; Katinas, G.S.; Schwartzkopff, O.; Otsuka, K. Cycles tipping the scale between death and survival (=“Life”). Prog. Theor. Phys. 2008, 173, 153–181. [Google Scholar] [CrossRef]
- Cornelissen, G.; Breus, T.K.; Bingham, C.; Zaslavskaya, R.; Varshitsky, M.; Mirsky, B.; Teibloom, M.; Tarquini, B.; Bakken, E.; Halberg, F. Beyond circadian chronorisk: Worldwide circaseptan-circasemiseptan patterns of myocardial infarctions, other vascular events, and emergencies. Chronobiologia 1993, 20, 87–115. [Google Scholar]
- Cornelissen, G.; Halberg, F.; Wendt, H.W.; Bingham, C.; Sothern, R.B.; Haus, E.; Kleitman, E.; Kleitman, N.; Revilla, M.A.; Revilla, M., Jr.; et al. Resonance of about-weekly human heart rate rhythm with solar activity change. Biologia (Bratisl) 1996, 51, 749–756. [Google Scholar] [PubMed]
- McCraty, R.; Atkinson, M.; Tomasino, D.; Bradley, R.T. The coherent heart: Heart-brain interactions, psychophysiological coherence, and the emergence of system-wide order. Integral. Rev. 2009, 5, 10–115. [Google Scholar]
- Halberg, F.; Cornelissen, G.; Regal, P.; Otsuka, K.; Wang, Z.; Katinas, G.S.; Siegelova, J.; Homolka, P.; Prikryl, P.; Chibisov, S.M.; et al. Chronoastrobiology: Proposal, nine conferences, heliogeomagnetics, transyears, near-weeks, near-decades, phylogenetic and ontogenetic memories. Biomed. Pharmacother. 2004, 58 (Suppl. S1), S150–S187. [Google Scholar] [CrossRef]
- Breus, T.; Cornelissen, G.; Halberg, F.; Levitin, A.E. Temporal associations of life with solar and geophysical activity. Ann. Geophys. 1995, 13, 1211–1222. [Google Scholar] [CrossRef]
- DiCarlo, A.L.; Farrell, J.M.; Litovitz, T.A. Myocardial protection conferred by electromagnetic fields. Circulation 1999, 99, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Breus, T.K.; Pimenov, K.Y.; Cornelissen, G.; Halberg, E.; Syutkina, E.V.; Baevsky, R.M.; Petrov, V.M.; Orth-Gómer, K.; Akerstedt, T.; Otsuka, K.; et al. The biological effects of solar activity. Biomed. Pharmacother. 2002, 56 (Suppl. S2), 273–283. [Google Scholar] [CrossRef]
- Cornelissen, G.; Masalov, A.; Halberg, F.; Richardson, J.D.; Katinas, G.S.; Sothern, R.B.; Watanabe, Y.; Syutkina, E.W.; Wendt, H.W.; Bakken, E.E.; et al. Multiple resonances among time structures chronomes around in, U.S. Is an about 1.3-year periodicity in solar wind built into the human cardiovascular chronome? Fiziol. Cheloveka. 2004, 30, 86–92. [Google Scholar]
- Dimitrova, S.; Stoilova, I.; Cholakov, I. Influence of Local Geomagnetic Storms on Arterial Blood Pressure. Bioelectromagnetics 2004, 25, 408–414. [Google Scholar] [CrossRef]
- Breus, T.; Baevskii, R.; Funtova, I.; Nikulina, G.A. Effect of geomagnetic field disturbances on the adaptive stress reaction of cosmonauts. Cosm. Res. 2008, 46, 367–372. [Google Scholar] [CrossRef]
- Breus, T.K.; Baevskii, R.M.; Chernikova, A.G. Effects of geomagnetic disturbances on humans functional state in space flight. J. Biomed. Sci. Eng. 2012, 5, 341–355. [Google Scholar] [CrossRef]
- Dimitrova, S.; Angelov, I.; Petrova, E. Solar and geomagnetic activity effects on heart rate variability. Nat. Hazards 2013, 69, 25–37. [Google Scholar] [CrossRef]
- Azcaratea, T.; Mendoza, B.; Levi, J.R. Influence of geomagnetic activity and atmospheric pressure on human arterial pressure during the solar cycle 24. Adv. Space Res. 2016, 58, 2116–2125. [Google Scholar] [CrossRef]
- Belyaev, I.; Dean, A.; Eger, H.; Hubmann, G.; Jandrisovits, R.; Kern, M.; Kundi, M.; Moshammer, H.; Lercher, P.; Muller, K.; et al. EUROPAEM EMF Guideline 2016 for the prevention, diagnosis and treatment of EMF-related health problems and illnesses. Rev. Environ. Health 2016, 31, 363–397. [Google Scholar] [CrossRef]
- McCraty, R.; Atkinson, M.; Stolc, V.; Alabdulgader, A.A.; Vainoras, A.; Ragulskis, M. Synchronization of Human Autonomic Nervous System Rhythms with Geomagnetic Activity in Human Subjects. Int. J. Environ. Res. Public Health 2017, 14, 770. [Google Scholar] [CrossRef] [PubMed]
- Ozheredov, V.A.; Chibisov, S.M.; Blagonravov, M.L.; Khodorovich, N.A.; Demurov, E.A.; Goryachev, V.A.; Kharlitskaya, E.V.; Eremina, I.S.; Meladze, Z.A. Influence of geomagnetic activity and earth weather changes on heart rate and blood pressure in young and healthy population. Int. J. Biometeorol. 2017, 61, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Alabdulgader, A.; McCraty, R.; Atkinson, M.; Dobyns, Y.; Vainoras, A.; Ragulskis, M.; Stolc, V. Long-Term Study of Heart Rate Variability Responses to Changes in the Solar and Geomagnetic Environment. Sci. Rep. 2018, 8, 2663. [Google Scholar] [CrossRef]
- Rosado, M.M.; Simkó, M.; Mattsson, M.O.; Pioili, C. Immune-modulating perspectives for low frequency electromagnetic fields in innate immunity. Front. Public Health 2018, 6, 85. [Google Scholar] [CrossRef]
- Mattoni, M.; Ahn, S.; Fröhlich, C.; Fröhlich, F. Exploring the relationship between geomagnetic activity and human heart rate variability. Eur. J. Appl. Physiol. 2020, 120, 1371–1381. [Google Scholar] [CrossRef] [PubMed]
- Cifra, M.; Apollonio, F.; Liberti, M.; García-Sánchez, T.; Mir, L.M. Possible molecular and cellular mechanisms at the basis of atmospheric electromagnetic field bioeffects. Int. J. Biometeorol. 2021, 65, 59–67. [Google Scholar] [CrossRef]
- Wang, V.A.; Zilli Vieira, C.L.; Garshick, E.; Schwartz, J.D.; Garshick, M.S.; Vokonas, P.; Koutrakis, P. Solar activity is associated with diastolic and systolic blood pressure in elderly adults. J. Am. Heart Assoc. 2021, 10, e021006. [Google Scholar] [CrossRef]
- Goldberg, R.B.; Creasey, W.A. A review of cancer induction by extremely low frequency electromagnetic fields. Is there a plausible mechanism? Med. Hypotheses 1991, 35, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Baevsky, R.M.; Petrov, V.M.; Cornelissen, G.; Halberg, F.; Orth-Gomer, K.; Akerstedt, T.; Otsuka, K.; Breus, T.; Siegelova, J.; Dusek, J.; et al. Meta-analyzed heart rate variability, exposure to geomagnetic storms, and the risk of ischemic heart disease. Scr. Med. (Brno) 1997, 70, 201–206. [Google Scholar] [PubMed]
- Cornelissen, G.; Otsuka, K.; Halberg, F. Remove and replace for a scrutiny of space weather and human affairs. In Proceedings of the International Conference, Space Weather Effects in Humans: In Space and on Earth, Moscow, Russia, 4–8 June 2012; Grigoriev, A.I., Zeleny, L.M., Eds.; Space Research Institute: Boulder, CO, USA, 2013; pp. 508–538. [Google Scholar]
- Cornelissen, G.; Watanabe, Y.; Otsuka, K.; Halberg, F. Influences of space and terrestrial weather on human physiology and pathology. In Bioelectromagnetic and Subtle Energy Medicine, 2nd ed.; Rosch, P.J., Ed.; CRC Press: Boca Raton, FL, USA, 2015; pp. 389–400. [Google Scholar]
- Palmer, S.J.; Rycroft, M.J.; Cermack, M. Solar and geomagnetic activity, extremely low frequency magnetic and electric fields and human health at the Earth’s surface. Surv. Geophys. 2006, 27, 557–595. [Google Scholar] [CrossRef]
- Chen, Q.; Lang, L.; Wu, W.; Xu, G.; Zhang, X.; Li, T.; Huang, H. A meta-analysis on the relationship between exposure to ELF-EMFs and the risk of female breast cancer. PLoS ONE 2013, 8, e69272. [Google Scholar] [CrossRef] [PubMed]
- Huss, A.; Koeman, T.; Kromhout, H.; Vermeulen, R. Extremely low frequency magnetic field exposure and Parkinson’s disease—A systematic review and meta-analysis of the data. Int. J. Environ. Res. Public Health 2015, 12, 7348–7356. [Google Scholar] [CrossRef]
- Zhang, Y.; Lai, J.; Ruan, G.; Chen, C.; Wang, D.W. Meta-analysis of extremely low frequency electromagnetic fields and cancer risk: A pooled analysis of epidemiologic studies. Environ. Int. 2016, 88, 36–43. [Google Scholar] [CrossRef]
- Huss, A.; Peters, S.; Vermeulen, R. Occupational exposure to extremely low-frequency magnetic fields and the risk of ALS: A systematic review and meta-analysis. Bioelectromagnetics 2018, 39, 156–163. [Google Scholar] [CrossRef]
- Choi, S.; Cha, W.; Park, J.; Kim, S.; Kim, W.; Yoon, C.; Park, J.H.; Ha, K.; Park, D. Extremely low frequency-magnetic field (ELF-MF) exposure characteristics among semiconductor workers. Int. J. Environ. Res. Public Health 2018, 15, 642. [Google Scholar] [CrossRef]
- Jalilian, H.; Teshnizi, S.H.; Röösli, M.; Neghab, M. Occupational exposure to extremely low frequency magnetic fields and risk of Alzheimer disease: A systematic review and meta-analysis. Neurotoxicology 2018, 69, 242–252. [Google Scholar] [CrossRef]
- Jalilian, H.; Najafi, K.; Khosravi, Y.; Röösli, M. Amyotrophic lateral sclerosis, occupational exposure to extremely low frequency magnetic fields and electric shocks: A systematic review and meta-analysis. Rev. Environ. Health 2020, 36, 129–142. [Google Scholar] [CrossRef]
- Nishimura, T.; Tsai, I.J.; Yamauchi, H.; Nakatani, E.; Fukushima, M.; Hsu, C.Y. Association of Geomagnetic Disturbances and Suicide Attempts in Taiwan, 1997-2013: A Cross-Sectional Study. Int. J. Environ. Res Public Health 2020, 17, 1154. [Google Scholar] [CrossRef] [PubMed]
- Gapon, L.I.; Shurkevich, N.P.; Vetoshkin, A.S.; Gubin, D.G. The rhythms of arterial pressure and heart rate in individuals with arterial hypertension under the conditions of Far North. Klin. Meditsina 2006, 84, 39–44. [Google Scholar]
- Staessen, J.A.; Fagard, R.; Thijs, L.; Amery, A. The Fourth International Consensus Conference on 24-Hour Ambulatory Blood Pressure Monitoring: A consensus view on the technique of ambulatory blood pressure monitoring. Hypertension 1995, 26, 912–918. [Google Scholar] [CrossRef]
- JCS Joint Working Group. Guidelines for the clinical use of 24 hour ambulatory blood pressure monitoring (ABPM) (JCS 2010): –digest version–. Circ. J. 2012, 76, 508–519. [Google Scholar] [CrossRef]
- Otsuka, K.; Watanabe, H.; Cornelissen, G.; Shinoda, M.; Uezono, K.; Kawasaki, T.; Halberg, F. Gender, age and circadian blood pressure variation of apparently healthy rural vs. metropolitan Japanese. Chronobiologia 1990, 17, 253–265. [Google Scholar] [PubMed]
- Otsuka, K.; Halberg, F. Circadian profiles of blood pressure and heart rate of apparently healthy metropolitan Japanese. Front. Med. Biol. Eng. 1994, 6, 149–155. [Google Scholar] [PubMed]
- Otsuka, K.; Cornelissen, G.; Halberg, F. Chronomics and Continuous Ambulatory Blood Pressure Monitoring: Vascular Chronomics: From 7-Day/24-Hour to Lifelong Monitoring; Springer: Tokyo, Japan, 2016; p. 870+lxxv. Available online: https://link.springer.com/book/10.1007/978-4-431-54631-3 (accessed on 5 January 2023).
- Otsuka, K.; Cornélissen, G.; Weydahl, A.; Holmeslet, B.; Hansen, T.L.; Shinagawa, M.; Kubo, Y.; Nishimura, Y.; Omori, K.; Yano, S.; et al. Geomagnetic disturbance associated with decrease in heart rate variability in a subarctic area. Biomed. Pharmacother. 2001, 55 (Suppl. S1), s51–s56. [Google Scholar] [CrossRef]
- Otsuka, K.; Oinuma, S.; Cornelissen, G.; Weydahl, A.; Ichimaru, Y.; Kobayashi, M.; Yano, S.; Holmeslet, B.; Hansen, T.L.; Mitsutake, G.; et al. Alternating-light-darkness-influenced human electrocardiographic magnetoreception in association with geomagnetic pulsations. Biomed. Pharmacother. 2001, 55 (Suppl. S1), s63–s75. [Google Scholar] [CrossRef]
- Oinuma, S.; Kubo, Y.; Otsuka, K.; Yamanaka, T.; Murakami, S.; Matsuoka, O.; Ohkawa, S.; Cornélissen, G.; Weydahl, A.; Holmeslet, B.; et al. Graded response of heart rate variability, associated with an alteration of geomagnetic activity in a subarctic area. Biomed. Pharmacother. 2002, 56 (Suppl. S2), 284–288. [Google Scholar] [CrossRef]
- Saito, K.; Koyama, A.; Yoneyama, K. A Recent Advances in Time Series Analysis by Maximum Entropy Method; Hokkaido University Press: Sapporo, Japan, 1994. [Google Scholar]
- Bingham, C.; Arbogast, B.; Guillaume, G.C.; Lee, J.K.; Halberg, F. Inferential statistical methods for estimating and comparing cosinor parameters. Chronobiologia 1982, 9, 397–439. [Google Scholar]
- Cornelissen, G. Cosinor-based rhythmometry. Theor. Biol. Med. Model. 2014, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Yellon, D.M.; Downey, J.M. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol. Rev. 2003, 83, 1113–1151. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Noyan Ashraf, M.H.; Facci, M.; Wang, R.; Paterson, P.G.; Ferrie, A.; Juurlink, B.H. Dietary approach to attenuate oxidative stress, hypertension, and inflammation in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2004, 101, 7094–7099. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Blain, R. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: An overview. Toxicol. Appl. Pharmacol. 2005, 202, 289–301. [Google Scholar] [CrossRef]
- Calabrese, E.J.; Bachmann, K.A.; Bailer, A.J.; Bolger, P.M.; Borak, J.; Cai, L.; Cedergreen, N.; Cherian, M.G.; Chiueh, C.C.; Clarkson, T.W.; et al. Biological stress response terminology: Integrating the concepts of adaptive response and preconditioning stress within a hormetic dose-response framework. Toxicol. Appl. Pharmacol. 2007, 222, 122–128. [Google Scholar] [CrossRef]
- Mattson, M.P. Hormesis defined. Ageing Res. Rev. 2008, 7, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.A.; Lee, Y.M.; Choi, J.Y.; Jacobs, D.R.; Lee, D.H. Evolutionarily adapted hormesis-inducing stressors can be a practical solution to mitigate harmful effects of chronic exposure to low dose chemical mixtures. Environ. Pollut. 2018, 233, 725–734. [Google Scholar] [CrossRef]
- Lee, Y.M.; Lee, D.H. Mitochondrial toxins and healthy lifestyle meet at the crossroad of hormesis. Diabetes Metab. J. 2019, 43, 568–577. [Google Scholar] [CrossRef]
- Epel, E.S. The geroscience agenda: Toxic stress, hormetic stress, and the rate of aging. Ageing Res. Rev. 2020, 63, 101167. [Google Scholar] [CrossRef]
- Jacome Burbano, M.S.; Gilson, E. The power of stress: The telo-hormesis hypothesis. Cells 2021, 10, 1156. [Google Scholar] [CrossRef]
- Murase, M. Environmental Pollution and Health: An Interdisciplinary Study of the Bioeffects of Electromagnetic Fields. SANSAI Environ. J. Glob. Community 2008, 3, 1–35. Available online: https://repository.kulib.kyoto-u.ac.jp/dspace/handle/2433/49793 (accessed on 5 January 2023).
- Bawin, S.M.; Adey, W.R. Sensitivity of calcium binding in cerebral tissuc to weak electric fields oscillating al low frequency. Proc. Natl. Acad. Sci. USA 1976, 73, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Murakami, S.; Kubo, Y.; Yamanaka, T.; Mitsutake, G.; Ohkawa, S.; Matsubayashi, K.; Yano, S.; Cornélissen, G.; Halberg, F. Chronomics for chronoastrobiology with immediate spin-offs for life quality and longevity. Biomed. Pharmacother. 2003, 57 (Suppl. S1), 1–18. [Google Scholar] [CrossRef]
- Shaffer, F.; Ginsberg, J.P. An overview of heart rate variability metrics and norms. Front Public Health 2017, 5, 258. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.A.; Carr, D.L.; Myers, C.W.; Eckberg, D.L. Mechanisms underlying very-low-frequency RR-interval oscillations in humans. Circulation 1998, 98, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Armour, J.A. Neurocardiology: Anatomical and Functional Principles; Publication No. 03-011; Institute of HeartMath: Boulder Creek, CA, USA, 2003. [Google Scholar]
- Schmidt, H.; Müller-Werdan, U.; Hoffmann, T.; Francis, D.P.; Piepoli, M.F.; Rauchhaus, M.; Prondzinsky, R.; Loppnow, H.; Buerke, M.; Hoyer, D.; et al. Autonomic dysfunction predicts mortality in patients with multiple organ dysfunction syndrome of different age groups. Crit. Care Med. 2005, 33, 1994–2002. [Google Scholar] [CrossRef]
- Shaffer, F.; McCraty, R.; Zerr, C.L. A healthy heart is not a metronome: An integrative review of the heart’s anatomy and heart rate variability. Front. Psychol. 2014, 5, 1040. [Google Scholar] [CrossRef]
- Halberg, F.; Guillaume, F.; Sanchez de la Peña, S.; Cavallini, M.; Cornélissen, G. Cephalo-adrenal interactions in the broader context of pragmatic and theoretical rhythm models. Chronobiologia 1986, 13, 137–154. [Google Scholar]
- Kaur, G.; Phillips, C.; Wong, K.; Saini, B. Timing is important in medication administration: A timely review of chronotherapy research. Int. J. Clin. Pharm. 2013, 35, 344–358. [Google Scholar] [CrossRef]
- Abbott, S.M.; Malkani, R.G.; Zee, P.C. Circadian disruption and human health: A bidirectional relationship. Eur. J. Neurosci. 2020, 51, 567–583. [Google Scholar] [CrossRef]
- Hill, R.J.W.; Innominato, P.F.; Lévi, F.; Ballesta, A. Optimizing circadian drug infusion schedules towards personalized cancer chronotherapy. PLoS Comput. Biol. 2020, 16, e1007218. [Google Scholar] [CrossRef]
- Antoch, M.P.; Kondratov, R.V.; Takahashi, J.S. Circadian clock genes as modulators of sensitivity to genotoxic stress. Cell Cycle 2005, 4, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Gabel, K.; Hoddy, K.K.; Haggerty, N.; Song, J.; Kroeger, C.M.; Trepanowski, J.F.; Panda, S.; Varady, K.A. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: A pilot study. Nutr. Healthy Aging 2018, 4, 345–353. [Google Scholar] [CrossRef]
- Tamaru, T.; Takamatsu, K. Circadian modification network of a core clock driver BMAL1 to harmonize physiology from brain to peripheral tissues. Neurochem. Int. 2018, 119, 11–16. [Google Scholar] [CrossRef]
- Xu, W.; Jain, M.K.; Zhang, L. Molecular link between circadian clocks and cardiac function: A network of core clock, slave clock, and effectors. Curr. Opin. Pharmacol. 2021, 57, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Myslivecek, J. Two Players in the Field: Hierarchical Model of Interaction between the Dopamine and Acetylcholine Signaling Systems in the Striatum. Biomedicines 2021, 9, 25. [Google Scholar] [CrossRef]
- Parnell, A.A.; De Nobrega, A.K.; Lyons, L.C. Translating around the clock: Multi-level regulation of post-transcriptional processes by the circadian clock. Cell Signal 2021, 80, 109904. [Google Scholar] [CrossRef]
- Anna, G.; Kannan, N.N. Post-transcriptional modulators and mediators of the circadian clock. Chronobiol. Int. 2021, 38, 1244–1261. [Google Scholar] [CrossRef]
- Halberg, F.; Cornélissen, G.; Katinas, G.; Watanabe, Y.; Otsuka, K.; Maggioni, C.; Perfetto, F.; Tarquini, R.; Schwartzkopff, O.; Bakken, E.E. Feedsidewards: Intermodulation (strictly) among time structures, chronomes, in and around us, and cosmo-vasculo-neuroimmunity. About ten-yearly changes: What Galileo missed and Schwabe found. Ann. N. Y. Acad. Sci. 2000, 917, 348–375. [Google Scholar] [CrossRef]
- Jozsa, R.; Halberg, F.; Cornelissen, G.; Zeman, M.; Kazsaki, J.; Csernus, V.; Katinas, G.S.; Wendt, H.W.; Schwartzkopff, O.; Stebelova, K.; et al. Chronomics, neuroendocrine feedsidewards and the recording and consulting of nowcasts—Forecasts of geomagnetics. Biomed. Pharmacother. 2005, 59, S24–S30. [Google Scholar] [CrossRef]
- Fu, S.; Watkins, S.M.; Hotamisligil, G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012, 15, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Zhang, Q.; Pan, Y.; Mace, E.M.; York, B.; Antoulas, A.C.; Dacso, C.C.; O’Malley, B.W. A Cell-Autonomous Mammalian 12 h Clock Coordinates Metabolic and Stress Rhythms. Cell Metab. 2017, 25, 1305–1319.e9. [Google Scholar] [CrossRef]
- Zhu, B.; Dacso, C.C.; O’Malley, B.W. Unveiling “Musica Universalis” of the Cell: A Brief History of Biological 12-Hour Rhythms. J. Endocr. Soc. 2018, 2, 727–752. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Ballance, H.; Meng, H.; Gonzalez, N.; Kim, S.M.; Abdurehman, L.; York, B.; Chen, X.; Schnytzer, Y.; Levy, O.; et al. 12-h clock regulation of genetic information flow by XBP1s. PLoS Biol. 2020, 18, e3000580. [Google Scholar] [CrossRef] [PubMed]
- Ballance, H.; Zhu, B. Revealing the hidden reality of the mammalian 12-h ultradian rhythms. Cell Mol. Life Sci. 2021, 78, 3127–3140. [Google Scholar] [CrossRef] [PubMed]
- Kamide, Y. Our life is protected by the Earth’s atmosphere and magnetic field: What aurora research tells us. Biomed. Pharmacother. 2001, 55, 21–24. [Google Scholar] [CrossRef]
- Baevsky, R.M.; Petrov, V.M.; Chernikova, A.G. Regulation of autonomic nervous system in space and magnetic storms. Adv. Space Res. 1998, 22, 227–234. [Google Scholar] [CrossRef]
- Mitsutake, G.; Otsuka, K.; Oinuma, S.; Ferguson, I.; Cornelissen, G.; Wanliss, J.; Halberg, F. Does exposure to an artifcial ULF magnetic feld afect blood pressure, heart rate variability and mood? Biomed. Pharmacother. 2004, 58 (Suppl. S1), S20–S27. [Google Scholar] [CrossRef]
- Stoupel, E.; Tamoshiunas, A.; Radishauskas, R.; Bernotiene, G.; Abramson, E.; Israelevich, P. Acute myocardial infarction (AMI) (n-11026) on days of zero geomagnetic activity (GMA) and the following week: Diferences at months of maximal and minimal solar activity (SA) in solar cycles 23 and 24. J. Basic Clin. Physiol. Pharmacol. 2012, 23, 5–9. [Google Scholar] [CrossRef]
- Otsuka, K.; Cornelissen, G.; Kubo, Y.; Shibata, K.; Hayashi, M.; Mizuno, K.; Ohshima, H.; Furukawa, S.; Mukai, C. Circadian challenge of astronauts’ unconscious mind adapting to microgravity in space, estimated by heart rate variability. Sci. Rep. 2018, 8, 10381. [Google Scholar] [CrossRef]
- Kassahun, B.T.; Bier, M.; Ding, J. Perturbing circadian oscillations in an in vitro suprachiasmatic nucleus with magnetic stimulation. Bioelectromagnetics 2020, 41, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Zadeh-Haghighi, H.; Simon, C. Radical pairs can explain magnetic field and lithium effects on the circadian clock. Sci. Rep. 2022, 12, 269. [Google Scholar] [CrossRef] [PubMed]
- Krylov, V.V. Influence of geomagnetic disturbances at different times of day on locomotor activity in Zebrafish (Danio Rerio). Clocks Sleep 2021, 3, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Burch, J.B.; Reif, J.B.; Yost, M.G. Geomagnetic disturbances are associated with reduced nocturnal secretion of a melatonin metabolite in humans. Neurosci. Lett. 1999, 266, 209–212. [Google Scholar] [CrossRef]
- Burch, J.B.; Reif, J.B.; Yost, M.G.; Keefe, T.J.; Pitrat, C.A. Reduced excretion of a melatonin metabolite in workers exposed to 60 Hz magnetic fields. Am. J. Epidemiol. 1999, 150, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Weydahl, A.; Sothern, R.B.; Cornelissen, G.; Wetterberg, L. Geomagnetic activity influences the melatonin secretion at latitude 70 degrees N. Biomed. Pharmacother. 2001, 55 (Suppl. S1), s57–s62. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, Y.; Hu, S.; Shi, C.; Zhu, P.; Ma, Q.; Jin, Q.; Cao, F.; Tian, F.; Chen, Y. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission-VDAC1-HK2-mPTP-mitophagy axis. J. Pineal Res. 2017, 63, e12413. [Google Scholar] [CrossRef]
- Pascual-Leone, A.; Tormos, J.M.; Keenan, J.; Tarazona, F.; Cañete, C.; Catalá, M.D. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J. Clin. Neurophysiol. 1998, 15, 333–343. [Google Scholar] [CrossRef]
- Bergmann, T.O.; Mölle, M.; Schmidt, M.A.; Lindner, C.; Marshall, L.; Born, J.; Siebner, H.R. EEG-guided transcranial magnetic stimulation reveals rapid shifts in motor cortical excitability during the human sleep slow oscillation. J. Neurosci. 2012, 32, 243–253. [Google Scholar] [CrossRef]
- Ly, J.Q.M.; Gaggioni, G.; Chellappa, S.L.; Papachilleos, S.; Brzozowski, A.; Borsu, C.; Rosanova, M.; Sarasso, S.; Middleton, B.; Luxen, A.; et al. Circadian regulation of human cortical excitability. Nat. Commun. 2016, 7, 11828. [Google Scholar] [CrossRef]
- Desideri, D.; Zrenner, C.; Ziemann, U.; Belardinelli, P. Phase of sensorimotor μ-oscillation modulates cortical responses to transcranial magnetic stimulation of the human motor cortex. J. Physiol. 2019, 597, 5671–5686. [Google Scholar] [CrossRef] [PubMed]
- Driessen, S.; Bodewein, L.; Dechent, D.; Graefrath, D.; Schmiedchen, K.; Stunder, D.; Kraus, T.; Petri, A.K. Biological and health-related effects of weak static magnetic fields (≤ 1 mT) in humans and vertebrates: A systematic review. PLoS ONE 2020, 15, e0230038. [Google Scholar] [CrossRef] [PubMed]
- Cornelissen, G.G.; Gubin, D.; Beaty, L.A.; Otsuka, K. Some near- and far-environmental effects on human health and disease with a focus on the cardiovascular system. Int. J. Environ. Res. Public Health 2020, 17, 3083. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.; Borland, S. Behavioural evidence for use of a light-dependent magnetoreception mechanism by a vertebrate. Nature 1992, 359, 142–144. [Google Scholar] [CrossRef]
- Griffin, E.A.; Staknis, D.; Weitz, C.J. Light-independent role of CRY1 and CRY2 in the mammalian circadian clock. Science 1999, 286, 768–771. [Google Scholar] [CrossRef]
- Those, F.; Bartsch, B.; Fritzsche, B.; Tellschaft, D.; Thoss, M. The magnetic field sensitivity of the human visual system shows resonance and compass characteristic. J. Comp. Physiol. A 2000, 186, 1007–1010. [Google Scholar] [CrossRef]
- Gegear, R.; Casselman, A.; Waddell, S.; Reppert, S.M. Cryptochrome mediates light-dependent magneto-sensitivity in Drosophila. Nature 2008, 454, 1014–1018. [Google Scholar] [CrossRef]
- Yoshii, U.; Ahmad, M.; Helfrich-Forster, C. Cryptochrome mediates light-dependent magnetosensitivity of Drosophila’s circadian clock. PLoS Biol. 2009, 7, e1000086. [Google Scholar] [CrossRef]
- Gegear, R.J.; Foley, L.E.; Casselman, A.; Reppert, S.M. Animal cryptochromes mediate magnetoreception by an unconventional photochemical mechanism. Nature 2010, 463, 804–807. [Google Scholar] [CrossRef]
- Foley, L.E.; Gegear, R.J.; Reppert, S.M. Human cryptochrome exhibits light-dependent magnetosensitivity. Nat. Commun. 2011, 2, 356. [Google Scholar] [CrossRef]
- Manzella, N.; Bracci, M.; Ciarapica, V.; Staffolani, S.; Strafella, E.; Rapisarda, V.; Valentino, M.; Amati, M.; Copertaro, A.; Santarelli, L. Circadian gene expression and extremely low-frequency magnetic fields: An in vitro study. Bioelectromagnetics 2015, 36, 294–301. [Google Scholar] [CrossRef]
- Nießner, C.; Denzau, S.; Malkemper, E.P.; Gross, J.C.; Burda, H.; Winklhofer, M.; Peichl, L. Cryptochrome 1 in retinal cone photoreceptors suggests a novel functional role in mammals. Sci. Rep. 2016, 6, 21848. [Google Scholar] [CrossRef] [PubMed]
- Ebrille, E.; Konecny, T.; Konecny, D.; Spacek, R.; Jones, P.; Ambroz, P.; DeSimone, C.V.; Powell, B.D.; Hayes, D.L.; Friedman, P.A.; et al. Correlation of geomagnetic activity with implantable cardioverter defibrillator shocks and antitachycardia pacing. Mayo Clin. Proc. 2015, 90, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Stoupel, E.G.; Petrauskiene, J.; Kalediene, R.; Sauliune, S.; Abramson, E.; Shochat, T. Space weather and human deaths distribution: 25 years’ observation (Lithuania, 1989-2013). J. Basic Clin. Physiol. Pharmacol. 2015, 26, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Stoupel, E.; Radishauskas, R.; Bernotiene, G.; Tamoshiunas, A.; Virvichiute, D. Blood troponin levels in acute cardiac events depends on space weather activity components (a correlative study). J. Basic Clin. Physiol. Pharmacol. 2018, 29, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Jaruševičius, G.; Rugelis, T.; McCraty, R.; Landauskas, M.; Berškienė, K.; Vainoras, A. Correlation between changes in local Earth’s magnetic field and cases of acute myocardial infarction. Int. J. Environ. Res. Public Health. 2018, 15, 399. [Google Scholar] [CrossRef]
- Vencloviene, J.; Radisauskas, R.; Vaiciulis, V.; Kiznys, D.; Bernotiene, G.; Kranciukaite-Butylkiniene, D.; Tamosiunas, A. Associations between Quasi-biennial Oscillation phase, solar wind, geomagnetic activity, and the incidence of acute myocardial infarction. Int. J. Biometeorol. 2020, 64, 1207–1220. [Google Scholar] [CrossRef]
- Kuljis, D.A.; Loh, D.H.; Truong, D.; Vosko, A.M.; Ong, M.L.; McClusky, R.; Arnold, A.P.; Colwell, C.S. Gonadal- and sex-chromosome-dependent sex differences in the circadian system. Endocrinology 2013, 154, 1501–1512. [Google Scholar] [CrossRef]
- Pozo, M.J.; Gomez-Pinilla, P.J.; Camello-Almaraz, C.; Martin-Cano, F.E.; Pascua, P.; Rol, M.A.; Acuña-Castroviejo, D.; Camello, P.J. Melatonin, a potential therapeutic agent for smooth muscle-related pathological conditions and aging. Curr. Med. Chem. 2010, 17, 4150–4165. [Google Scholar] [CrossRef]
| State of Geomagnetic Activity | Quiet (n = 16) | Disturbed (n = 16) | Paired t-Test | ||||
|---|---|---|---|---|---|---|---|
| Variable (Units) | Mean | SD | Mean | SD | t-Value | p-Value | |
| Circadian Characteristics of BP and HR | SBP-M (mmHg) | 119.8 | 9.7425 | 119.8 | 11.8 | 0.023 | 0.982 |
| SBP-A(24 h) (mmHg) | 13.8 | 4.3158 | 17.9 (129.1%) | 5.8 | 2.52 | 0.024 | |
| SBP-ϕ(24 h) (hour:min) | 15:08 | 1:37 | 16:14 | 2:11 | 2.058 | 0.057 | |
| DBP-M (mmHg) | 74.1 | 6.8 | 72.1 | 6.1 | −1.745 | 0.102 | |
| DBP-A(24 h) (mmHg) | 11.3 | 4.8 | 10.6 | 5.9 | −0.38 | 0.709 | |
| DBP-ϕ(24 h) (hour:min) | 15:05 | 1:56 | 16:55 | 3:54 | 2.172 | 0.046 | |
| HR-M (bpm) | 67.2 | 7.1 | 68.2 | 7.4 | 1.035 | 0.317 | |
| HR-A(24 h) (bpm) | 8.3 | 3.8 | 9.7 | 3.4 | 1.567 | 0.138 | |
| HR-ϕ(24 h) (hour:min) | 14:17 | 2:33 | 14:46 | 3:57 | 0.471 | 0.644 | |
| Circasemidian Characteristics of BP and HR | SBP-M (mmHg) | 118.4 | 9.7 | 119.8 | 11.7 | 0.827 | 0.421 |
| SBP-A(12 h) (mmHg) | 10.5 | 2.9 | 7.6 (72.4%) | 4.7 | −2.101 | 0.053 | |
| SBP-ϕ(12 h) (hour:min) | 19:08 | 6:36 | 17:51 | 5:02 | −0.815 | 0.428 | |
| DBP-M (mmHg) | 73.3 | 6.8 | 72.7 | 6.6 | −0.478 | 0.640 | |
| DBP-A(12 h) (mmHg) | 9.0 | 4.2 | 6.0 (66.7%) | 4.3 | −2.029 | 0.061 | |
| DBP-ϕ(12 h) (hour:min) | 19:18 | 6:38 | 16:30 | 5:52 | −1.428 | 0.174 | |
| HR-M (bpm) | 67.1 | 7.0 | 67.9 | 7.3 | 0.827 | 0.421 | |
| HR-A(12 h) (bpm) | 5.0 | 3.1 | 4.2 | 2.6 | −0.7 | 0.495 | |
| HR-ϕ(12 h) (hour:min) | 12:57 | 7:58 | 16:03 | 5:34 | 1.412 | 0.179 | |
| Circadian Characteristics of Geomagnetics | M: Declination (degree) | 3.65 | 0.19 | 3.69 (101.0%) | 0.2 | 3.564 | 0.003 |
| A(24 h): Declination (degree) | 0.09 | 0.05 | 0.18 (207.0%) | 0.1 | 4.499 | 0.0004 | |
| ϕ(24 h): Declination (hour:min) | 2:01 | 1:55 | 1:54 | 1:47 | −0.328 | 0.747 | |
| M: Horizontal component (nT) | 11,020.5 | 26.0252 | 10,997.3 (99.8%) | 39.9 | −2.499 | 0.025 | |
| A(24 h): Horizontal component (nT) | 52.3 | 61.58 | 108.4 (207.4%) | 75.0 | 3.754 | 0.002 | |
| ϕ(24 h): Horizontal component (hour:min) | 15:13 | 4:23 | 13:06 | 3:08 | −3.027 | 0.009 | |
| M: Vertical component (nT) | 51,980.8 | 39.8312 | 51,984.6 | 46.4 | 0.965 | 0.35 | |
| A(24 h): Vertical component (nT) | 17.1 | 15.1 | 33.8 (197.6%) | 23.0 | 3.994 | 0.001 | |
| ϕ(24 h): Vertical component (hour:min) | 12:44 | 6:26 | 18:49 | 7:44 | 3.227 | 0.006 | |
| M: Inclination (degree) | 78.03 | 0.03 | 78.06 (100.03%) | 0.1 | 2.5 | 0.025 | |
| A(24 h): Inclination (degree) | 0.05 | 0.07 | 0.12 (223.3%) | 0.1 | 3.856 | 0.002 | |
| ϕ(24 h): Inclination (hour:min) | 12:20 | 9:44 | 10:08 | 10:13 | −2.957 | 0.001 | |
| M: Total field intensity (nT) | 53,136.2 | 36.8631 | 53,135.4 | 42.1 | −0.241 | 0.813 | |
| A(24 h): Total field intensity (nT) | 20.3 | 15.6 | 26.2 | 13.1 | 1.191 | 0.252 | |
| ϕ(24 h): Total field intensity (hour:min) | 14:57 | 3:38 | 17:47 | 6:48 | 1.71 | 0.108 | |
| Variable (Circadian Parameter) | Two 48-h Spans of BP and HR | |||||
|---|---|---|---|---|---|---|
| Quiet Days (n = 5) | Disturbed Days (n = 5) | Paired t-Test | ||||
| Mean | SD | Mean | SD | p-Value | ||
| SBP (mmHg) | τ | 23.4 | 1.5 | 24.122 | 2.0 | 0.584 |
| M | 122.1 | 12.2 | 121.7 | 13.1 | 0.929 | |
| A | 11.1 | 3.3 | 18.8 (168.5%) | 3.3 | 0.002 | |
| ϕ | 15:10 | 1:16 | 15:37 | 1:53 | 0.497 | |
| DBP (mmHg) | τ | 24.4 | 0.9 | 24.8 | 3.8 | 0.812 |
| M | 77.3 | 6.5 | 75.9 | 7.4 | 0.641 | |
| A | 8.6 | 2.9 | 9.8 | 3.9 | 0.570 | |
| ϕ | 15:13 | 1:10 | 15:48 | 1:14 | 0.420 | |
| HR (bpm) | τ | 23.0 | 1.8 | 26.8 (116.8%) | 4.8 | 0.089 |
| M | 74.0 | 10.3 | 73.5 | 8.7 | 0.873 | |
| A | 7.6 | 5.5 | 9.4 (123.8%) | 4.5 | 0.022 | |
| ϕ | 14:10 | 1:35 | 13:37 | 5:24 | 0.784 | |
| D (°) | M | 3.576 | 0.254 | 3.598 | 0.28 | 0.324 |
| A | 0.095 | 0.029 | 0.23 (243.5%) | 0.08 | 0.008 | |
| H (nT) | M | 11,026.1 | 26.04 | 11,006.3 (99.8%) | 39.22 | 0.070 |
| A | 40.28 | 58.56 | 93.0 (230.9%) | 50.08 | 0.054 | |
| Z (nT) | M | 51,956.0 | 55.89 | 51,952.9 | 46.23 | 0.645 |
| A | 8.19 | 5.44 | 25.35 (309.6%) | 12.98 | 0.012 | |
| Inc (°) | M | 78.02 | 0.03 | 78.04 | 0.05 | 0.121 |
| A | 0.041 | 0.06 | 0.10 (247.3%) | 0.05 | 0.060 | |
| F (nT) | M | 53,113.1 | 51.27 | 53,106.1 | 39.28 | 0.322 |
| A | 15.32 | 10.58 | 24.13 (157.5%) | 9.98 | 0.025 | |
| Variable (Circadian Parameter) | Comparison of Response of Circadian Profiles of BP and HR to Low- or Higher-Intensity Magnetic Stimulation | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quiet Days (Q) (n = 2) | Moderately Disturbed Days (MD) (n = 2) | Extremely Disturbed Days (ED) (n = 2) | Paired t-Test (MD vs. Q) | Paired t-Test (ED vs. Q) | Paired t-Test (ED vs. MD) | ||||||||
| Mean | SD | Mean | MD/Q (%) | SD | Mean | ED/Q (%) | ED/MD (%) | SD | p-Value | p-Value | p-Value | ||
| SBP (mmHg) | M | 124.5 | 3.408 | 132.95 | 8.471 | 121.96 | 2.828 | 0.254 | 0.103 | 0.222 | |||
| A | 13.9 | 1.937 | 21.38 | (154.2%) | 4.36 | 10.62 | (76.6%) | (49.7%) | 4.468 | 0.143 | 0.321 | 0.004 | |
| ϕ | 14:35 | 1:42 | 14:31 | 2:31 | 15:59 | 0:31 | 0.919 | 0.339 | 0.482 | ||||
| DBP (mmHg) | M | 77.5 | 4.476 | 81.865 | 0.191 | 76.42 | 2.320 | 0.415 | 0.598 | 0.200 | |||
| A | 9.9 | 3.80 | 12.07 | 2.065 | 10.83 | 0.078 | 0.357 | 0.798 | 0.538 | ||||
| ϕ | 14:35 | 14:09 | 14:55 | 1:39 | 16:15 | 0:58 | 0.844 | 0.195 | 0.605 | ||||
| HR (bpm) | M | 66.0 | 14.602 | 65.06 | 4.469 | 65.62 | 11.95 | 0.921 | 0.887 | 0.933 | |||
| A | 5.5 | 4.12 | 8.06 | 3.295 | 11.10 | 6.306 | 0.141 | 0.170 | 0.389 | ||||
| ϕ | 15:01 | 1:09 | 15:12 | 0:00 | 15:31 | 0:38 | 0.865 | 0.400 | 0.608 | ||||
| D (°) | M | 3.370 | 3.361 | (99.7%) | 3.413 | (101.3%) | (101.6%) | ||||||
| A | 0.099 | 0.199 | (199.8%) | 0.436 | (438.7%) | (219.6%) | |||||||
| H (nT) | M | 11,037.0 | 11,033.3 | (100.0%) | 11,011.6 | (99.8%) | (99.8%) | ||||||
| A | 15.62 | 58.86 | (376.8%) | 189.8 | (1215.1%) | (322.5%) | |||||||
| Z (nT) | M | 51,904.4 | 51,906.6 | (100.0%) | 51,896.4 | (100.0%) | (100.0%) | ||||||
| A | 5.75 | 17.3 | (300.9%) | 66.68 | (1159.7%) | (385.4%) | |||||||
| Inc (°) | M | 78.00 | 77.999 | (100.0%) | 78.020 | (100.0%) | (100.0%) | ||||||
| A | 0.016 | 0.062 | (386.3%) | 0.215 | (1341.4%) | (347.3%) | |||||||
| F (nT) | M | 53,064.9 | 53,066.3 | (100.0%) | 53,052.2 | (100.0%) | (100.0%) | ||||||
| A | 7.49 | 21.63 | (288.9%) | 27.47 | (367.0%) | (127.0%) | |||||||
| Group A (n = 10) (Geomagnetic Stimulation Started in the Evening: 14:00–20:00) | Group B (n = 6) (Geomagnetic Stimulation Started in the Morning: 06:00–12:00) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Quiet Day | Disturbed Day | Paired t-Test | Quiet Day | Disturbed Day | Paired T-Test | ||||||||
| Mean | SD | Mean | SD | t-Value | p-Value | Mean | SD | Mean | SD | t-Value | p-Value | ||
| SBP (mm Hg) | M | 120.82 | 8.19 | 118.65 | 11.15 | −1.112 | 0.2951 | 118.09 | 12.59 | 121.80 (103.1%) | 13.71 | 3.048 | 0.0285 |
| A | 13.48 | 4.79 | 20.11 (149.2%) | 4.12 | 5.683 | 0.0003 | 14.43 | 3.74 | 14.11 | 6.69 | −0.100 | 0.9243 | |
| ϕ | 14:49 | 1:41 | 15:50 | 2:04 | 1.625 | 0.1385 | 15:39 | 1:28 | 16:54 | 2:25 | 1.177 | 0.2921 | |
| DBP (mm Hg) | M | 75.14 | 6.47 | 71.21 (94.8%) | 7.23 | −3.379 | 0.0081 | 72.39 | 7.47 | 73.58 | 3.84 | 0.657 | 0.5401 |
| A | 11.93 | 5.17 | 11.73 | 6.51 | −0.087 | 0.9329 | 10.21 | 4.36 | 8.68 | 4.75 | −0.467 | 0.6599 | |
| ϕ | 15:13 | 1:57 | 16:05 | 1:48 | 1.336 | 0.2144 | 14:53 | 2:05 | 14:17 | 5:28 | −0.200 | 0.8495 | |
| HR (bpm) | M | 67.82 | 7.72 | 68.12 | 8.32 | 0.205 | 0.8419 | 66.21 | 6.57 | 68.5 (103.4%) | 6.45 | 2.178 | 0.0813 |
| A | 8.17 | 4.06 | 8.37 | 2.76 | 0.199 | 0.8468 | 8.64 | 3.60 | 11.92 (138.0%) | 3.43 | 2.427 | 0.0596 | |
| ϕ | 14:51 | 2:54 | 14:15 | 4:12 | −0.514 | 0.6199 | 13:21 | 1:38 | 15:37 | 3:43 | 1.257 | 0.2642 | |
| D (degrees) | M | 3.615 | 0.182 | 3.666 (101.4%) | 0.202 | 3.484 | 0.0069 | 3.711 | 0.201 | 3.728 | 0.211 | 1.471 | 0.2012 |
| A | 0.092 | 0.060 | 0.202 (219.6%) | 0.087 | 3.639 | 0.0054 | 0.083 | 0.044 | 0.152 (183.1%) | 0.079 | 2.845 | 0.0360 | |
| ϕ | 9:22 | 9:46 | 4:16 | 6:25 | −1.773 | 0.1100 | 5:46 | 8:50 | 5:58 | 8:40 | 0.034 | 0.9741 | |
| H (nT) | M | 11,022.5 | 27.03 | 11,001.0 (99.8%) | 31.50 | −2.291 | 0.0477 | 11,017.3 | 26.38 | 10,991.2 | 54.09 | −1.266 | 0.2612 |
| A | 44.08 | 57.97 | 102.28 (232.0%) | 59.34 | 3.692 | 0.0050 | 65.97 | 70.47 | 118.73 | 101.72 | 1.635 | 0.1629 | |
| ϕ | 15:09 | 4:55 | 13:15 | 2:19 | −2.124 | 0.0626 | 15:18 | 3:46 | 12:51 | 4:25 | −2.044 | 0.0963 | |
| Z (nT) | M | 51,972.3 | 38.04 | 51,974.0 | 44.41 | 0.471 | 0.6490 | 51,994.9 | 42.10 | 52,002.3 | 47.96 | 0.810 | 0.4550 |
| A | 14.72 | 11.46 | 37.32 (253.5%) | 21.64 | 4.557 | 0.0014 | 21.02 | 20.44 | 57.23 | 72.68 | 1.148 | 0.3027 | |
| ϕ | 14:37 | 4:54 | 12:57 | 9:21 | −0.483 | 0.6408 | 13:35 | 6:36 | 12:36 | 8:47 | −0.212 | 0.8405 | |
| Inc (degrees) | M | 78.03 | 0.03 | 78.05 (100.03%) | 0.04 | 2.330 | 0.0448 | 78.04 | 0.03 | 78.07 | 0.06 | 1.273 | 0.2591 |
| A | 0.045 | 0.064 | 0.112 (248.9%) | 0.068 | 3.809 | 0.0042 | 0.068 | 0.076 | 0.135 | 0.119 | 1.734 | 0.1434 | |
| ϕ | 12:50 | 9:54 | 5:57 | 9:01 | −2.454 | 0.0365 | 3:30 | 4:07 | 9:06 | 9:20 | 1.162 | 0.2976 | |
| F (nT) | M | 53,128.4 | 34.95 | 53,125.7 | 41.65 | −0.663 | 0.5242 | 53,149.4 | 39.32 | 53,151.6 | 41.15 | 0.307 | 0.7715 |
| A | 17.14 | 7.52 | 31.52 (183.9%) | 11.58 | 3.687 | 0.0050 | 25.58 | 24.07 | 40.83 | 57.65 | 0.543 | 0.6107 | |
| ϕ | 14:56 | 4:13 | 12:11 | 7:38 | −1.202 | 0.2600 | 14:59 | 2:43 | 11:06 | 7:17 | −1.060 | 0.3378 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otsuka, K.; Cornelissen, G.; Weydahl, A.; Gubin, D.; Beaty, L.A.; Murase, M. Rules of Heliogeomagnetics Diversely Coordinating Biological Rhythms and Promoting Human Health. Appl. Sci. 2023, 13, 951. https://doi.org/10.3390/app13020951
Otsuka K, Cornelissen G, Weydahl A, Gubin D, Beaty LA, Murase M. Rules of Heliogeomagnetics Diversely Coordinating Biological Rhythms and Promoting Human Health. Applied Sciences. 2023; 13(2):951. https://doi.org/10.3390/app13020951
Chicago/Turabian StyleOtsuka, Kuniaki, Germaine Cornelissen, Andi Weydahl, Denis Gubin, Larry A. Beaty, and Masatoshi Murase. 2023. "Rules of Heliogeomagnetics Diversely Coordinating Biological Rhythms and Promoting Human Health" Applied Sciences 13, no. 2: 951. https://doi.org/10.3390/app13020951
APA StyleOtsuka, K., Cornelissen, G., Weydahl, A., Gubin, D., Beaty, L. A., & Murase, M. (2023). Rules of Heliogeomagnetics Diversely Coordinating Biological Rhythms and Promoting Human Health. Applied Sciences, 13(2), 951. https://doi.org/10.3390/app13020951








