Does Silver Diamine Fluoride Affect the Adaptation of High-Viscosity Resin-Modified Glass Ionomer to Dentin? An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
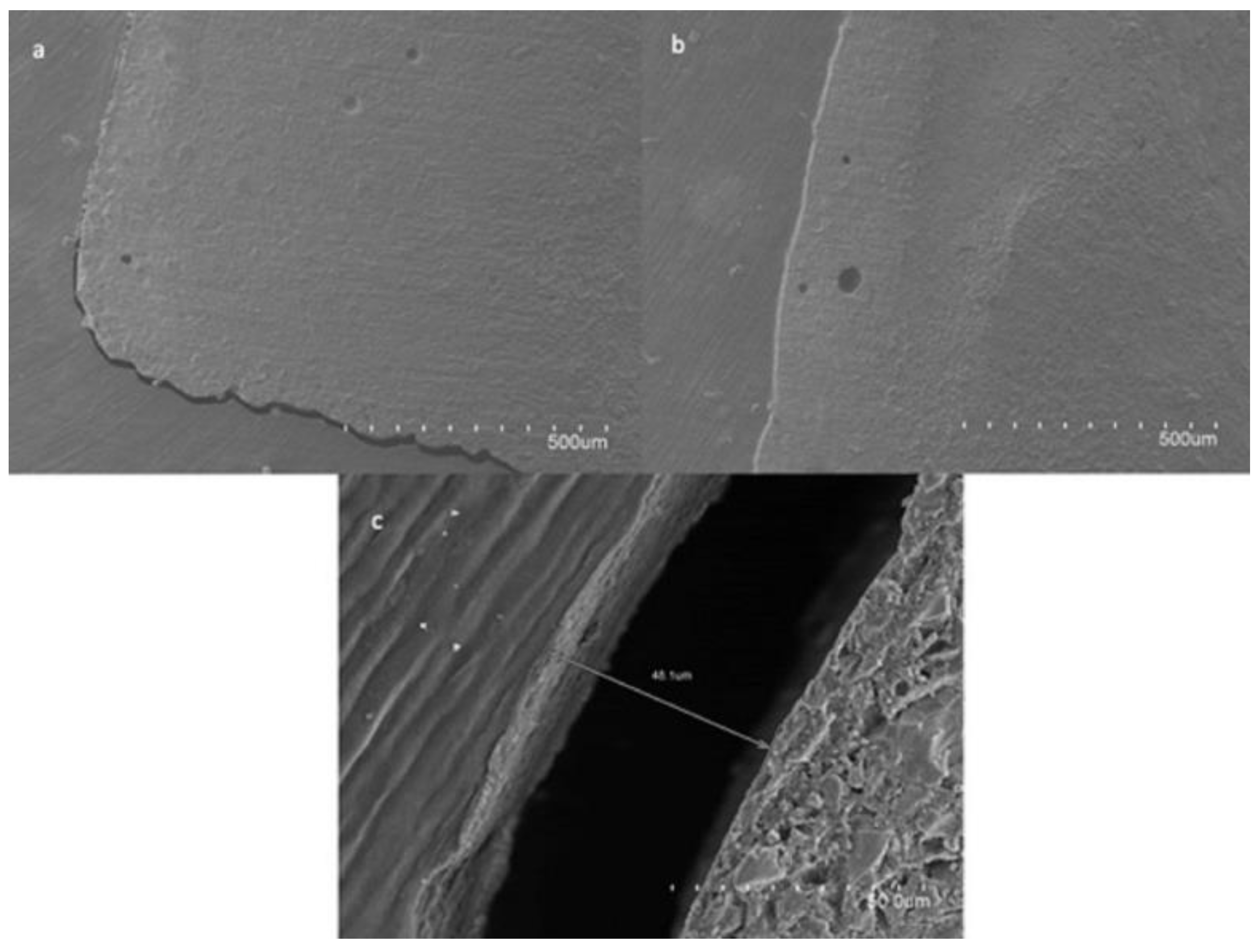
2.2. Sample Visualization under Scanning Electron Microscopy (SEM)
2.3. Statistical Analysis
3. Results
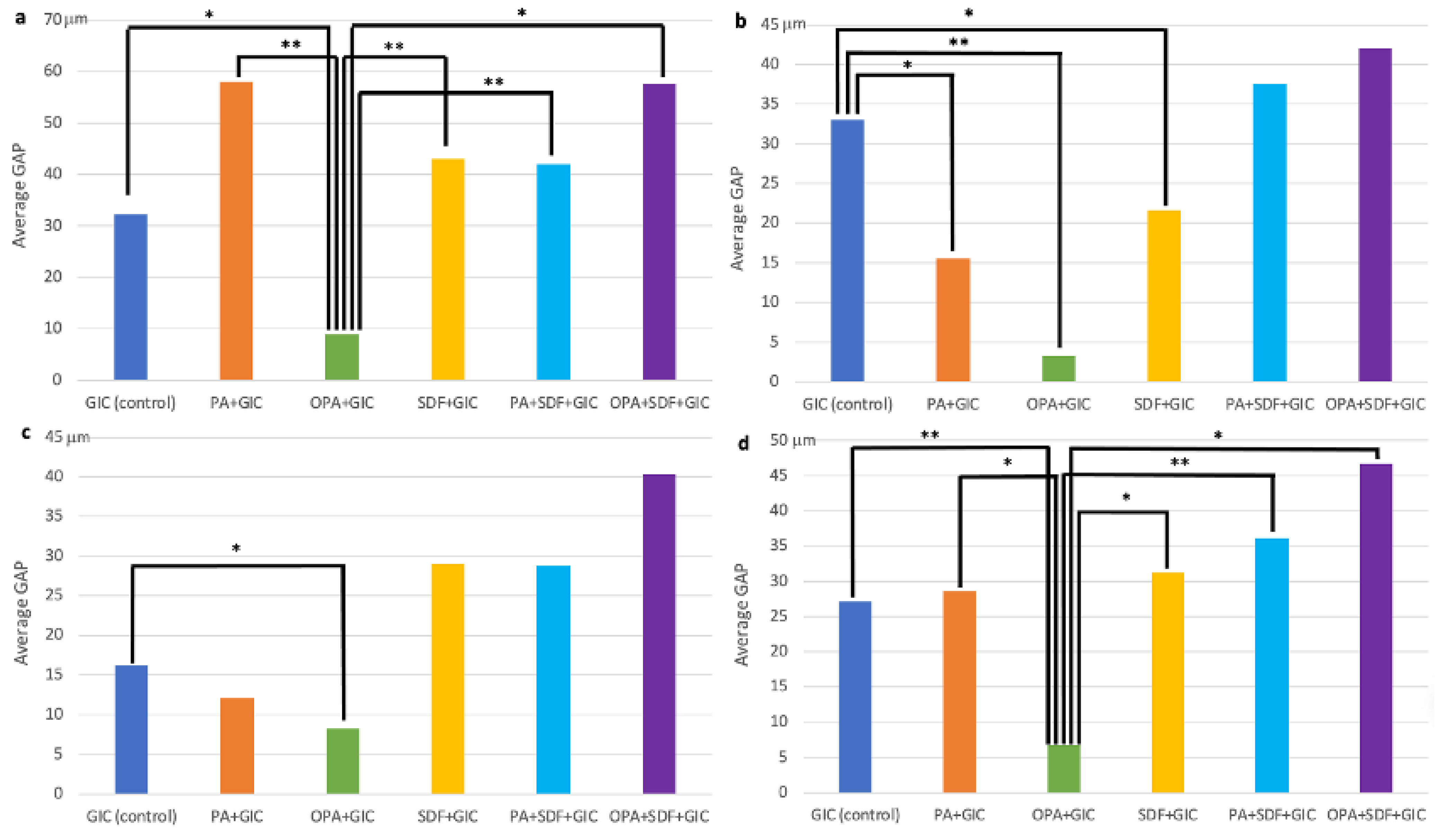
3.1. Influence of SDF/KI in RLC Adaptation
3.2. Comparison between Groups
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arola, D.D.; Gao, S.; Zhang, H.; Masri, R. The Tooth: Its Structure and Properties. Dent. Clin. N. Am. 2017, 61, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Perdigão, J. Dentin bonding as a function of dentin structure. Dent. Clin. N. Am. 2002, 46, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Tian, K.V.; Mahmoud, M.Z.; Cozza, P.; Licoccia, S.; Fang, D.C.; Di Tommaso, D.; Chass, G.A.; Neville Greaves, G. Periodic vs. molecular cluster approaches to resolving glass structure and properties: Anorthite a case study. J. Non Cryst. Soli. 2016, 451, 138–145. [Google Scholar] [CrossRef]
- Powis, D.R.; Follerås, T.; Merson, S.A.; Wilson, A.D. Improved Adhesion of a Glass Ionomer Cement to Dentin and Enamel. J. Dent. Res. 1982, 61, 1416–1422. [Google Scholar] [CrossRef] [PubMed]
- Tay, F.R.; Smales, R.J.; Ngo, H.; Wei, S.H.; Pashley, D.H. Effect of different conditioning protocols on adhesion of a GIC to dentin. J. Adhes. Dent. 2001, 3, 153–167. [Google Scholar]
- Ebaya, M.M.; Ali, A.I.; Mahmoud, S.H. Evaluation of Marginal Adaptation and Microleakage of Three Glass Ionomer-Based Class V Restorations: In Vitro Study. Eur. J. Dent. 2019, 13, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Eskandarizadeh, A.; Parizi, M.T.; Goroohi, H.; Badrian, H.; Asadi, A.; Khalighinejad, N. Histological assessment of pulpal responses to resin modified glass ionomer cements in human teeth. Dent. Res. J. 2015, 12, 144–149. [Google Scholar]
- Fröhlich, T.T.; Botton, G.; Rocha, R.O. Bonding of Glass-Ionomer Cement and Adhesives to Silver Diamine Fluoride-treated Dentin, An Updated Systematic Review and Meta-Analysis. J. Adhes. Dent. 2022, 24, 29–38. [Google Scholar]
- Fung, M.H.T.; Duangthip, D.; Wong, M.C.M.; Lo, E.C.M.; Chu, C.H. Randomized Clinical Trial of 12% and 38% Silver Diamine Fluoride Treatment. J. Dent. Res. 2017, 97, 171–178. [Google Scholar] [CrossRef]
- Gupta, J.; Thomas, M.S.; Radhakrishna, M.; Srikant, N.; Ginjupalli, K. Effect of silver diamine fluoride-potassium iodide and 2% chlorhexidine gluconate cavity cleansers on the bond strength and microleakage of resin-modified glass ionomer cement. J. Conserv. Dent. 2019, 22, 201–206. [Google Scholar]
- Hamama, H.H.; Burrow, M.F.; Yiu, C. Effect of dentine conditioning on adhesion of resin-modified glass ionomer adhesives. Aust. Dent. J. 2014, 59, 193–200. [Google Scholar] [CrossRef]
- Pedersen, M.T.; Tian, K.V.; Dobó-Nagy, C.; Chass, G.A.; Neville Greaves, G.; Yue, Y. Phase separation in an ionomer glass: Insight from calorimetry and phase transition. J. Non Cryst. Soli. 2015, 415, 24–29. [Google Scholar] [CrossRef]
- Tian, K.V.; Chass, G.A.; Di Tommaso, D. Simulations reveal the role of composition into the atomic-level flexibility of bioactive glass cements. Phys. Chem. Chem. Phys. 2016, 18, 837–845. [Google Scholar] [CrossRef]
- Kumari, P.D.; Khijmatgar, S.; Chowdhury, A.; Lynch, E.; Chowdhury, C.R. Factors influencing fluoride release in atraumatic restorative treatment (ART) materials: A review. J. Oral Biol. Craniofac. Res. 2019, 9, 315–320. [Google Scholar] [CrossRef]
- Bahsi, E.; Sagmak, S.; Dayi, B.; Cellik, O.; Akkus, Z. The evaluation of microleakage and fluoride release of different types of glass ionomer cements. Niger. J. Clin. Pract. 2019, 22, 961–970. [Google Scholar] [CrossRef]
- Najeeb, S.; Khurshid, Z.; Zafar, M.; Khan, A.; Zohaib, S.; Martí, J.M.N.; Sauro, S.; Matinlinna, J.P.; Rehman, I.U. Modifications in Glass Ionomer Cements: Nano-Sized Fillers and Bioactive Nanoceramics. Int. J. Mol. Sci. 2016, 17, 1134. [Google Scholar] [CrossRef] [Green Version]
- Horst, J.A.; Ellenikiotis, H.; Milgrom, P.L. UCSF Protocol for Caries Arrest Using Silver Diamine Fluoride: Rationale.; Indications and Consent. J. Calif. Dent. Assoc. 2016, 44, 16–28. [Google Scholar]
- Zhao, I.S.; Gao, S.S.; Hiraishi, N.; Burrow, M.F.; Duangthip, D.; Mei, M.L.; Lo, E.C.M.; Chu, C.H. Mechanisms of silver diamine fluoride on arresting caries: A literature review. Int. Dent. J. 2018, 68, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Crystal, Y.O.; Niederman, R. Evidence-Based Dentistry Update on Silver Diamine Fluoride. Dent. Clin. N. Am. 2019, 63, 45–68. [Google Scholar] [CrossRef]
- Jiang, M.; Mei, M.L.; Wong, M.C.M.; Chu, C.H.; Lo, E.C.M. Effect of silver diamine fluoride solution application on the bond strength of dentine to adhesives and to glass ionomer cements: A systematic review. BMC Oral Health 2020, 20, 40. [Google Scholar] [CrossRef]
- Tüzüner, T.; Dimkov, A.; Nicholson, J.W. The effect of antimicrobial additives on the properties of dental glass-ionomer cements: A review. Acta Biomater. Odontol. Scand. 2019, 5, 9–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjørndal, L.; Simon, S.; Tomson, P.L.; Duncan, H.F. Management of deep caries and the exposed pulp. Int. Endod. J. 2019, 52, 949–973. [Google Scholar] [CrossRef] [PubMed]
- Oliva, A.; della Ragione, F.; Salerno, A.; Riccio, V.; Tartaro, G.; Cozzolino, A.; D'amato, S.; Pontoni, G.; Zappia, V. Biocompatibility studies on glass ionomer cements by primary cultures of human osteoblasts. Biomaterials 1996, 17, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Tanumiharja, M.; Burrow, M.F.; Tyas, M.J. Microtensile bond strengths of glass ionomer (polyalkenoate) cements to dentine using four conditioners. J. Dent. 2000, 28, 361–366. [Google Scholar] [CrossRef]
- Soares, C.J.; Celiberto, L.; Dechichi, P.; Fonseca, R.B.; Martins, L.R. Marginal integrity and microleakage of direct and indirect composite inlays: SEM and stereomicroscopic evaluation. Braz. Oral Res. 2005, 7, 295–301. [Google Scholar] [CrossRef]
- Pontes, D.G.; Guedes-Neto, M.V.; Cabral, M.F.; Cohen-Carneiro, F. Microleakage evaluation of class V restorations with conventional and resin-modified glass ionomer cements. Oral Health Dent. Manag. 2014, 13, 642–646. [Google Scholar]
- Soubhagya, M.; Goud, K.M.; Deepak, B.S.; Thakur, S.; Nandini, T.N.; Arun, J. Comparative in vitro evaluation of internal adaptation of resin-modified glass ionomer.; flowable composite and bonding agent applied as a liner under composite restoration: A scanning electron microscope study. J. Int. Oral Health 2015, 7, 27–31. [Google Scholar]
- Tanumiharja, M.; Burrow, M.F.; Cimmino, A.; Tyas, M.J. The evaluation of four conditioners for glass ionomer cements using field-emission scanning electron microscopy. J. Dent. 2001, 29, 131–138. [Google Scholar] [CrossRef]
- Zhao, I.S.; Chu, S.; Yu, O.Y.; Mei, M.L.; Chu, C.H.; Lo, E.C.M. Effect of silver diamine fluoride and potassium iodide on shear bond strength of glass ionomer cements to caries-affected dentine. Int. Dent. J. 2019, 69, 341–347. [Google Scholar] [CrossRef]

| Material | Composition | Manufacturer, City, Country | Batch n° |
|---|---|---|---|
| Riva Light Cure | Liquid: Polyacrylic acid, tartaric acid, HEMA Powder: Bioactive glass (Ionglass™), contains fluoride and strontium ions | SDI, Bayswater, Australia | 873003 |
| Riva Conditioner | Polyacrylic acid 25–30% in weight | SDI, Bayswater, Australia | 334601 |
| Gel Etchant | Orthophosphoric acid 37.5% in weight | Kerr, Bioggio, Switzerland | 200317 |
| Riva Star | Silver diamine fluoride: 38% silver fluoride, 15–20% anhydrous ammonia, and water. Potassium iodide | SDI, Bayswater, Australia | 11557551 |
| GIC (Control) | Glass Ionomer Cement. Application in One Increment and Light Curing for 20 s |
|---|---|
| SDF/KI + GIC | SDF application for 1 min, KI application for 2 min, cavity rinsing, GIC application in one increment and light curing for 20 s. |
| PA + GIC | 25% Polyacrylic acid. Dentin conditioning for 10 s, cavity rinsing and mild drying, GMIC application in one increment and light curing for 20 s. |
| PA + SDF/KI + GIC | SDF application for 1 min, KI application for 2 min, cavity rinsing, dentin conditioning with 25% PA for 10 s, cavity rinsing and mild drying, GIC application in one increment and light curing for 20 s. |
| OPA + GIC | 37% Orthophosphoric acid. Dentin conditioning for 5 s, cavity rinsing and mild drying, GIC application in one increment and light curing for 20 s. |
| OPA + SDF/KI + GIC | SDF application for 1 min, KI application for 2 min, cavity rinsing, dentin conditioning with 37% OPA for 5 s, cavity rinsing and mild drying, GIC application in one increment and light curing for 20 s. |
| Median | IQR | Mean | SD | p | ||
|---|---|---|---|---|---|---|
| Coronal | GIC | 25.30 | 37.95 | 32.26 | 22.44 | 0.72 |
| SDF/KI + GIC | 34.25 | 54.17 | 43.04 | 34.23 | ||
| PA + GIC | 52.50 | 68.05 | 58.04 | 44.03 | 0.11 | |
| PA + SDF/KI + GIC | 40.10 | 26.85 | 41.99 | 32.27 | ||
| OPA + GIC | 00.00 | 24.58 | 8.95 | 14.05 | 0.04 | |
| OPA + SDF/KI + GIC | 63.50 | 103.2 | 57.59 | 54.91 | ||
| Axial | GIC | 29.10 | 14.70 | 34.17 | 17.09 | 0.04 |
| SDF/kI + GIC | 04.93 | 32.37 | 21.70 | 37.19 | ||
| PA + GIC | 00.00 | 36.70 | 15.58 | 20.05 | 0.35 | |
| PA + SDF/KI + GIC | 12.50 | 50.30 | 37.54 | 57.77 | ||
| OPA + GIC | 00.00 | 00.00 | 03.25 | 09.19 | 0.42 | |
| OPA + SDF/KI + GIC | 00.00 | 110.7 | 42.04 | 59.94 | ||
| Cervical | GIC | 19.30 | 15.71 | 16.19 | 08.56 | 0.63 |
| SDF/kI + GIC | 07.38 | 51.87 | 29.05 | 41.15 | ||
| PA + GIC | 00.00 | 34.15 | 12.14 | 18.39 | 0.84 | |
| PA + SDF/kI + GIC | 00.00 | 83.20 | 28.76 | 43.25 | ||
| OPA + GIC | 00.00 | 19.22 | 8.20 | 11.61 | 0.37 | |
| OPA + SDF/kI + GIC | 00.00 | 99.50 | 40.34 | 58.05 | ||
| Total | GIC | 21.44 | 20.42 | 27.15 | 12.70 | 0.54 |
| SDF/KI + GIC | 18.47 | 43.02 | 31.26 | 30.37 | ||
| PA + GIC | 37.40 | 31.00 | 28.59 | 17.70 | 0.66 | |
| PA + SDF/KI + GIC | 29.46 | 33.02 | 36.10 | 36.73 | ||
| OPA + GIC | 04.70 | 13.82 | 06.80 | 07.61 | 0.04 | |
| OPA + SDF/KI + GIC | 46.00 | 80.18 | 46.66 | 42.15 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghilotti, J.; Alzina-Cendra, A.; Sanz, J.L.; Forner, L.; Llena, C. Does Silver Diamine Fluoride Affect the Adaptation of High-Viscosity Resin-Modified Glass Ionomer to Dentin? An In Vitro Study. Appl. Sci. 2023, 13, 991. https://doi.org/10.3390/app13020991
Ghilotti J, Alzina-Cendra A, Sanz JL, Forner L, Llena C. Does Silver Diamine Fluoride Affect the Adaptation of High-Viscosity Resin-Modified Glass Ionomer to Dentin? An In Vitro Study. Applied Sciences. 2023; 13(2):991. https://doi.org/10.3390/app13020991
Chicago/Turabian StyleGhilotti, James, Arnau Alzina-Cendra, José Luis Sanz, Leopoldo Forner, and Carmen Llena. 2023. "Does Silver Diamine Fluoride Affect the Adaptation of High-Viscosity Resin-Modified Glass Ionomer to Dentin? An In Vitro Study" Applied Sciences 13, no. 2: 991. https://doi.org/10.3390/app13020991





