Effect of Antimony Content on Transition Behavior and Electric Properties of (K0.44Na0.52Li0.04)(Nb0.9−xTa0.10Sbx)O3 Ceramics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation Process
2.2. Structural Characterization
2.3. Microstructural Characterization
2.4. Electrical Characterization
3. Results and Discussion
Structural and Microstructural Characterization
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gao, X.; Cheng, Z.; Chen, Z.; Liu, Y.; Meng, X.; Zhang, X.; Wang, J.; Guo, Q.; Li, B.; Sun, H.; et al. The Mechanism for the Enhanced Piezoelectricity in Multi-Elements Doped (K,Na)NbO3Ceramics. Nat. Commun. 2021, 12, 881. [Google Scholar] [CrossRef]
- Zhang, S.; Li, F.; Jiang, X.; Kim, J.; Luo, J.; Geng, X. Advantages and Challenges of Relaxor-PbTiO3 Ferroelectric Crystals for Electroacoustic Transducers—A Review. Prog. Mater. Sci. 2015, 68, 1–66. [Google Scholar] [CrossRef] [Green Version]
- Luo, H.; Ke, H.; Zhang, H.; Zhang, L.; Li, F.; Cao, L.; Jia, D.; Zhou, Y. Enhanced Ferroelectric and Energy-Storage Properties of Nb-Doped 0.94Na0.5Bi0.5TiO3-0.06BaTiO3 Ceramics Prepared by a Multi-Ionic Sol-Gel Method. Phys. B Condens. Matter. 2019, 567, 17–24. [Google Scholar] [CrossRef]
- Cortés, J.A.; Camargo, J.; Rachia, M.F.; Rubio-Marcos, F.; Ramajo, L.; Castro, M.; Ramírez, M.A. Influence of the Sintering Temperature on Ferroelectric Properties of Potassium-Sodium Niobate Piezoelectric Ceramics. J. Adv. Dielect. 2021, 11, 2140002. [Google Scholar] [CrossRef]
- Kang, W.-S.; Lee, S.-K.; Koh, J.-H. AC Conductivity and Dielectric Properties of (Bi,Na)TiO3–BaTiO3 Lead Free Ceramics. Ceram. Int. 2015, 41, 6925–6932. [Google Scholar] [CrossRef]
- Prado, A.; Ramajo, L.; Camargo, J.; del Campo, A.; Öchsner, P.; Rubio-Marcos, F.; Castro, M. Stabilization of the Morphotropic Phase Boundary in (1−x)Bi0.5Na0.5TiO3-XBaTiO3 Ceramics through Two Alternative Synthesis Pathways. J. Mater. Sci. Mater. Electron. 2019, 30, 18405–18412. [Google Scholar] [CrossRef]
- Hao, J.; Xu, Z.; Chu, R.; Zhang, Y.; Chen, Q.; Fu, P.; Li, W.; Li, G.; Yin, Q. Characterization of (K0.5Na0.5)NbO3 Powders and Ceramics Prepared by a Novel Hybrid Method of Sol–Gel and Ultrasonic Atomization. Mater. Des. 2010, 31, 3146–3150. [Google Scholar] [CrossRef]
- Shrout, T.R.; Zhang, S.J. Lead-Free Piezoelectric Ceramics: Alternatives for PZT? J. Electroceram. 2007, 19, 113–126. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Fernandez, J.F.; Ochoa, D.A.; García, J.E.; Rojas-Hernandez, R.E.; Castro, M.; Ramajo, L. Understanding the Piezoelectric Properties in Potassium-Sodium Niobate-Based Lead-Free Piezoceramics: Interrelationship between Intrinsic and Extrinsic Factors. J. Eur. Ceram. Soc. 2017, 37, 3501–3509. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Gou, G.; Li, J. Ruddlesden–Popper Perovskite Sulfides A3B2S7: A New Family of Ferroelectric Photovoltaic Materials for the Visible Spectrum. Nano Energy 2016, 22, 507–513. [Google Scholar] [CrossRef]
- Wendari, T.P.; Arief, S.; Mufti, N.; Blake, G.R.; Baas, J.; Suendo, V.; Prasetyo, A.; Insani, A.; Zulhadjri, Z. Lead-Free Aurivillius Phase Bi2LaNb1.5Mn0.5O9: Structure, Ferroelectric, Magnetic, and Magnetodielectric Effects. Inorg. Chem. 2022, 61, 8644–8652. [Google Scholar] [CrossRef]
- Chang, Y.; Poterala, S.; Yang, Z.; Messing, G.L. Enhanced Electromechanical Properties and Temperature Stability of Textured (K0.5Na0.5)NbO3-Based Piezoelectric Ceramics: Properties and Temperature Stability of Textured (K0.5Na0.5)NbO3-Based Piezoelectric Ceramics. J. Am. Ceram. Soc. 2011, 94, 2494–2498. [Google Scholar] [CrossRef]
- Chae, M.-S.; Lee, K.-S.; Koo, S.-M.; Ha, J.-G.; Jeon, J.-H.; Koh, J.-H. Improved Piezoelectric Properties of Ag Doped 0.94(K0.5–ΒNa0.5–δ)NbO3–0.06Li1–γNbO3 Ceramics by Templated Grain Growth Method. J. Electroceram. 2013, 30, 60–65. [Google Scholar] [CrossRef]
- Zhang, S.; Xia, R.; Hao, H.; Liu, H.; Shrout, T.R. Mitigation of Thermal and Fatigue Behavior in K0.5Na0.5NbO3-Based Lead Free Piezoceramics. Appl. Phys. Lett. 2008, 92, 152904. [Google Scholar] [CrossRef] [Green Version]
- Moure, A. Review and Perspectives of Aurivillius Structures as a Lead-Free Piezoelectric System. Appl. Sci. 2018, 8, 62. [Google Scholar] [CrossRef] [Green Version]
- Saito, Y.; Takao, H.; Toshihiko, T.; Toshihiko, N. Lead-Free Piezoceramics. Nature 2004, 432, 84–87. [Google Scholar] [CrossRef]
- Fuentes, J.; Portelles, J.; Pérez, A.; Durruthy-Rodríguez, M.D.; Ostos, C.; Raymond, O.; Heiras, J.; Cruz, M.P.; Siqueiros, J.M. Structural and Dielectric Properties of La- and Ti-Modified K0.5Na0.5NbO3 Ceramics. Appl. Phys. A 2012, 107, 733–738. [Google Scholar] [CrossRef]
- Carreño-Jiménez, B.; Villafuerte-Castrejón, M.E.; Reyes-Montero, A.; López-Juárez, R. Effect of Antimony Content on Electrical and Structural Properties of 0.98(K0.48Na0.52)0.95Li0.05Nb1−xSbx O3–0.02Ba0.5(Bi0.5Na0.5)0.5ZrO3 Ceramics. Bol. Soc. Esp. Ceram. V. 2021, 60, 266–272. [Google Scholar] [CrossRef]
- Carreño-Jiménez, B.; Reyes-Montero, A.; Villafuerte-Castrejón, M.E.; López-Juárez, R. Piezoelectric, Dielectric and Ferroelectric Properties of (1−x)(K0.48Na0.52)0.95Li0.05Nb0.95Sb0.05O3-XBa0.5(Bi0.5Na0.5)0.5ZrO3 Lead-Free Solid Solution. J. Electron. Mater. 2018, 47, 6053–6058. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, Y.; Pan, D.; Duan, H.; Chen, Y.; Li, H.; Liu, H. Phase Transition and Piezoelectric Properties of Dense (K0.48Na0.52)0.95Li0.05SbxNb1−xO3-0.03Ca0.5(Bi0.5,Na0.5)0.5ZrO3 Lead Free Ceramics. J. Alloys Compd. 2016, 664, 503–509. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Romero, J.J.; Fernandez, J.F. Effect of the Temperature on the Synthesis of (K,Na)NbO3-Modified Nanoparticles by a Solid State Reaction Route. J. Nanoparticle Res. 2010, 12, 2495–2502. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Romero, J.J.; Ochoa, D.A.; García, J.E.; Perez, R.; Fernandez, J.F. Effects of Poling Process on KNN-Modified Piezoceramic Properties. J. Am. Ceram. Soc. 2010, 93, 318–321. [Google Scholar] [CrossRef]
- Bortolani, F.; del Campo, A.; Fernandez, J.F.; Clemens, F.; Rubio-Marcos, F. High Strain in (K,Na)NbO3 -Based Lead-Free Piezoelectric Fibers. Chem. Mater. 2014, 26, 3838–3848. [Google Scholar] [CrossRef]
- Trodahl, H.J.; Klein, N.; Damjanovic, D.; Setter, N.; Ludbrook, B.; Rytz, D.; Kuball, M. Raman Spectroscopy of (K,Na)NbO3 and (K,Na)1−xLixNbO3. Appl. Phys. Lett. 2008, 93, 262901. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Glazer, A.M.; Baker, D.; Thomas, P.A. Structures of K0.05Na0.95NbO3 (50–300 K) and K0.30Na0.70NbO3 (100–200 K). Acta Crystallogr. B Struct. Sci. 2009, 65, 291–299. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Ang, C.; Guo, R.; Bhalla, A.S. Piezoelectric and Strain Properties of Ba(Ti1−xZrx)O3 Ceramics. J. Appl. Phys. 2002, 92, 1489–1493. [Google Scholar] [CrossRef]
- Rubio-Marcos, F.; Romero, J.J.; Martín-Gonzalez, M.S.; Fernández, J.F. Effect of Stoichiometry and Milling Processes in the Synthesis and the Piezoelectric Properties of Modified KNN Nanoparticles by Solid State Reaction. J. Eur. Ceram. Soc. 2010, 30, 2763–2771. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zhang, B.; Yang, W.; Ma, N. Influence of Sintering Temperature on the Structure and Piezoelectric Properties of ZnO-Modified (Li, Na, K)NbO3 Lead-Free Ceramics. Int. J. Miner. Metall. Mater. 2012, 19, 843–848. [Google Scholar] [CrossRef]
- Wendari, T.P.; Arief, S.; Mufti, N.; Baas, J.; Blake, G.R.; Zulhadjri, Z. Ratio Effect of Salt Fluxes on Structure, Dielectric and Magnetic Properties of La,Mn-Doped PbBi2Nb2O9 Aurivillius Phase. Ceram. Int. 2020, 46, 14822–14827. [Google Scholar] [CrossRef]


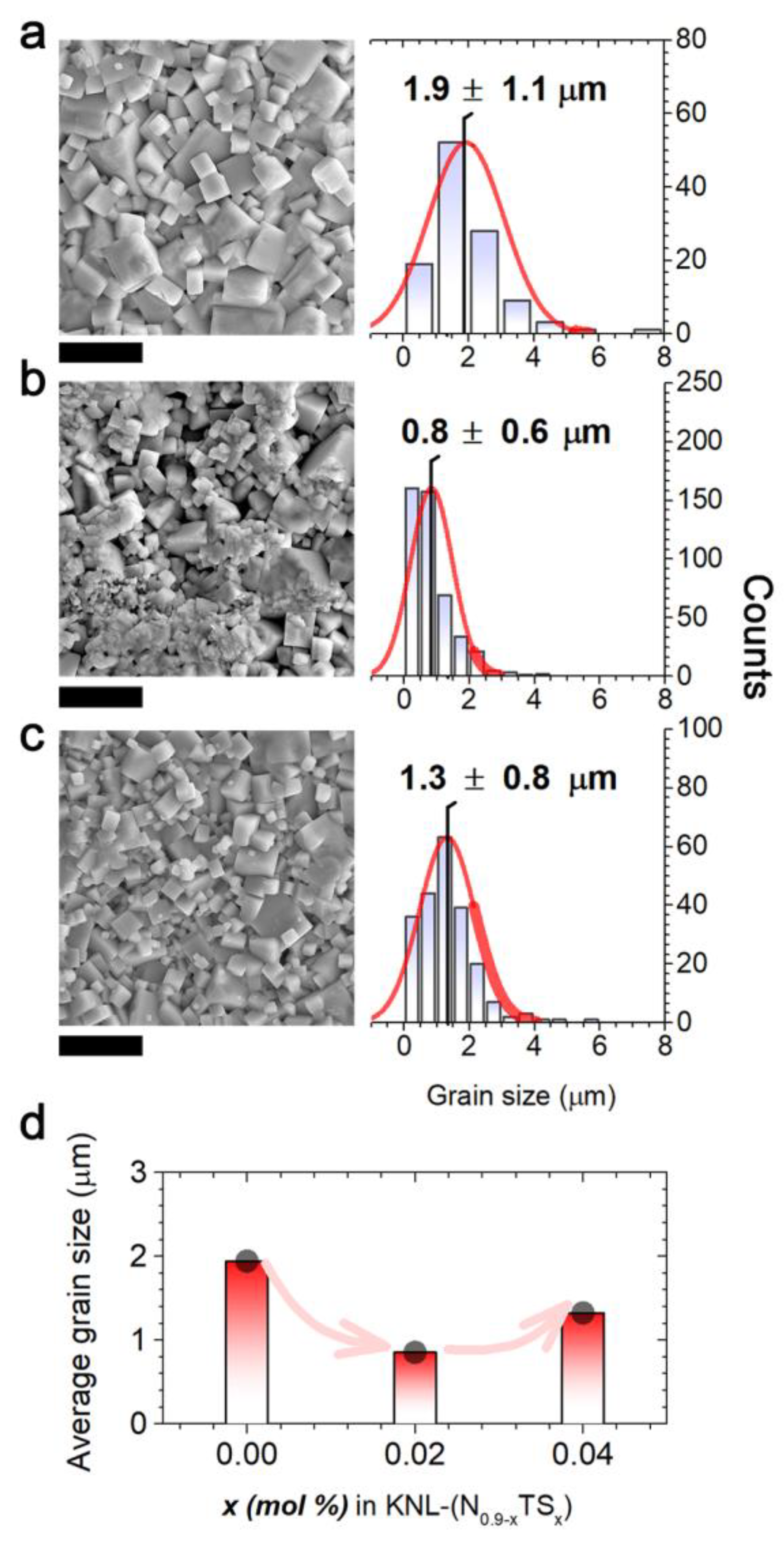

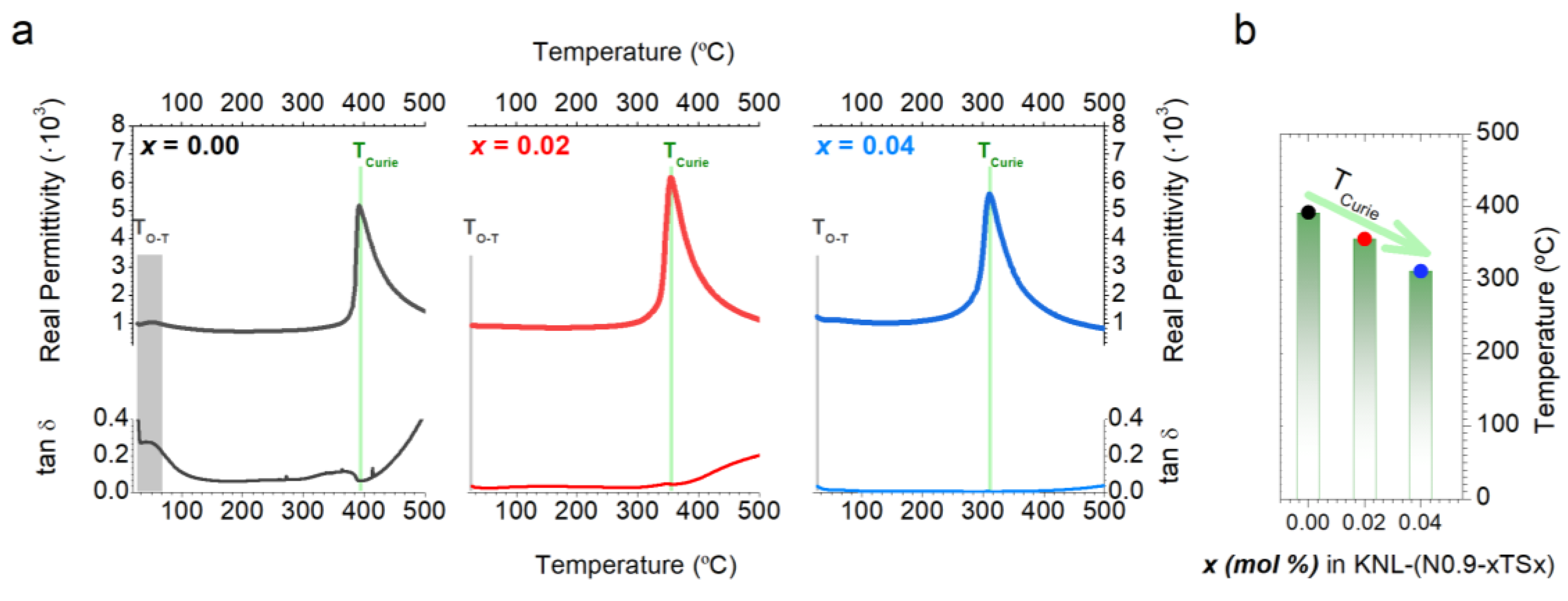
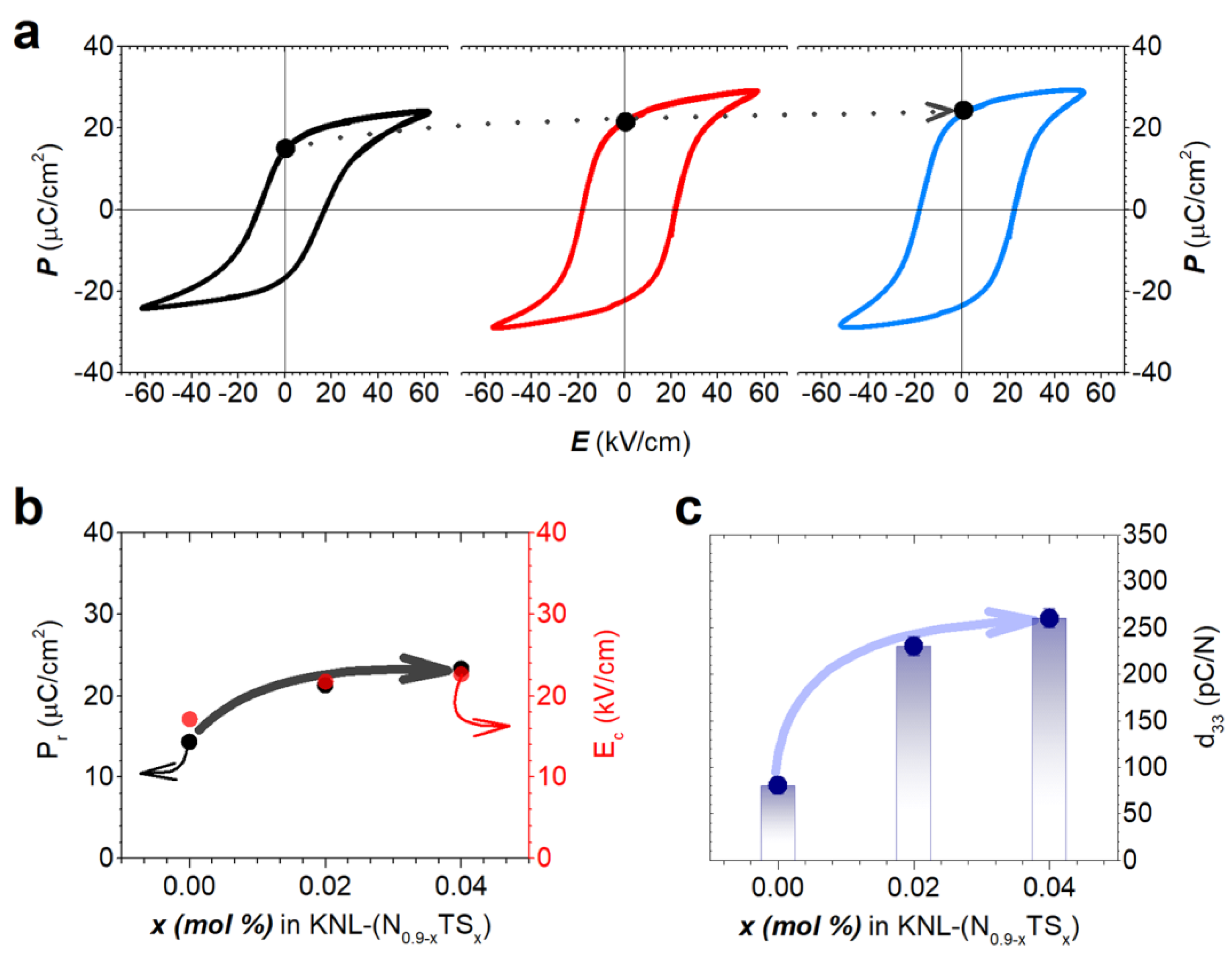
| Sb Content (x) | ρ (g/cm3) | d33 (pC/N) | ε’ | tanδ | Pr (μC/cm2) | Ec (kV/cm) |
|---|---|---|---|---|---|---|
| 0.00 | 4.57 | 80 | 933.8 | 0.28 | 14.3 | 17.1 |
| 0.02 | 4.56 | 230 | 935.1 | 0.04 | 21.3 | 21.7 |
| 0.04 | 4.55 | 260 | 1211.2 | 0.03 | 23.3 | 22.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Difeo, M.; Rubio-Marcos, F.; Gibbs, F.; Castro, M.; Ramajo, L. Effect of Antimony Content on Transition Behavior and Electric Properties of (K0.44Na0.52Li0.04)(Nb0.9−xTa0.10Sbx)O3 Ceramics. Appl. Sci. 2023, 13, 992. https://doi.org/10.3390/app13020992
Difeo M, Rubio-Marcos F, Gibbs F, Castro M, Ramajo L. Effect of Antimony Content on Transition Behavior and Electric Properties of (K0.44Na0.52Li0.04)(Nb0.9−xTa0.10Sbx)O3 Ceramics. Applied Sciences. 2023; 13(2):992. https://doi.org/10.3390/app13020992
Chicago/Turabian StyleDifeo, Mauro, Fernando Rubio-Marcos, Florencia Gibbs, Miriam Castro, and Leandro Ramajo. 2023. "Effect of Antimony Content on Transition Behavior and Electric Properties of (K0.44Na0.52Li0.04)(Nb0.9−xTa0.10Sbx)O3 Ceramics" Applied Sciences 13, no. 2: 992. https://doi.org/10.3390/app13020992






