Influence of Silver Nanoparticles on Color Stability of Room-Temperature-Vulcanizing Maxillofacial Silicone Subjected to Accelerated Artificial Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
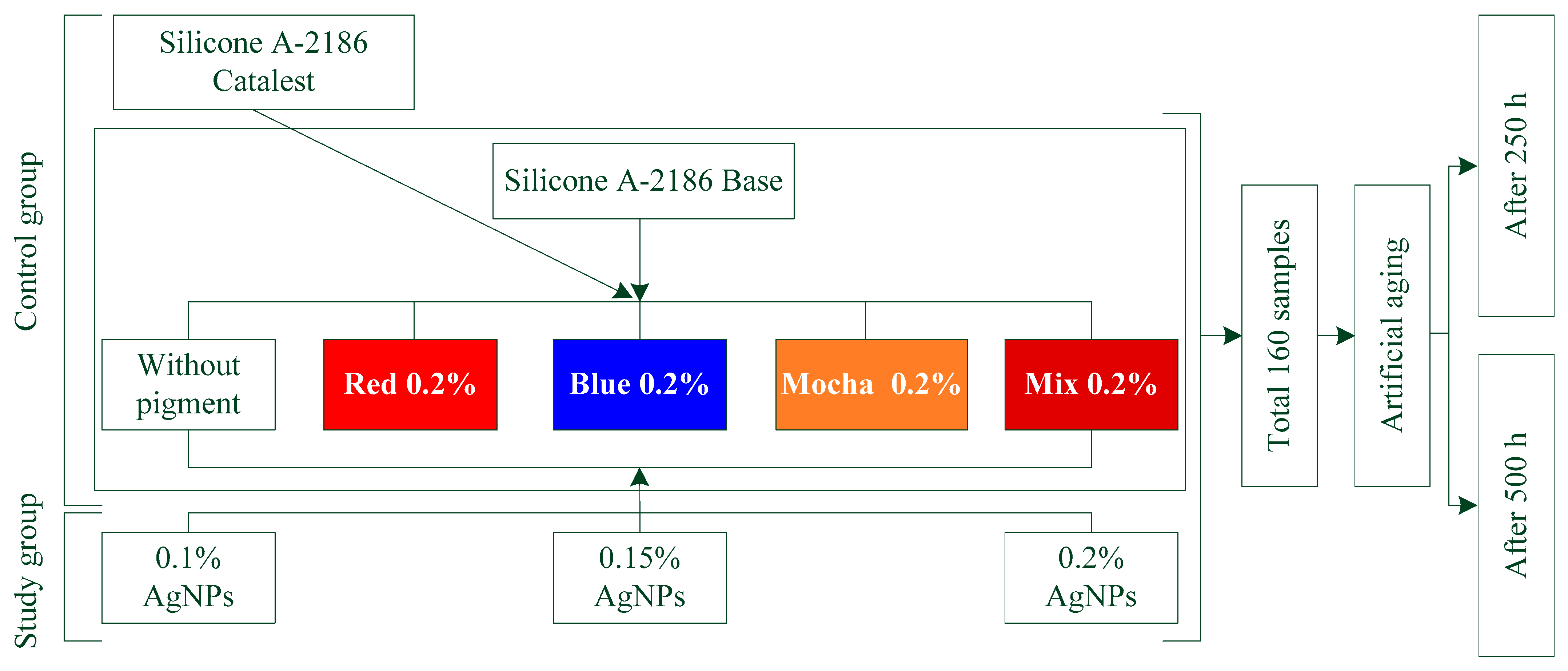
2.2. Experimental Design and Sample Preparation
2.3. Characterization and Statistical Analysis
3. Results
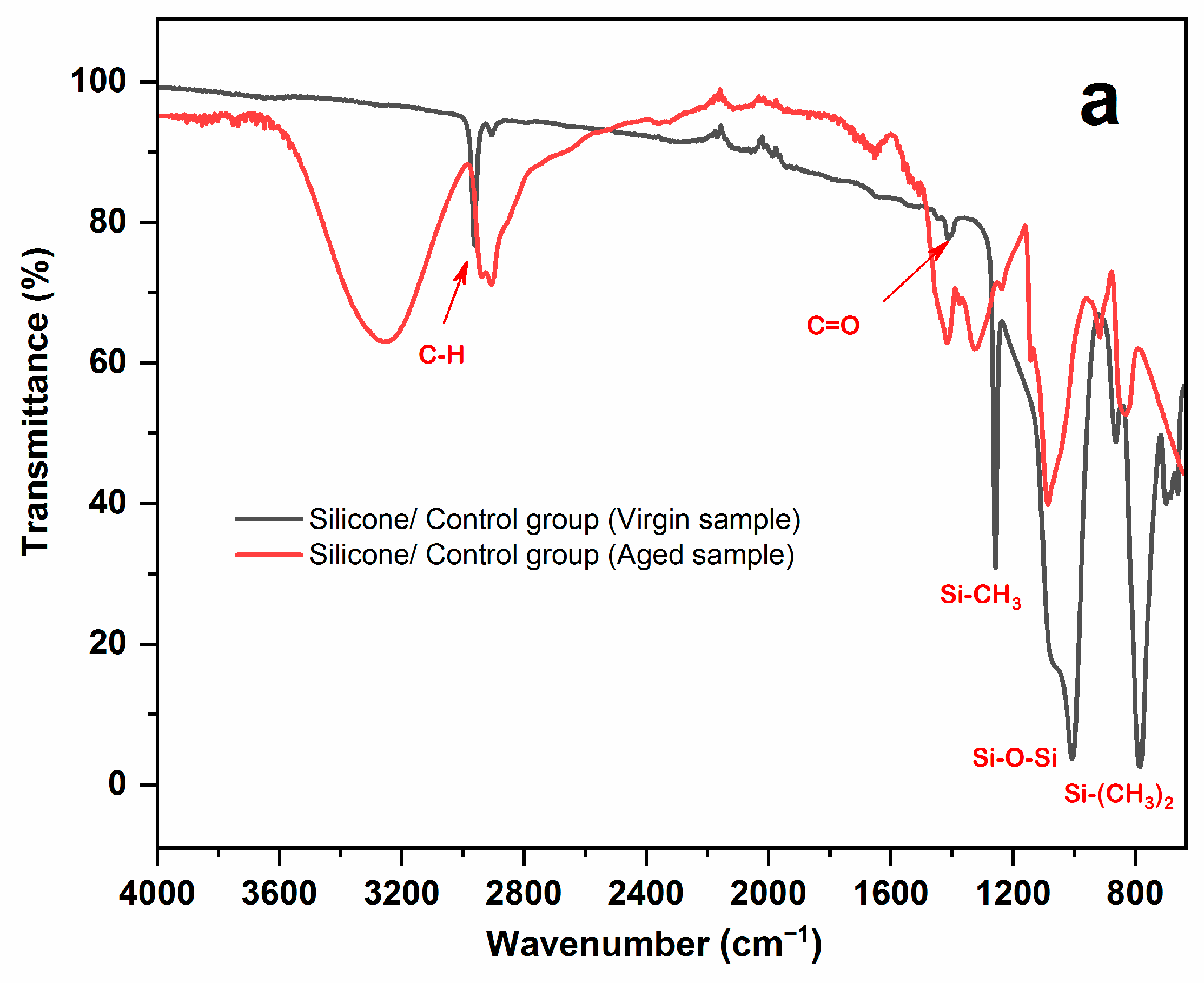
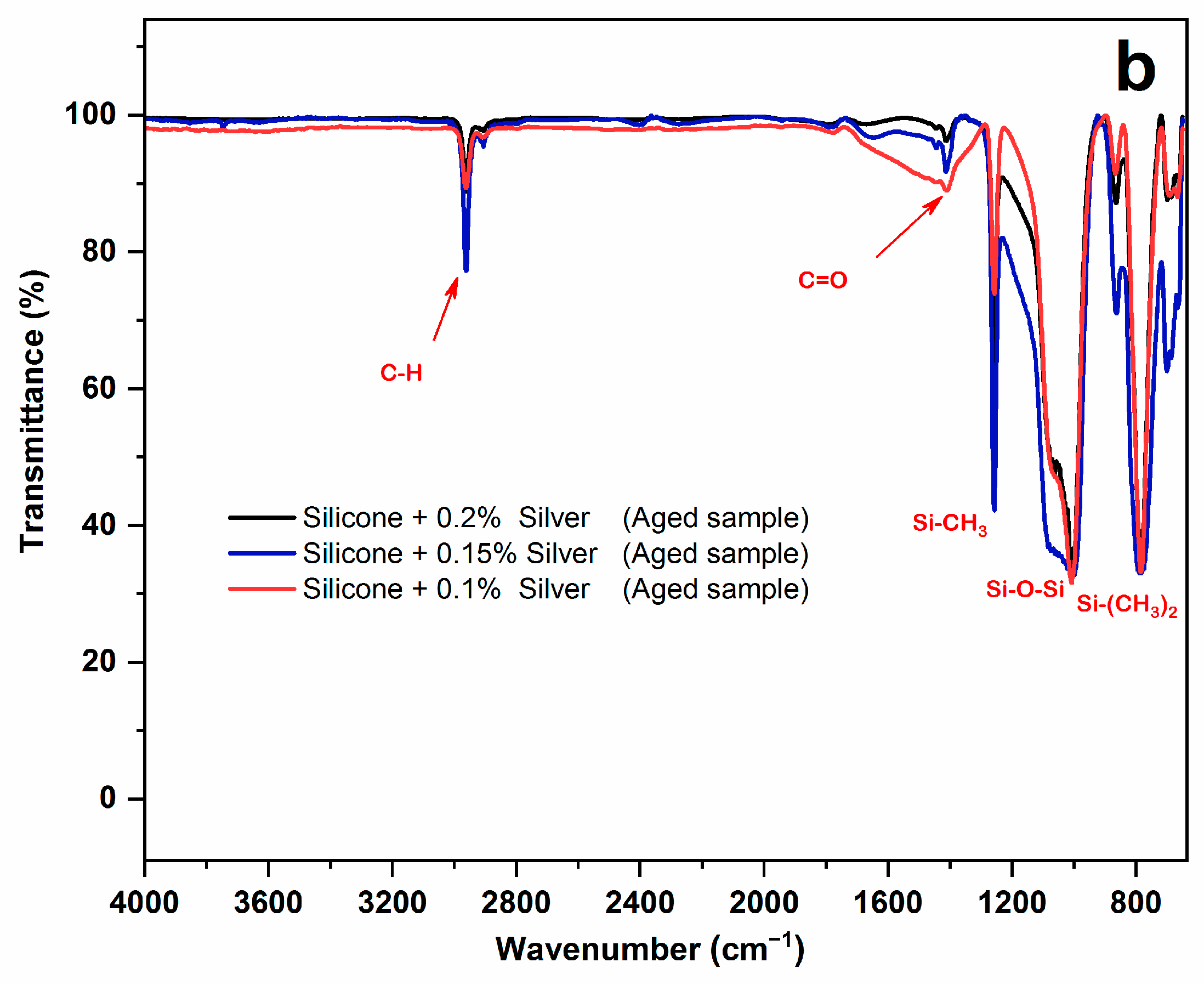
3.1. FTIR-ATR Result
3.2. Color Change Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roberts, A.C. Facial Reconstruction by Prosthetic Means. Br. J. Oral Surg. 1966, 4, 157–182. [Google Scholar] [CrossRef] [PubMed]
- Beatty, M.W.; Mahanna, G.K.; Dick, K.; Jia, W. Color Changes in Dry-Pigmented Maxillofacial Elastomer Resulting from Ultraviolet Light Exposure. J. Prosthet. Dent. 1995, 74, 493–498. [Google Scholar] [CrossRef]
- Kiat-amnuay, S.; Johnston, D.A.; Powers, J.M.; Jacob, R.F. Color Stability of Dry Earth Pigmented Maxillofacial Silicone A-2186 Subjected to Microwave Energy Exposure. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2005, 14, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Pesqueira, A.A.; dos Santos, D.M.; Zavanelli, A.C.; do Prado Ribeiro, P. Color Stability Comparison of Silicone Facial Prostheses Following Disinfection. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2009, 18, 242–244. [Google Scholar] [CrossRef] [PubMed]
- Hecker, D.M. Maxillofacial Rehabilitation of a Large Facial Defect Resulting from an Arteriovenous Malformation Utilizing a Two-Piece Prosthesis. J. Prosthet. Dent. 2003, 89, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Pesqueira, A.A.; Ramos da Silva, C.; Gennari Filho, H.; Micheline Dos Santos, D. Patient Satisfaction with Maxillofacial Prosthesis. Literature Review. J. Plast. Reconstr. Aesthet. Surg. 2009, 62, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, D.N.; Goiato, M.C.; dos Santos, D.M. Color Stability after Accelerated Aging of Two Silicones, Pigmented or Not, for Use in Facial Prostheses. Braz. Oral Res. 2009, 23, 144–148. [Google Scholar] [CrossRef]
- Barnhart, G.W. A New Material and Technic in the Art of Somato-Prosthesis. J. Dent. Res. 1960, 39, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.C.; Fischer, T.E. Evaluation of Sunlight Stability of Polyurethane Elastomers for Maxillofacial Use. I. J. Biomed. Mater. Res. 1978, 12, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, R.A.; Moore, D.J.; Cruz, D.L.; Chappell, R. Comparison of the Physical Properties of Two Types of Polydimethyl Siloxane for Fabrication of Facial Prostheses. J. Prosthet. Dent. 1992, 67, 679–682. [Google Scholar] [CrossRef] [PubMed]
- Polyzois, G.L. Evaluation of a New Silicone Elastomer for Maxillofacial Prostheses. J. Prosthodont. 1995, 4, 38–41. [Google Scholar] [CrossRef] [PubMed]
- Andres, C.J.; Haug, S.P.; Munoz, C.A.; Bernal, G. Effects of Environmental Factors on Maxillofacial Elastomers: Part I-Literature Review. J. Prosthet. Dent. 1992, 68, 327–330. [Google Scholar] [CrossRef] [PubMed]
- Aziz, T.; Waters, M.; Jagger, R. Analysis of the Properties of Silicone Rubber Maxillofacial Prosthetic Materials. J. Dent. 2003, 31, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Polyzois, G.L. Color Stability of Facial Silicone Prosthetic Polymers after Outdoor Weathering. J. Prosthet. Dent. 1999, 82, 447–450. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Polyzois, G.L.; Nuseir, A.; Hatamleh, K.; Alnazzawi, A. Mechanical Properties and Simulated Aging of Silicone Maxillofacial Elastomers: Advancements in the Past 45 Years. J. Prosthodont. 2016, 25, 418–426. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-L.; Garrett, N.; Roumanas, E.; Beumer, J., 3rd. Treatment Satisfaction with Facial Prostheses. J. Prosthet. Dent. 2005, 94, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Ariani, N.; Visser, A.; van Oort, R.P.; Kusdhany, L.; Rahardjo, T.B.W.; Krom, B.P.; van der Mei, H.C.; Vissink, A. Current State of Craniofacial Prosthetic Rehabilitation. Int. J. Prosthodont. 2013, 26, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Nemli, S.K.; Aydin, C.; Yilmaz, H.; Bal, B.T.; Arici, Y.K. Quality of Life of Patients with Implant-Retained Maxillofacial Prostheses: A Prospective and Retrospective Study. J. Prosthet. Dent. 2013, 109, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Brandão, T.B.; Vechiato Filho, A.J.; de Souza Batista, V.E.; Ribeiro, A.C.P.; Nary Filho, H.; Chilvarquer, I.; Nunn, M.E.; Santos-Silva, A.R.; Barão, V.A.R.; Wee, A.G. Assessment of Treatment Outcomes for Facial Prostheses in Patients with Craniofacial Defects: A Pilot Retrospective Study. J. Prosthet. Dent. 2017, 118, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Lemon, J.C.; Chambers, M.S.; Jacobsen, M.L.; Powers, J.M. Color Stability of Facial Prostheses. J. Prosthet. Dent. 1995, 74, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Ishigami, T.; Tanaka, Y.; Kishimoto, Y.; Okadac, M. A Facial Prosthesis Made of Porcelain Fused to Metal: A Clinical Report. J. Prosthet. Dent. 1997, 77, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Al-Harbi, F.A.; Ayad, N.M.; Saber, M.A.; ArRejaie, A.S.; Morgano, S.M. Mechanical Behavior and Color Change of Facial Prosthetic Elastomers after Outdoor Weathering in a Hot and Humid Climate. J. Prosthet. Dent. 2015, 113, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Eleni, P.N.; Krokida, M.K.; Polyzois, G.L.; Gettleman, L. Effect of Different Disinfecting Procedures on the Hardness and Color Stability of Two Maxillofacial Elastomers over Time. J. Appl. Oral Sci. 2013, 21, 278–283. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Watts, D.C. Porosity and Color of Maxillofacial Silicone Elastomer. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2011, 20, 60–66. [Google Scholar] [CrossRef]
- Gary, J.J.; Huget, E.F.; Powell, L.D. Accelerated Color Change in a Maxillofacial Elastomer with and without Pigmentation. J. Prosthet. Dent. 2001, 85, 614–620. [Google Scholar] [CrossRef]
- Guiotti, A.M.; Goiato, M.C. Dimensional Changing and Maintenance of Details Evaluations of a Silicone for Use in Maxillofacial Prosthesis. J. Dent. Res. 2003, 82, 250. [Google Scholar]
- Kiat-Amnuay, S.; Mekayarajjananonth, T.; Powers, J.M.; Chambers, M.S.; Lemon, J.C. Interactions of Pigments and Opacifiers on Color Stability of MDX4-4210/Type A Maxillofacial Elastomers Subjected to Artificial Aging. J. Prosthet. Dent. 2006, 95, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.Ú.R.; Goiato, M.C.; Batista, M.A.J.; Santos, D.M. dos Color Alteration of the Paint Used for Iris Painting in Ocular Prostheses. Braz. Oral Res. 2009, 23, 386–392. [Google Scholar] [CrossRef]
- Kiat-amnuay, S.; Lemon, J.C.; Powers, J.M. Effect of Opacifiers on Color Stability of Pigmented Maxillofacial Silicone A-2186 Subjected to Artificial Aging. J. Prosthodont. 2002, 11, 109–116. [Google Scholar] [PubMed]
- Bangera, B.S.; Guttal, S.S. Evaluation of Varying Concentrations of Nano-Oxides as Ultraviolet Protective Agents When Incorporated in Maxillofacial Silicones: An in Vitro Study. J. Prosthet. Dent. 2014, 112, 1567–1572. [Google Scholar] [CrossRef]
- Paravina, R.D.; Majkic, G.; del Mar Perez, M.; Kiat-amnuay, S. Color Difference Thresholds of Maxillofacial Skin Replications. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2009, 18, 618–625. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D.; Powers, J.M. Esthetic Color Training in Dentistry; Elsevier Mosby: St. Louis, MO, USA, 2004; ISBN 9780323028387. [Google Scholar]
- Han, Y.; Kiat-amnuay, S.; Powers, J.M.; Zhao, Y. Effect of Nano-Oxide Concentration on the Mechanical Properties of a Maxillofacial Silicone Elastomer. J. Prosthet. Dent. 2008, 100, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Akash, R.N.; Guttal, S.S. Effect of Incorporation of Nano-Oxides on Color Stability of Maxillofacial Silicone Elastomer Subjected to Outdoor Weathering. J. Prosthodont. Off. J. Am. Coll. Prosthodont. 2015, 24, 569–575. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.; Ahmad, A.; Mandal, D.; Senapati, S.; Sainkar, S.R.; Khan, M.I.; Parishcha, R.; Ajaykumar, P.V.; Alam, M.; Kumar, R. Fungus-Mediated Synthesis of Silver Nanoparticles and Their Immobilization in the Mycelial Matrix: A Novel Biological Approach to Nanoparticle Synthesis. Nano Lett. 2001, 1, 515–519. [Google Scholar] [CrossRef]
- Li, W.-R.; Xie, X.-B.; Shi, Q.-S.; Zeng, H.-Y.; Ou-Yang, Y.-S.; Chen, Y.-B. Antibacterial Activity and Mechanism of Silver Nanoparticles on Escherichia coli. Appl. Microbiol. Biotechnol. 2010, 85, 1115–1122. [Google Scholar] [CrossRef]
- Gurunathan, S.; Park, J.H.; Han, J.W.; Kim, J.-H. Comparative Assessment of the Apoptotic Potential of Silver Nanoparticles Synthesized by Bacillus Tequilensis and Calocybe Indica in MDA-MB-231 Human Breast Cancer Cells: Targeting P53 for Anticancer Therapy. Int. J. Nanomed. 2015, 10, 4203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Sonnahalli, N.K.; Chowdhary, R. Effect of Nanoparticles on Color Stability and Mechanical and Biological Properties of Maxillofacial Silicone Elastomer: A Systematic Review. J. Indian Prosthodont. Soc. 2020, 20, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Filié Haddad, M.; Coelho Goiato, M.; Micheline Dos Santos, D.; Moreno, A.; Filipe D’almeida, N.; Alves Pesqueira, A. Color Stability of Maxillofacial Silicone with Nanoparticle Pigment and Opacifier Submitted to Disinfection and Artificial Aging. J. Biomed. Opt. 2011, 16, 95004. [Google Scholar] [CrossRef] [PubMed]
- Goiato, M.C.; Haddad, M.F.; Pesqueira, A.A.; Moreno, A.; Dos Santos, D.M.; Bannwart, L.C. Effect of Chemical Disinfection and Accelerated Aging on Color Stability of Maxillofacial Silicone with Opacifiers. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2011, 20, 566–569. [Google Scholar] [CrossRef]
- Han, Y.; Zhao, Y.; Xie, C.; Powers, J.M.; Kiat-amnuay, S. Color Stability of Pigmented Maxillofacial Silicone Elastomer: Effects of Nano-Oxides as Opacifiers. J. Dent. 2010, 38 (Suppl. S2), e100–e105. [Google Scholar] [CrossRef] [PubMed]
- Guiotti, A.M.; Goiato, M.C.; Dos Santos, D.M.; Vechiato-Filho, A.J.; Cunha, B.G.; Paulini, M.B.; Moreno, A.; de Almeida, M.T.G. Comparison of Conventional and Plant-Extract Disinfectant Solutions on the Hardness and Color Stability of a Maxillofacial Elastomer after Artificial Aging. J. Prosthet. Dent. 2016, 115, 501–508. [Google Scholar] [CrossRef]
- Pesqueira, A.A.; Goiato, M.C.; dos Santos, D.M.; Haddad, M.F.; do Prado Ribeiro, P.; Coelho Sinhoreti, M.A.; Sundefeld, M.L.M.M. Effect of Disinfection and Accelerated Aging on Color Stability of Colorless and Pigmented Facial Silicone. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2011, 20, 305–309. [Google Scholar] [CrossRef]
- Abdalqadir, M.; Faraj, S.; Azhdar, B. An Evaluation of a Technique to Improve the Mechanical Properties of Maxillofacial Silicone Elastomers with Zinc Oxide Nanoparticles. J. Prosthet. Dent. 2021, 128, 531–538. [Google Scholar] [CrossRef] [PubMed]
- ASTM G-154-06; Standard Practice for Operating Fluorescent Light Apparatus for UV Exposure of Nonmetallic Materials. ASTM Internation: West Conshohocken, PA, USA, 2016; pp. 646–654.
- Kulkarni, R.S.; Nagda, S.J. Colour Stability of Maxillofacial Silicone Elastomers: A Review of the Literature. Eur. J. Prosthodont. Restor. Dent. 2014, 22, 108–115. [Google Scholar] [PubMed]
- Khashayar, G.; Bain, P.A.; Salari, S.; Dozic, A.; Kleverlaan, C.J.; Feilzer, A.J. Perceptibility and Acceptability Thresholds for Colour Differences in Dentistry. J. Dent. 2014, 42, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Hatamleh, M.M.; Watts, D.C. Effect of Extraoral Aging Conditions on Color Stability of Maxillofacial Silicone Elastomer. J. Prosthodont. Implant. Esthet. Reconstr. Dent. 2010, 19, 536–543. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, D.M.; Goiato, M.C.; Sinhoreti, M.A.C.; Fernandes, A.U.R.; Ribeiro, P.d.P.; Dekon, S.F.d.C. Color Stability of Polymers for Facial Prosthesis. J. Craniofac. Surg. 2010, 21, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.; Pérez Gómez, M.d.M.; Ghinea, R.I. Acceptability and Perceptibility Thresholds in Dentistry: A Comprehensive Review of Clinical and Research Applications. J. Esthet. Restor. Dent. 2019, 31, 103–112. [Google Scholar] [CrossRef]
- Gómez-Polo, C.; María, M.; Gomez-Polo, M.; Montero, J. Colour Thresholds of the Gingival Chromatic Space. J. Dent. 2020, 103, 103502. [Google Scholar] [CrossRef]
- Nada, H.E.; Ahmad, M.A.; Moustafa, N.A. Evaluation of intrinsic color stability of facial silicone elastomer reinforced with different nanoparticles. Alex. Dent. J. 2016, 41, 50–54. [Google Scholar] [CrossRef]
- Li, R.; Yabe, S.; Yamashita, M.; Momose, S.; Yoshida, S.; Yin, S.; Sato, T. UV-Shielding Properties of Zinc Oxide-Doped Ceria Fine Powders Derived via Soft Solution Chemical Routes. Mater. Chem. Phys. 2002, 75, 39–44. [Google Scholar] [CrossRef]
| Materials | Lot No. | Manufacturer |
|---|---|---|
| A-2186 maxillofacial silicon elastomer (part A) | T 72533 | Factor II Inc., Lakeside, AZ, USA |
| A-2186 maxillofacial silicon elastomer (part B) | T 72533 | Factor II Inc., Lakeside, AZ, USA |
| Silver nanoparticles 99.99% purity, 30–50 nm, SSA: ~16–20 m2/g, density: 10.5 g/cm3 spherical, black | CAS: 7440-22-4 | Us Research Nanomaterials, Inc., Houston, TX, USA |
| Dry pigment (brilliant red) P212 | B19D | Technovent Ltd., Bridgend, UK |
| Dry pigment (blue) P216 | B19A | Technovent Ltd., Bridgend, UK |
| Dry pigment (mocha) P213 | B19D | Technovent Ltd., Bridgend, UK |
| Control Groups | A-2186 Silicone (Without Color) | A-2186 + (Red 0.2%) | A-2186 + (Blue 0.2%) | A-2186 + (Mocha 0.2%) | A-2186 + (Mix 0.2%) |
| Study groups | A-2186 + (0.1% Ag NPs) | A-2186 + (0.2% Red) + (0.1% Ag NPs) | A-2186 + (0.2% Blue) + (0.1% Ag NPs) | A-2186 + (0.2% Mocha) + (0.1% Ag NPs) | A-2186 + (0.2% Mix) + (0.1% Ag NPs) |
| A-2186 + (0.15% Ag NPs) | A-2186 + (0.2% Red) + (0.15% Ag NPs) | A-2186 + (0.2% Blue) + (0.15% Ag NPs) | A-2186 + (0.2% Mocha) + (0.15% Ag NPs) | A-2186 + (0.2% Mix) + (0.15% Ag NPs) | |
| A-2186 + (0.2% Ag NPs) | A-2186 + (0.2% Red) + (0.2% Ag NPs) | A-2186 + (0.2% Blue) + (0.2% Ag NPs) | A-2186 + (0.2% Mocha) + (0.2% Ag NPs) | A-2186 + (0.2% Mix) + (0.2% Ag NPs) |
| Pigments | Control | AgNPs (%) | ||
|---|---|---|---|---|
| 0.1 AgNPs | 0.15 AgNPs | 0.2 AgNPs | ||
| Colorless (Silicone only) | 1.98 (0.33) | 1.77 (0.47) | 1.67 (0.58) | 1.47 (0.28) |
| Red | 26.71 (2.24) | 14.03 (0.40) | 13.11 (0.35) | 11.65 (0.70) |
| Blue | 2.03 (0.40) | 1.71 (0.32) | 1.58 (0.39) | 1.12 (0.33) |
| Mocha | 1.41 (0.31) | 1.22 (0.29) | 1.17 (0.31) | 0.97 (0.23) |
| Mix (Red + Blue + Mocha) | 2.36 (0.40) | 2.14 (0.36) | 2.05 (0.19) | 1.92 (0.24) |
| Pigments | Control | AgNPs (%) | ||
|---|---|---|---|---|
| 0.1 AgNPs | 0.15 AgNPs | 0.2 AgNPs | ||
| Colorless (Silicone only) | 2.41 (0.22) | 2.13 (0.60) | 2.03 (0.51) | 1.85 (0.72) |
| Red | 28.32 (0.83) | 15.47 (0.76) | 14.65 (1.32) | 12.88 (1.58) |
| Blue | 3.15 (0.44) | 2.14 (0.55) | 1.93 (0.53) | 1.49 (0.28) |
| Mocha | 2.44 (0.24) | 2.02 (0.41) | 1.74 (0.46) | 1.44 (0.49) |
| Mix (Red + Blue + Mocha) | 2.70 (0.26) | 2.37 (0.58) | 2.16 (0.24) | 1.92 (0.22) |
| Sum of Squares | df | Mean Square | F | Sig. | |
|---|---|---|---|---|---|
| Between Groups | 14418.484 | 40 | 360.462 | 894.406 | 0.000 |
| Within Groups | 115.666 | 287 | 0.403 | ||
| Total | 14534.150 | 327 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohammed, K.; Zardawi, F.; Azhdar, B. Influence of Silver Nanoparticles on Color Stability of Room-Temperature-Vulcanizing Maxillofacial Silicone Subjected to Accelerated Artificial Aging. Appl. Sci. 2023, 13, 11201. https://doi.org/10.3390/app132011201
Mohammed K, Zardawi F, Azhdar B. Influence of Silver Nanoparticles on Color Stability of Room-Temperature-Vulcanizing Maxillofacial Silicone Subjected to Accelerated Artificial Aging. Applied Sciences. 2023; 13(20):11201. https://doi.org/10.3390/app132011201
Chicago/Turabian StyleMohammed, Kaml, Faraedon Zardawi, and Bruska Azhdar. 2023. "Influence of Silver Nanoparticles on Color Stability of Room-Temperature-Vulcanizing Maxillofacial Silicone Subjected to Accelerated Artificial Aging" Applied Sciences 13, no. 20: 11201. https://doi.org/10.3390/app132011201






